Association between Inflammatory and Metabolic Biomarkers and Common Mental Disorders among Adults: 2015 Health Survey of São Paulo, SP, Brazil
Abstract
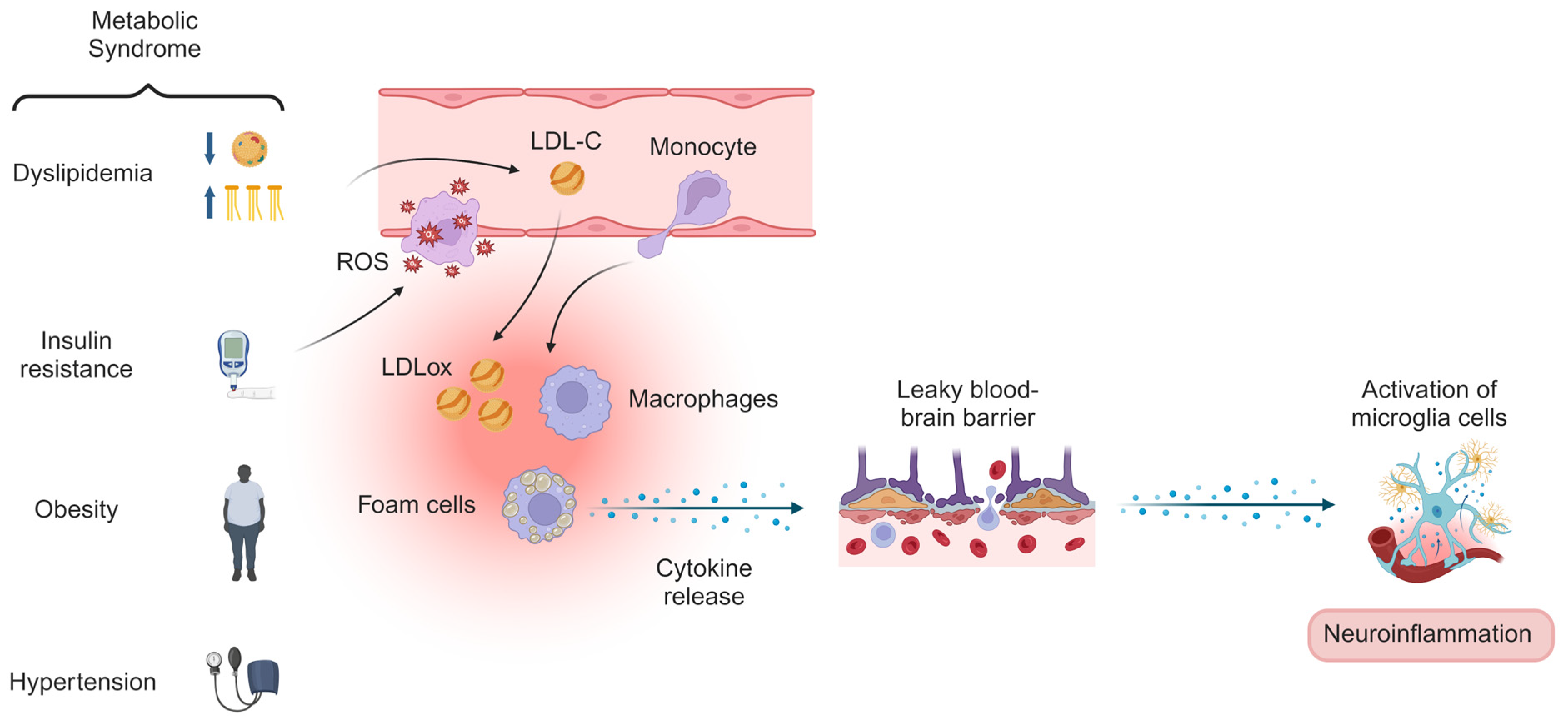
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.2.1. Common Mental Disorders
2.2.2. Blood Samples
2.2.3. Inflammatory Biomarkers
2.2.4. Systemic Low-Grade Inflammation Score
2.2.5. Cardiometabolic Biomarkers
2.2.6. Metabolic Syndrome
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuring, J.K.; Mathias, J.L.; Ward, L.; Tachas, G. Inflammatory Markers in Persons with Clinically-Significant Depression, Anxiety or PTSD: A Systematic Review and Meta-Analysis. J. Psychiatr. Res. 2023, 168, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Thylur, D.S.; Goldsmith, D.R. Brick by Brick: Building a Transdiagnostic Understanding of Inflammation in Psychiatry. Harv. Rev. Psychiatry 2022, 30, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.E.; Teixeira, A.L. Inflammation in Psychiatric Disorders: What Comes First? Ann. N. Y. Acad. Sci. 2019, 1437, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Angiulli, F.; Conti, E.; Zoia, C.P.; Da Re, F.; Appollonio, I.; Ferrarese, C.; Tremolizzo, L. Blood-Based Biomarkers of Neuroinflammation in Alzheimer’s Disease: A Central Role for Periphery? Diagnostics 2021, 11, 1525. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Raison, C.L. The Role of Inflammation in Depression: From Evolutionary Imperative to Modern Treatment Target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Hasheminasabgorji, E.; Jha, J.C. Dyslipidemia, Diabetes and Atherosclerosis: Role of Inflammation and Ros-Redox-Sensitive Factors. Biomedicines 2021, 9, 1602. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, Metaflammation and Immunometabolic Disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Stein, D.J.; Benjet, C.; Gureje, O.; Lund, C.; Scott, K.M.; Poznyak, V.; Van Ommeren, M. Integrating Mental Health with Other Non-Communicable Diseases. BMJ 2019, 364, l295. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Fahed, G.; Aoun, L.; Zerdan, M.B.; Allam, S.; Zerdan, M.B.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Fisberg, R.M.; Sales, C.H.; De Mello Fontanelli, M.; Pereira, J.L.; Alves, M.C.G.P.; Escuder, M.M.L.; César, C.L.G.; Goldbaum, M. 2015 Health Survey of São Paulo with Focus in Nutrition: Rationale, Design, and Procedures. Nutrients 2018, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Obesity: Preventing and Managing the Global Epidemic; Report of a WHO Consultation (WHO Technical Report Series); World Health Organization (WHO): Geneva, Switzerland, 2000. [Google Scholar]
