A Rapid and Reliable Absorbance Assay to Identify Drug–Drug Interactions with Thiopurine Drugs
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Materials, and Reagents
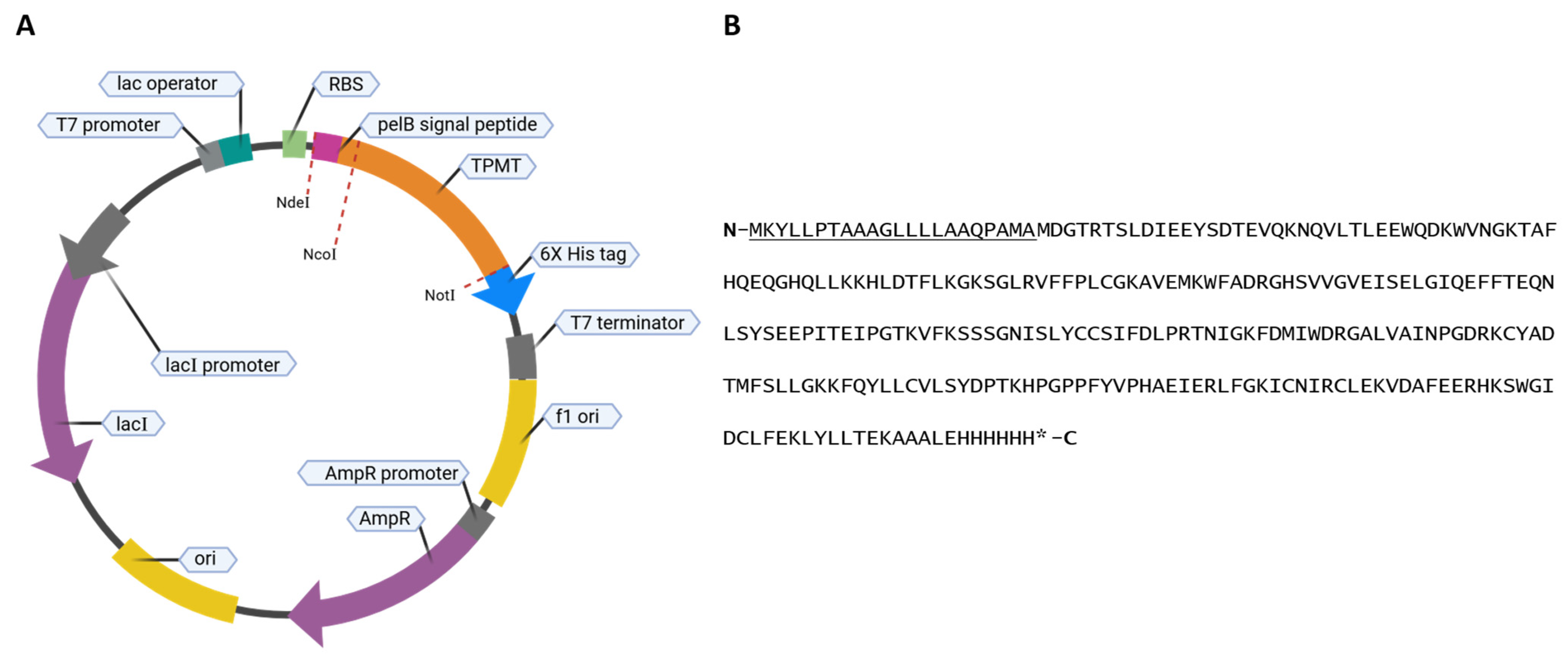
2.2. Expression and Purification of TPMT
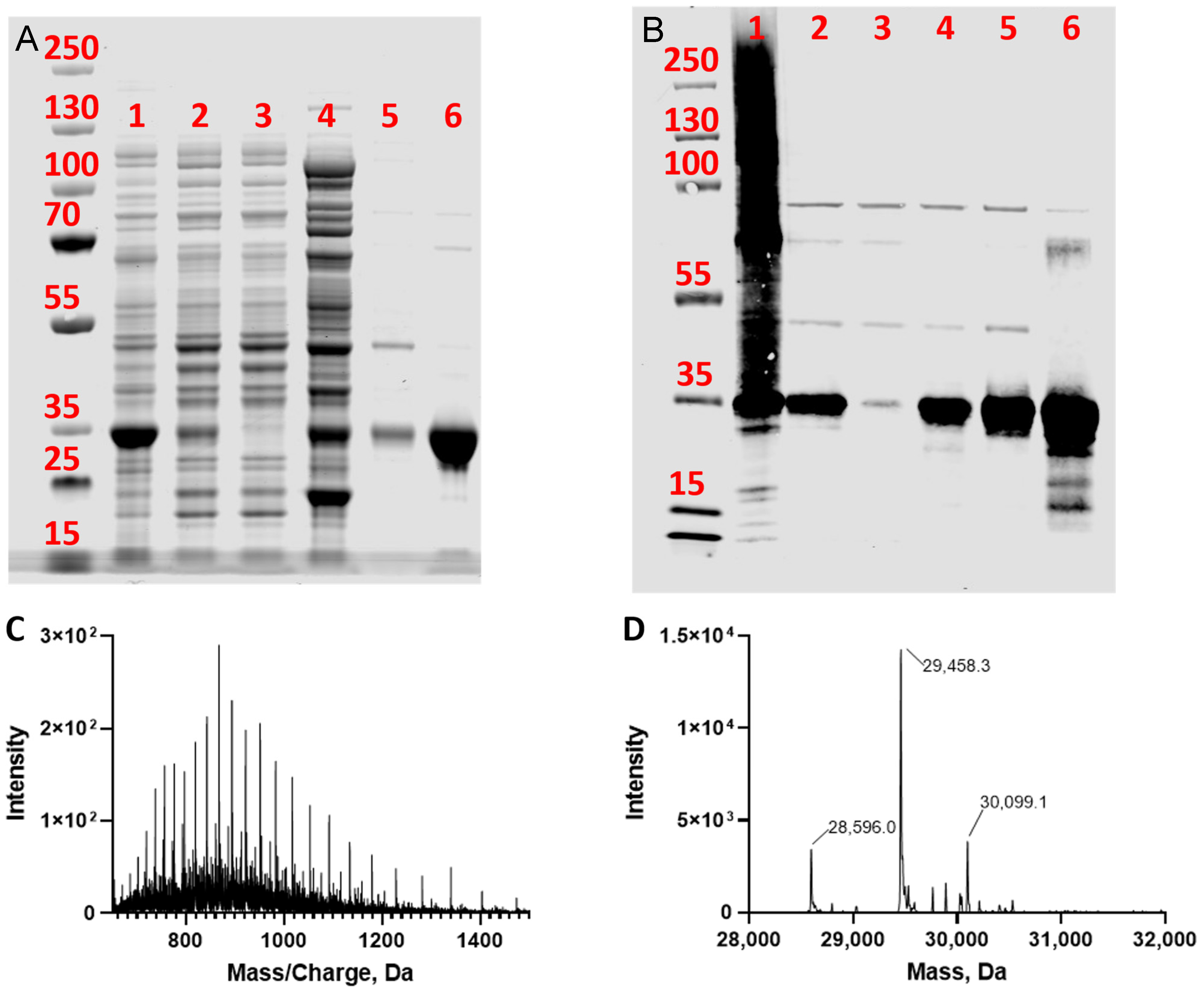
2.3. Purity Analysis of TPMT
2.4. High-Resolution Proteomic Analysis of Intact TPMT
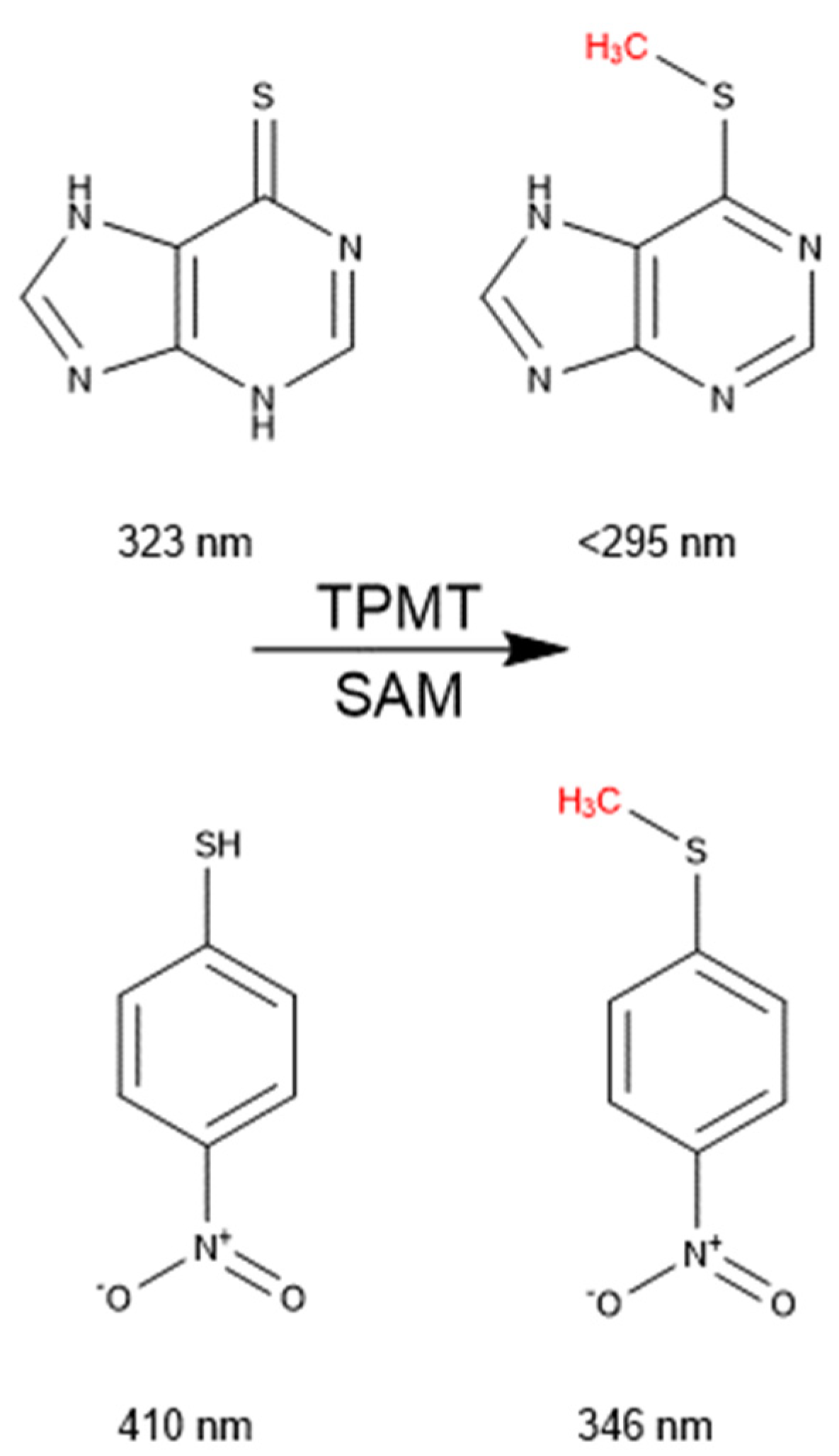
2.5. Absorbance Activity Assay Using Recombinant-Purified TPMT
2.6. Absorbance Inhibition Assay Using 4-NBT as a Probe Substrate
2.7. Mass Spectrometry Activity Assay Validation Using Recombinant-Purified TPMT
2.8. Data Analysis
3. Results
3.1. Expression of TPMT with an N-Terminal PelB Signal Peptide
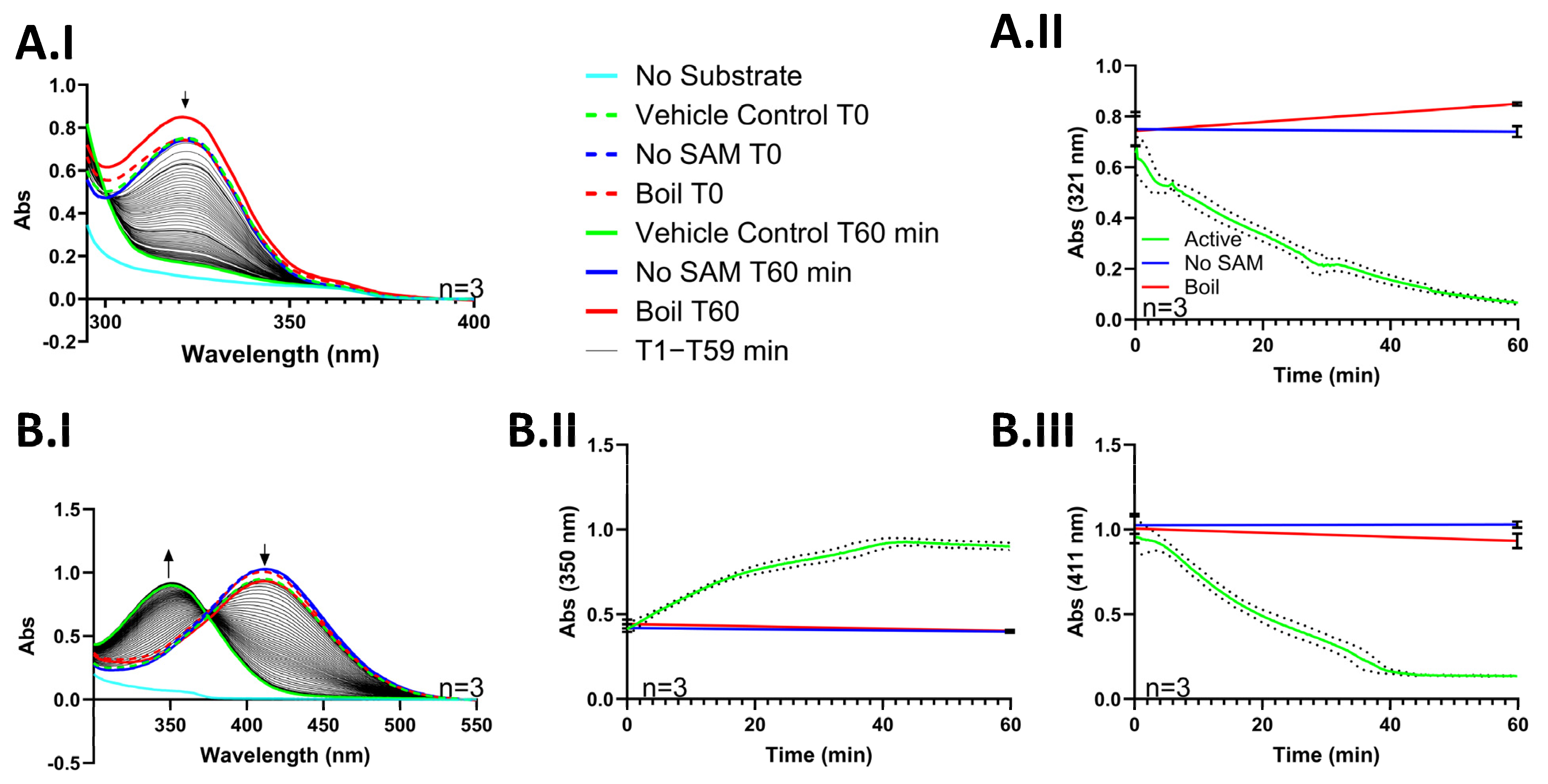
3.2. An Absorbance Assay to Determine the Activity of Purified TPMT
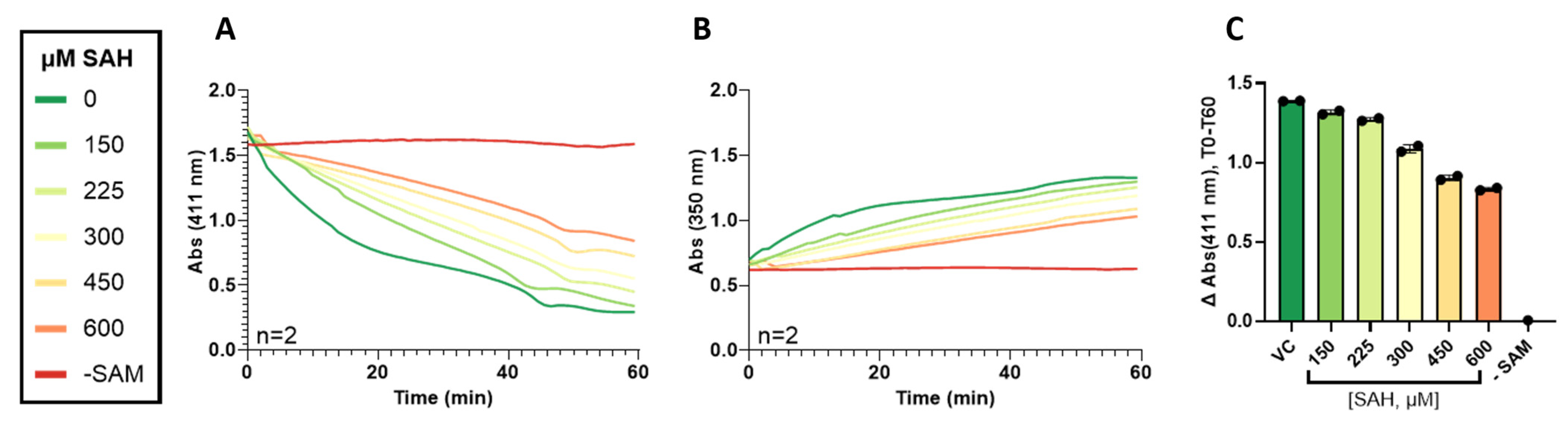
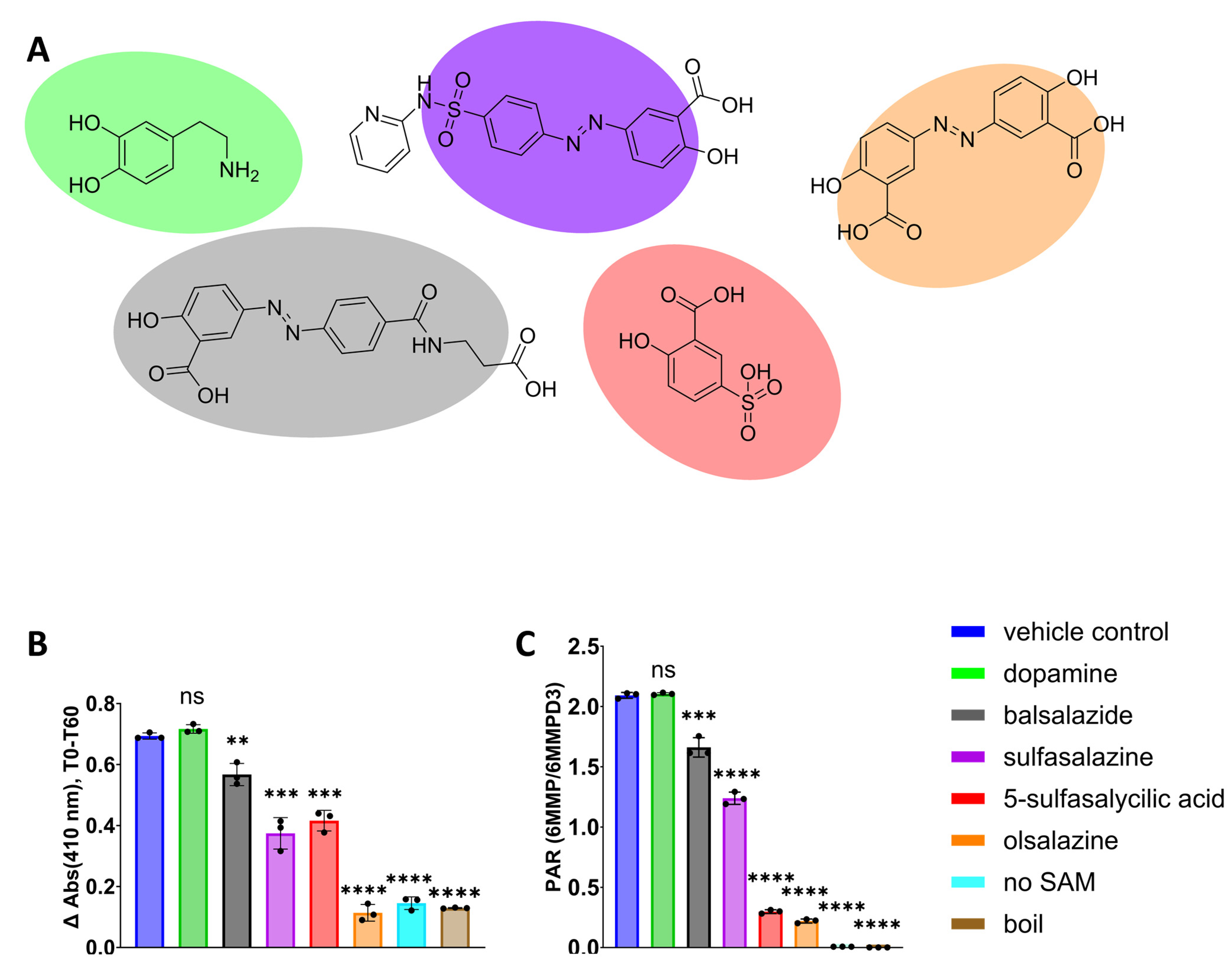
