The Kidney–Gut Axis as a Novel Target for Nutritional Intervention to Counteract Chronic Kidney Disease Progression
Abstract
1. Introduction
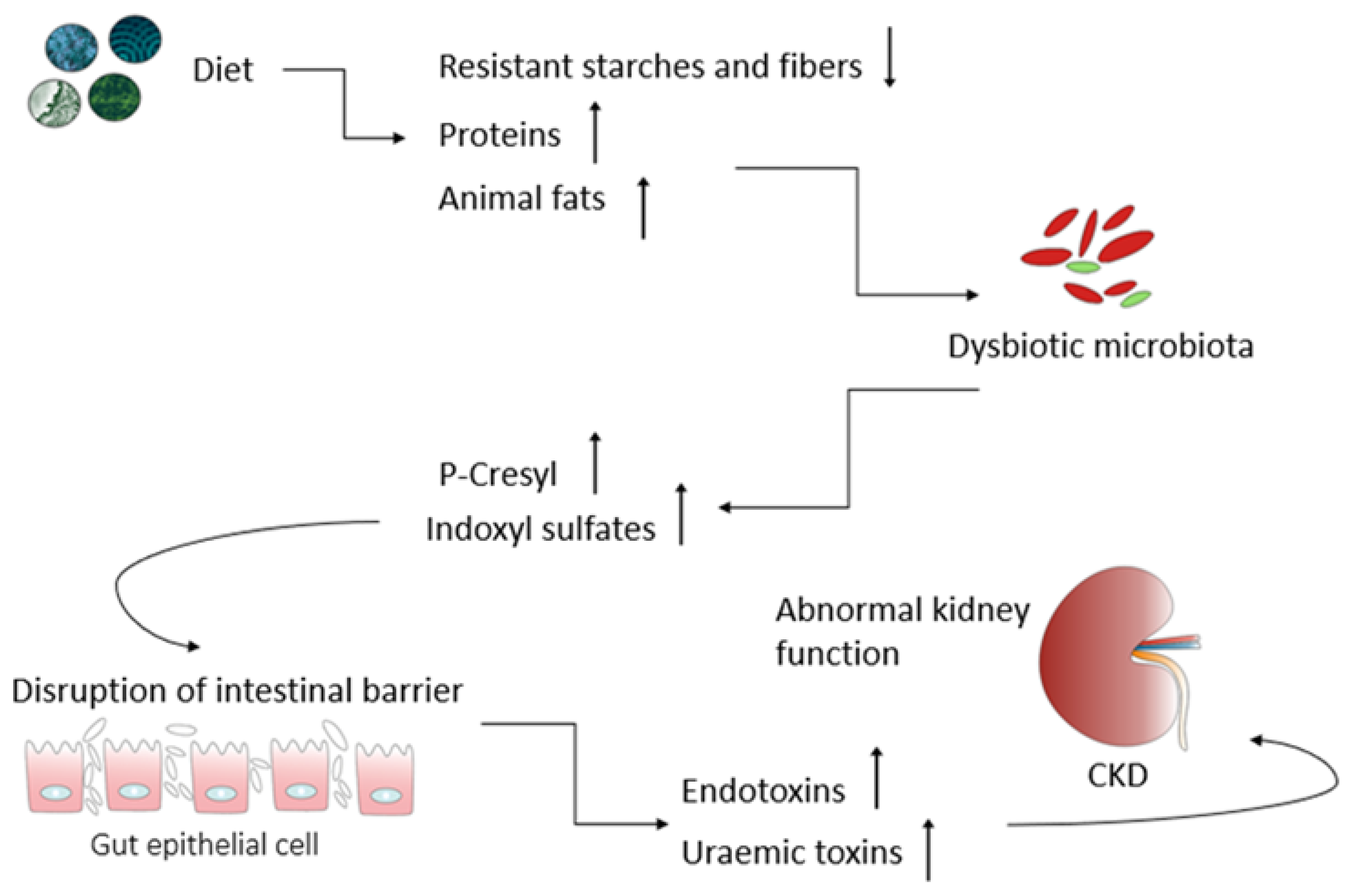
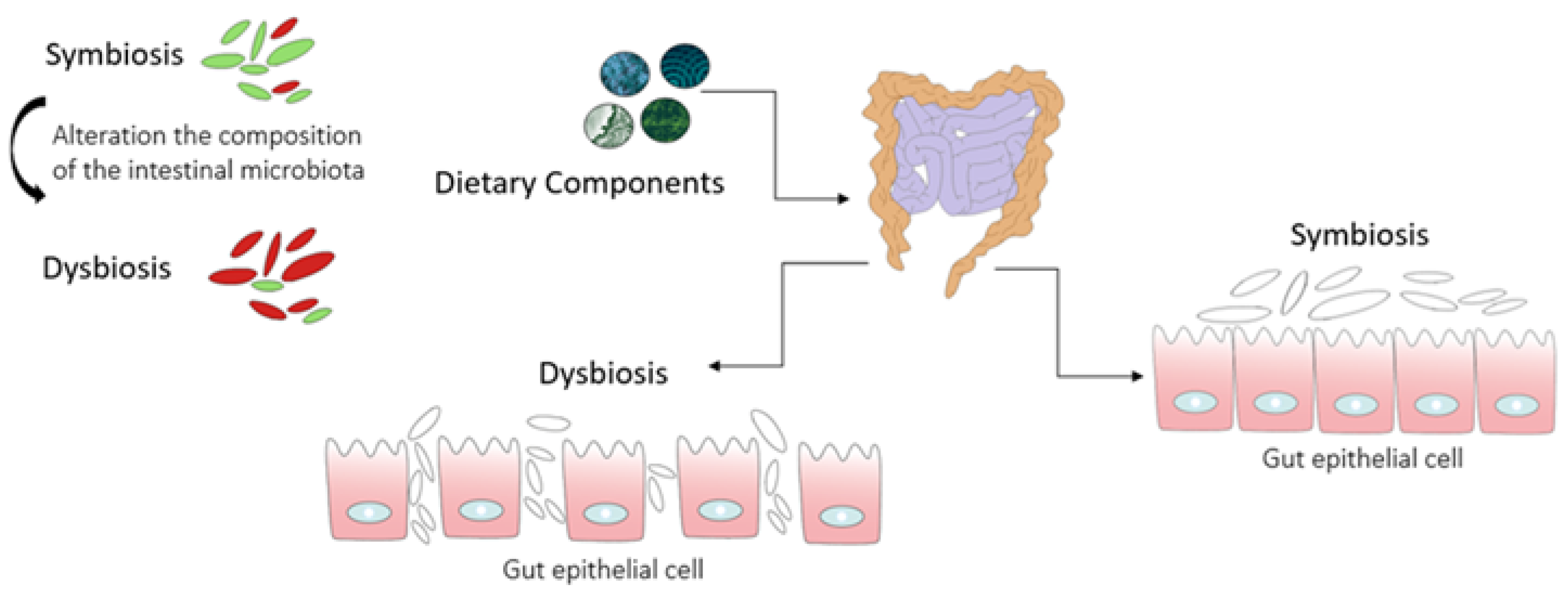
2. The Kidney–Gut Axis: A Potential Connection between Gut Dysbiosis and CKD
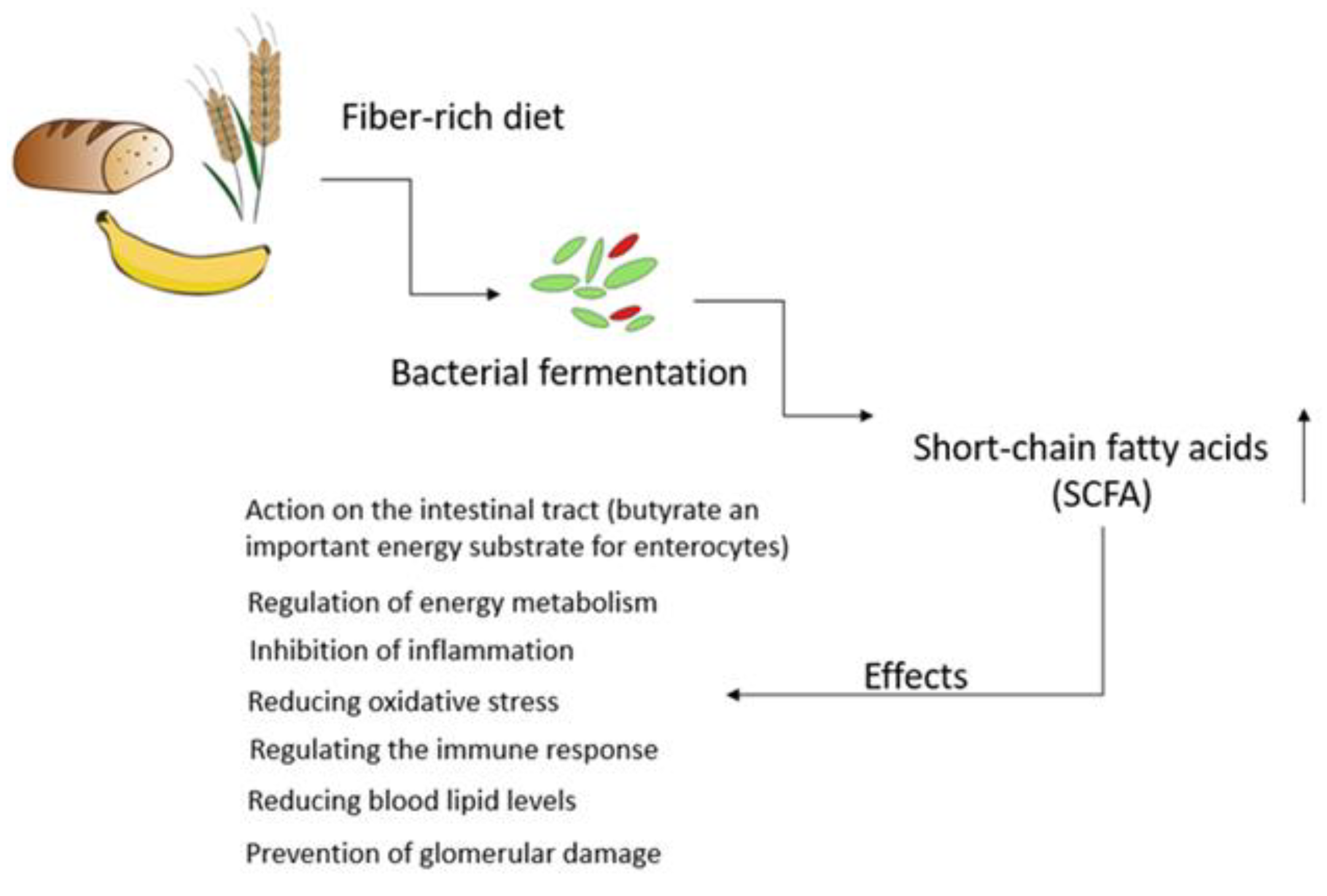
3. Nutritional Strategies Focusing on the Gut Microbiota as a Novel Treatment for Counteracting CKD Progression
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global impacts of western diet and its effects on metabolism and health: A narrative review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef] [PubMed]
- Dobrek, Ł. The kidney-gut axis in chronic kidney disease. Pol. Merkur. Lekarski 2022, 50, 323–327. [Google Scholar] [PubMed]
- Podkowińska, A.; Formanowicz, D. Chronic kidney disease as oxidative stress-and inflammatory-mediated cardiovascular disease. Antioxidants 2020, 9, 752. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulou, E.; Kantartzi, K.; Tsigalou, C.; Konstantinidis, T.; Romanidou, G.; Voidarou, C.; Bezirtzoglou, E. Focus on the gut–kidney axis in health and disease. Front. Med. 2021, 7, 620102. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.M.; Hard, G.C.; Alden, C.L. Chapter 47—Kidney. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology; Academic Press: Cambridge, MA, USA, 2013; pp. 1667–1773. [Google Scholar]
- Bhat, P.V.; Manolescu, D.C. Role of vitamin A in determining nephron mass and possible relationship to hypertension. J. Nutr. 2008, 138, 1407–1410. [Google Scholar] [CrossRef] [PubMed]
- Bonvalet, J.P.; Champion, M.; Courtalon, A.; Farman, N.; Vandewalle, A.; Wanstok, F. Number of glomeruli in normal and hypertrophied kidneys of mice and guinea pigs. J. Physiol. 1977, 269, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Johnson, A.C.; Williams, J.M.; White, T.; Chade, A.R.; Zhang, J.; Liu, R.; Roman, R.J.; Lee, J.W.; Kyle, P.B. Nephron deficiency and predisposition to renal injury in a novel one-kidney genetic model. J. Am. Soc. Nephrol. 2015, 26, 1634–1646. [Google Scholar] [CrossRef]
- van Vuuren, S.H.; Sol, C.M.; Broekhuizen, R.; Lilien, M.R.; Oosterveld, M.J.S.; Nguyen, T.Q.; Goldschmeding, R.; de Jong, T.P.V.M. Compensatory growth of congenital solitary kidneys in pigs reflects increased nephron numbers rather than hypertrophy. PLoS ONE 2012, 7, e49735. [Google Scholar] [CrossRef][Green Version]
- Wintour, E.M.; Moritz, K.M.; Johnson, K.; Ricardo, S.; Samuel, C.S.; Dodic, M. Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J. Physiol. 2003, 549, 929–935. [Google Scholar] [CrossRef]
- Cooper, T.E.; Khalid, R.; Craig, J.C.; Hawley, C.M.; Howell, M.; Johnson, D.W.; Teixeira-Pinto, A.; Tong, A.; Wong, G. Synbiotics, prebiotics and probiotics for people with chronic kidney disease. Cochrane Database Syst. Rev. 2023, 10, CD013631. [Google Scholar] [PubMed]
- Levey, A.S.; Stevens, L.A.; Coresh, J. Conceptual model of CKD: Applications and implications. Am. J. Kid. Dis. 2009, 53, S4–S16. [Google Scholar] [CrossRef] [PubMed]
- Falodia, J.; Singla, M.K. CKD epidemiology and risk factors. Clin. Queries Nephrol. 2012, 1, 249–252. [Google Scholar] [CrossRef]
- Horowitz, B.; Miskulin, D.; Zager, P. Epidemiology of hypertension in CKD. Adv. Chronic Kidney Dis. 2015, 22, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Bleyer, A.J.; Shemanski, L.R.; Burke, G.L.; Hansen, K.J.; Appel, R.G. Tobacco, hypertension, and vascular disease: Risk factors for renal functional decline in an older population. Kidney Int. 2000, 57, 2072–2079. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.S.; Patel, N.R.; Patel, J.V.; Chavda, A.B. A cross-sectional study to evaluate etiopathogenesis, clinical features, complications, and treatment of patients of chronic kidney disease. Natl. J. Physiol. Pharm. Pharmacol. 2022, 12, 680–683. [Google Scholar] [CrossRef]
- Magliocca, G.; Mone, P.; Di Iorio, B.R.; Heidland, A.; Marzocco, S. Short-chain fatty acids in chronic kidney disease: Focus on inflammation and oxidative stress regulation. Int. J. Mol. Sci. 2022, 23, 5354. [Google Scholar] [CrossRef]