- Lebrão, M.L.; Duarte, Y.A.O. SABE-Saúde, Bem-Estar e Envelhecimento-Projeto Sabe No Município de São Paulo: Uma Abordagem Inicial; Pan American Health Organization: Brasília, Brazil, 2003. [Google Scholar]
- Matsudo, S.; Araújo, T.; Matsudo, V.; Andrade, D.; Andrade, E.; Oliveira, L.C.; Braggion, G. Questionário Internacional de Atividade Física (IPAQ): Estudo de Validade e Reprodutibilidade No Brasil. Rev. Bras. Ativ. Fís. Saúde 2001, 6, 5–18. [Google Scholar] [CrossRef]
- Goldberg, D. A bio-social model for common mental disorders. Acta Psychiatr. Scand. Suppl. 1994, 385, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Regier, D.A.; Kuhl, E.A.; Kupfer, D.J. The DSM-5: Classification and criteria changes. World Psychiatry 2013, 12, 92–98. [Google Scholar] [CrossRef]
- Harding, T.W.; Climent, C.E.; De Arango, M.V.; Baltazar, J.; Ibrahim, H.H.A.; Ladrido-Ignacio, L.; Murthy, R.S.; Wig, N.N. Mental Disorders in Primary Health Care: A Study of Their Frequency and Diagnosis in Four Developing Countries. Psychol. Med. 1980, 10, 231–241. [Google Scholar] [CrossRef]
- Mari, J.d.J.; Williams, P. A Validity Study of a Psychiatric Screening Questionnaire (SRQ-20) in Primary Care in the City of São Paulo. Br. J. Psychiatry 1986, 148, 23–26. [Google Scholar] [CrossRef]
- Scazufca, M.; Menezes, P.R.; Vallada, H.; Araya, R. Validity of the Self Reporting Questionnaire-20 in Epidemiological Studies with Older Adults: Results from the Sao Paulo Ageing & Health Study. Soc. Psychiatry Psychiatr. Epidemiol. 2009, 44, 247–254. [Google Scholar] [CrossRef]
- Tabung, F.K.; Smith-Warner, S.A.; Chavarro, J.E.; Wu, K.; Fuchs, C.S.; Hu, F.B.; Chan, A.T.; Willett, W.C.; Giovannucci, E.L. Development and Validation of an Empirical Dietary Inflammatory Index. J. Nutr. 2016, 146, 1560–1570. [Google Scholar] [CrossRef]
- Editorial, C. Diretrizes Sociedade Brasileira de Diabetes 2019–2020. Alamedas 2021, 9, 215–217. [Google Scholar] [CrossRef]
- Précoma, D.B.; de Oliveira, G.M.M.; Simão, A.F.; Dutra, O.P.; Coelho, O.R.; Izar, M.C.d.O.; Póvoa, R.M.D.S.; Giuliano, I.d.C.B.; Filho, A.C.d.A.; Machado, C.A.; et al. Updated Cardiovascular Prevention Guideline of the Brazilian Society of Cardiology—2019. Arq. Bras. Cardiol. 2019, 113, 787–891. [Google Scholar] [CrossRef]
- Faludi, A.; Izar, M.; Saraiva, J.; Chacra, A.; Bianco, H.; Afiune Neto, A.; Bertolami, A.; Pereira, A.; Lottenberg, A.; Sposito, A.; et al. Atualização da Diretriz Brasileira de Dislipidemias e Prevenção da Aterosclerose—2017. Arq. Bras. Cardiol. 2017, 109, 1–76. [Google Scholar] [CrossRef] [PubMed]