3.3. Validation of 4-NBT Absorbance Assay for TPMT Inhibitor Screening
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Woodson, L.C.; Weinshilboum, R.M. Human kidney thiopurine methyltransferase. Purification and biochemical properties. Biochem. Pharmacol. 1983, 32, 819–826. [Google Scholar] [CrossRef]
- Ames, M.M.; Selassie, C.D.; Woodson, L.C.; Van Loon, J.A.; Hansch, C.; Weinshilboum, R.M. Thiopurine methyltransferase: Structure-activity relationships for benzoic acid inhibitors and thiophenol substrates. J. Med. Chem. 1986, 29, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Deininger, M.; Szumlanski, C.L.; Otterness, D.M.; Van Loon, J.; Ferber, W.; Weinshilboum, R.M. Purine substrates for human thiopurine methyltransferase. Biochem. Pharmacol. 1994, 48, 2135–2138. [Google Scholar] [CrossRef] [PubMed]
- Sahasranaman, S.; Howard, D.; Roy, S. Clinical pharmacology and pharmacogenetics of thiopurines. Eur. J. Clin. Pharmacol. 2008, 64, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Swann, P.F.; Waters, T.R.; Moulton, D.C.; Xu, Y.Z.; Zheng, Q.; Edwards, M.; Mace, R. Role of postreplicative DNA mismatch repair in the cytotoxic action of thioguanine. Science 1996, 273, 1109–1111. [Google Scholar] [CrossRef] [PubMed]
- Inamochi, H.; Higashigawa, M.; Shimono, Y.; Nagata, T.; Cao, D.C.; Mao, X.Y.; M’Soka, T.; Hori, H.; Kawasaki, H.; Sakurai, M. Delayed cytotoxicity of 6-mercaptopurine is compatible with mitotic death caused by DNA damage due to incorporation of 6-thioguanine into DNA as 6-thioguanine nucleotide. J. Exp. Clin. Cancer Res. 1999, 18, 417–424. [Google Scholar]
- Lennard, L.; Lilleyman, J.S. Variable mercaptopurine metabolism and treatment outcome in childhood lymphoblastic leukemia. J. Clin. Oncol. 1989, 7, 1816–1823. [Google Scholar] [CrossRef]
