Detailed Profiling of 17-Hydroxygeranyllinalool Diterpene Glycosides from Nicotiana Species Reveals Complex Reaction Networks of Conjugation Isomers
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Metabolite Preparation
2.3. LC-MS Analysis
2.4. Metabolomic Data Processing
2.5. HGL-DTG Annotation
3. Results
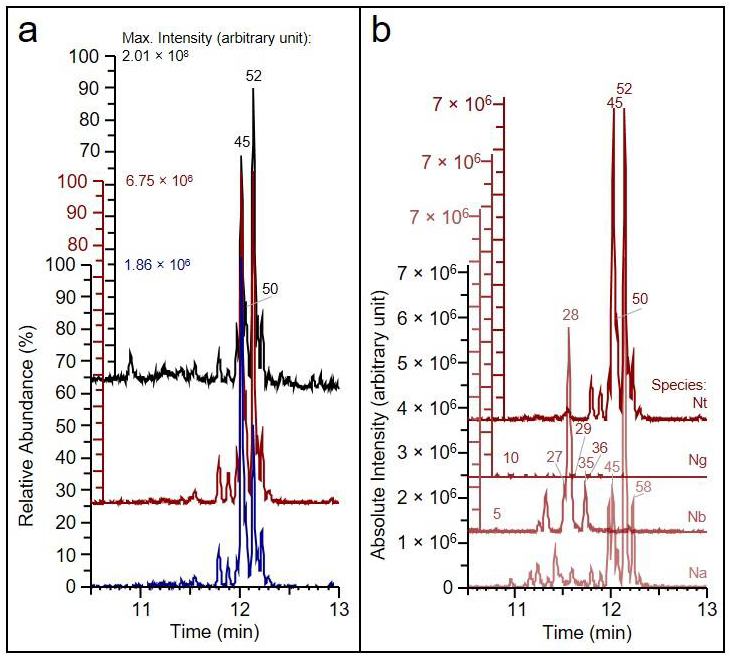
3.1. Detection of HGL-DTGs in Four Nicotiana Species via Presence of the Aglycone
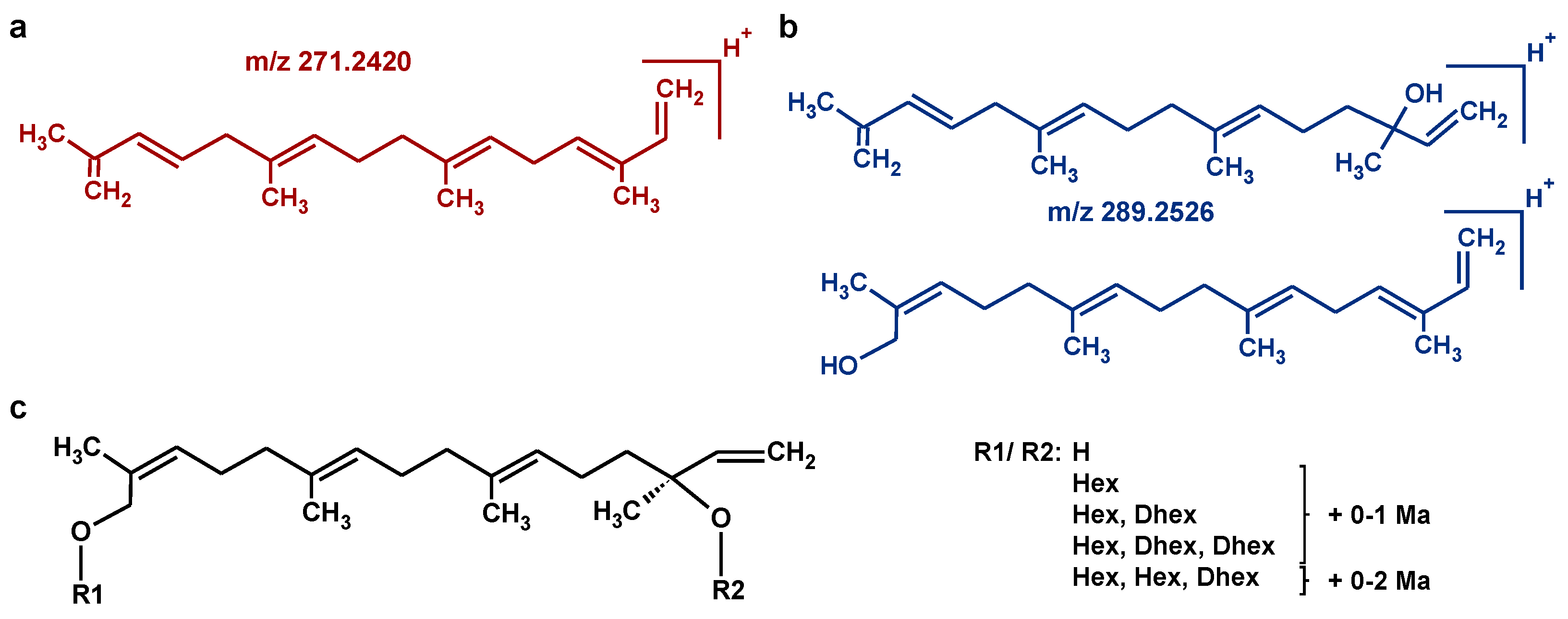
3.2. Characteristic Fragmentation Reactions of HGL-DTGs
3.3. Comprehensive Annotation of HGL-DTGs from N. tabacum, N. glauca and N. benthamiana with Reference to N. attenuata
3.4. Occurrence of N. tabacum, N. glauca, N. benthamiana HGL-DTGs in the Analysed Nicotiana Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fiesel, P.D.; Parks, H.M.; Last, R.L.; Barry, C.S. Fruity, Sticky, Stinky, Spicy, Bitter, Addictive, and Deadly: Evolutionary Signatures of Metabolic Complexity in the Solanaceae. Nat. Prod. Rep. 2022, 39, 1438–1464. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, L.H.; Verpoorte, R. Secondary Metabolism in Tobacco. Plant Cell Tissue Organ Cult. 2002, 68, 105–125. [Google Scholar] [CrossRef]
- Jassbi, A.R.; Zare, S.; Asadollahi, M.; Schuman, M.C. Ecological Roles and Biological Activities of Specialized Metabolites from the Genus Nicotiana. Chem. Rev. 2017, 117, 12227–12280. [Google Scholar] [CrossRef]
- Elser, D.; Pflieger, D.; Villette, C.; Moegle, B.; Miesch, L.; Gaquerel, E. Evolutionary Metabolomics of Specialized Metabolism Diversification in the Genus Nicotiana Highlights N-Acylnornicotine Innovations. Sci. Adv. 2023, 9, eade8984. [Google Scholar] [CrossRef] [PubMed]
- Yahara, S.; Shigeyama, C.; Ura, T.; Wakamatsu, K.; Yasuhara, T.; Nohara, T. Cyclic Peptides, Acyclic Diterpene Glycosides and Other Compounds from Lycium chinense MILL. Chem. Pharm. Bull. 1993, 41, 703–709. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Tobita, T.; Mizutani, M.; Matsuzaki, T. Isolation and Identification of Two New Diterpene Glycosides from Nicotiana tabacum. Biosci. Biotechnol. Biochem. 1996, 60, 903–905. [Google Scholar] [CrossRef]
- Snook, M.E.; Johnson, A.W.; Severson, R.F.; Teng, Q.; White, R.A.; Sisson, V.A.; Jackson, D.M. Hydroxygeranyllinalool Glycosides from Tobacco Exhibit Antibiosis Activity in the Tobacco Budworm [Heliothis virescens (F.)]. J. Agric. Food Chem. 1997, 45, 2299–2308. [Google Scholar] [CrossRef]
- Terauchi, M.; Kanamori, H.; Nobuso, M.; Yahara, S.; Yamasaki, K. New Acyclic Diterpene Glycosides, Lyciumoside IV-IX from Lycium chinese Mill. Nat. Med. 1998, 52, 167–171. [Google Scholar]
- Wallin, I.; Narbonne, C.; Wahlberg, I.; Nishida, T.; Enzell, C.R. Two New Acyclic Diterpenoids from Nicotiana sylvestris. Acta Chem. Scand. 1980, 34, 391–396. [Google Scholar] [CrossRef]