- Geloneze, B.; Vasques, A.C.J.; Stabe, C.F.C.; Pareja, J.C.; de Lima Rosado, L.E.F.P.; de Queiroz, E.C.; Tambascia, M.A. Índices HOMA1-IR e HOMA2-IR Para Identificação de Resistência à Insulina e Síndrome Metabólica—Estudo Brasileiro de Síndrome Metabólica (BRAMS). Arq. Bras. Endocrinol. Metabol. 2009, 53, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef] [PubMed]
- Lü, P.; Liu, Y.; Yu, X.; Shi, C.L.; Liu, X. The Right Microbe-Associated Molecular Patterns for Effective Recognition by Plants. Front. Microbiol. 2022, 13, 1019069. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front. Immunol. 2021, 11, 594150. [Google Scholar] [CrossRef]
- Kim, H.K.; Andreazza, A.C.; Elmi, N.; Chen, W.; Young, L.T. Nod-like Receptor Pyrin Containing 3 (NLRP3) in the Post-Mortem Frontal Cortex from Patients with Bipolar Disorder: A Potential Mediator between Mitochondria and Immune-Activation. J. Psychiatr. Res. 2016, 72, 43–50. [Google Scholar] [CrossRef]
- Kim, H.K.; Chen, W.; Andreazza, A.C. The Potential Role of the NLRP3 Inflammasome as a Link between Mitochondrial Complex I Dysfunction and Inflammation in Bipolar Disorder. Neural Plast. 2015, 2015, 408136. [Google Scholar] [CrossRef]
- Wieck, A.; Grassi-Oliveira, R.; do Prado, C.H.; Viola, T.W.; Petersen, L.E.; Porto, B.; Teixeira, A.L.; Bauer, M.E. Toll-like Receptor Expression and Function in Type I Bipolar Disorder. Brain. Behav. Immun. 2016, 54, 110–121. [Google Scholar] [CrossRef]
- Xu, J.; Núñez, G. The NLRP3 Inflammasome: Activation and Regulation. Trends Biochem. Sci. 2023, 48, 331–344. [Google Scholar] [CrossRef]
- Osier, N.; Motamedi, V.; Edwards, K.; Puccio, A.; Diaz-Arrastia, R.; Kenney, K.; Gill, J. Exosomes in Acquired Neurological Disorders: New Insights into Pathophysiology and Treatment. Mol. Neurobiol. 2018, 55, 9280–9293. [Google Scholar] [CrossRef]
- Neurauter, G.; Schröcksnadel, K.; Scholl-Bürgi, S.; Sperner-Unterweger, B.; Schubert, C.; Ledochowski, M.; Fuchs, D. Chronic Immune Stimulation Correlates with Reduced Phenylalanine Turnover. Curr. Drug Metab. 2008, 9, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Leonard, B.E.; Myint, A.M.; Kubera, M.; Verkerk, R. The New “5-HT” Hypothesis of Depression: Cell-Mediated Immune Activation Induces Indoleamine 2,3-Dioxygenase, Which Leads to Lower Plasma Tryptophan and an Increased Synthesis of Detrimental Tryptophan Catabolites (TRYCATs), Both of Which Contribute to the Onset of Depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 702–721. [Google Scholar]
- Zhu, C.B.; Lindler, K.M.; Owens, A.W.; Daws, L.C.; Blakely, R.D.; Hewlett, W.A. Interleukin-1 Receptor Activation by Systemic Lipopolysaccharide Induces Behavioral Despair Linked to MAPK Regulation of CNS Serotonin Transporters. Neuropsychopharmacology 2010, 35, 2510–2520. [Google Scholar] [CrossRef] [PubMed]
