The Role of Beta-Hydroxybutyrate in Mitigating the Inflammatory and Metabolic Consequences of Uric Acid
Abstract
1. Introduction
2. Experimental Design
2.1. Cell Culture
2.2. Cell Viability
2.3. NLRP3 Activity
2.4. Mitochondrial Respiration
2.5. H2O2 Emission
2.6. Protein Analysis
2.7. Statistical Methods
3. Results
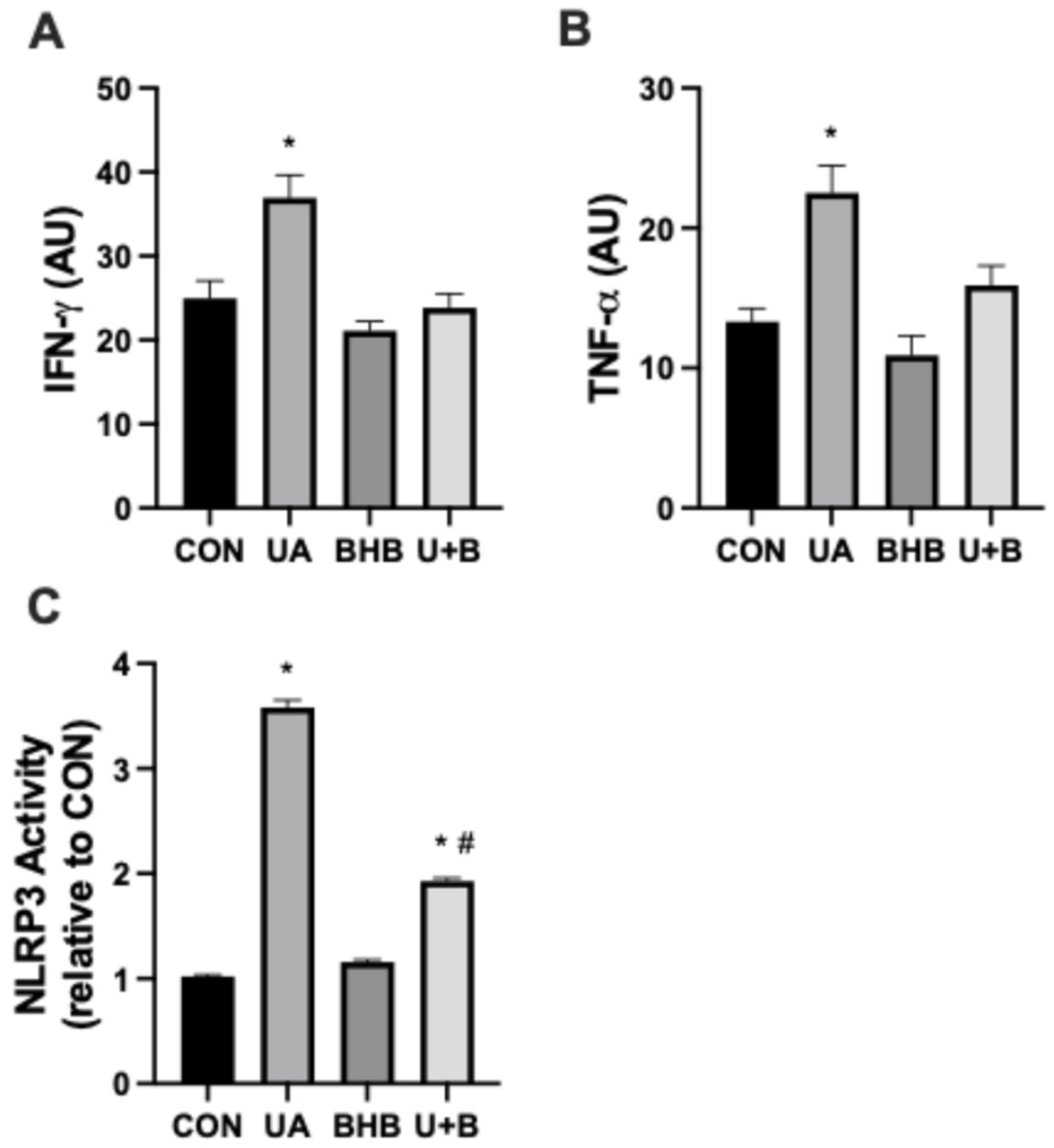
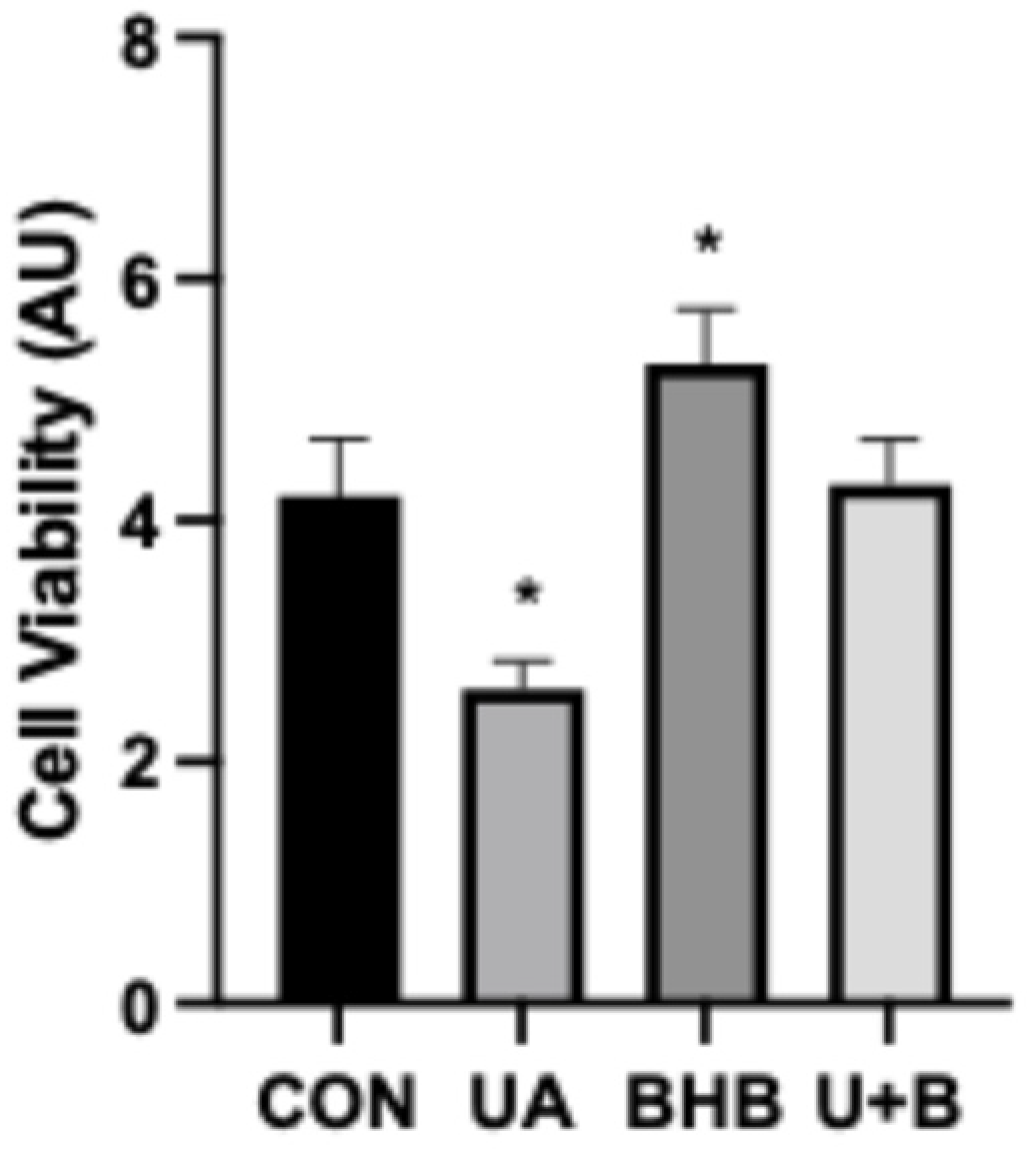
3.1. Beta-Hydroxybutyrate Mitigates Uric Acid-Induced Changes in Inflammatory Activation and Cell Viability
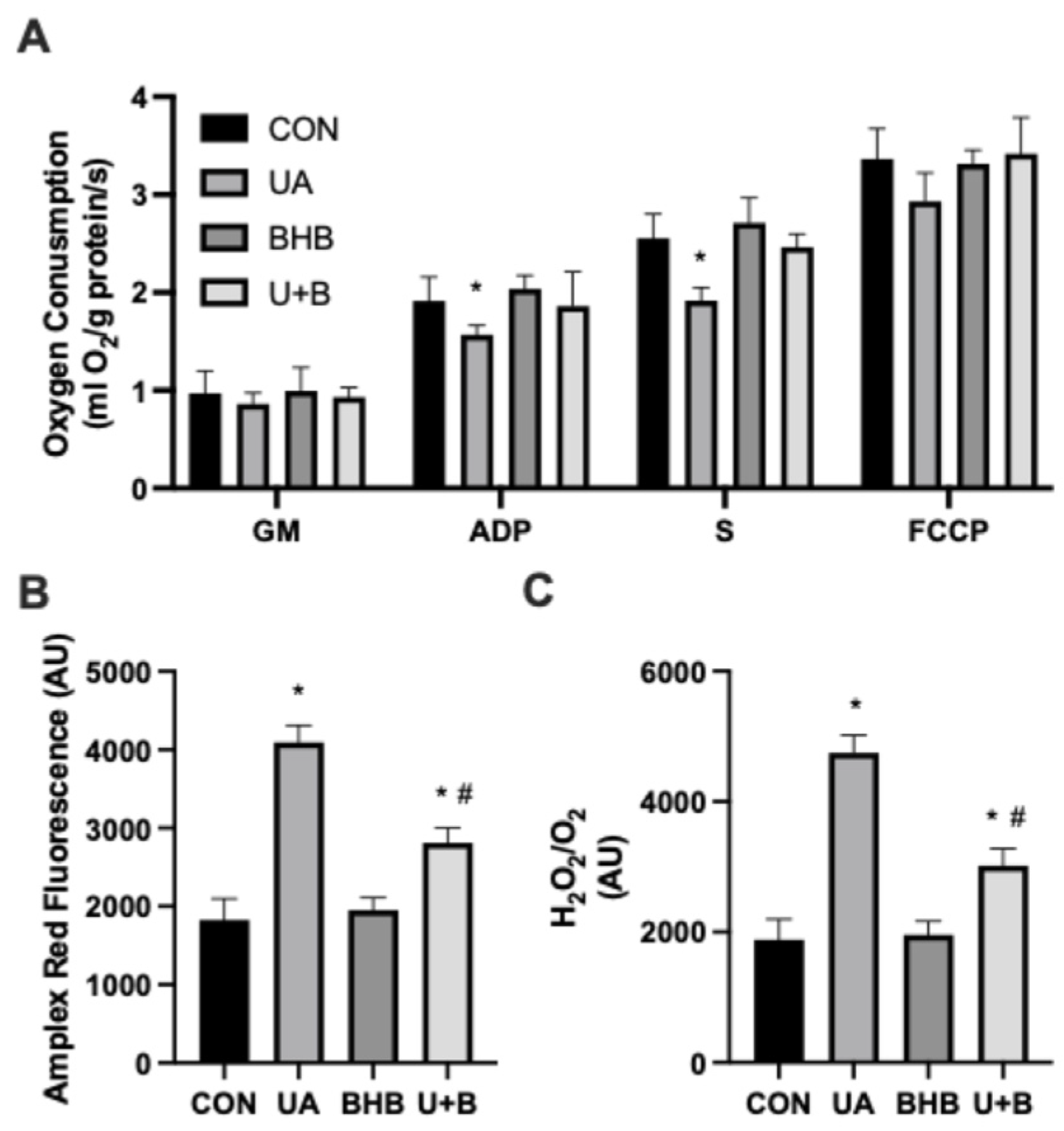
3.2. Uric Acid and Ketones Have Differential Effects on Mitochondrial Bioenergetics
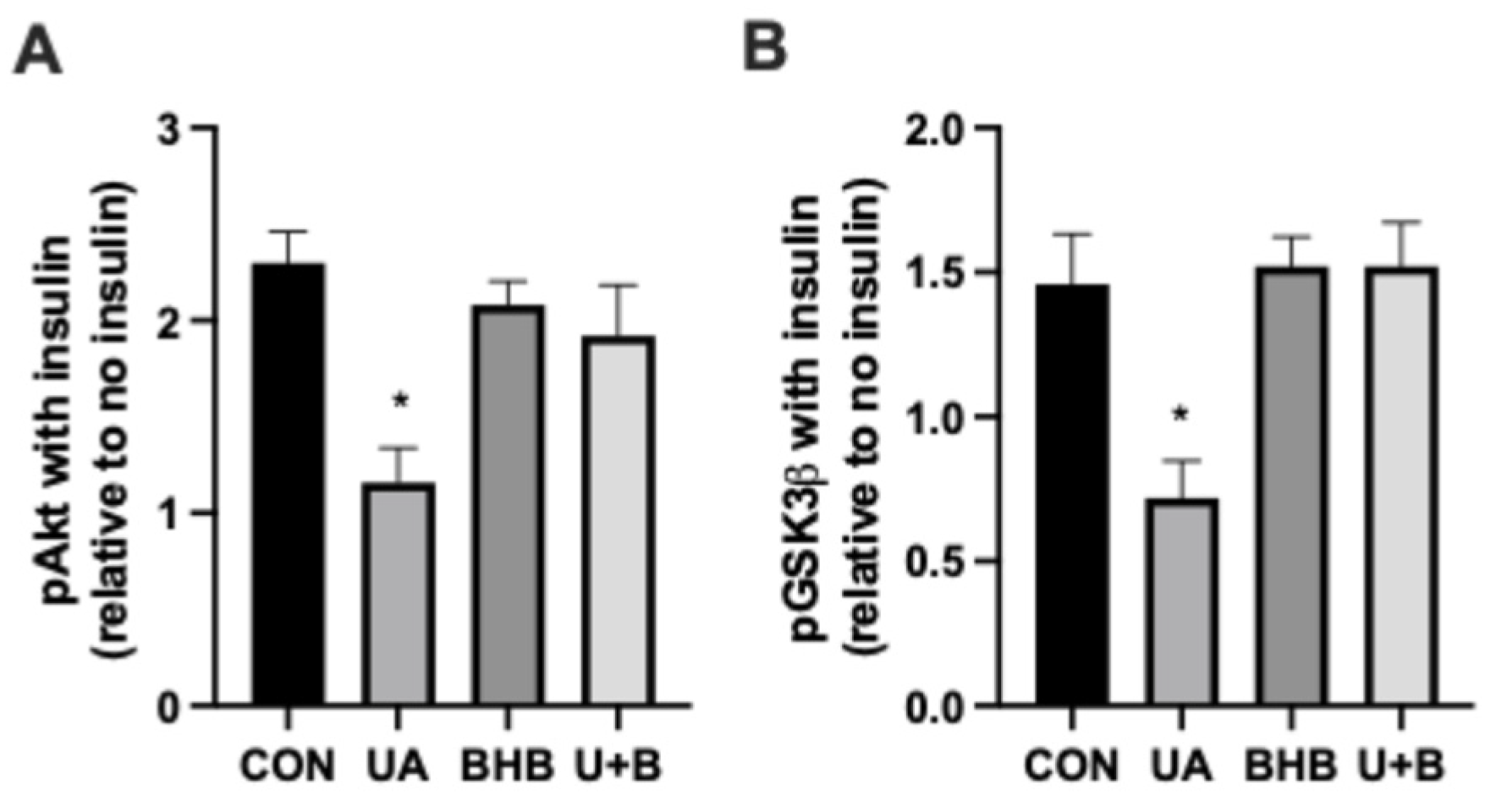
3.3. β-Hydroxybutyrate Protects Insulin Signaling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kushiyama, A.; Nakatsu, Y.; Matsunaga, Y.; Yamamotoya, T.; Mori, K.; Ueda, K.; Inoue, Y.; Sakoda, H.; Fujishiro, M.; Ono, H.; et al. Role of Uric Acid Metabolism-Related Inflammation in the Pathogenesis of Metabolic Syndrome Components Such as Atherosclerosis and Nonalcoholic Steatohepatitis. Mediat. Inflamm. 2016, 2016, 8603164. [Google Scholar] [CrossRef] [PubMed]
- Braga, T.T.; Forni, M.F.; Correa-Costa, M.; Ramos, R.N.; Barbuto, J.A.; Branco, P.; Castoldi, A.; Hiyane, M.I.; Davanso, M.R.; Latz, E.; et al. Soluble Uric Acid Activates the NLRP3 Inflammasome. Sci. Rep. 2017, 7, 39884. [Google Scholar] [CrossRef] [PubMed]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Kurajoh, M.; Fukumoto, S.; Yoshida, S.; Akari, S.; Murase, T.; Nakamura, T.; Ishii, H.; Yoshida, H.; Nagata, Y.; Morioka, T.; et