Transcriptomic and Metabolomic Analysis Reveals Multifaceted Impact of Gcn5 Knockdown in Drosophila Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Drosophila Husbandry
2.2. Collection of 96 h Larvae
2.3. Transcriptome and Metabolome Data Analysis
2.4. Semi-Intact Drosophila Heart Preparation and Cardiac Function Analysis
2.5. Behavioral Trajectory Tracking Analysis
2.6. Lifespan Assay
2.7. Western Blot
2.8. Data Analysis and Visualization
3. Results
3.1. Gcn5 Is Essential for Drosophila Metamorphosis
3.2. Transcriptome Analysis
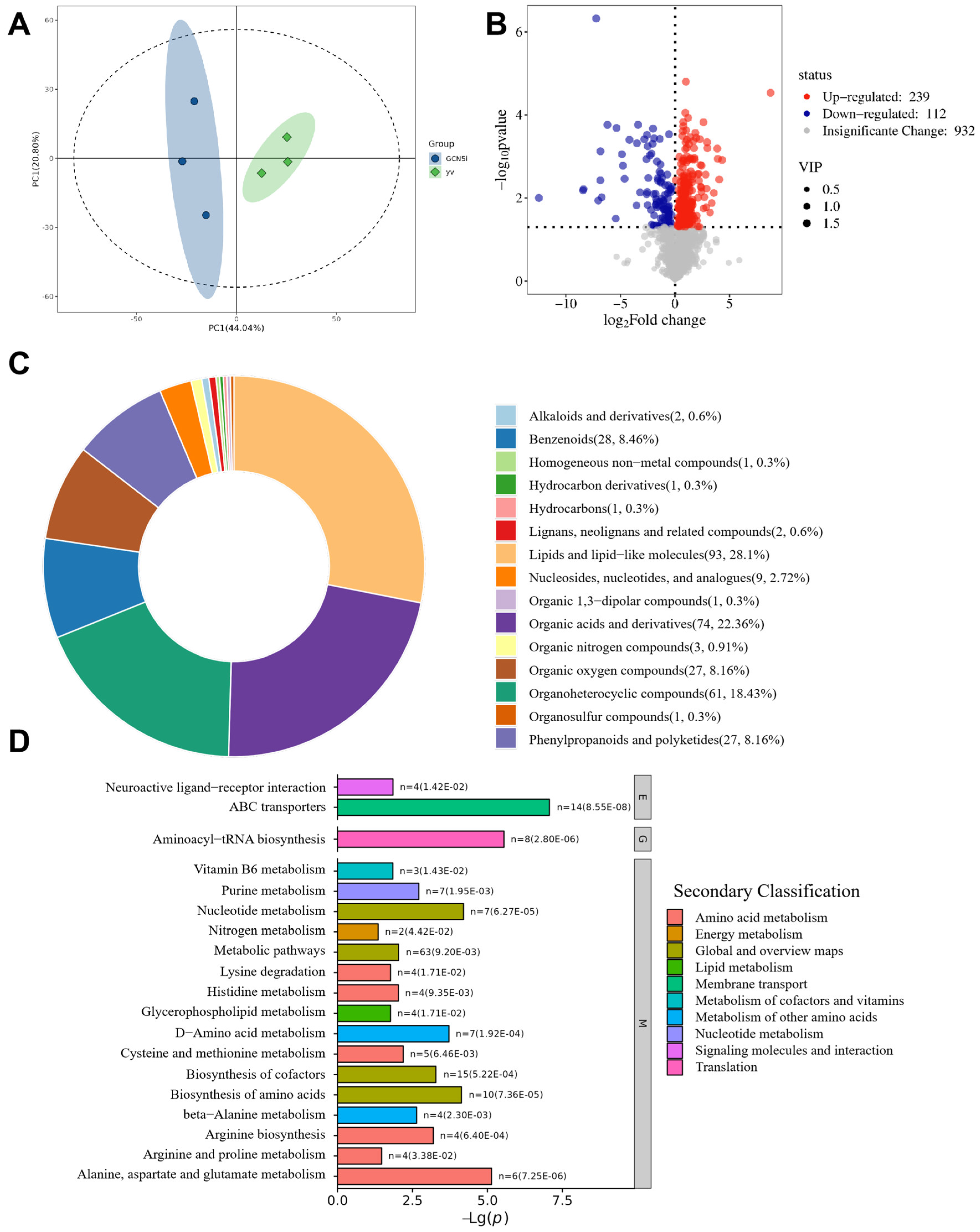
3.3. Metabolome Analysis
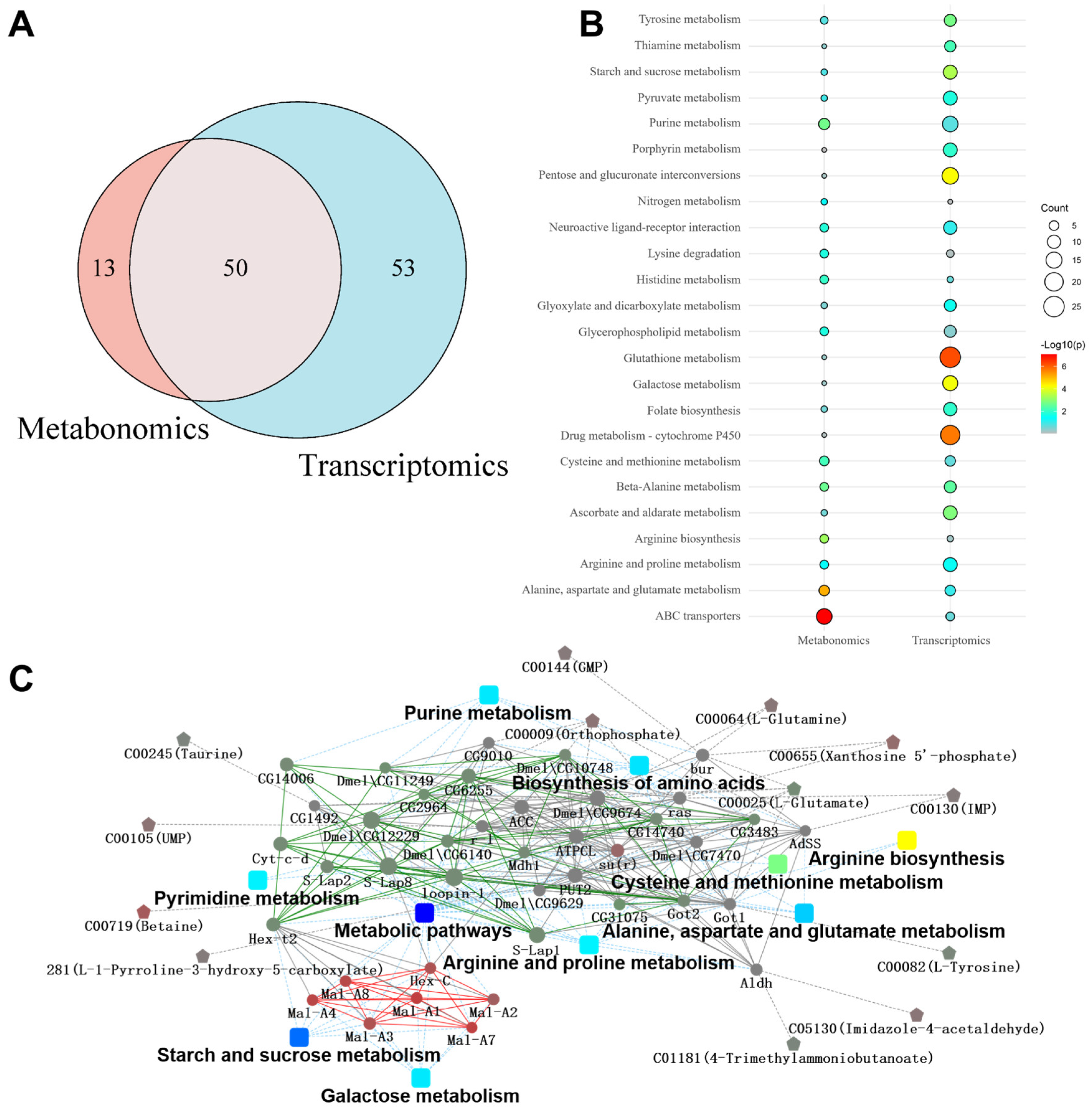
3.4. Correlation Analysis of Transcriptome and Metabolome
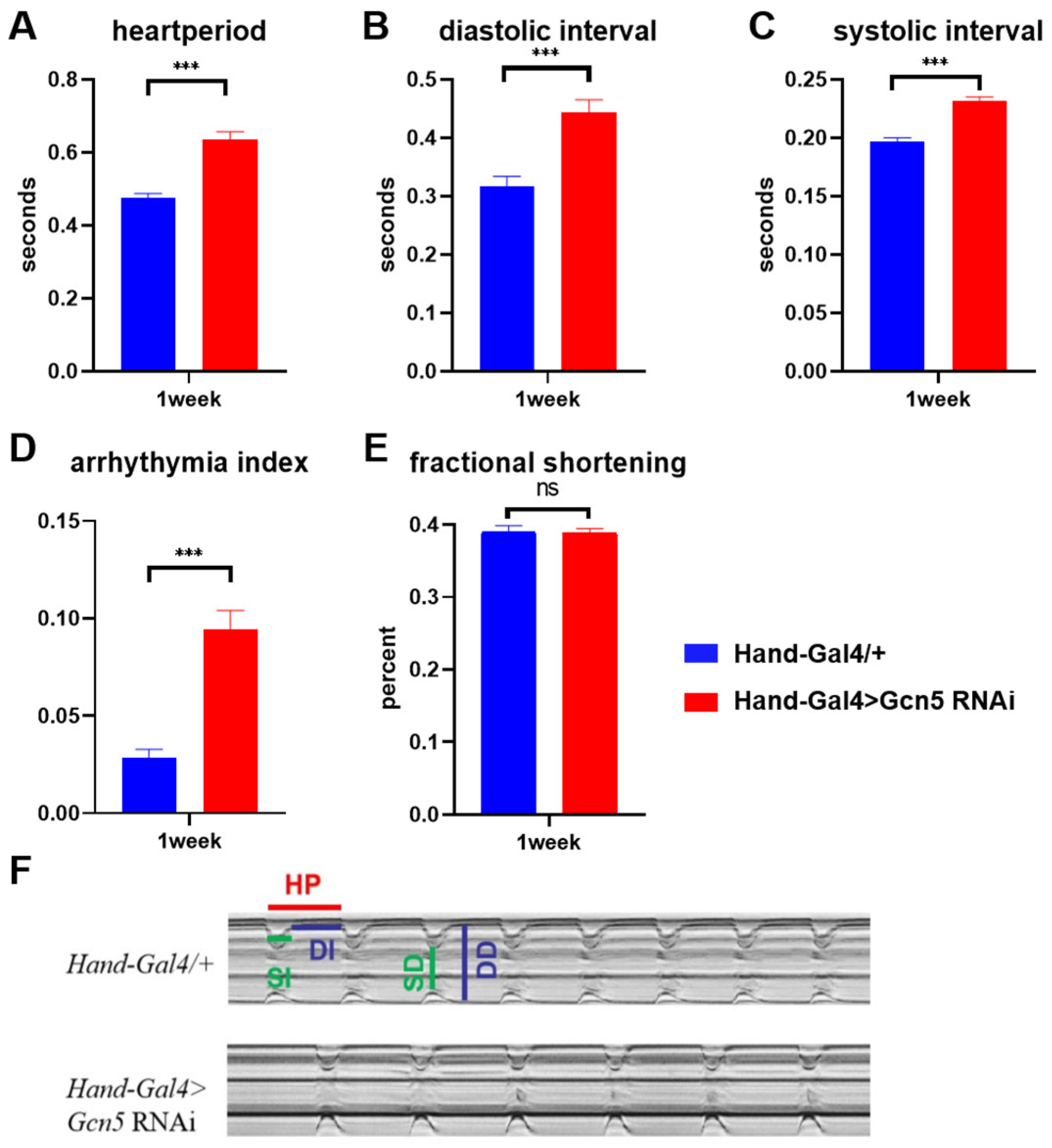
3.5. Gcn5 Is Required to Maintain Normal Heart Physiology
3.6. Heart-Specific Gcn5 Knockdown Makes Aged Flies More Active and Live Longer
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brownell, J.E.; Zhou, J.; Ranalli, T.; Kobayashi, R.; Edmondson, D.G.; Roth, S.Y.; Allis, C.D. Tetrahymena histone acetyltransferase A: A homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell 1996, 84, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Belote, J.M.; Schiltz, R.L.; Yang, X.J.; Moore, P.A.; Berger, S.L.; Nakatani, Y.; Allis, C.D. Cloning of Drosophila GCN5: Conserved features among metazoan GCN5 family members. Nucleic Acids Res. 1998, 26, 2948–2954. [Google Scholar] [CrossRef] [PubMed]
- Allis, C.D.; Berger, S.L.; Cote, J.; Dent, S.; Jenuwien, T.; Kouzarides, T.; Pillus, L.; Reinberg, D.; Shi, Y.; Shiekhattar, R.; et al. New nomenclature for chromatin-modifying enzymes. Cell 2007, 131, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Marmorstein, R.; Roth, S.Y. Histone acetyltransferases: Function, structure, and catalysis. Curr. Opin. Genet. Dev. 2001, 11, 155–161. [Google Scholar] [CrossRef]