- Tavares, R.G.; Tasca, C.I.; Santos, C.E.S.; Alves, L.B.; Porciúncula, L.O.; Emanuelli, T.; Souza, D.O. Quinolinic Acid Stimulates Synaptosomal Glutamate Release and Inhibits Glutamate Uptake into Astrocytes. Neurochem. Int. 2002, 40, 621–627. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Fukunaga, Y.; Bading, H. Extrasynaptic NMDARs Oppose Synaptic NMDARs by Triggering CREB Shut-off and Cell Death Pathways. Nat. Neurosci. 2002, 5, 405–414. [Google Scholar] [CrossRef]
- Ng, A.; Tam, W.W.; Zhang, M.W.; Ho, C.S.; Husain, S.F.; McIntyre, R.S.; Ho, R.C. IL-1β, IL-6, TNF-α and CRP in Elderly Patients with Depression or Alzheimer’s Disease: Systematic Review and Meta-Analysis. Sci. Rep. 2018, 8, 12050. [Google Scholar] [CrossRef]
- Paugh, S.W.; Bonten, E.J.; Savic, D.; Ramsey, L.B.; Thierfelder, W.E.; Gurung, P.; Malireddi, R.K.S.; Actis, M.; Mayasundari, A.; Min, J.; et al. NALP3 Inflammasome Upregulation and CASP1 Cleavage of the Glucocorticoid Receptor Cause Glucocorticoid Resistance in Leukemia Cells. Nat. Genet. 2015, 47, 607–614. [Google Scholar] [CrossRef]
- Raison, C.L.; Miller, A.H. Reviews and Overviews When Not Enough Is Too Much: The Role of Insufficient Glucocorticoid Signaling in the Pathophysiology of Stress-Related Disorders. Am. J. Psychiatry 2003, 160, 1554–1565. [Google Scholar] [CrossRef]
- Agrawal, A.; Wu, Y. Editorial: Biology of C-Reactive Protein. Front. Immunol. 2024, 15, 1445001. [Google Scholar] [CrossRef]
- Windgassen, E.B.; Funtowicz, L.; Lunsford, T.N.; Harris, L.A.; Mulvagh, S.L. C-Reactive Protein and High-Sensitivity C-Reactive Protein: An Update for Clinicians. Postgrad. Med. 2011, 123, 114–119. [Google Scholar] [CrossRef]
- Orsolini, L.; Pompili, S.; Valenta, S.T.; Salvi, V.; Volpe, U. C-Reactive Protein as a Biomarker for Major Depressive Disorder? Int. J. Mol. Sci. 2022, 23, 1616. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, L.R.; Hong, S.; Tarraf, W.; Perreira, K.; Camacho, Á.; Kohn, J.N.; Jimenez, D.E.; Talavera, G.A.; Gallo, L.; Allison, M.A.; et al. Association of Anxiety and Depressive Symptoms with C-Reactive Protein in Diverse Latinos: Results from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). PLoS ONE 2023, 18, e0289833. [Google Scholar] [CrossRef] [PubMed]
- Costello, H.; Gould, R.L.; Abrol, E.; Howard, R. Systematic Review and Meta-Analysis of the Association between Peripheral Inflammatory Cytokines and Generalised Anxiety Disorder. BMJ Open 2019, 9, e027925. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, S.; Nagy, C.; Ibrahim, P.; Théroux, J.F.; Wakid, M.; Fiori, L.M.; Yang, J.; Rotzinger, S.; Foster, J.A.; Mechawar, N.; et al. Neuron-Derived Extracellular Vesicles Enriched from Plasma Show Altered Size and MiRNA Cargo as a Function of Antidepressant Drug Response. Mol. Psychiatry 2021, 26, 7417–7424. [Google Scholar] [CrossRef]