al. Uric acid shown to contribute to increased oxidative stress level independent of xanthine oxidoreductase activity in MedCity21 health examination registry. Sci. Rep. 2021, 11, 7378. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.A.; Mathew, T.C.; Dashti, A.A.; Asfar, S.; Al-Zaid, N.; Dashti, H.M. Effect of low-calorie versus low-carbohydrate ketogenic diet in type 2 diabetes. Nutrition 2012, 28, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, C.E.; Phinney, S.D.; Fernandez, M.L.; Quann, E.E.; Wood, R.J.; Bibus, D.M.; Kraemer, W.J.; Feinman, R.D.; Volek, J.S. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids 2008, 43, 65–77. [Google Scholar] [CrossRef]
- Parker, B.A.; Walton, C.M.; Carr, S.T.; Andrus, J.L.; Cheung, E.C.K.; Duplisea, M.J.; Wilson, E.K.; Draney, C.; Lathen, D.R.; Kenner, K.B.; et al. beta-Hydroxybutyrate Elicits Favorable Mitochondrial Changes in Skeletal Muscle. Int. J. Mol. Sci. 2018, 19, 2247. [Google Scholar] [CrossRef]
- Llorente-Folch, I.; Dussmann, H.; Watters, O.; Connolly, N.M.C.; Prehn, J.H.M. Ketone body beta-hydroxybutyrate (BHB) preserves mitochondrial bioenergetics. Sci. Rep. 2023, 13, 19664. [Google Scholar] [CrossRef]
- Dallon, B.W.; Parker, B.A.; Hodson, A.E.; Tippetts, T.S.; Harrison, M.E.; Appiah, M.M.A.; Witt, J.E.; Gibbs, J.L.; Gray, H.M.; Sant, T.M.; et al. Insulin selectively reduces mitochondrial uncoupling in brown adipose tissue in mice. Biochem. J. 2018, 475, 561–569. [Google Scholar] [CrossRef]