- Lambert, K.; Rinninella, E.; Biruete, A.; Sumida, K.; Stanford, J.; Raoul, P.; Mele, M.C.; Wang, A.Y.; Mafra, D. Targeting the gut microbiota in kidney disease: The future in renal nutrition and metabolism. J. Ren. Nutr. 2023, 33, S30–S39. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. Chronic kidney disease and gut microbiota: What is their connection in early life? Int. J. Mol. Sci. 2022, 23, 3954. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Stanford, J.; Charlton, K.; Stefoska-Needham, A.; Ibrahim, R.; Lambert, K. The gut microbiota profile of adults with kidney disease and kidney stones: A systematic review of the literature. BMC Nephrol. 2020, 21, 215. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S. Factors influencing development of the infant microbiota: From prenatal period to early infancy. Clin. Exp. Pediatr. 2022, 65, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Turroni, F.; Milani, C.; Ventura, M.; van Sinderen, D. The human gut microbiota during the initial stages of life: Insights from bifidobacteria. Curr. Opin. Biotechnol. 2022, 73, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Guldris, S.C.; Parra, E.G.; Amenos, A.C. Gut microbiota in chronic kidney disease. Nefrologia 2017, 37, 9–19. [Google Scholar] [CrossRef]
- Liu, J.; Tan, Y.; Cheng, H.; Zhang, D.; Feng, W.; Peng, C. Functions of gut microbiota metabolites, current status and future perspectives. Aging Dis. 2022, 13, 1106–1126. [Google Scholar] [CrossRef]
- Yao, C.K.; Muir, J.G.; Gibson, P.R. Insights into colonic protein fermentation, its modulation and potential health implications. Aliment. Pharmacol. Ther. 2016, 43, 181–196. [Google Scholar] [CrossRef]
- Wong, J.M.W.; De Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Vinolo, M.A.R.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Li, L.Z.; Ma, L.; Fu, P. Gut microbiota-derived short-chain fatty acids and kidney diseases. Drug Des. Devel. Ther. 2017, 11, 3531–3542. [Google Scholar] [CrossRef]
- Tao, P.Y.; Ji, J.; Wang, Q.; Cui, M.M.; Cao, M.F.; Xu, Y.Z. The role and mechanism of gut microbiota-derived short-chain fatty in the prevention and treatment of diabetic kidney disease. Front. Immunol. 2022, 13, 1080456. [Google Scholar] [CrossRef]
- Evenepoel, P.; Poesen, R.; Meijers, B. The gut-kidney axis. Pediatr. Nephrol. 2017, 32, 2005–2014. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Xin, W.; Xiong, J.C.; Yao, M.Y.; Zhang, B.; Zhao, J.H. The intestinal microbiota and metabolites in the gut-kidney-heart axis of chronic kidney disease. Front. Pharmacol. 2022, 13, 837500. [Google Scholar] [CrossRef] [PubMed]
- Filipska, I.; Winiarska, A.; Knysak, M.; Stompór, T. Contribution of gut microbiota-derived uremic toxins to the cardiovascular system mineralization. Toxins 2021, 13, 274. [Google Scholar] [CrossRef] [PubMed]
- Ondrussek-Sekac, M.; Navas-Carrillo, D.; Orenes-Piñero, E. Intestinal microbiota alterations in chronic kidney disease and the influence of dietary components. Crit. Rev. Food Sci. Nutr. 2021, 61, 1490–1502. [Google Scholar] [CrossRef]
- Kim, S.M.; Song, I.H. The clinical impact of gut microbiota in chronic kidney disease. Korean J. Intern. Med. 2020, 35, 1305. [Google Scholar] [CrossRef] [PubMed]
- Moraes, C.; Fouque, D.; Amaral, A.C.F.; Mafra, D. Trimethylamine N-oxide from gut microbiota in chronic kidney disease patients: Focus on diet. J. Ren. Nutr. 2015, 25, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Canyelles, M.; Tondo, M.; Cedó, L.; Farràs, M.; Escolà-Gil, J.C.; Blanco-Vaca, F. Trimethylamine N-oxide: A link among diet, gut microbiota, gene regulation of liver and intestine cholesterol homeostasis and HDL function. Int. J. Mol. Sci. 2018, 19, 3228. [Google Scholar] [CrossRef] [PubMed]
- Nallu, A.; Sharma, S.; Ramezani, A.; Muralidharan, J.; Raj, D. Gut microbiome in chronic kidney disease: Challenges and opportunities. Transl. Res. 2017, 179, 24–37. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Yuan, J.; Rahimi, A.; Ni, Z.; Said, H.; Subramanian, V.S. Disintegration of colonic epithelial tight junction in uremia: A likely cause of CKD-associated inflammation. Nephrol. Dial. Transplant. 2012, 27, 2686–2693. [Google Scholar] [CrossRef]