- Heiling, S.; Schuman, M.C.; Schoettner, M.; Mukerjee, P.; Berger, B.; Schneider, B.; Jassbi, A.R.; Baldwin, I.T. Jasmonate and PpHsystemin Regulate Key Malonylation Steps in the Biosynthesis of 17-Hydroxygeranyllinalool Diterpene Glycosides, an Abundant and Effective Direct Defense Against Herbivores in Nicotiana attenuata. Plant Cell 2010, 22, 273–292. [Google Scholar] [CrossRef]
- Heiling, S.; Khanal, S.; Barsch, A.; Zurek, G.; Baldwin, I.T.; Gaquerel, E. Using the Knowns to Discover the Unknowns: MS-Based Dereplication Uncovers Structural Diversity in 17-Hydroxygeranyllinalool Diterpene Glycoside Production in the Solanaceae. Plant J. 2016, 85, 561–577. [Google Scholar] [CrossRef]
- Heiling, S.; Llorca, L.C.; Li, J.; Gase, K.; Schmidt, A.; Schäfer, M.; Schneider, B.; Halitschke, R.; Gaquerel, E.; Baldwin, I.T. Specific Decorations of 17-Hydroxygeranyllinalool Diterpene Glycosides Solve the Autotoxicity Problem of Chemical Defense in Nicotiana attenuata. Plant Cell 2021, 33, 1748–1770. [Google Scholar] [CrossRef]
- Falara, V.; Alba, J.M.; Kant, M.R.; Schuurink, R.C.; Pichersky, E. Geranyllinalool Synthases in Solanaceae and Other Angiosperms Constitute an Ancient Branch of Diterpene Synthases Involved in the Synthesis of Defensive Compounds. Plant Physiol. 2014, 166, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Halitschke, R.; Li, D.; Paetz, C.; Su, H.; Heiling, S.; Xu, S.; Baldwin, I.T. Controlled Hydroxylations of Diterpenoids Allow for Plant Chemical Defense without Autotoxicity. Science 2021, 371, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Schuman, M.C.; Halitschke, R.; Li, X.; Guo, H.; Grabe, V.; Hammer, A.; Baldwin, I.T. The Decoration of Specialized Metabolites Influences Stylar Development. Elife 2018, 7, e38611. [Google Scholar] [CrossRef] [PubMed]
- Jassbi, A.R.; Zamanizadehnajari, S.; Kessler, D.; Baldwin, I.T. A New Acyclic Diterpene Glycoside from Nicotiana attenuata with a Mild Deterrent Effect on Feeding Manduca sexta Larvae. Z. Naturforschung Sect. B J. Chem. Sci. 2006, 61, 1138–1142. [Google Scholar] [CrossRef]
- Jassbi, A.R.; Gase, K.; Hettenhausen, C.; Schmidt, A.; Baldwin, I.T. Silencing Geranylgeranyl Diphosphate Synthase in Nicotiana attenuata Dramatically Impairs Resistance to Tobacco Hornworm. Plant Physiol. 2008, 146, 974–986. [Google Scholar] [CrossRef]
- Clarkson, J.J.; Dodsworth, S.; Chase, M.W. Time-Calibrated Phylogenetic Trees Establish a Lag between Polyploidisation and Diversification in Nicotiana (Solanaceae). Plant Syst. Evol. 2017, 303, 1001–1012. [Google Scholar] [CrossRef]
- Schiavinato, M.; Marcet-Houben, M.; Dohm, J.C.; Gabaldón, T.; Himmelbauer, H. Parental Origin of the Allotetraploid Tobacco Nicotiana benthamiana. Plant J. 2020, 102, 541–554. [Google Scholar] [CrossRef]
