Effects of Branched-Chain Amino Acids on the Inflammatory Response Induced by LPS in Caco-2 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Caco-2 Cells Culture
2.2. Treatment Protocol
2.3. MTT Assay
2.4. Intracellular Concentrations of Reduced and Oxidized Glutathione
2.5. Glutathione Peroxidase (GPX) Enzyme Activity
2.6. Cytokine Concentration in Cell Culture Supernatant
2.7. Protein Extraction and Western Blotting
2.8. Statistical Analysis
3. Results
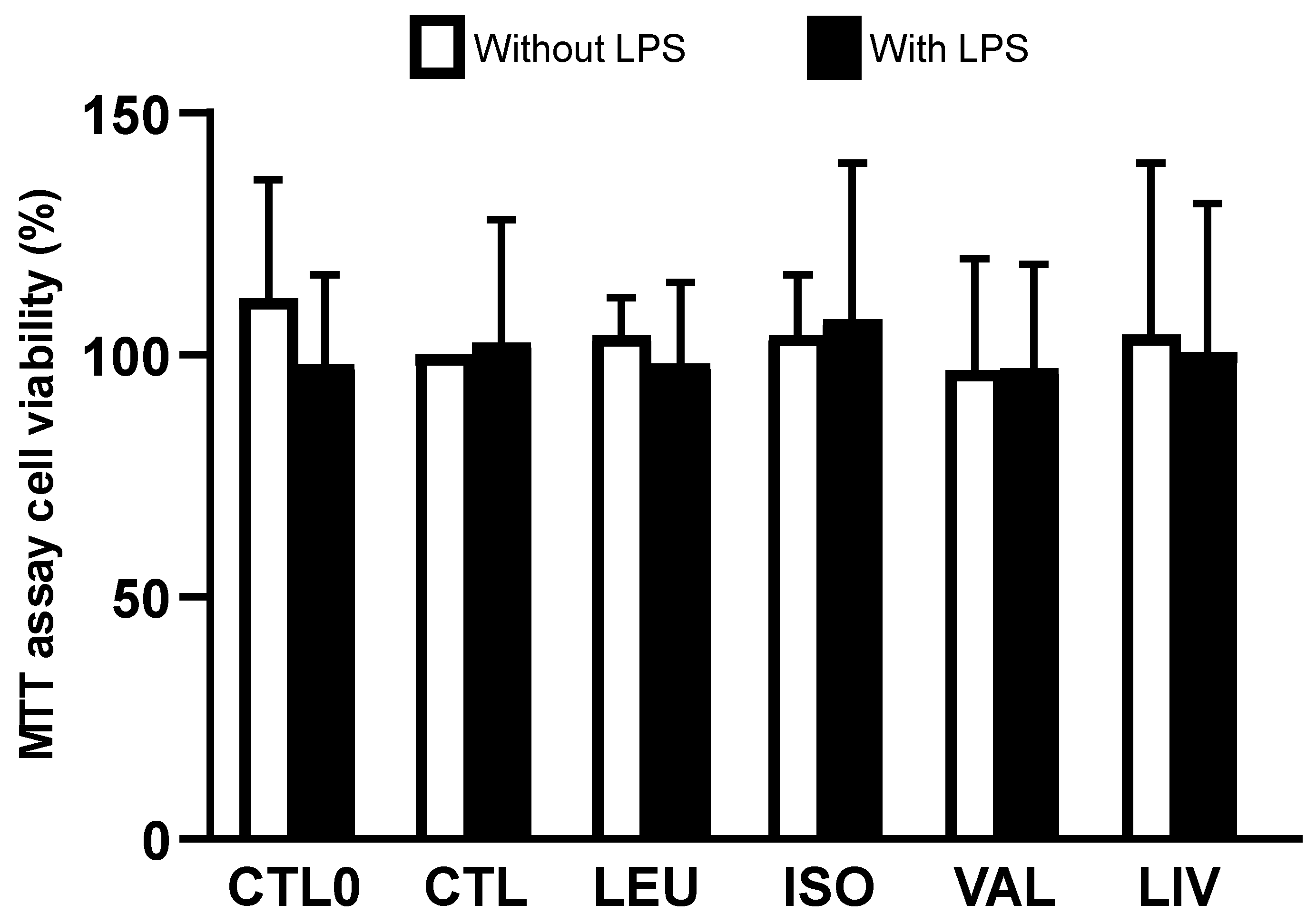
3.1. Cell Viability
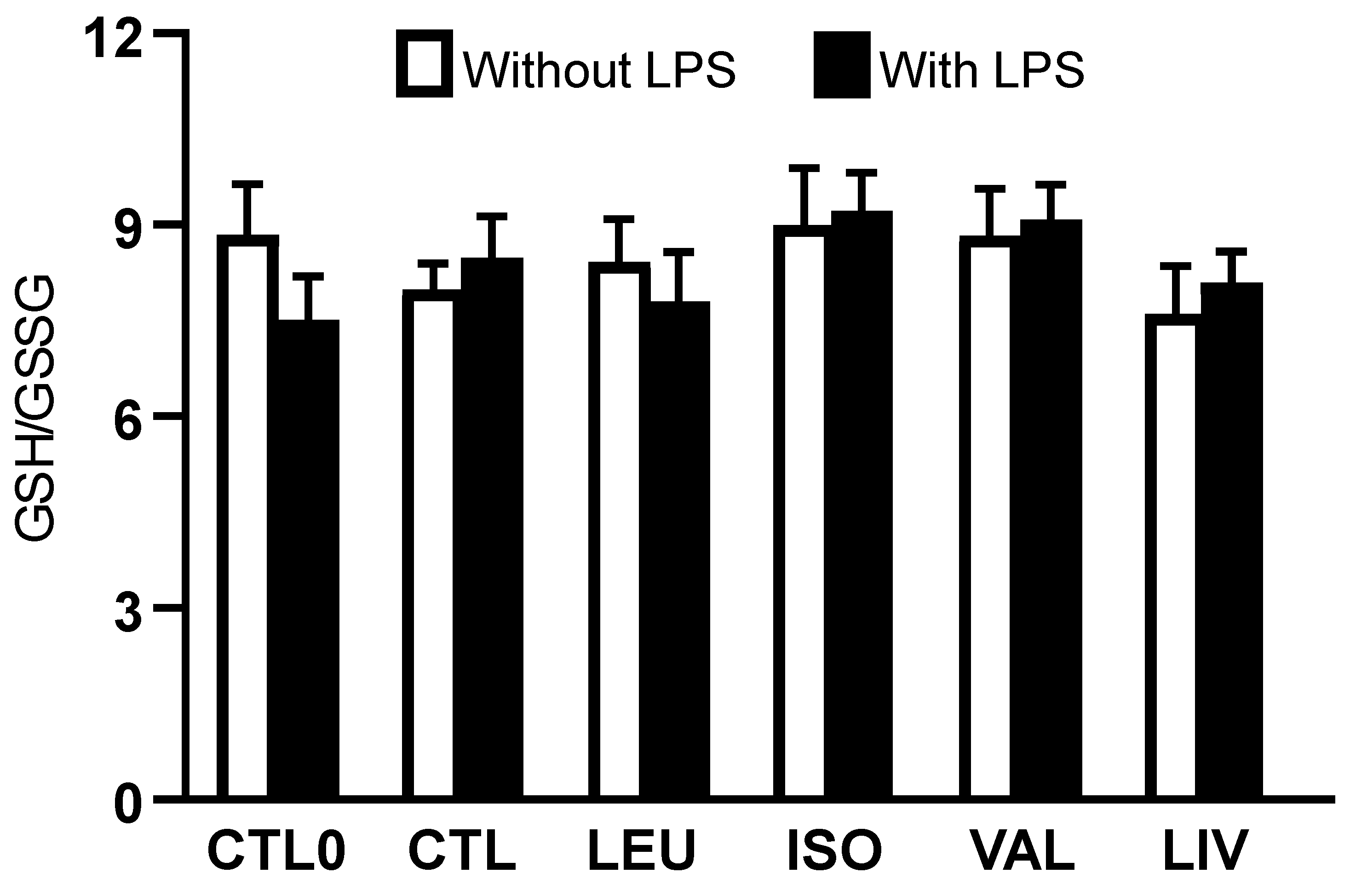
3.2. Intracellular Concentrations of Reduced and Oxidized Glutathione
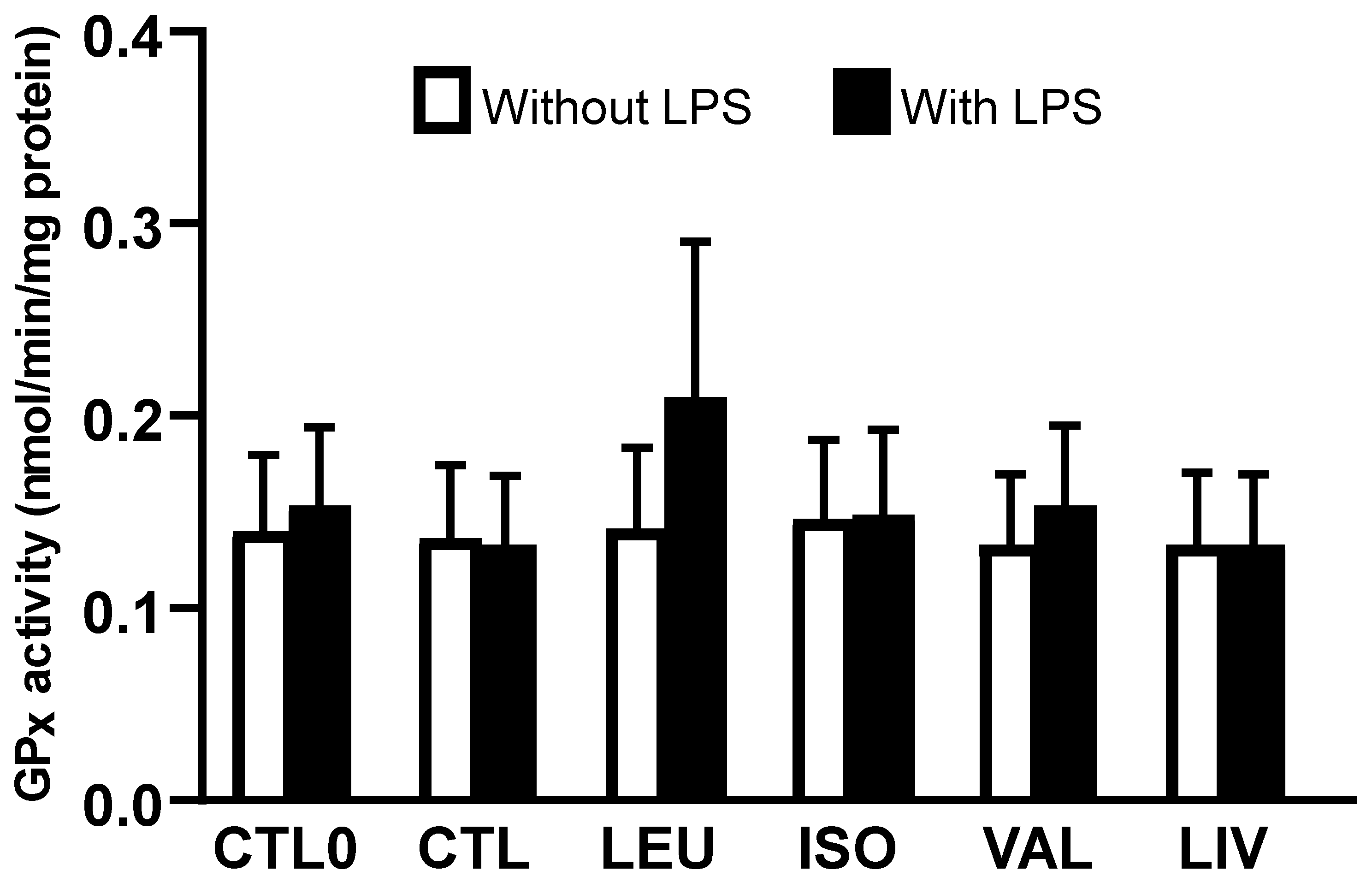
3.3. Activity of Glutathione Peroxidase (GPx)
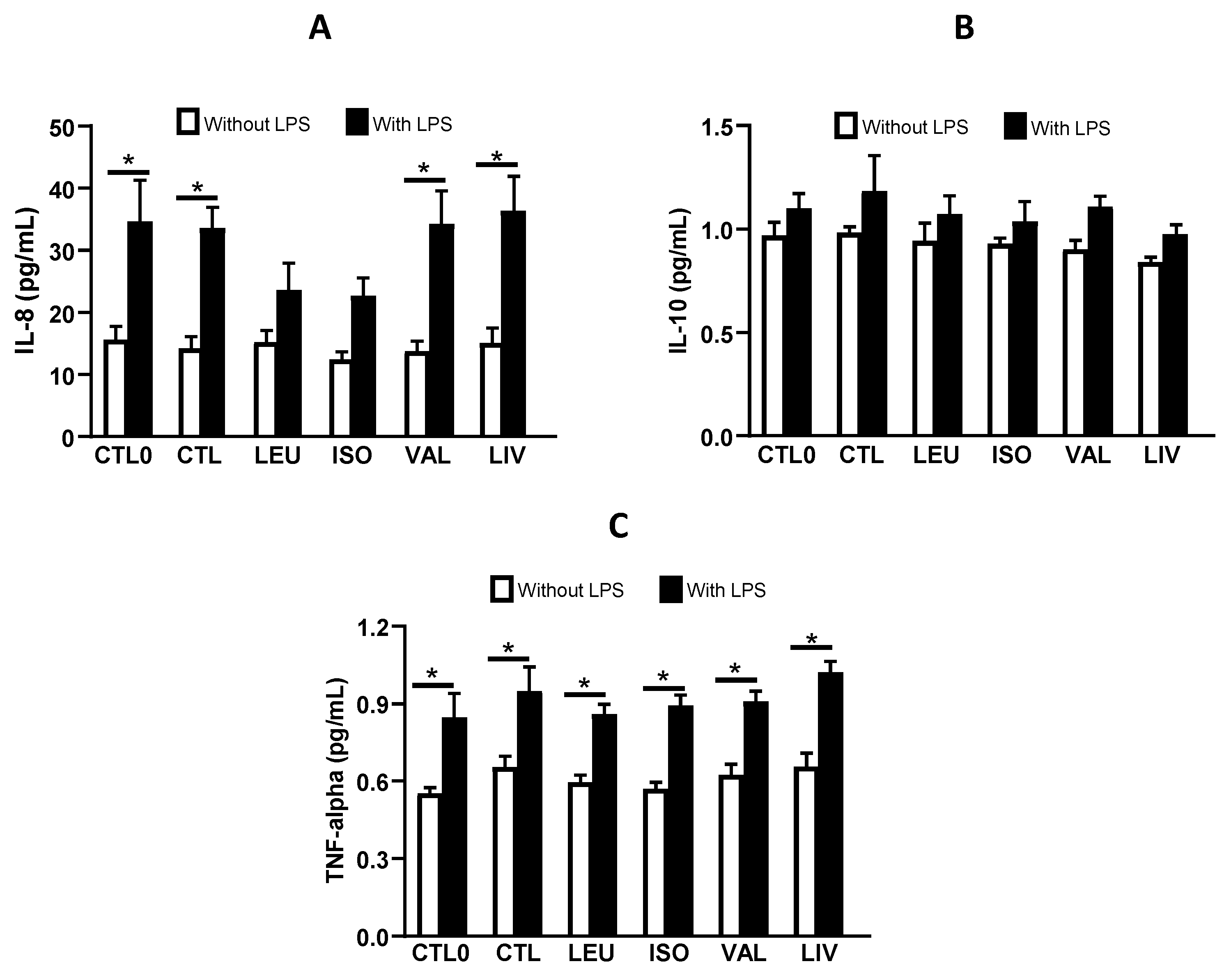
3.4. Cytokine Concentration in Cell Culture Supernatant
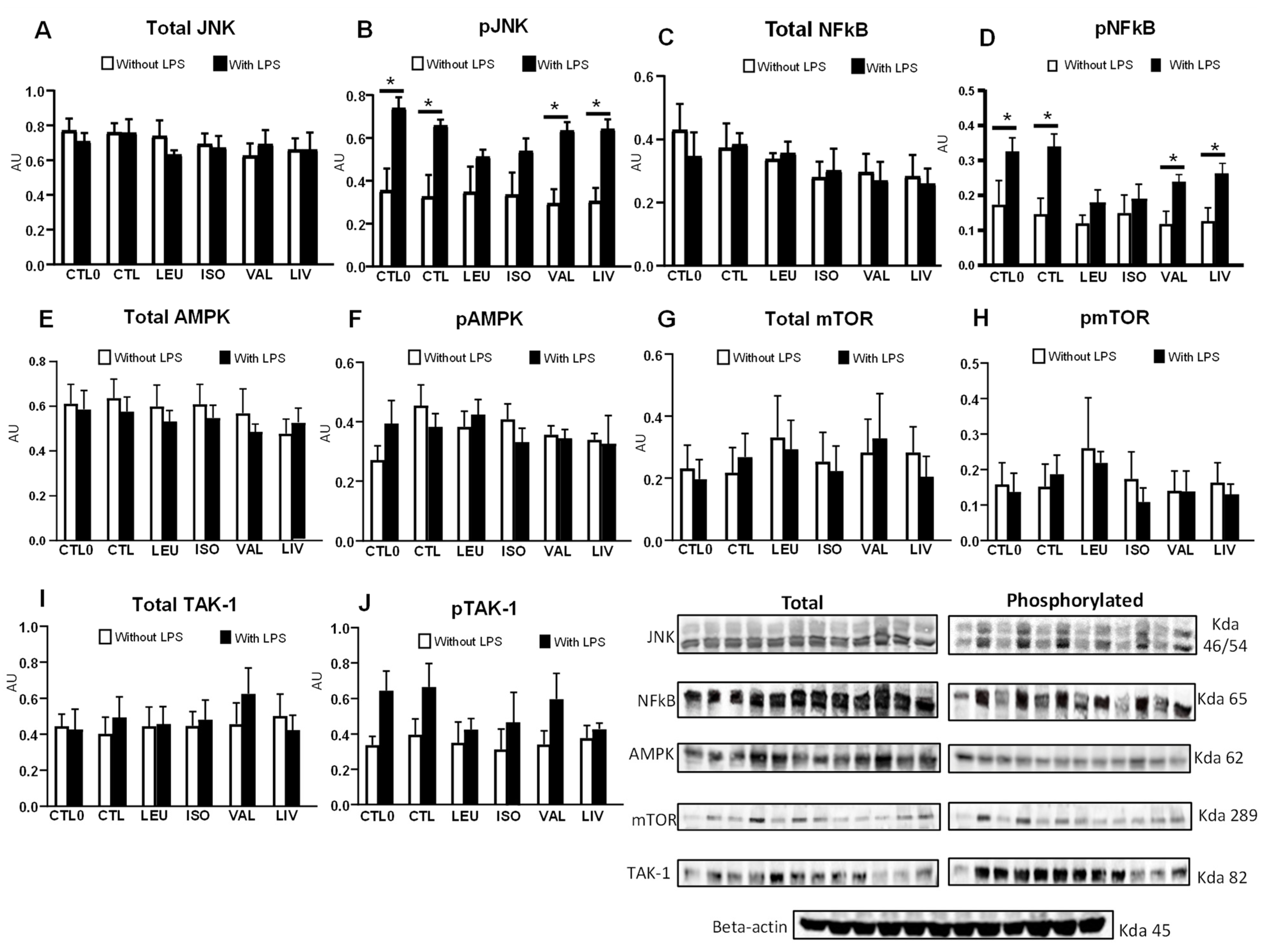
3.5. Content and Phosphorylation of Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tamanna, N.; Mahmood, N. Emerging Roles of Branched-Chain Amino Acid Supplementation in Human Diseases. Int. Sch. Res. Not. 2014, 2014, 235619. [Google Scholar] [CrossRef] [PubMed]
- De Bandt, J.P.; Coumoul, X.; Barouki, R. Branched-Chain Amino Acids and Insulin Resistance, from Protein Supply to Diet-Induced Obesity. Nutrients 2022, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- Le Couteur, D.G.; Solon-Biet, S.M.; Cogger, V.C.; Ribeiro, R.; de Cabo, R.; Raubenheimer, D.; Cooney, G.J.; Simpson, S.J. Branched Chain Amino Acids, Aging and Age-Related Health. Ageing Res. Rev. 2020, 64, 101198. [Google Scholar] [CrossRef] [PubMed]
