Species Differences in Ezetimibe Glucuronidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Method
3. Results
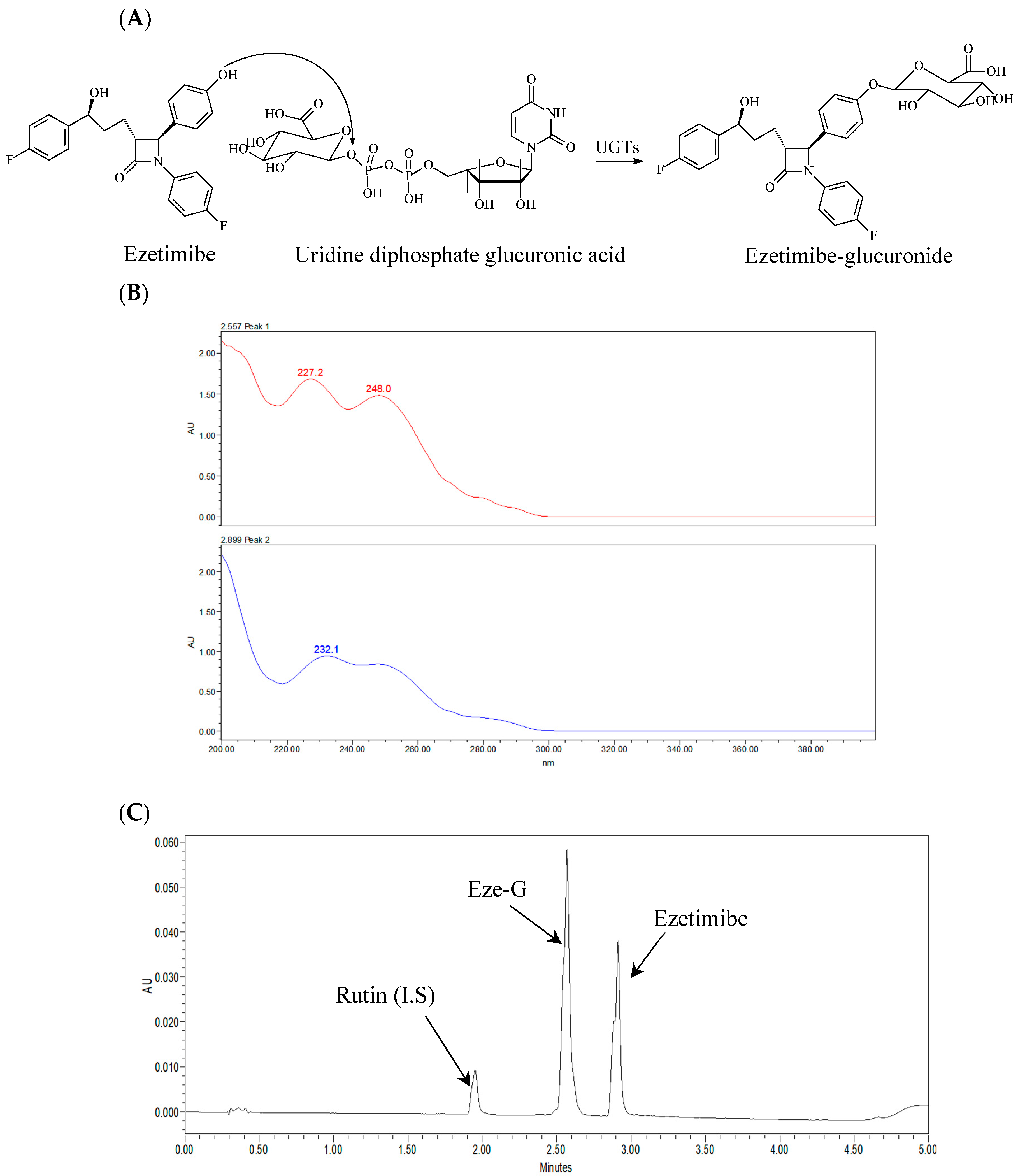
3.1. Ezetimibe Glucuronidation
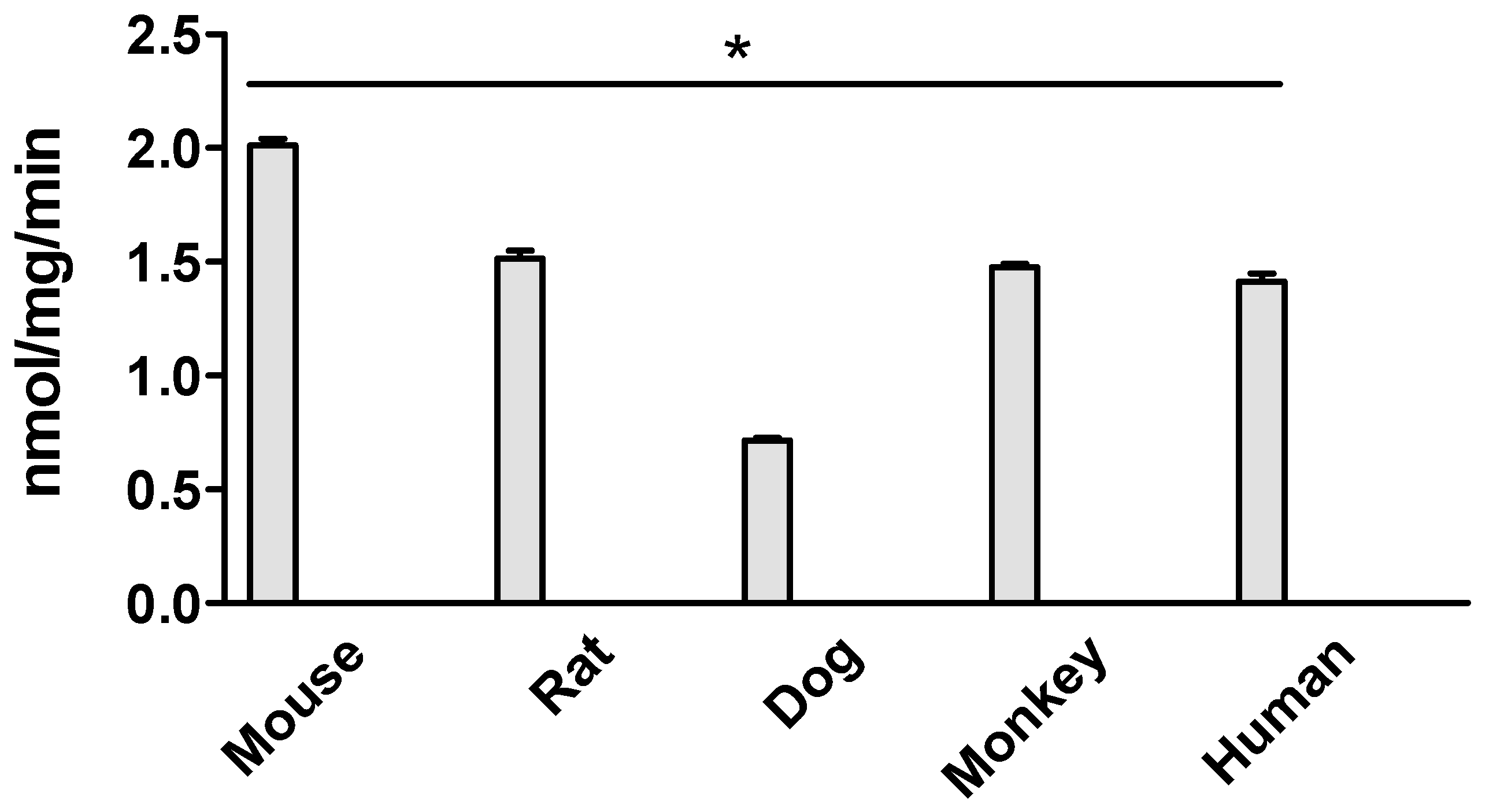
3.2. Ezetimibe Glucuronidation Activities in Intestinal Microsomes of Different Species at 5 μM
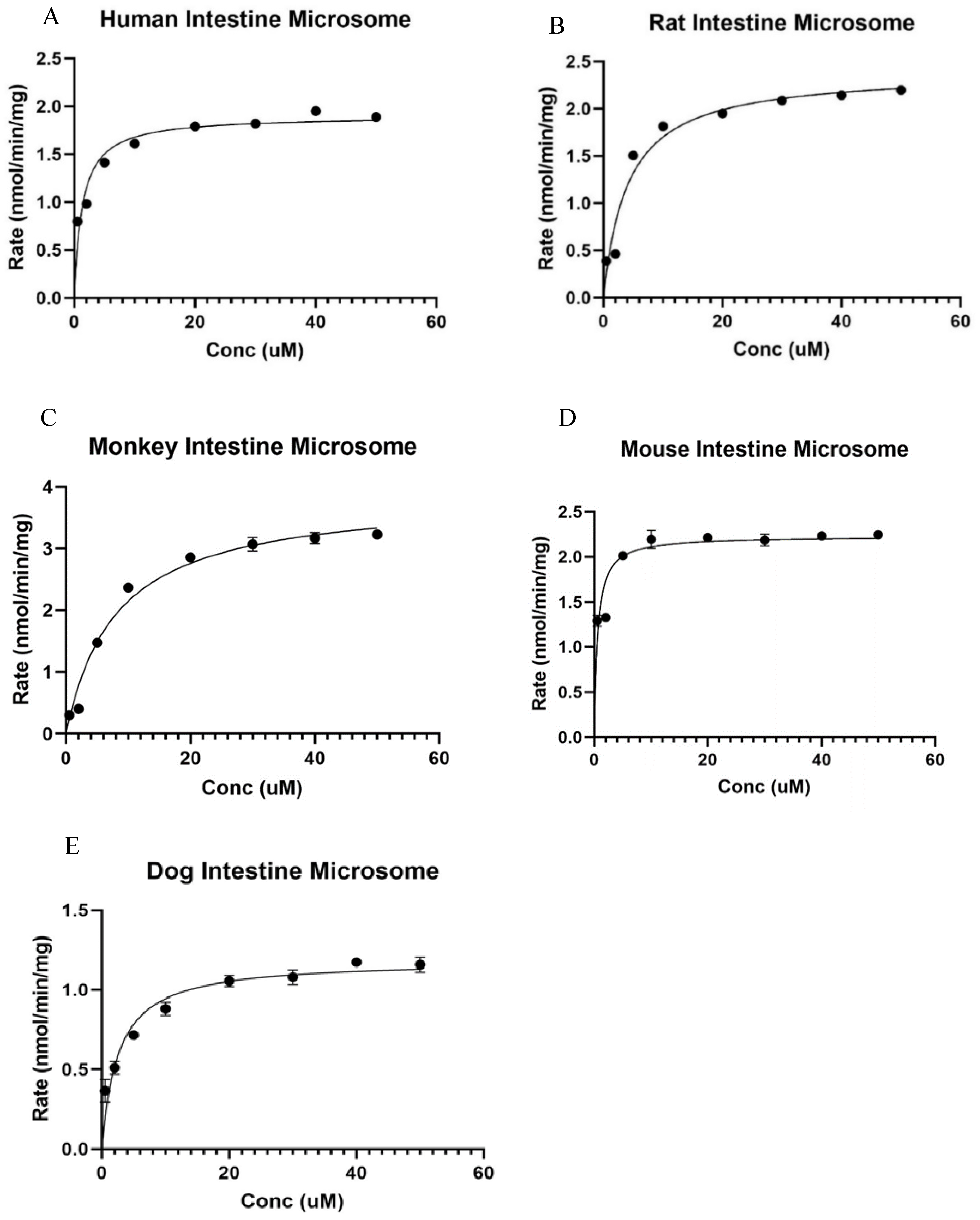
3.3. Kinetics for Ezetimibe Glucuronidation by Intestinal Microsomes of Different Species
3.3.1. Kinetics of Ezetimibe Glucuronidation in Human Intestine Microsomes
3.3.2. Kinetics of Ezetimibe Glucuronidation in Rat Intestine Microsomes
3.3.3. Kinetics of Ezetimibe Glucuronidation in Male Cyno Monkey Intestine Microsomes
3.3.4. Kinetics of Ezetimibe Glucuronidation in Male CDI Mouse Intestinal Microsomes
3.3.5. Kinetics of Ezetimibe Glucuronidation in Beagle Dog Intestine Microsomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boutari, C.; Karagiannis, A.; Athyros, V.G. Rosuvastatin and ezetimibe for the treatment of dyslipidemia and hypercholesterolemia. Expert Rev. Cardiovasc. Ther. 2021, 19, 575–580. [Google Scholar] [CrossRef]
- Yu, M.; Liang, C.; Kong, Q.; Wang, Y.; Li, M. Efficacy of combination therapy with ezetimibe and statins versus a double dose of statin monotherapy in participants with hypercholesterolemia: A meta-analysis of literature. Lipids Health Dis. 2020, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Popovic, D.S.; Papachristou, S.; Stokic, E.; Papanas, N. Ezetimibe and Insulin Resistance. Curr. Vasc. Pharmacol. 