- Lewis, R.S.; Nicholson, J.S. Aspects of the Evolution of Nicotiana tabacum L. and the Status of the United States Nicotiana Germplasm Collection. Genet. Resour. Crop Evol. 2007, 54, 727–740. [Google Scholar] [CrossRef]
- Bally, J.; Jung, H.; Mortimer, C.; Naim, F.; Philips, J.G.; Hellens, R.; Bombarely, A.; Goodin, M.M.; Waterhouse, P.M. The Rise and Rise of Nicotiana benthamiana: A Plant for All Reasons. Annu. Rev. Phytopathol. 2018, 56, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Ollerton, J.; Watts, S.; Connerty, S.; Lock, J.; Parker, L.; Wilson, I.; Schueller, S.; Nattero, J.; Cocucci, A.A.; Izhaki, I.; et al. Pollination Ecology of the Invasive Tree Tobacco Nicotiana glauca: Comparisons across Native and Non-Native Ranges. J. Pollinat. Ecol. 2012, 9, 85–95. [Google Scholar] [CrossRef]
- Stegemann, S.; Bock, R. Exchange of Genetic Material Between Cells in Plant Tissue Grafts. Science 2009, 324, 649–651. [Google Scholar] [CrossRef] [PubMed]
- Stegemann, S.; Keuthe, M.; Greiner, S.; Bock, R. Horizontal Transfer of Chloroplast Genomes between Plant Species. Proc. Natl. Acad. Sci. USA 2012, 109, 2434–2438. [Google Scholar] [CrossRef]
- Fuentes, I.; Stegemann, S.; Golczyk, H.; Karcher, D.; Bock, R. Horizontal Genome Transfer as an Asexual Path to the Formation of New Species. Nature 2014, 511, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Giavalisco, P.; Li, Y.; Matthes, A.; Eckhardt, A.; Hubberten, H.M.; Hesse, H.; Segu, S.; Hummel, J.; Köhl, K.; Willmitzer, L. Elemental Formula Annotation of Polar and Lipophilic Metabolites Using 13C, 15N and 34S Isotope Labelling, in Combination with High-Resolution Mass Spectrometry. Plant J. 2011, 68, 364–376. [Google Scholar] [CrossRef]
- Rohn, H.; Junker, A.; Hartmann, A.; Grafahrend-Belau, E.; Treutler, H.; Klapperstück, M.; Czauderna, T.; Klukas, C.; Schreiber, F. VANTED v2: A Framework for Systems Biology Applications. BMC Syst. Biol. 2012, 6, 139. [Google Scholar] [CrossRef]
- Alseekh, S.; Aharoni, A.; Brotman, Y.; Contrepois, K.; D’Auria, J.; Ewald, J.; Ewald, J.C.; Fraser, P.D.; Giavalisco, P.; Hall, R.D.; et al. Mass Spectrometry-Based Metabolomics: A Guide for Annotation, Quantification and Best Reporting Practices. Nat. Methods 2021, 18, 747–756. [Google Scholar] [CrossRef]
- Jassbi, A.R.; Zamanizadehnajari, S.; Baldwin, I.T. 17-Hydroxygeranyllinalool Glycosides Are Major Resistance Traits of Nicotiana obtusifolia Against Attack from Tobacco Hornworm Larvae. Phytochemistry 2010, 71, 1115–1121. [Google Scholar] [CrossRef]
- D’Andrea, L.; Sierro, N.; Ouadi, S.; Hasing, T.; Rinaldi, E.; Ivanov, N.V.; Bombarely, A. Polyploid Nicotiana Section Suaveolentes Originated by Hybridization of Two Ancestral Nicotiana Clades. Front. Plant Sci. 2023, 14, 999887. [Google Scholar] [CrossRef]