- Lowenstein, J.; Nigam, S.K. Uremic toxins in organ crosstalk. Front. Med. 2021, 8, 592602. [Google Scholar] [CrossRef]
- Nigam, S.K.; Bush, K.T. Uraemic syndrome of chronic kidney disease: Altered remote sensing and signalling. Nat. Rev. Nephrol. 2019, 15, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Masereeuw, R. The dual roles of protein-bound solutes as toxins and signaling molecules in uremia. Toxins 2022, 14, 402. [Google Scholar] [CrossRef]
- Hagos, Y.; Wolff, N.A. Assessment of the role of renal organic anion transporters in drug-induced nephrotoxicity. Toxins 2010, 2, 2055–2082. [Google Scholar] [CrossRef] [PubMed]
- Naud, J.; Michaud, J.; Beauchemin, S.; Hébert, M.J.; Roger, M.; Lefrancois, S.; Leblond, F.A.; Pichette, V. Effects of chronic renal failure on kidney drug transporters and cytochrome P450 in rats. Drug Metab. Dispos. 2011, 39, 1363–1369. [Google Scholar] [CrossRef]
- Zheng, H.J.; Guo, J.; Wang, Q.H.; Wang, L.S.; Wang, Y.H.; Zhang, F.; Huang, W.J.; Zhang, W.T.; Liu, W.J.; Wang, Y.X. Probiotics, prebiotics, and synbiotics for the improvement of metabolic profiles in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 61, 577–598. [Google Scholar] [CrossRef]
- Naber, T.; Purohit, S. Chronic kidney disease: Role of diet for a reduction in the severity of the disease. Nutrients 2021, 13, 3277. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Qin, S.; Zhang, H. Precise strategies for selecting probiotic bacteria in treatment of intestinal bacterial dysfunctional diseases. Front. Immunol. 2022, 13, 1034727. [Google Scholar] [CrossRef]
- Tian, N.; Li, L.; Ng, J.K.-C.; Li, P.K.-T. The potential benefits and controversies of probiotics use in patients at different stages of chronic kidney disease. Nutrients 2022, 14, 4044. [Google Scholar] [CrossRef]
- Soleimani, A.; Mojarrad, M.Z.; Bahmani, F.; Taghizadeh, M.; Ramezani, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Esmaillzadeh, A.; Asemi, Z. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney Int. 2017, 91, 435–442. [Google Scholar] [CrossRef]
- Yu, Z.X.; Zhao, J.; Qin, Y.L.; Wang, Y.W.; Zhang, Y.M.; Sun, S.R. Probiotics, prebiotics, and synbiotics improve uremic, inflammatory, and gastrointestinal symptoms in end-stage renal disease with dialysis: A network meta-analysis of randomized controlled trials. Front. Nutr. 2022, 9, 850425. [Google Scholar] [CrossRef]
- Rule, A.D.; Bergstralh, E.J.; Melton, L.J., 3rd; Li, X.; Weaver, A.L.; Lieske, J.C. Kidney stones and the risk for chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Goldfarb, D.S.; Nazzal, L. The role of the microbiome in kidney stone formation. Int. Surg. J. 2016, 36, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Lieske, J.C. Probiotics for prevention of urinary stones. Ann. Transl. Med. 2017, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Wigner, P.; Bijak, M.; Saluk-Bijak, J. Probiotics in the prevention of the calcium oxalate urolithiasis. Cells 2022, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, N.; Friedman, E.A.; Tam, P.; Rao, V.; Ranganathan, P.; Dheer, R. Probiotic dietary supplementation in patients with stage 3 and 4 chronic kidney disease: A 6-month pilot scale trial in Canada. Curr. Med. Res. Opin. 2009, 25, 1919–1930. [Google Scholar] [CrossRef]
- Ranganathan, N.; Ranganathan, P.; Friedman, E.A.; Joseph, A.; Delano, B.; Goldfarb, D.S.; Tam, P.; Venketeshwer Rao, A.; Anteyi, E.; Guido Musso, C. Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Adv. Ther. 2010, 27, 634–647. [Google Scholar] [CrossRef] [PubMed]
- Alatriste, P.V.M.; Arronte, R.U.; Espinosa, C.O.G.; Cuevas, M.D.E. Effect of probiotics on human blood urea levels in patients with chronic renal failure. Nutr. Hosp. 2014, 29, 582–590. [Google Scholar]
- Eidi, F.; Gholi, F.P.-r.; Ostadrahimi, A.; Dalili, N.; Samadian, F.; Barzegari, A. Effect of Lactobacillus Rhamnosus on serum uremic toxins (phenol and P-Cresol) in hemodialysis patients: A double blind randomized clinical trial. Clin. Nutr. 2018, 28, 158–164. [Google Scholar] [CrossRef]