2022, 20, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Mercep, I.; Strikic, D.; Sliskovic, A.M.; Reiner, Z. New Therapeutic Approaches in Treatment of Dyslipidaemia—A Narrative Review. Pharmaceuticals 2022, 15, 839. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, A.; Hapangama, N.; Yuan, Y.; Achanfuo-Yeboah, J.; Iannucci, R.; Chowdhury, S.; Alton, K.; Patrick, J.E.; Zbaida, S. Identification of human UDP-glucuronosyltransferase enzyme(s) responsible for the glucuronidation of ezetimibe (Zetia). Drug Metab. Dispos. 2004, 32, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Torrado-Salmeron, C.; Guarnizo-Herrero, V.; Gallego-Arranz, T.; Del Val-Sabugo, Y.; Torrado, G.; Morales, J.; Torrado-Santiago, S. Improvement in the Oral Bioavailability and Efficacy of New Ezetimibe Formulations-Comparative Study of a Solid Dispersion and Different Micellar Systems. Pharmaceutics 2020, 12, 617. [Google Scholar] [CrossRef] [PubMed]
- Kosoglou, T.; Statkevich, P.; Johnson-Levonas, A.O.; Paolini, J.F.; Bergman, A.J.; Alton, K.B. Ezetimibe: A review of its metabolism, pharmacokinetics and drug interactions. Clin. Pharmacokinet. 2005, 44, 467–494. [Google Scholar] [CrossRef] [PubMed]