- Clarkson, J.J.; Kelly, L.J.; Leitch, A.R.; Knapp, S.; Chase, M.W. Nuclear Glutamine Synthetase Evolution in Nicotiana: Phylogenetics and the Origins of Allotetraploid and Homoploid (Diploid) Hybrids. Mol. Phylogenet. Evol. 2010, 55, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Kelly, L.J.; Leitch, A.R.; Clarkson, J.J.; Knapp, S.; Chase, M.W. Reconstructing the Complex Evolutionary Origin of Wild Allopolyploid Tobaccos (Nicotiana Section Suaveolentes). Evolution 2013, 67, 80–94. [Google Scholar] [CrossRef]
- Bazzini, A.A.; Manacorda, C.A.; Tohge, T.; Conti, G.; Rodriguez, M.C.; Nunes-Nesi, A.; Villanueva, S.; Fernie, A.R.; Carrari, F.; Asurmendi, S. Metabolic and MiRNa Profiling of TMV Infected Plants Reveals Biphasic Temporal Changes. PLoS ONE 2011, 6, e28466. [Google Scholar] [CrossRef]
- Zimmermann, M.M.; Grundmann, L.; Känel, A.; Schwarze, A.; Wiedmann, D.R.; Muth, J.; Twyman, R.M.; Prüfer, D.; Noll, G.A. Unraveling the Mystery behind the Short-Day-Specific Flowering of Tobacco Cultivar Maryland Mammoth. bioRxiv 2022, 37, 140–152. [Google Scholar] [CrossRef]
- Keinänen, M.; Oldham, N.J.; Baldwin, I.T. Rapid HPLC Screening of Jasmonate-Induced Increases in Tobacco Alkaloids, Phenolics, and Diterpene Glycosides in Nicotiana attenuata. J. Agric. Food Chem. 2001, 49, 3553–3558. [Google Scholar] [CrossRef]
- He, Z.; Webster, S.; He, S.Y. Growth–Defense Trade-Offs in Plants. Curr. Biol. 2022, 32, R634–R639. [Google Scholar] [CrossRef]
- Mhinana, Z.; Mayekiso, B.; Magwa, M.L. Anatomy and Morphology of Nicotiana glauca with Regard to Its Crystals Characterization. Afr. J. Plant Sci. 2010, 4, 172–178. [Google Scholar]
- Negin, B.; Hen-Avivi, S.; Almekias-Siegl, E.; Shachar, L.; Jander, G.; Aharoni, A. Tree Tobacco (Nicotiana glauca) Cuticular Wax Composition Is Essential for Leaf Retention during Drought, Facilitating a Speedy Recovery Following Rewatering. New Phytol. 2023, 237, 1574–1589. [Google Scholar] [CrossRef] [PubMed]
- Negin, B.; Shachar, L.; Meir, S.; Ramirez, C.C.; Rami Horowitz, A.; Jander, G.; Aharoni, A. Fatty Alcohols, a Minor Component of the Tree Tobacco Surface Wax, Are Associated with Defence Against Caterpillar Herbivory. Plant Cell Environ. 2024, 47, 664–681. [Google Scholar] [CrossRef]
- Feng, H.; Acosta-Gamboa, L.; Kruse, L.H.; Tracy, J.D.; Chung, S.H.; Nava Fereira, A.R.; Shakir, S.; Xu, H.; Sunter, G.; Gore, M.A.; et al. Acylsugars Protect Nicotiana benthamiana Against Insect Herbivory and Desiccation. Plant Mol. Biol. 2022, 109, 505–522. [Google Scholar] [CrossRef]
- Poreddy, S.; Mitra, S.; Schöttner, M.; Chandran, J.; Schneider, B.; Baldwin, I.T.; Kumar, P.; Pandit, S.S. Detoxification of Hostplant’s Chemical Defence Rather than Its Anti-Predator Co-Option Drives β-Glucosidase-Mediated Lepidopteran Counteradaptation. Nat. Commun. 2015, 6, 8525. [Google Scholar] [CrossRef] [PubMed]