- De Bandt, J.P. Leucine and Mammalian Target of Rapamycin-Dependent Activation of Muscle Protein Synthesis in Aging. J. Nutr. 2016, 146, 2616S–2624S. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R. Branched-Chain Amino Acids and Muscle Protein Synthesis in Humans: Myth or Reality? J. Int. Soc. Sports Nutr. 2017, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Dimou, A.; Tsimihodimos, V.; Bairaktari, E. The Critical Role of the Branched Chain Amino Acids (BCAAs) Catabolism-Regulating Enzymes, Branched-Chain Aminotransferase (BCAT) and Branched-Chain α-Keto Acid Dehydrogenase (BCKD), in Human Pathophysiology. Int. J. Mol. Sci. 2022, 23, 4022. [Google Scholar] [CrossRef]
- Morrison, H.A.; Trusiano, B.; Rowe, A.J.; Allen, I.C. Negative regulatory NLRs mitigate inflammation via NF-κB pathway signaling in inflammatory bowel disease. Biomed. J. 2023, 46, 100616. [Google Scholar] [CrossRef]
- Moens, U.; Kostenko, S.; Sveinbjørnsson, B. The Role of Mitogen-Activated Protein Kinase-Activated Protein Kinases (MAPKAPKs) in Inflammation. Genes 2013, 4, 101–133. [Google Scholar] [CrossRef]