- Salgado, M.M.; Manchado, A.; Nieto, C.T.; Diez, D.; Garrido, N.M. Synthesis and Modeling of Ezetimibe Analogues. Molecules 2021, 26, 3107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, W.; Zeng, J.; Meng, J.; Jiang, H.; Wang, J.; Xing, D. Niemann-Pick C1-Like 1 inhibitors for reducing cholesterol absorption. Eur. J. Med. Chem. 2022, 230, 114111. [Google Scholar] [PubMed]
- Hawes, B.E.; O’Neill, K.A.; Yao, X.; Crona, J.H.; Davis, H.R., Jr.; Graziano, M.P.; Altmann, S.W. In vivo responsiveness to ezetimibe correlates with niemann-pick C1 like-1 (NPC1L1) binding affinity: Comparison of multiple species NPC1L1 orthologs. Mol. Pharmacol. 2007, 71, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Hitomi, O.; Aiko, H.; Kaoru, K.; Kureha, G.; Ran, A.; Takumi, K.; Shinogu, H.; Toshinori, S.; Akihiro, M. Treatment of Ezetimibe lowers total and low-density lipoprotein cholesterol in hypercholesterolemic dogs with hyperadorenocorticism. J. Vet. Med. Sci. 2024, 86, 363–367. [Google Scholar]
- Tomlinson, B.; Chan, P.; Zhang, Y.; Liu, Z.; Lam, C.W.K. Pharmacokinetics of current and emerging treatments for hypercholesterolemia. Expert Opin. Drug Metab. Toxicol. 2020, 16, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Elsby, R.; Atkinson, H.; Butler, P.; Riley, R.J. Studying the right transporter at the right time: An in vitro strategy for assessing drug-drug interaction risk during drug discovery and development. Expert Opin. Drug Metab. Toxicol. 2022, 18, 619–655. [Google Scholar] [CrossRef] [PubMed]