| # | Name | Formula | Putative Decorations | Previous Nomenclature [11] |
|---|---|---|---|---|
| 37 | 700.4 a | C35H56O14 | 1 Hex, 1 DHex, 1 Ma | DTG 718′ |
| 59 | 700.4 b | C35H56O14 | 1 Hex, 1 DHex, 1 Ma | DTG 718″ |
| 64 | 700.4 c | C35H56O14 | 1 Hex, 1 DHex, 1 Ma | DTG 718‴ |
| 43 | 630.4 | C32H54O12 | 2 Hex | Lyciumoside I |
| 24 | 776.4 a | C38H64O16 | 2 Hex, 1 DHex | DTG 794′ |
| 39 | 776.4 b | C38H64O16 | 2 Hex, 1 DHex | Lyciumoside IV |
| 47 | 776.4 c | C38H64O16 | 2 Hex, 1 DHex | DTG 794‴ |
| 29 | 862.4 a | C41H66O19 | 2 Hex, 1 DHex, 1Ma | DTG 880′ |
| 38 | 862.4 b | C41H66O19 | 2 Hex, 1 DHex, 1Ma | DTG 880″ |
| 45 | 862.4 d | C41H66O19 | 2 Hex, 1 DHex, 1Ma | Nicotianoside Ib |
| 46 | 862.4 c + d + e * | C41H66O19 | 2 Hex, 1 DHex, 1Ma | Nicotianoside Ia + b + c |
| 50 | 862.4 e | C41H66O19 | 2 Hex, 1 DHex, 1Ma | Nicotianoside Ic |
| 36 | 948.4 a | C44H68O22 | 2 Hex, 1 Dhex, 2 Ma | DTG 966 |
| 52 | 948.4 b | C44H68O22 | 2 Hex, 1 Dhex, 2 Ma | Nicotianoside IIb |
| 58 | 948.4 c | C44H68O22 | 2 Hex, 1 Dhex, 2 Ma | Nicotianoside IIc |
| 3 | 716.4 a | C35H56O15 | 2 Hex, 1 Ma | DTG 734′ |
| 15 | 716.4 b | C35H56O15 | 2 Hex, 1 Ma | DTG 734″ |
| 49 | 716.4 c | C35H56O15 | 2 Hex, 1 Ma | Nicotianoside IXc |
| 54 | 716.4 d + e * | C35H56O15 | 2 Hex, 1 Ma | DTG 734‴ + DTG 734⁗ |
| 57 | 716.4 e | C35H56O15 | 2 Hex, 1 Ma | DTG 734⁗ |
| 60 | 716.4 e + f * | C35H56O15 | 2 Hex, 1 Ma | DTG 734⁗ + 734′′′′′ |
| 22 | 922.5 a | C44H74O20 | 2 Hex, 2 Dhex | DTG 940 |
| 31 | 922.5 b | C44H74O20 | 2 Hex, 2 Dhex | Nicotianoside III |
| 28 | 1008.5 a | C47H76O23 | 2 Hex, 2 DHex, 1 Ma | DTG 1026′ |
| 35 | 1008.5 b | C47H76O23 | 2 Hex, 2 DHex, 1 Ma | DTG 1026″ |
| 42 | 1008.5 c | C47H76O23 | 2 Hex, 2 DHex, 1 Ma | Nicotianoside IV |
| 34 | 1094.5 a | C50H78O26 | 2 Hex, 2 DHex, 2 Ma | DTG 1112′ |
| 41 | 1094.5 b | C50H78O26 | 2 Hex, 2 DHex, 2 Ma | DTG 1112″ |
| 44 | 1094.5 c | C50H78O26 | 2 Hex, 2 DHex, 2 Ma | DTG 1112‴ |
| 51 | 1094.5 d | C50H78O26 | 2 Hex, 2 Dhex, 2 Ma | Nicotianoside V |
| 53 | 802.4 a | C38H58O18 | 2 Hex, 2 Ma | Nicotianoside Xb |
| 55 | 802.4 a + b * | C38H58O18 | 2 Hex, 2 Ma | Nicotianoside Xb + c |
| 62 | 802.4 c | C38H58O18 | 2 Hex, 2 Ma | Nicotianoside Xd |
| 63 | 802.4 d | C38H58O18 | 2 Hex, 2 Ma | Nicotianoside X |
| 21 | 1068.5 | C50H84O24 | 2 Hex, 3 Dhex | DTG 1086 |
| 27 | 1154.5 | C53H86O27 | 2 Hex, 3 DHex, 1 Ma | DTG 1172 |
| 6 | 938.5 a | C44H74O21 | 3 Hex, 1 Dhex | HGL-DTG 956 |
| 9 | 938.5 b | C44H74O21 | 3 Hex, 1 Dhex | Attenoside |
| 7 | 1024.5 a | C47H76O24 | 3 Hex, 1 DHex, 1 Ma | DTG 1042 |
| 12 | 1024.5 b | C47H76O24 | 3 Hex, 1 Dhex, 1 Ma | HGL-DTG 1042 |