- Dhalluin, C.; Carlson, J.E.; Zeng, L.; He, C.; Aggarwal, A.K.; Zhou, M.M. Structure and ligand of a histone acetyltransferase bromodomain. Nature 1999, 399, 491–496. [Google Scholar] [CrossRef]
- Hudson, B.P.; Martinez-Yamout, M.A.; Dyson, H.J.; Wright, P.E. Solution structure and acetyl-lysine binding activity of the GCN5 bromodomain. J. Mol. Biol. 2000, 304, 355–370. [Google Scholar] [CrossRef]
- Josling, G.A.; Selvarajah, S.A.; Petter, M.; Duffy, M.F. The role of bromodomain proteins in regulating gene expression. Genes 2012, 3, 320–343. [Google Scholar] [CrossRef]
- Downey, M.; Johnson, J.R.; Davey, N.E.; Newton, B.W.; Johnson, T.L.; Galaang, S.; Seller, C.A.; Krogan, N.; Toczyski, D.P. Acetylome profiling reveals overlap in the regulation of diverse processes by sirtuins, gcn5, and esa1. Mol. Cell. Proteom. MCP 2015, 14, 162–176. [Google Scholar] [CrossRef]
- Li, J.; Cao, Y.; Yang, Y.; Ma, H.; Zhao, J.; Zhang, Y.; Liu, N. Quantitative Acetylomics Reveals Substrates of Lysine Acetyltransferase GCN5 in Adult and Aging Drosophila. J. Proteome Res. 2023, 22, 2909–2924. [Google Scholar] [CrossRef]
- Haque, M.E.; Jakaria, M.; Akther, M.; Cho, D.Y.; Kim, I.S.; Choi, D.K. The GCN5: Its biological functions and therapeutic potentials. Clin. Sci. 2021, 135, 231–257. [Google Scholar] [CrossRef]
- Xiao, H.T.; Jin, J.; Zheng, Z.G. Emerging role of GCN5 in human diseases and its therapeutic potential. Biomed. Pharmacother. 2023, 165, 114835. [Google Scholar] [CrossRef]