- Ahire, D.; Kruger, L.; Sharma, S.; Mettu, V.S.; Basit, A.; Prasad, B. Quantitative Proteomics in Translational Absorption, Distribution, Metabolism, and Excretion and Precision Medicine. Pharmacol. Rev. 2022, 74, 769–796. [Google Scholar] [PubMed]
- Badee, J.; Qiu, N.; Parrott, N.; Collier, A.C.; Schmidt, S.; Fowler, S. Optimization of Experimental Conditions of Automated Glucuronidation Assays in Human Liver Microsomes Using a Cocktail Approach and Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry. Drug Metab. Dispos. 2019, 47, 124–134. [Google Scholar] [CrossRef] [PubMed]
- van Heek, M.; Farley, C.; Compton, D.S.; Hoos, L.; Alton, K.B.; Sybertz, E.J.; Davis, H.R., Jr. Comparison of the activity and disposition of the novel cholesterol absorption inhibitor, SCH58235, and its glucuronide, SCH60663. Br. J. Pharmacol. 2000, 129, 1748–1754. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Yoo, P.; Lee, T.; Klopf, W.; Takao, D. The role of pH in the glucuronidation of raloxifene, mycophenolic acid and ezetimibe. Mol. Pharm. 2009, 6, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Wang, L.; Rong, Y.; Tam, V.; Yin, T.; Gao, S.; Singh, R.; Hu, M. Hepatoenteric recycling is a new disposition mechanism for orally administered phenolic drugs and phytochemicals in rats. Elife 2021, 10, e58820. [Google Scholar] [CrossRef] [PubMed]