- Bubb, K.J.; Drummond, G.R.; Figtree, G.A. New Opportunities for Targeting Redox Dysregulation in Cardiovascular Disease. Cardiovasc. Res. 2020, 116, 532–544. [Google Scholar] [CrossRef]
- Katakami, N. Mechanism of Development of Atherosclerosis and Cardiovascular Disease in Diabetes Mellitus. J. Atheroscler. Thromb. 2018, 25, 27–39. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef]
- Molendijk, M.; Molero, P.; Sánchez-Pedreño, F.O.; Van der Does, W.; Martínez-González, M.A. Diet quality and depression risk: A systematic review and dose-response meta-analysis of prospective studies. J. Affect. Disord. 2018, 226, 346–354. [Google Scholar] [CrossRef]
- Tolkien, K.; Bradburn, S.; Murgatroyd, C. An anti-inflammatory diet as a potential intervention for depressive disorders: A systematic review and meta-analysis. Clin. Nutr. 2019, 38, 2045–2052. [Google Scholar] [CrossRef]
- Bastos, A.A.; Nogueira, L.R.; Neto, J.V.; Fisberg, R.M.; Yannakoulia, M.; Ribeiro, S.M.L. Association between the adherence to the Mediterranean dietary pattern and common mental disorders among community-dwelling elders: 2015 Health Survey of São Paulo, SP, Brazil. J. Affect. Disord. 2020, 265, 389–394. [Google Scholar] [CrossRef]
- Hosseinzadeh, M.; Vafa, M.; Esmaillzadeh, A.; Feizi, A.; Majdzadeh, R.; Afshar, H.; Keshteli, A.H.; Adibi, P. Empirically derived dietary patterns in relation to psychological disorders. Public Health Nutr. 2016, 19, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.M.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
| Variables (n) | n (%)/Median (Q1–Q3) |
|---|---|
| Age (years) (575) | 59 (43–68) |
| Sex assigned at birth (575) | |
| Male | 290 (50.4) |
| Female | 285 (49.6) |
| Alcohol (574) | |
| No | 402 (70) |
| Yes | 172 (30) |
| Smoking (575) | |
| No | 479 (83.3) |
| Yes | 96 (16.7) |
| Physical activity (564) | |
| Minutes/week | 480 (160–1205) |
| Nutritional status (570) | |
| Underweight | 61 (10.7) |
| Normal weight | 212 (37.2) |
| Overweight | 144 (25.3) |
| Obese | 153 (26.8) |
| Type 2 diabetes (575) | |
| No | 453 (78.8) |
| Yes | 122 (21.2) |
| Systemic arterial hypertension (570) | |
| No | 259 (45.4) |
| Yes | 311 (54.6) |
| Dyslipidemia (566) | |
| No | 164 (29) |
| Yes | 402 (71) |
| Common mental disorders (575) | |
| No | 445 (77.4) |
| Yes | 130 (22.6) |
| Inflammatory Biomarkers | Common Mental Disorders | p-Value | |
|---|---|---|---|
| No | Yes | ||
| IL-6 (pg/mL) | 1.40 | 1.30 | 0.71 |