| 14 | 1024.5 c | C47H76O24 | 3 Hex, 1 Dhex, 1 Ma | Nicotianoside VIa |
| 17 | 1024.5 d | C47H76O24 | 3 Hex, 1 Dhex, 1 Ma | Nicotianoside VIb |
| 18 | 1024.5 e | C47H76O24 | 3 Hex, 1 Dhex, 1 Ma | Nicotianoside VIc |
| 20 | 1024.5 f | C47H76O24 | 3 Hex, 1 Dhex, 1 Ma | DTG 1042 |
| 10 | 1110.5 a | C50H78O27 | 3 Hex, 1 DHex, 2 Ma | DTG 1128 |
| 23 | 1110.5 b | C50H78O27 | 3 Hex, 1 Dhex, 2 Ma | Nicotianoside VIIa |
| 25 | 1110.5 c | C50H78O27 | 3 Hex, 1 Dhex, 2 Ma | Nicotianoside VIIb |
| 30 | 1196.5 a | C53H80O30 | 3 Hex, 1 Dhex, 3 Ma | Nicotianoside VIIIa |
| 33 | 1196.5 b | C53H80O30 | 3 Hex, 1 Dhex, 3 Ma | Nicotianoside VIIIb |
| 16 | 878.4 a | C41H66O20 | 3 Hex, 1 Ma | DTG 896′ |
| 19 | 878.4 b + c * | C41H66O20 | 3 Hex, 1 Ma | Nicotianoside XIb+c |
| 40 | 878.4 d | C41H66O20 | 3 Hex, 1 Ma | DTG 896″ |
| 1 | 1084.5 a | C50H84O25 | 3 Hex, 2 DHex | DTG 1102′ |
| 2 | 1084.5 b | C50H84O25 | 3 Hex, 2 DHex | DTG 1102″ |
| 11 | 1084.5 c | C50H84O25 | 3 Hex, 2 DHex | DTG 1102‴ |
| 4 | 1170.5 a | C53H86O28 | 3 Hex, 2 DHex, 1 Ma | DTG 1188′ |
| 5 | 1170.5 b | C53H86O28 | 3 Hex, 2 DHex, 1 Ma | DTG 1188″ |
| 8 | 1256.5 a | C56H88O31 | 3 Hex, 2 DHex, 2 Ma | DTG 1274′ |
| 13 | 1256.5 b | C56H88O31 | 3 Hex, 2 DHex, 2 Ma | DTG 1274″ |
| 26 | 964.4 a + b * | C44H68O23 | 3 Hex, 2 Ma | Nicotianoside XIIa + b |
| 48 | 964.4 c | C44H68O23 | 3 Hex, 2 Ma | Nicotianoside XII |
| 56 | 964.4 d + e * | C44H68O23 | 3 Hex, 2 Ma | DTG 982″ + DTG 982‴ |
| 61 | 964.4 e | C44H68O23 | 3 Hex, 2 Ma | DTG 982‴ |
| 32 | 1050.4 | C47H70O26 | 3 Hex, 3 Ma | Nicotianoside XIIIa |
| # | Species Name | Ref. | Structure Details [11] | Detected in N. attenuata | 1. IL [28] | 2. IL |
|---|---|---|---|---|---|---|
| 37 | Nicotiana tomentosiformis | [11] | no | D | II | |
| 59 | n. d. | no | B(i) | II | ||
| 64 | n. d. | no | D | III | ||
| 43 | Nicotiana attenuata, Lycium chinense | [5] | Glc, Glc | yes | D | I |
| 24 | Nicotiana alata, Capsicum spp. | [11] | no | B(i) | II | |
| 39 | Nicotiana attenuata, N. africana, N. cavicola, N. obtusifolia, N. tomentosiformis, Lycium chinense | [8] | yes | B(i) | I | |
| 47 | Nicotiana africana, N. tomentosiformis | [11] | no | D | II | |
| 29 | n. d. | no | B(i) | III | ||
| 38 | Capsicum annuum | [11] | no | B(i) | II | |
| 45 | Nicotiana attenuata, N. acuminata, N. africana, N. cavicola, N. clevelandii, N. obtusifolia, N. pauciflora, N. tomentosiformis | [10] | Glc Ma (6-1), Glcc Rha (4-1) | yes | D | I |
| 46 | Nicotiana attenuata, N. acuminata, N. africana, N. cavicola, N. clevelandii, N. obtusifolia, N. pauciflora, N. tomentosiformis | [10] | Glc-Ma (6-1), Glc-Rha (4-1) | yes, ambigous | B(i) | II/I |
| 50 | Nicotiana attenuata | [10] | Glc-Ma (6-1), Glc-Rha (4-1) | yes | B(i) | I |
| 36 | n. d. | no | D | IV | ||