- McDaniel, D.K.; Eden, K.; Ringel, V.M.; Allen, I.C. Emerging Roles for Noncanonical NF-κB Signaling in the Modulation of Inflammatory Bowel Disease Pathobiology. Inflamm. Bowel Dis. 2016, 22, 2265–2279. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, C.; Yao, M.; Wu, D.; Chen, M.; Guo, T.; Mo, J. Research progress of NF-κB signaling pathway and thrombosis. Front. Immunol. 2023, 14, 1257988. [Google Scholar] [CrossRef]
- Aghamohamadi, E.; Asri, N.; Odak, A.; Rostami-Nejad, M.; Chaleshi, V.; Hajinabi, Y.; Eslami, M.; Mohammadian Haftcheshmeh, S.; Gholam-Mostafaei, F.S.; Asadzadeh-Aghdaei, H.; et al. Gene expression analysis of intestinal IL-8, IL-17 A and IL-10 in patients with celiac and inflammatory bowel diseases. Mol. Biol. Rep. 2022, 49, 6085–6091. [Google Scholar] [CrossRef] [PubMed]
- Zobeiri, M.; Momtaz, S.; Parvizi, F.; Tewari, D.; Farzaei, M.H.; Nabavi, S.M. Targeting Mitogen-Activated Protein Kinases by Natural Products: A Novel Therapeutic Approach for Inflammatory Bowel Diseases. Curr. Pharm. Biotechnol. 2020, 21, 1342–1353. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liao, S.F. Physiological Effects of Dietary Amino Acids on Gut Health and Functions of Swine. Front. Vet. Sci. 2019, 6, 169. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Intestinal mucosal amino acid catabolism. J. Nutr. 1998, 128, 1249–1252. [Google Scholar] [CrossRef]