- Mutlu, B.; Puigserver, P. GCN5 acetyltransferase in cellular energetic and metabolic processes. Biochim. Biophys. Acta Gene Regul. Mech. 2021, 1864, 194626. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Zhong, D.; Zhu, J.; An, Y.; Gao, M.; Zhu, S.; Dang, W.; Wang, X.; Yang, B.; Xie, Z. Inhibition of histone acetyltransferase GCN5 extends lifespan in both yeast and human cell lines. Aging Cell 2020, 19, e13129. [Google Scholar] [CrossRef] [PubMed]
- Carradori, S.; Secci, D.; Mai, A. Epigenetic modulation of PGC-1α activity by GCN5 inhibitors: WO2010007085. Expert Opin. Ther. Pat. 2011, 21, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Wang, J.; Lu, W.; Zhang, R.; Xie, Y.; Liu, Y.C.; Liu, R.; Yue, L.; Chen, K.; Jiang, H.; et al. Discovery of trisubstituted nicotinonitrile derivatives as novel human GCN5 inhibitors through AlphaScreen-based high throughput screening. RSC Adv. 2019, 9, 4917–4924. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Han, J.; Wang, J.; Lu, W.; Wang, C.; Chen, Y.; Fulin, L.; Zhang, N.; Liu, Y.C.; Zhang, C.; et al. Discovery of 1,8-acridinedione derivatives as novel GCN5 inhibitors via high throughput screening. Eur. J. Med. Chem. 2018, 151, 740–751. [Google Scholar] [CrossRef]
- Bassi, Z.I.; Fillmore, M.C.; Miah, A.H.; Chapman, T.D.; Maller, C.; Roberts, E.J.; Davis, L.C.; Lewis, D.E.; Galwey, N.W.; Waddington, K.E.; et al. Modulating PCAF/GCN5 Immune Cell Function through a PROTAC Approach. ACS Chem. Biol. 2018, 13, 2862–2867. [Google Scholar] [CrossRef]
- Carré, C.; Szymczak, D.; Pidoux, J.; Antoniewski, C. The histone H3 acetylase dGcn5 is a key player in Drosophila melanogaster metamorphosis. Mol. Cell. Biol. 2005, 25, 8228–8238. [Google Scholar] [CrossRef]
- Ciabrelli, F.; Rabbani, L.; Cardamone, F.; Zenk, F.; Löser, E.; Schächtle, M.A.; Mazina, M.; Loubiere, V.; Iovino, N. CBP and Gcn5 drive zygotic genome activation independently of their catalytic activity. Sci. Adv. 2023, 9, eadf2687. [Google Scholar] [CrossRef]