- Dubinsky, M.C.; Lamothe, S.; Yang, H.Y.; Targan, S.R.; Sinnett, D.; Théorêt, Y.; Seidman, E.G. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology 2000, 118, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Formea, C.M.; Myers-Huentelman, H.; Wu, R.; Crabtree, J.; Fujita, S.; Hemming, A.; Reed, A.; Howard, R.; Karlix, J.L. Thiopurine S-methyltransferase genotype predicts azathioprine-induced myelotoxicity in kidney transplant recipients. Am. J. Transplant. 2004, 4, 1810–1817. [Google Scholar] [CrossRef]
- Hao, Q.S.; Wang, Z.; Fang, Q.Y.; Gong, X.Y.; Liu, K.Q.; Li, Y.; Wei, H.; Wang, Y.; Li, Q.H.; Wang, M.; et al. Effect of genetic polymorphism of TPMT and NUDT15 on the tolerance of 6-mercaptopurine therapy in adult acute lymphoblastic leukemia. Chin. J. Hematol. 2021, 42, 911–916. [Google Scholar] [CrossRef]
- Evans, W.E.; Horner, M.; Chu, Y.Q.; Kalwinsky, D.; Roberts, W.M. Altered mercaptopurine metabolism, toxic effects, and dosage requirement in a thiopurine methyltransferase-deficient child with acute lymphocytic leukemia. J. Pediatr. 