- De Araújo, É.M.R.; Meneses, G.C.; Carioca, A.A.F.; Martins, A.M.C.; Daher, E.D.F.; da Silva Junior, G.B. Use of probiotics in patients with chronic kidney disease on hemodialysis: A randomized clinical trial. J. Bras. Nefrol. 2022, 45, 152–161. [Google Scholar] [CrossRef]
- De Mauri, A.; Carrera, D.; Bagnati, M.; Rolla, R.; Vidali, M.; Chiarinotti, D.; Pane, M.; Amoruso, A.; Del Piano, M. Probiotics-supplemented low-protein diet for microbiota modulation in patients with advanced chronic kidney disease (ProLowCKD): Results from a placebo-controlled randomized trial. Nutrients 2022, 14, 1637. [Google Scholar] [CrossRef] [PubMed]
- Tayebi-Khosroshahi, H.; Habibzadeh, A.; Niknafs, B.; Ghotaslou, R.; Sefidan, F.Y.; Ghojazadeh, M.; Moghaddaszadeh, M.; Parkhide, S. The effect of lactulose supplementation on fecal microflora of patients with chronic kidney disease; a randomized clinical trial. J. Ren. Inj. Prev. 2016, 5, 162–167. [Google Scholar] [CrossRef]
- Younes, H.; Egret, N.; Hadj-Abdelkader, M.; Rémésy, C.; Demigné, C.; Gueret, C.; Deteix, P.; Alphonse, J.-C. Fermentable carbohydrate supplementation alters nitrogen excretion in chronic renal failure. J. Ren. Nutr. 2006, 16, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, Z.; Proost, S.; Tito, R.Y.; Raes, J.; Glorieux, G.; Moosa, M.R.; Blaauw, R. The Effect of ß-Glucan Prebiotic on Kidney Function, Uremic Toxins and Gut Microbiome in Stage 3 to 5 Chronic Kidney Disease (CKD) Predialysis Participants: A Randomized Controlled Trial. Nutrients 2022, 14, 805. [Google Scholar] [CrossRef] [PubMed]
- Kuskunov, T.; Tilkiyan, E.; Doykov, D.; Boyanov, K.; Bivolarska, A.; Hristov, B. The effect of synbiotic supplementation on uremic toxins, oxidative stress, and inflammation in hemodialysis patients-results of an uncontrolled prospective single-arm study. Medicina 2023, 59, 1383. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Johnson, D.W.; Morrison, M.; Pascoe, E.M.; Coombes, J.S.; Forbes, J.M.; Szeto, C.-C.; McWhinney, B.C.; Ungerer, J.P.J.; Campbell, K.L. Synbiotics easing renal failure by improving gut microbiology (SYNERGY): A randomized trial. Clin. J. Am. Soc. Nephrol. CJASN 2016, 11, 223. [Google Scholar] [CrossRef] [PubMed]
- Armani, R.G.; Carvalho, A.B.; Ramos, C.I.; Hong, V.; Bortolotto, L.A.; Cassiolato, J.L.; Oliveira, N.F.; Cieslarova, Z.; do Lago, C.L.; Klassen, A. Effect of fructooligosaccharide on endothelial function in CKD patients: A randomized controlled trial. Nephrol. Dial. Transplant. 2022, 37, 85–91. [Google Scholar] [CrossRef]
- Cosola, C.; Rocchetti, M.T.; di Bari, I.; Acquaviva, P.M.; Maranzano, V.; Corciulo, S.; Di Ciaula, A.; Di Palo, D.M.; La Forgia, F.M.; Fontana, S. An innovative synbiotic formulation decreases free serum indoxyl sulfate, small intestine permeability and ameliorates gastrointestinal symptoms in a randomized pilot trial in stage IIIb-IV CKD patients. Toxins 2021, 13, 334. [Google Scholar] [CrossRef]
- Guida, B.; Germano, R.; Trio, R.; Russo, D.; Memoli, B.; Grumetto, L.; Barbato, F.; Cataldi, M. Effect of short-term synbiotic treatment on plasma p-cresol levels in patients with chronic renal failure: A randomized clinical trial. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1043–1049. [Google Scholar] [CrossRef]
- Teixeira, K.T.R.; Moreira, L.d.S.G.; Borges, N.A.; Brum, I.; de Paiva, B.R.; Alvarenga, L.; Nakao, L.S.; Leal, V.d.O.; Carraro-Eduardo, J.C.; Rodrigues, S.D. Effect of cranberry supplementation on toxins produced by the gut microbiota in chronic kidney disease patients: A pilot randomized placebo-controlled trial. Clin. Nutr. ESPEN 2022, 47, 63–69. [Google Scholar] [CrossRef]