- Duan, Y.; Tan, B.; Li, J.; Liao, P.; Huang, B.; Li, F.; Xiao, H.; Liu, Y.; Yin, Y. Optimal branched-chain amino acid ratio improves cell proliferation and protein metabolism of porcine enterocytes in in vivo and in vitro. Nutrition 2018, 54, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Bonvini, A.; Rogero, M.M.; Coqueiro, A.Y.; Raizel, R.; Bella, L.M.; Fock, R.A.; Borelli, P.; Tirapegui, J. Effects of different branched-chain amino acids supplementation protocols on the inflammatory response of LPS-stimulated RAW 264.7 macrophages. Amino Acids 2021, 53, 597–607. [Google Scholar] [CrossRef]
- Sartori, T.; Santos, A.C.A.; Oliveira da Silva, R.; Kodja, G.; Rogero, M.M.; Borelli, P.; Fock, R.A. Branched-chain amino acids improve mesenchymal stem cell proliferation, reducing nuclear factor kappa B expression and modulating some inflammatory properties. Nutrition 2020, 78, 110935. [Google Scholar] [CrossRef]
- Liu, S.Q.; Wang, L.Y.; Liu, G.H.; Tang, D.Z.; Fan, X.X.; Zhao, J.P.; Jiao, H.C.; Wang, X.J.; Sun, S.H.; Lin, H. Leucine alters immunoglobulin a secretion and inflammatory cytokine expression induced by lipopolysaccharide via the nuclear factor-κB pathway in intestine of chicken embryos. Animal 2018, 12, 1903–1911. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef]
- Zhenyukh, O.; González-Amor, M.; Rodrigues-Diez, R.R.; Esteban, V.; Ruiz-Ortega, M.; Salaices, M.; Mas, S.; Briones, A.M.; Egido, J. Branched-chain amino acids promote endothelial dysfunction through increased reactive oxygen species generation and inflammation. J. Cell. Mol. Med. 2018, 22, 4948–4962. [Google Scholar] [CrossRef]