1991, 119, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Ogungbenro, K.; Aarons, L. Physiologically based pharmacokinetic model for 6-mercpatopurine: Exploring the role of genetic polymorphism in TPMT enzyme activity. Br. J. Clin. Pharmacol. 2015, 80, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Weinshilboum, R.M.; Sladek, S.L. Mercaptopurine pharmacogenetics: Monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am. J. Hum. Genet. 1980, 32, 651–662. [Google Scholar]
- Appell, M.L.; Berg, J.; Duley, J.; Evans, W.E.; Kennedy, M.A.; Lennard, L.; Marinaki, T.; McLeod, H.L.; Relling, M.V.; Schaeffeler, E.; et al. Nomenclature for alleles of the thiopurine methyltransferase gene. Pharmacogenet Genom. 2013, 23, 242–248. [Google Scholar] [CrossRef]
- Relling, M.V.; Schwab, M.; Whirl-Carrillo, M.; Suarez-Kurtz, G.; Pui, C.H.; Stein, C.M.; Moyer, A.M.; Evans, W.E.; Klein, T.E.; Antillon-Klussmann, F.G.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for Thiopurine Dosing Based on TPMT and NUDT15 Genotypes: 2018 Update. Clin. Pharmacol. Ther. 2019, 105, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- FDA. Table of Pharmacogenetic Associations. Available online: https://www.fda.gov/medical-devices/precision-medicine/table-pharmacogenetic-associations (accessed on 28 October 2024).
- Woodson, L.C.; Ames, M.M.; Selassie, C.D.; Hansch, C.; Weinshilboum, R.M. Thiopurine methyltransferase. Aromatic thiol substrates and inhibition by benzoic acid derivatives. Mol. Pharmacol. 1983, 24, 471–478. [Google Scholar]
- Present, D.H.; Korelitz, B.I.; Wisch, N.; Glass, J.L.; Sachar, D.B.; Pasternack, B.S. Treatment of Crohn’s disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N. Engl. J. Med. 1980, 302, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.D.; Benin, A.; Szumlanski, C.L.; Otterness, D.M.; Lennard, L.; Weinshilboum, R.M.; Nierenberg, D.W. Olsalazine and 6-mercaptopurine-related bone marrow suppression: A possible drug-drug interaction. Clin. Pharmacol. Ther. 1997, 62, 464–475. [Google Scholar] [CrossRef]
- Morikubo, H.; Kobayashi, T.; Ozaki, R.; Okabayashi, S.; Kuronuma, S.; Takeuchi, O.; Shiba, T.; Kiyohara, H.; Matsubayashi, M.; Sagami, S.; et al. Differential effects of mesalazine formulations on thiopurine metabolism through thiopurine S-methyltransferase inhibition. J. Gastroenterol. Hepatol. 2021, 36, 2116–2124. [Google Scholar] [CrossRef]
- Karyolaimos, A.; de Gier, J.W. Strategies to Enhance Periplasmic Recombinant Protein Production Yields in Escherichia coli. Front. Bioeng. Biotechnol. 2021, 9, 797334. [Google Scholar] [CrossRef]
- Expasy. Expasy Compute pI/Mw. Available online: https://web.expasy.org/compute_pi/ (accessed on 5 August 2024).