- Alvarenga, L.; Cardozo, L.F.M.F.; Leal, V.d.O.; Kemp, J.A.; Saldanha, J.F.; Ribeiro-Alves, M.; Meireles, T.; Nakao, L.S.; Mafra, D. Can resveratrol supplementation reduce uremic toxin Plasma levels from the Gut Microbiota in Nondialyzed patients with chronic kidney disease? J. Ren. Nutr. 2022, 32, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Valle Flores, J.A.; Fariño Cortéz, J.E.; Mayner Tresol, G.A.; Perozo Romero, J.; Blasco Carlos, M.; Nestares, T. Oral supplementation with omega-3 fatty acids and inflammation markers in patients with chronic kidney disease in hemodialysis. Appl. Physiol. Nutr. Metab. 2020, 45, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.; Mori, T.; Chew, G.; Beilin, L.J.; Puddey, I.; Watts, G.F.; Irish, A.; Dogra, G.; Boudville, N.; Lim, W. The effects of OMEGA-3 fatty acid supplementation upon interleukin-12 and interleukin-18 in chronic kidney disease patients. J. Ren. Nutr. 2019, 29, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-L.; Lin, T.-L.; Chang, C.-J.; Wu, T.-R.; Lai, W.-F.; Lu, C.-C.; Lai, H.-C. Probiotics, prebiotics and amelioration of diseases. J. Biomed. Sci. 2019, 26, 3. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota-introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Probert, H.M.; Van Loo, J.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Fao, F.; Organisation, A. FAO technical meeting on prebiotics. In Food Quality and Standards Services (AGNIS); Food and Agricultural Organisation of the United Nations: San Francisco, CA, USA, 2007. [Google Scholar]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Wang, X.; Yang, S.; Li, S.; Zhao, L.; Hao, Y.; Qin, J.; Zhang, L.; Zhang, C.; Bian, W.; Zuo, L.I. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut 2020, 69, 2131–2142. [Google Scholar] [CrossRef]
- Lobel, L.; Cao, Y.G.; Fenn, K.; Glickman, J.N.; Garrett, W.S. Diet posttranslationally modifies the mouse gut microbial proteome to modulate renal function. Science 2020, 369, 1518–1524. [Google Scholar] [CrossRef]
- Martynowicz, H.; Jodkowska, A.; Nowacki, D.; Mazur, G. A closer look at polyunsaturated fatty acids and hypertension. Postępy Hig. Med. Dośw. 2019, 73, 102–108. [Google Scholar] [CrossRef]
- Fassett, R.G.; Gobe, G.C.; Peake, J.M.; Coombes, J.S. Omega-3 polyunsaturated fatty acids in the treatment of kidney disease. Am. J. Kidney Dis. 2010, 56, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Hirahashi, J. Omega-3 polyunsaturated fatty acids for the treatment of IgA nephropathy. J. Clin. Med. 2017, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Saglimbene, V.M.; Wong, G.; van Zwieten, A.; Palmer, S.C.; Ruospo, M.; Natale, P.; Campbell, K.; Teixeira-Pinto, A.; Craig, J.C.; Strippoli, G.F.M. Effects of omega-3 polyunsaturated fatty acid intake in patients with chronic kidney disease: Systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2020, 39, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Epure, A.; Pârvu, A.E.; Vlase, L.; Benedec, D.; Hanganu, D.; Gheldiu, A.-M.; Toma, V.A.; Oniga, I. Phytochemical profile, antioxidant, cardioprotective and nephroprotective activity of romanian chicory extract. Plants 2020, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.T.; Wong, T.Y.; Wei, C.I.; Huang, Y.W.; Lin, Y. Tannins and human health: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef] [PubMed]
- Mafra, D.; Borges, N.A.; Lindholm, B.; Shiels, P.G.; Evenepoel, P.; Stenvinkel, P. Food as medicine: Targeting the uraemic phenotype in chronic kidney disease. Nat. Rev. Nephrol. 2021, 17, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liu, L.; Ji, H.-F. Regulative effects of curcumin spice administration on gut microbiota and its pharmacological implications. Food Nutr. Res. 2017, 61, 1361780. [Google Scholar] [CrossRef]
- Zirker, L. Benefit and Use of Prebiotics in Patients with Chronic Kidney Disease. J. Ren. Nutr. 2015, 25, e9–e10. [Google Scholar] [CrossRef]
- Al-Sheraji, S.H.; Ismail, A.; Manap, M.Y.; Mustafa, S.; Yusof, R.M.; Hassan, F.A. Prebiotics as functional foods: A review. J. Funct. Foods 2013, 5, 1542–1553. [Google Scholar] [CrossRef]
- Douglas, L.C.; Sanders, M.E. Probiotics and prebiotics in dietetics practice. J. Am. Diet. Assoc. 2008, 108, 510–521. [Google Scholar] [CrossRef]