- Papait, R.; Greco, C.; Kunderfranco, P.; Latronico, M.V.; Condorelli, G. Epigenetics: A new mechanism of regulation of heart failure? Basic Res. Cardiol. 2013, 108, 361. [Google Scholar] [CrossRef]
- Volani, C.; Pagliaro, A.; Rainer, J.; Paglia, G.; Porro, B.; Stadiotti, I.; Foco, L.; Cogliati, E.; Paolin, A.; Lagrasta, C.; et al. GCN5 contributes to intracellular lipid accumulation in human primary cardiac stromal cells from patients affected by Arrhythmogenic cardiomyopathy. J. Cell. Mol. Med. 2022, 26, 3687–3701. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.; Patat, J.; Guida, M.C.; Lachaussée, N.; Arrondel, C.; Helmstädter, M.; Boyer, O.; Gribouval, O.; Gubler, M.C.; Mollet, G.; et al. Correction: A homozygous KAT2B variant modulates the clinical phenotype of ADD3 deficiency in humans and flies. PLoS Genet. 2018, 14, e1007748. [Google Scholar] [CrossRef] [PubMed]
- Vogler, G.; Ocorr, K. Visualizing the beating heart in Drosophila. J. Vis. Exp. JoVE 2009, 31, 1425. [Google Scholar]
- Souidi, A.; Jagla, K. Drosophila Heart as a Model for Cardiac Development and Diseases. Cells 2021, 10, 3078. [Google Scholar] [CrossRef] [PubMed]
- Barlev, N.A.; Liu, L.; Chehab, N.H.; Mansfield, K.; Harris, K.G.; Halazonetis, T.D.; Berger, S.L. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol. Cell 2001, 8, 1243–1254. [Google Scholar] [CrossRef]
- Patel, J.H.; Du, Y.; Ard, P.G.; Phillips, C.; Carella, B.; Chen, C.J.; Rakowski, C.; Chatterjee, C.; Lieberman, P.M.; Lane, W.S.; et al. The c-MYC oncoprotein is a substrate of the acetyltransferases hGCN5/PCAF and TIP60. Mol. Cell. Biol. 2004, 24, 10826–10834. [Google Scholar] [CrossRef]
- Bradshaw, P.C. Acetyl-CoA Metabolism and Histone Acetylation in the Regulation of Aging and Lifespan. Antioxidants 2021, 10, 572. [Google Scholar] [CrossRef]