- Smith, M.E.; Tippetts, T.S.; Brassfield, E.S.; Tucker, B.J.; Ockey, A.; Swensen, A.C.; Anthonymuthu, T.S.; Washburn, T.D.; Kane, D.A.; Prince, J.T.; et al. Mitochondrial fission mediates ceramide-induced metabolic disruption in skeletal muscle. Biochem. J. 2013, 456, 427–439. [Google Scholar] [CrossRef]
- Sen, S.; Roy, S.; Bandyopadhyay, G.; Scott, B.; Xiao, D.; Ramadoss, S.; Mahata, S.K.; Chaudhuri, G. gamma-Aminobutyric Acid Is Synthesized and Released by the Endothelium: Potential Implications. Circ. Res. 2016, 119, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Maarman, G.J.; Andrew, B.M.; Blackhurst, D.M.; Ojuka, E.O. Melatonin protects against uric acid-induced mitochondrial dysfunction, oxidative stress, and triglyceride accumulation in C(2)C(12) myotubes. J. Appl. Physiol. 2017, 122, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Zong, Q.; Ma, G.; Wang, T. Uric acid lowering improves insulin sensitivity and lowers blood pressure: A meta-analysis of randomized parallel-controlled clinical trials. Afr. Health Sci. 2021, 21, 82–95. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, J.; He, F.; Wang, M.; Yan, Y.; Chen, B.; Xie, D.; Xu, C.; Wang, Q.; Liu, W.; et al. Hyperuricemia contributes to glucose intolerance of hepatic inflammatory macrophages and impairs the insulin signaling pathway via IRS2-proteasome degradation. Front. Immunol. 2022, 13, 931087. [Google Scholar] [CrossRef]