- Liboni, K.; Li, N.; Neu, J. Mechanism of glutamine-mediated amelioration of lipopolysaccharide-induced IL-8 production in Caco-2 cells. Cytokine 2004, 26, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Zhao, P.; Shen, A.; Liu, L.; Chen, H.; Chen, Y.; Peng, J.; Sferra, T.J.; Sankararaman, S.; Luo, Y.; et al. Effects of Qing Hua Chang Yin on lipopolysaccharide induced intestinal epithelial tight junction injury in Caco 2 cells. Mol. Med. Rep. 2021, 23, 205. [Google Scholar] [CrossRef] [PubMed]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blázquez-Castro, A. Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Schnur, S.; Wahl, V.; Metz, J.K.; Gillmann, J.; Hans, F.; Rotermund, K.; Zäh, R.K.; Brück, D.A.; Schneider, M.; Hittinger, M. Inflammatory bowel disease addressed by Caco-2 and monocyte-derived macrophages: An opportunity for an in vitro drug screening assay. Vitr. Model. 2022, 1, 365–383. [Google Scholar] [CrossRef] [PubMed]
- De Simone, R.; Vissicchio, F.; Mingarelli, C.; De Nuccio, C.; Visentin, S.; Ajmone-Cat, M.A.; Minghetti, L. Branched-chain amino acids influence the immune properties of microglial cells and their responsiveness to pro-inflammatory signals. Biochim. Biophys. Acta. 2013, 1832, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; Morgan, B.; Riemer, J. Mitochondrial Glutathione: Regulation and Functions. Antioxid. Redox Signal. 