- Lerin, C.; Rodgers, J.T.; Kalume, D.E.; Kim, S.H.; Pandey, A.; Puigserver, P. GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1alpha. Cell Metab. 2006, 3, 429–438. [Google Scholar] [CrossRef]
- Sakai, M.; Tujimura-Hayakawa, T.; Yagi, T.; Yano, H.; Mitsushima, M.; Unoki-Kubota, H.; Kaburagi, Y.; Inoue, H.; Kido, Y.; Kasuga, M.; et al. The GCN5-CITED2-PKA signalling module controls hepatic glucose metabolism through a cAMP-induced substrate switch. Nat. Commun. 2016, 7, 13147. [Google Scholar] [CrossRef]
- Coste, A.; Louet, J.F.; Lagouge, M.; Lerin, C.; Antal, M.C.; Meziane, H.; Schoonjans, K.; Puigserver, P.; O’Malley, B.W.; Auwerx, J. The genetic ablation of SRC-3 protects against obesity and improves insulin sensitivity by reducing the acetylation of PGC-1α. Proc. Natl. Acad. Sci. USA 2008, 105, 17187–17192. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Liu, J.; Zhang, J.; Xu, M.; You, Z.; Peng, C.; Gong, Z.; Liu, W. Acetyltransferase GCN5 regulates autophagy and lysosome biogenesis by targeting TFEB. EMBO Rep. 2020, 21, e48335. [Google Scholar] [CrossRef] [PubMed]
- Payre, F. Genetic control of epidermis differentiation in Drosophila. Int. J. Dev. Biol. 2004, 48, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Middlebrooks, B.W.; Alexander, S.; Wasserman, S.A. Mutation of TweedleD, a member of an unconventional cuticle protein family, alters body shape in Drosophila. Proc. Natl. Acad. Sci. USA 2006, 103, 16794–16799. [Google Scholar] [CrossRef] [PubMed]
- Doenst, T.; Nguyen, T.D.; Abel, E.D. Cardiac metabolism in heart failure: Implications beyond ATP production. Circ. Res. 2013, 113, 709–724. [Google Scholar] [CrossRef] [PubMed]