- Shao, J.; Yamashita, H.; Qiao, L.; Friedman, J.E. Decreased Akt kinase activity and insulin resistance in C57BL/KsJ-Leprdb/db mice. J. Endocrinol. 2000, 167, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.; Jansson, P.A.; Nagaev, I.; Wenthzel, A.M.; Smith, U. Insulin resistance with low cellular IRS-1 expression is also associated with low GLUT4 expression and impaired insulin-stimulated glucose transport. FASEB J. 2001, 15, 1101–1103. [Google Scholar] [CrossRef]
- Wan, X.; Xu, C.; Lin, Y.; Lu, C.; Li, D.; Sang, J.; He, H.; Liu, X.; Li, Y.; Yu, C. Uric acid regulates hepatic steatosis and insulin resistance through the NLRP3 inflammasome-dependent mechanism. J. Hepatol. 2016, 64, 925–932. [Google Scholar] [CrossRef]
- Dadkhah, M.; Sharifi, M. The NLRP3 inflammasome: Mechanisms of activation, regulation, and role in diseases. Int. Rev. Immunol. 2024, 1–14. [Google Scholar] [CrossRef]
- Ghaemi-Oskouie, F.; Shi, Y. The role of uric acid as an endogenous danger signal in immunity and inflammation. Curr. Rheumatol. Rep. 2011, 13, 160–166. [Google Scholar] [CrossRef]
- Sampson, M.; Lathen, D.; Dallon, B.; Draney, C.; Ray, J.; Kener, K.; Parker, B.; Witt, J.; Gibbs, J.; Tessem, J.S.; et al. Beta-hydroxybutyrate favorably alters b-cell survival and mitochondrial bioenergetics. FASEB J. 2017, 31, 883–887. [Google Scholar] [CrossRef]