- Nakabayashi, I.; Nakamura, M.; Kawakami, K.; Ohta, T.; Kato, I.; Uchida, K.; Yoshida, M. Effects of synbiotic treatment on serum level of p-cresol in haemodialysis patients: A preliminary study. Nephrol. Dial. Transplant. 2011, 26, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Tienda-Vazquez, M.A.; Morreeuw, Z.P.; Sosa-Hernandez, J.E.; Cardador-Martinez, A.; Sabath, E.; Melchor-Martinez, E.M.; Iqbal, H.M.N.; Parra-Saldivar, R. Nephroprotective plants: A review on the use in pre-renal and post-renal diseases. Plants 2022, 11, 818. [Google Scholar] [CrossRef] [PubMed]
- Basist, P.; Parveen, B.; Zahiruddin, S.; Gautam, G.; Parveen, R.; Khan, M.A.; Krishnan, A.; Shahid, M.; Ahmad, S. Potential nephroprotective phytochemicals: Mechanism and future prospects. J. Ethnopharmacol. 2022, 283, 114743. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, A.; Anyangwe, N.; Carlacci, L.; Casper, S.; Danam, R.P.; Enongene, E.; Erives, G.; Fabricant, D.; Gudi, R.; Hilmas, C.J. The safety and regulation of natural products used as foods and food ingredients. Toxicol. Sci. 2011, 123, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Edeoga, H.O.; Okwu, D.E.; Mbaebie, B.O. Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 2005, 4, 685–688. [Google Scholar] [CrossRef]
- Sansores-España, D.; Pech-Aguilar, A.G.; Cua-Pech, K.G.; Medina-Vera, I.; Guevara-Cruz, M.; Gutiérrez-Solis, A.L.; Reyes-García, J.G.; Avila-Nava, A. plants used in mexican traditional medicine for the management of urolithiasis: A review of preclinical evidence, bioactive compounds, and molecular mechanisms. Molecules 2022, 27, 2008. [Google Scholar] [CrossRef] [PubMed]
- Eugenio-Pérez, D.; Medina-Fernández, L.Y.; Saldivar-Anaya, J.A.; Molina-Jijón, E.; Pedraza-Chaverri, J. Role of Dietary Antioxidant Agents in Chronic Kidney Disease. In Free Radicals and Diseases; InTech: Rijeka, Croatia, 2016. [Google Scholar] [CrossRef]
- Koppe, L.; Fouque, D. Microbiota and prebiotics modulation of uremic toxin generation. Panminerva Med. 2017, 59, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.N.S.; Agarwala, M. Phytochemical analysis of some medicinal plants. J. Phytol. 2011, 3, 10–14. [Google Scholar]
- Vargas, F.; Romecín, P.; García-Guillén, A.I.; Wangesteen, R.; Vargas-Tendero, P.; Paredes, M.D.; Atucha, N.M.; García-Estañ, J. Flavonoids in kidney health and disease. Front. Physiol. 2018, 9, 394. [Google Scholar] [CrossRef]
- Oluwole, O.; Fernando, W.; Lumanlan, J.; Ademuyiwa, O.; Jayasena, V. Role of phenolic acid, tannins, stilbenes, lignans and flavonoids in human health—A review. Int. J. Food Sci. Technol. 2022, 57, 6326–6335. [Google Scholar] [CrossRef]
- Zhang, X.N.; Huang, H.Z.; Zhao, X.Y.; Lv, Q.; Sun, C.D.; Li, X.; Chen, K.S. Effects of flavonoids-rich Chinese bayberry (Myrica rubra Sieb. et Zucc.) pulp extracts on glucose consumption in human HepG2 cells. J. Funct. Foods 2015, 14, 144–153. [Google Scholar] [CrossRef]
- Fang, J. Bioavailability of anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, S.; Rafieian-Kopaei, M. Herbs, health and hazards; a nephrology viewpoint on current concepts and new trends. Ann. Res. Antioxid. 2016, 1, e05. [Google Scholar]
- Singh, R.; Rathore, S.; Kumar, R.; Agarwal, A.; Dubey, G. Nephroprotective role of Salacia chinensis in diabetic CKD patients: A pilot study. Indian J. Med. Sci. 2010, 64, 378–384. [Google Scholar] [PubMed]
- Narayanan, M.; Gopi, A.; Natarajan, D.; Kandasamy, S.; Saravanan, M.; El Askary, A.; Elfasakhany, A.; Pugazhendhi, A. Hepato and nephroprotective activity of methanol extract of Hygrophila spinosa and its antibacterial potential against multidrug resistant Pandoraea sputorum. Environ. Res. 2021, 201, 111594. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, N.; Wang, K.; Zhang, L.; Li, D.; Wang, Z.; Xu, G.; Liu, Y.; Xu, Q. Rosamultin from Potentilla anserine L. exhibits nephroprotection and antioxidant activity by regulating the reactive oxygen species/C/EBP homologous protein signaling pathway. Phytother. Res. 2021, 35, 6343–6358. [Google Scholar] [CrossRef]
- Basist, P.; Zahiruddin, S.; Khan, M.U.; Gautam, G.; Jan, B.; Khan, M.A.; Parveen, R.; Ahmad, S. Metabolite profiling and nephroprotective potential of Glycyrrhiza glabra L. roots against cisplatin-induced nephrotoxicity in vitro and in vivo. Iran. J. Basic Med. Sci. 2022, 25, 1286. [Google Scholar]