2017, 27, 1162–1177. [Google Scholar] [CrossRef] [PubMed]
- Csányi, G.; Miller, F.J., Jr. Oxidative stress in cardiovascular disease. Int. J. Mol. Sci. 2014, 15, 6002–6008. [Google Scholar] [CrossRef]
- Erden-Inal, M.; Sunal, E.; Kanbak, G. Age-related changes in the glutathione redox system. Cell Biochem. Funct. 2002, 20, 61–66. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Marí, M.; Morales, A.; Colell, A.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondrial glutathione, a key survival antioxidant. Antioxid. Redox Signal. 2009, 11, 2685–2700. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, L.; Jung, H.C.; Schürer-Maly, C.; Panja, A.; Morzycka-Wroblewska, E.; Kagnoff, M.F. Differential cytokine expression by human intestinal epithelial cell lines: Regulated expression of interleukin 8. Gastroenterology 1993, 105, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.A. Role of nutrients and bacterial colonization in the development of intestinal host defense. J. Pediatr. Gastroenterol. Nutr. 2000, 30 (Suppl. S2), S2–S7. [Google Scholar] [CrossRef] [PubMed]
- Baggiolini, M. Chemokines in pathology and medicine. J. Intern. Med. 2001, 250, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, K.; Yang, D.; Oppenheim, J.J. Interleukin-8: An evolving chemokine. Cytokine 2022, 153, 155828. [Google Scholar] [CrossRef] [PubMed]