| Species | Km (µM) | Vmax (nmol/mg/min) | CLint (µL/min/mg) |
|---|---|---|---|
| Human | 1.33 ± 0.36 | 1.90 ± 0.08 | 1.43 ± 0.01 |
| Rat | 4.10 ± 1.03 * | 2.40 ± 0.14 * | 0.58 ± 0.01 # |
| Monkey | 8.01 ± 1.60 * | 3.87 ± 0.22 * | 0.47 ± 0.02 # |
| Mouse | 0.58 ± 0.19 # | 2.23 ± 0.10 * | 3.84 ± 0.01 * |
| Beagle dog | 2.59 ± 0.64 * | 1.19 ± 0.06 # | 2.17 ± 0.01 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emmanuel, S.; Asare, E.A.; Du, T.; Xie, H.; Liang, D.; Gao, S. Species Differences in Ezetimibe Glucuronidation. Metabolites 2024, 14, 569. https://doi.org/10.3390/metabo14110569
Emmanuel S, Asare EA, Du T, Xie H, Liang D, Gao S. Species Differences in Ezetimibe Glucuronidation. Metabolites. 2024; 14(11):569. https://doi.org/10.3390/metabo14110569
Chicago/Turabian StyleEmmanuel, Shalom, Eric A. Asare, Ting Du, Huan Xie, Dong Liang, and Song Gao. 2024. "Species Differences in Ezetimibe Glucuronidation" Metabolites 14, no. 11: 569. https://doi.org/10.3390/metabo14110569
APA StyleEmmanuel, S., Asare, E. A., Du, T., Xie, H., Liang, D., & Gao, S. (2024). Species Differences in Ezetimibe Glucuronidation. Metabolites, 14(11), 569. https://doi.org/10.3390/metabo14110569