- Shah, N.A.; Khan, M.R.; Nigussie, D. Phytochemical investigation and nephroprotective potential of Sida cordata in rat. BMC Complement. Altern. Med. 2017, 17, 388. [Google Scholar] [CrossRef]
- Pérez-Ramírez, I.F.; Enciso-Moreno, J.A.; Guevara-González, R.G.; Gallegos-Corona, M.A.; Loarca-Piña, G.; Reynoso-Camacho, R. Modulation of renal dysfunction by Smilax cordifolia and Eryngium carlinae, and their effect on kidney proteome in obese rats. J. Funct. Foods 2016, 20, 545–555. [Google Scholar] [CrossRef]
| Study Design | Intervention | Results | References |
|---|---|---|---|
| Probiotic; randomized clinical trial; 42 hemodialysis patients; 4 weeks | 1.6 × 107 CFU/day of L. Rhamnosus | ↓ Phenol and p-cresol serum levels | [60] |
| Probiotic; randomized, double-blind, placebo-controlled study; 46 outpatients with stage 3 and 4 CKD; 6-month | 1010 CFU/day of a probiotic mix: S. thermophilus, L. acidophilus, and B. longum | ↓ BUN No change in Cr and uric acid | [58] |
| Probiotic; randomized, double-blind, clinical trial; 70 hemodialysis patients; 3-month; one capsule a day | Gram-positive mix: Lactobacillus Plantarum A87, Lactobacillus rhamnosus, Bifidobacterium bifidum A218 and Bifidobacterium longum A101 | ↓ Syndecan-1 ↓ Blood glucose | [61] |
| Probiotic; randomized, double-blind, placebo-controlled study; low-protein diet; 60 patients; two doses daily for one month; one dose daily for another two months | 5 × 109 Bifidobacterium longum; 1 × 109 Lactobacillus reuteri; maltodextrin | ↓ Microflora toxins ↓ Blood urea nitrogen ↓ Total cholesterol ↓ Triglycerides | [62] |
| Prebiotic; randomized clinical trial; 32 patients with stage 3 and 4 CKD; non-dialysis; 8 weeks; | 30 mm thrice/day of lactulose syrup | ↓ Cr ↑ Bifidobacteria ↑ Lactobacillus | [63] |
| Prebiotic; chronic renal failure (CRF) cross-over method after randomization; 5 weeks | 40 g/day fermentable carbohydrate (25 g wholemeal bread + 4.5 g inulin + 10.5 g crude potato starch) | ↑ Nitrogen (N) in stool ↓ Nitrogen (N) excreted in the urine ↓ Plasma urea concentration | [64] |
| Prebiotic; randomized control trial, single-center, single-blind study; 59 patients with stage 3–5 CKD; | 13.5 g of prebiotic fiber supplement with ß-glucan (GlucaChol-22®,, Bryanston, South Africa) daily | ↓ Uremic toxins ↓ Total pCG and free pCG | [65] |
| Prebiotics; prospective, quasi-experimental single-center study; 30 hemodialysis patients; 8 weeks; | 75 mg Lactobacillus acidophilus La-14 2 × 1011 CFU/g and 65 mg prebiotic fructooligosaccharides; once daily | ↓ IS and p-CS in plasma ↓ IL-6 | [66] |
| Synbiotics; randomized, double-blind, placebo-controlled; crossover study of symbiotic therapy; 37 patients; 6 weeks | SYNERGY; 15 g prebiotic, mix of 3 different types of fiber, probiotic 1 × 109 (CFU) of 9 different strains of Lactobacillus, Bifidobacteria and Streptococcus; daily dose | ↓ PCS in serum ↑ Bifidobacterium ↓ Ruminococcaceae | [67] |
| Prebiotics; double-blind, controlled study; 46 patients with CKD; 3 months | 12 g FOS per day | ↓ IL-6 | [68] |
| Synbiotics; randomized, single-blind, placebo-controlled; 23 patients with stage 3b-4 CKD; 2 months | NATUREN G®(Canosa di Puglia, Italy) mix Lactobacillus, Bifidobacteria, FOS, inulin and natural antioxidants | ↓ IS ↓ Small intestinal permeability, ↓ Abdominal pain and constipation syndromes | [69] |
| Synbiotics; randomized, double-blind, placebo-controlled trial; 30 patients in stages 3–4 of CKD; 4 weeks | Probinul neutro® (Rome, Italy); 5 g three times a day; Lactobacillus plantarum; 5 × 109 CFU Lactobacillus casei subsp. Rhamnosus and gasseri 2 × 109 CFU Bifidobacterium infantis and longum 1 × 109 CFU Lactobacillus acidophilus, salivarius and sporogenes 1 × 109 CFU Streptococcus thermophilus 5 × 109 CFU Prebiotic inulin 2.2 g and 1.3 g resistant starch | ↓ Total plasma concentration of p-cresol | [70] |