- Cheng, B.; Yang, X.; Hou, Z.; Lin, X.; Meng, H.; Li, Z.; Liu, S. D-beta-hydroxybutyrate inhibits the apoptosis of PC12 cells induced by 6-OHDA in relation to up-regulating the ratio of Bcl-2/Bax mRNA. Auton. Neurosci. 2007, 134, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hu, Y.; Huang, T.; Zhang, Y.; Li, Z.; Luo, C.; Luo, Y.; Yuan, H.; Hisatome, I.; Yamamoto, T.; et al. High uric acid directly inhibits insulin signalling and induces insulin resistance. Biochem. Biophys. Res. Commun. 2014, 447, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.J.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M.; et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: Potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Takir, M.; Kostek, O.; Ozkok, A.; Elcioglu, O.C.; Bakan, A.; Erek, A.; Mutlu, H.H.; Telci, O.; Semerci, A.; Odabas, A.R.; et al. Lowering Uric Acid with Allopurinol Improves Insulin Resistance and Systemic Inflammation in Asymptomatic Hyperuricemia. J. Investig. Med. 2015, 63, 924–929. [Google Scholar] [CrossRef]
- Puchalska, P.; Crawford, P.A. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef]
- Bikman, B.T. A role for sphingolipids in the pathophysiology of obesity-induced inflammation. Cell. Mol. Life Sci. 2012, 69, 2135–2146. [Google Scholar] [CrossRef]
- Hansen, M.E.; Simmons, K.J.; Tippetts, T.S.; Thatcher, M.O.; Saito, R.R.; Hubbard, S.T.; Trumbull, A.M.; Parker, B.A.; Taylor, O.J.; Bikman, B.T. Lipopolysaccharide Disrupts Mitochondrial Physiology in Skeletal Muscle via Disparate Effects on Sphingolipid Metabolism. Shock 2015, 44, 