| 52 | Nicotiana attenuata, N. acuminata, N. cavicola, N. clevelandii, N. obtusifolia, N. pauciflora, N. spegazzini | [10] | Glc-Ma (6-1), Glc-Rha (4-1)-Ma (6-1) | yes | B(i) | I |
| 58 | Nicotiana attenuata, N. acuminata, N. cavicola, N. clevelandii, N. obtusifolia, N. pauciflora | [10] | Glc-Ma (6-1), Glc-Rha (4-1)-Ma (6-1) | yes, ambigous | B(i) | II/I |
| 3 | n. d. | no | D | III | ||
| 15 | n. d. | no | D | III | ||
| 49 | Nicotiana attenuata | [11] | Glc, Glc, Ma | yes | B(i) | I |
| 54 | Lycium barbarum | [11] | no | B(i) | II | |
| 57 | Lycium barbarum | [11] | no | B(i) | II | |
| 60 | Lycium barbarum | [11] | no | B(i) | II | |
| 22 | Nicotiana benthamiana, N. alata, Capsicum spp. | [11] | no | B(i) | II | |
| 31 | Nicotiana attenuata, N. africana, N. cavicola, N. clevelandii, N. linearis, N. pauciflora, N. spegazzini | [10] | Glc-Rha (4-1), Glc-Rha (4-1) | yes | D | I |
| 28 | Nicotiana benthamiana | [11] | no | B(i) | II | |
| 35 | Nicotiana benthamiana, Capsicum spp. | [11] | no | B(i) | II | |
| 42 | Nicotiana attenuata/Nicotiana obtusifolia, N. acuminata, N. africana, N. cavicola, N. clevelandii, N. linearis, N. pauciflora, N. spegazzini, N. tomentosiformis | [10]/[29] | Glc-Rha (4-1), Glc-Rha (4-1), Ma | yes | B(i) | I |
| 34 | n. d. | no | D | III | ||
| 41 | n. d. | no | D | III | ||
| 44 | n. d. | no | B(i) | III | ||
| 51 | Nicotiana attenuata/Nicotiana obtusifolia, N. acuminata, N. africana, N. alata, N. cavicola, N. clevelandii, N. linearis, N. miersii, N. pauciflora, N. spegazzini, N. tomentosiformis | [10]/[29] | Glc-Rha (4-1), Glc-Rha (4-1), 2 Ma | yes | B(i) | I |
| 53 | Nicotiana attenuata, N. cavicola, N. obtusifolia | [11] | Glc, Glc, 2 Ma | yes | B(i) | I |
| 55 | Nicotiana attenuata, N. cavicola, N. obtusifolia | [11] | Glc, Glc, 2 Ma | yes, ambigous | B(i) | II/I |
| 62 | Nicotiana attenuata | [11] | Glc, Glc, 2 Ma | yes | B(i) | I |
| 63 | n. d. | yes | D | III | ||
| 21 | [6] | no | B(i) | IV | ||
| 27 | Nicotiana benthamiana | [11] | no | B(i) | II | |
| 6 | Nicotiana attenuata, N. alata, N. cavicola, N. obtusifolia | [11] | Glc-Glc (2-1), Glc-Rha (4-1) | yes | D | I |
| 9 | Nicotiana attenuata | [16] | Glc-Glc (2-1), Glc-Rha (4-1) | yes | D | I |
| 7 | Capsicum annuum | [11] | no | D | II | |
| 12 | Nicotiana attenuata, N. cavicola, Lycium barbarum | [11] | Glc-Glc-Glc-Rha#, Ma | yes | D | I |
| 14 | Nicotiana attenuata, N. acuminata, N. clevelandii, N. pauciflora, N. spegazzini | [10] | Glc-Glc (2-1), Glc-Rha (4-1), 1 Ma | yes | D | I |
| 17 | Nicotiana attenuata, N. acuminata, N. clevelandii, N. pauciflora, N. spegazzini | [11] | Glc-Glc (2-1), Glc-Rha (4-1), 1 Ma | yes | D | I |
| 18 | Nicotiana attenuata, N. clevelandii | [11] | Glc-Glc (2-1), Glc-Rha (4-1), 1 Ma | yes | B(i) | I |
| 20 | n. d. | no | D | III | ||