- McGarrah, R.W.; Crown, S.B.; Zhang, G.F.; Shah, S.H.; Newgard, C.B. Cardiovascular Metabolomics. Circ. Res. 2018, 122, 1238–1258. [Google Scholar] [CrossRef]
- Peleg, S.; Feller, C.; Forne, I.; Schiller, E.; Sévin, D.C.; Schauer, T.; Regnard, C.; Straub, T.; Prestel, M.; Klima, C.; et al. Life span extension by targeting a link between metabolism and histone acetylation in Drosophila. EMBO Rep. 2016, 17, 455–469. [Google Scholar] [CrossRef]
- Koutelou, E.; Farria, A.T.; Dent, S.Y.R. Complex functions of Gcn5 and Pcaf in development and disease. Biochim. Biophys. Acta Gene Regul. Mech. 2021, 1864, 194609. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Xu, Y.; Li, R.; Huang, S.; Wu, Q.; Yan, J.; Jiang, Z.; Wu, X.; Li, F.; Wang, Y.; et al. Transcriptomic and Metabolomic Analysis Reveals Multifaceted Impact of Gcn5 Knockdown in Drosophila Development. Metabolites 2024, 14, 680. https://doi.org/10.3390/metabo14120680
Li Y, Xu Y, Li R, Huang S, Wu Q, Yan J, Jiang Z, Wu X, Li F, Wang Y, et al. Transcriptomic and Metabolomic Analysis Reveals Multifaceted Impact of Gcn5 Knockdown in Drosophila Development. Metabolites. 2024; 14(12):680. https://doi.org/10.3390/metabo14120680
Chicago/Turabian StyleLi, Youfeng, Yue Xu, Ruike Li, Sirui Huang, Qiong Wu, Jing Yan, Zhigang Jiang, Xiushan Wu, Fang Li, Yuequn Wang, and et al. 2024. "Transcriptomic and Metabolomic Analysis Reveals Multifaceted Impact of Gcn5 Knockdown in Drosophila Development" Metabolites 14, no. 12: 680. https://doi.org/10.3390/metabo14120680
APA StyleLi, Y., Xu, Y., Li, R., Huang, S., Wu, Q., Yan, J., Jiang, Z., Wu, X., Li, F., Wang, Y., Li, Y., Fan, X., & Yuan, W. (2024). Transcriptomic and Metabolomic Analysis Reveals Multifaceted Impact of Gcn5 Knockdown in Drosophila Development. Metabolites, 14(12), 680. https://doi.org/10.3390/metabo14120680