| IL-1β (pg/mL) | 1.13 | 1.15 | 0.38 |
| IL-10 (pg/mL) | 4.02 | 4.15 | 0.24 |
| TNF-α (pg/mL) | 11.10 | 11.16 | 0.77 |
| PAI-1 (ng/mL) | 23.66 | 29.11 | 0.02 * |
| MCP-1 (ng/mL) | 0.29 | 0.31 | 0.36 |
| sICAM-1 (ng/mL) | 245 | 258 | 0.09 |
| sVCAM-1 (ng/mL) | 806 | 813 | 0.24 |
| CRP (mg/L) | 0.35 | 0.46 | 0.01 * |
| Leptin (ng/mL) | 1.76 | 2.31 | 0.08 |
| Adiponectin (ng/mL) | 19,212 | 18,340 | 0.80 |
| SIS | 1.29 | 1.47 | 0.02 * |
| Inflammatory Biomarkers | OR | 95% CI | p-Value |
|---|---|---|---|
| CRP (mg/L) | 1.54 | 1.09–2.19 | 0.01 * |
| PAI-1 (ng/mL) | 1.00 | 1.00–1.02 | 0.14 |
| SIS | 1.43 | 0.96–2.11 | 0.08 |
| Cardiometabolic Markers (n) | CRP (mg/L) | p-Value |
|---|---|---|
| Fasting glycemia | 0.38 | |
| <100 mg/dL (385) | 0.37 | |
| 100–126 mg/dL (190) | 0.40 | |
| HOMA-IR | <0.001 * | |
| <2.71 (289) | 0.30 | |
| >2.71 (285) | 0.53 | |
| Serum triglycerides | <0.001 * | |
| <150 mg/dL (404) | 0.32 | |
| >150 mg/dL (160) | 0.56 | |
| Total cholesterol | 0.05 | |
| <190 mg/dL (334) | 0.34 | |
| >190 mg/dL (230) | 0.43 | |
| LDL-c | 0.25 | |
| <130 mg/dL (396) | 0.37 | |
| >130 mg/dL (167) | 0.41 | |
| HDL-c | <0.001 * | |
| Male > 40 mg/dL (154) Female > 50 mg/dL (109) | 0.31 | |
| Male < 40 mg/dL (130) Female < 50 mg/dL (170) | 0.43 | |
| non-HDL-c | 0.03 * | |
| <160 mg/dL (405) | 0.35 | |
| >160 mg/dL (158) | 0.45 | |
| Metabolic syndrome | <0.001 * | |
| Yes (186) | 0.42 | |
| No (185) | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, L.d.N.M.; Fisberg, R.M.; Sarti, F.M.; Rogero, M.M. Association between Inflammatory and Metabolic Biomarkers and Common Mental Disorders among Adults: 2015 Health Survey of São Paulo, SP, Brazil. Metabolites 2024, 14, 535. https://doi.org/10.3390/metabo14100535
Ferreira LdNM, Fisberg RM, Sarti FM, Rogero MM. Association between Inflammatory and Metabolic Biomarkers and Common Mental Disorders among Adults: 2015 Health Survey of São Paulo, SP, Brazil. Metabolites. 2024; 14(10):535. https://doi.org/10.3390/metabo14100535
Chicago/Turabian StyleFerreira, Letícia do Nascimento Maximiano, Regina Mara Fisberg, Flavia Mori Sarti, and Marcelo Macedo Rogero. 2024. "Association between Inflammatory and Metabolic Biomarkers and Common Mental Disorders among Adults: 2015 Health Survey of São Paulo, SP, Brazil" Metabolites 14, no. 10: 535. https://doi.org/10.3390/metabo14100535
APA StyleFerreira, L. d. N. M., Fisberg, R. M., Sarti, F. M., & Rogero, M. M. (2024). Association between Inflammatory and Metabolic Biomarkers and Common Mental Disorders among Adults: 2015 Health Survey of São Paulo, SP, Brazil. Metabolites, 14(10), 535. https://doi.org/10.3390/metabo14100535