| Polyphenol in cranberry; randomized, double-blind, placebo-controlled study; 25 patients with CKD; 2 months | 500 mg of dry cranberry extract (2 times daily), and the placebo group received 500 mg of cornstarch (2 times daily) | No change in LPS and uremic toxins plasma levels | [71] |
| Trans-resveratrol; placebo-controlled crossover study; 20 nondialyzed patients with CKD; 16 weeks | 500 mg trans-resveratrol (one capsule/day) for 8 weeks | ↓ IS, ↓ p-CS ↓ IAA | [72] |
| Omega-3 fatty acids; prospective, randomized, double-blind study; patients with CKD undergoing hemodialysis; 12-week | 4 capsules (2.4 g) of omega-3 fatty acids daily; placebo group 4 capsules of paraffin oil | ↓ C-reactive protein ↓ IL-6 ↓ TNF-α, | [73] |
| Omega-3 fatty acids; randomized placebo-controlled trial; 73 nondiabetic patients with stage 3–4 CKD; 8 weeks | Omega-3 fatty acids 4 g daily | ↓ IL-18 No change highly sensitive C-reactive protein and IL-12 | [74] |
| Plant | Study Design | Research Results | References |
|---|---|---|---|
| Salacia chinensis | Pilot study; 30 stable diabetic CKD patients; Salacia chinensis 1000 mg twice daily | ↓ homocysteine ↓ IL-6 | [107] |
| Hygrophila spinosa | Analysis of the phytochemical profile, bactericidal activity of Hygrophila spinosa against multidrug-resistant Pandoraea sputorum, and examination of their hepatoprotective and nephroprotective activities on HepG2 and HEK 293 cell lines. | Methanol extract shows hepato- and nephroprotective effects against CCl4 and cisplatin induced cytotoxicity on HepG2 and HEK 293 cell lines, respectively. Bactericidal efficacy of phytohormones against multidrug-resistant P. sputorum was demonstrated. | [108] |
| Potentilla anserine L. | Investigating the protective effect of rosamultin against cisplatin-induced nephrotoxicity. | ↓ BUN ↑ In vitro viability of HEK293 cells Inhibition of cisplatin-induced apoptosis, in vivo amelioration of renal dysfunction, and reduction in renal tubular damage. | [109] |
| Glycyrrhiza glabra L. | Phytochemical analysis and investigation of the nephroprotective potential of Glycyrrhiza glabra L. root extract against cisplatin in vitro and in vivo. | The nephroprotective effect of G. glabra roots can be attributed to antioxidant, anti-inflammatory and anti-apoptotic activities. Therefore, it has promising potential in the treatment of nephrotoxicity. | [110] |
| Sida cordata | Evaluation of antioxidant activity against CCL4-induced nephrotoxicity and analysis of phytochemicals of ethyl acetate from Sida cordata | The results indicate a protective role for ethyl acetate from S.cordat against CCl4-induced nephrotoxicity in rats, which is related to the antioxidant compounds it contains. | [111] |
| Smilax cordifolia Eryngium carlinae | To evaluate the effects of decoction of two plants, Smilax cordifolia and Eryngium carlinae, on renal dysfunction in rats. | Herbal decoctions decreased serum uric acid, albumin, and urea concentrations, accumulation of proteins associated with the formation of glomerular sclerosis and renal tubular fibrosis, increased creatinine clearance and concentration of pro-inflammatory and protective proteins | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabała, S.; Ożgo, M.; Herosimczyk, A. The Kidney–Gut Axis as a Novel Target for Nutritional Intervention to Counteract Chronic Kidney Disease Progression. Metabolites 2024, 14, 78. https://doi.org/10.3390/metabo14010078
Cabała S, Ożgo M, Herosimczyk A. The Kidney–Gut Axis as a Novel Target for Nutritional Intervention to Counteract Chronic Kidney Disease Progression. Metabolites. 2024; 14(1):78. https://doi.org/10.3390/metabo14010078
Chicago/Turabian StyleCabała, Sandra, Małgorzata Ożgo, and Agnieszka Herosimczyk. 2024. "The Kidney–Gut Axis as a Novel Target for Nutritional Intervention to Counteract Chronic Kidney Disease Progression" Metabolites 14, no. 1: 78. https://doi.org/10.3390/metabo14010078
APA StyleCabała, S., Ożgo, M., & Herosimczyk, A. (2024). The Kidney–Gut Axis as a Novel Target for Nutritional Intervention to Counteract Chronic Kidney Disease Progression. Metabolites, 14(1), 78. https://doi.org/10.3390/metabo14010078