| 10 | Lycium barbarum | [11] | no | B(i) | II | |
| 23 | Nicotiana attenuata, N. acuminata, N. clevelandii, N. pauciflora, N. spegazzini | [10] | Glc-Glc (2-1), Glc-Rha (4-1), 2 Ma | yes | D | I |
| 25 | Nicotiana attenuata, N. acuminata, N. cavicola, N. pauciflora, N. spegazzini | [11] | Glc-Glc (2-1), Glc-Rha (4-1), 2 Ma | yes | B(i) | I |
| 30 | Nicotiana attenuata, N. acuminata, N. clevelandii, N. pauciflora, N. spegazzini | [11] | Glc-Glc (2-1), Glc-Rha (4-1), 3 Ma | yes | D | I |
| 33 | Nicotiana attenuata, N. acuminata | [11] | Glc-Glc (2-1), Glc-Rha (4-1), 3 Ma | yes | D | I |
| 16 | n. d. | no | B(i) | III | ||
| 19 | Nicotiana attenuata | [11] | Glc-Glc (2-1), Glc, Ma | yes | B(i) | I |
| 40 | n. d. | yes | B(i) | III | ||
| 1 | Nicotiana alata, Capsicum spp. | [11] | no | B(i) | II | |
| 2 | Capsicum spp. | [11] | no | D | II | |
| 11 | n. d. | no | D | III | ||
| 4 | Nicotiana alata, Capsicum spp. | [11] | no | D | II | |
| 5 | Nicotiana alata, Capsicum spp. | [11] | no | B(i) | II | |
| 8 | Nicotiana alata | [11] | no | D | II | |
| 13 | Nicotiana alata | [11] | no | D | II | |
| 26 | Nicotiana attenuata, N. clevelandii, N. corymbosa, N. pauciflora | [11] | Glc-Glc (2-1), Glc, 2 Ma | yes | B(i) | I |
| 48 | Nicotiana acuminata, N. pauciflora | [11] | Glc-Glc (2-1), Glc, 2 Ma | yes | D | II |
| 56 | n. d. | no | B(i) | III | ||
| 61 | n. d. | no | B(i) | III | ||
| 32 | Nicotiana attenuata, N. quadrivalvis | [11] | Glc-Glc (2-1), Glc, 3 Ma | yes | D | I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebert, A.; Alseekh, S.; D’Andrea, L.; Roessner, U.; Bock, R.; Kopka, J. Detailed Profiling of 17-Hydroxygeranyllinalool Diterpene Glycosides from Nicotiana Species Reveals Complex Reaction Networks of Conjugation Isomers. Metabolites 2024, 14, 562. https://doi.org/10.3390/metabo14100562
Ebert A, Alseekh S, D’Andrea L, Roessner U, Bock R, Kopka J. Detailed Profiling of 17-Hydroxygeranyllinalool Diterpene Glycosides from Nicotiana Species Reveals Complex Reaction Networks of Conjugation Isomers. Metabolites. 2024; 14(10):562. https://doi.org/10.3390/metabo14100562
Chicago/Turabian StyleEbert, Alina, Saleh Alseekh, Lucio D’Andrea, Ute Roessner, Ralph Bock, and Joachim Kopka. 2024. "Detailed Profiling of 17-Hydroxygeranyllinalool Diterpene Glycosides from Nicotiana Species Reveals Complex Reaction Networks of Conjugation Isomers" Metabolites 14, no. 10: 562. https://doi.org/10.3390/metabo14100562
APA StyleEbert, A., Alseekh, S., D’Andrea, L., Roessner, U., Bock, R., & Kopka, J. (2024). Detailed Profiling of 17-Hydroxygeranyllinalool Diterpene Glycosides from Nicotiana Species Reveals Complex Reaction Networks of Conjugation Isomers. Metabolites, 14(10), 562. https://doi.org/10.3390/metabo14100562