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar] [CrossRef] [PubMed]
- Bjellqvist, B.; Hughes, G.J.; Pasquali, C.; Paquet, N.; Ravier, F.; Sanchez, J.C.; Frutiger, S.; Hochstrasser, D. The focusing positions of polypeptides in immobilized pH gradients can be predicted from their amino acid sequences. Electrophoresis 1993, 14, 1023–1031. [Google Scholar] [CrossRef]
- Bjellqvist, B.; Basse, B.; Olsen, E.; Celis, J.E. Reference points for comparisons of two-dimensional maps of proteins from different human cell types defined in a pH scale where isoelectric points correlate with polypeptide compositions. Electrophoresis 1994, 15, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Sockolosky, J.T.; Szoka, F.C. Periplasmic production via the pET expression system of soluble, bioactive human growth hormone. Protein Expr. Purif. 2013, 87, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Bi, N.; Hu, M.; Xu, J.; Jia, L. Colorimetric determination of mercury(II) based on the inhibition of the aggregation of gold nanorods coated with 6-mercaptopurine. Microchim. Acta 2017, 184, 3961–3967. [Google Scholar] [CrossRef]
- Sudharsanam, R.; Chandrasekaran, S.; Das, P.K. Unsymmetrical aryl disulfides with excellent transparency in the visible region for second order nonlinear optics. J. Mater. Chem. 2002, 12, 2904–2908. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Huang, X.; Mao, S.Z.; Liang, K.; Liu, J.Q.; Luo, G.M.; Shen, J.C. Cyclodextrin-derived mimic of glutathione peroxidase exhibiting enzymatic specificity and high catalytic efficiency. Chemistry 2006, 12, 3575–3579. [Google Scholar] [CrossRef] [PubMed]
- Lowry, P.W.; Szumlanski, C.L.; Weinshilboum, R.M.; Sandborn, W.J. Balsalazide and azathiprine or 6-mercaptopurine: Evidence for a potentially serious drug interaction. Gastroenterology 1999, 116, 1505–1506. [Google Scholar] [CrossRef]
- Szumlanski, C.L.; Weinshilboum, R.M. Sulphasalazine inhibition of thiopurine methyltransferase: Possible mechanism for interaction with 6-mercaptopurine and azathioprine. Br. J. Clin. Pharmacol. 1995, 39, 456–459. [Google Scholar] [CrossRef]
- Michaelis, L.; Menten, M.L.; Johnson, K.A.; Goody, R.S. The original Michaelis constant: Translation of the 1913 Michaelis-Menten paper. Biochemistry 2011, 50, 8264–8269. [Google Scholar] [CrossRef] [PubMed]
- Cannon, L.M.; Butler, F.N.; Wan, W.; Zhou, Z.S. A stereospecific colorimetric assay for (S,S)-adenosylmethionine quantification based on thiopurine methyltransferase-catalyzed thiol methylation. Anal. Biochem. 2002, 308, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Lin, W.; Wang, C.; Dong, J.; Zheng, W.; Zeng, D.; Liu, Y.; Lin, C.; Jiao, Z.; Huang, P. Population pharmacokinetics of azathioprine active metabolite in patients with inflammatory bowel disease and dosage regimens optimisation. Basic. Clin. Pharmacol. Toxicol. 2021, 128, 482–492. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russell, D.A.; Stafford, C.; Totah, R.A. A Rapid and Reliable Absorbance Assay to Identify Drug–Drug Interactions with Thiopurine Drugs. Metabolites 2024, 14, 715. https://doi.org/10.3390/metabo14120715
Russell DA, Stafford C, Totah RA. A Rapid and Reliable Absorbance Assay to Identify Drug–Drug Interactions with Thiopurine Drugs. Metabolites. 2024; 14(12):715. https://doi.org/10.3390/metabo14120715
Chicago/Turabian StyleRussell, Drake A., Carson Stafford, and Rheem A. Totah. 2024. "A Rapid and Reliable Absorbance Assay to Identify Drug–Drug Interactions with Thiopurine Drugs" Metabolites 14, no. 12: 715. https://doi.org/10.3390/metabo14120715
APA StyleRussell, D. A., Stafford, C., & Totah, R. A. (2024). A Rapid and Reliable Absorbance Assay to Identify Drug–Drug Interactions with Thiopurine Drugs. Metabolites, 14(12), 715. https://doi.org/10.3390/metabo14120715