585–592. [Google Scholar] [CrossRef]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Remund, N.P.; Larsen, J.G.; Shin, M.J.; Warren, C.E.; Palmer, I.L.; Kim, I.J.; Cooper-Leavitt, E.T.; Clarke, D.M.; Beus, C.G.; Johnson, R.J.; et al. The Role of Beta-Hydroxybutyrate in Mitigating the Inflammatory and Metabolic Consequences of Uric Acid. Metabolites 2024, 14, 679. https://doi.org/10.3390/metabo14120679
Remund NP, Larsen JG, Shin MJ, Warren CE, Palmer IL, Kim IJ, Cooper-Leavitt ET, Clarke DM, Beus CG, Johnson RJ, et al. The Role of Beta-Hydroxybutyrate in Mitigating the Inflammatory and Metabolic Consequences of Uric Acid. Metabolites. 2024; 14(12):679. https://doi.org/10.3390/metabo14120679
Chicago/Turabian StyleRemund, Nicole P., John G. Larsen, Marley J. Shin, Cali E. Warren, Isabelle L. Palmer, Iris J. Kim, Elijah T. Cooper-Leavitt, Derek M. Clarke, Colson G. Beus, Richard J. Johnson, and et al. 2024. "The Role of Beta-Hydroxybutyrate in Mitigating the Inflammatory and Metabolic Consequences of Uric Acid" Metabolites 14, no. 12: 679. https://doi.org/10.3390/metabo14120679
APA StyleRemund, N. P., Larsen, J. G., Shin, M. J., Warren, C. E., Palmer, I. L., Kim, I. J., Cooper-Leavitt, E. T., Clarke, D. M., Beus, C. G., Johnson, R. J., Arroyo, J. A., Reynolds, P. R., & Bikman, B. T. (2024). The Role of Beta-Hydroxybutyrate in Mitigating the Inflammatory and Metabolic Consequences of Uric Acid. Metabolites, 14(12), 679. https://doi.org/10.3390/metabo14120679