- Katayama, S.; Mine, Y. Antioxidative activity of amino acids on tissue oxidative stress in human intestinal epithelial cell model. J. Agric. Food Chem. 2007, 55, 8458–8464. [Google Scholar] [CrossRef] [PubMed]
- Kunsch, C.; Rosen, C.A. NF-kappa B subunit-specific regulation of the interleukin-8 promoter. Mol. Cell. Biol. 1993, 13, 6137–6146. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, M.B.; Ford, A.E.; Liu, Y.; Zhang, J.Y. The JNK Signaling Pathway in Inflammatory Skin Disorders and Cancer. Cells 2020, 9, 857. [Google Scholar] [CrossRef]
- Antonioli, L.; Colucci, R.; Pellegrini, C.; Giustarini, G.; Sacco, D.; Tirotta, E.; Caputi, V.; Marsilio, I.; Giron, M.C.; Németh, Z.H.; et al. The AMPK enzyme-complex: From the regulation of cellular energy homeostasis to a possible new molecular target in the management of chronic inflammatory disorders. Expert Opin. Ther. Targets 2016, 20, 179–191. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Schertzer, J.D. AMPK promotes macrophage fatty acid oxidative metabolism to mitigate inflammation: Implications for diabetes and cardiovascular disease. Immunol. Cell Biol. 2014, 92, 340–345. [Google Scholar] [CrossRef]
- Salt, I.P.; Palmer, T.M. Exploiting the anti-inflammatory effects of AMP-activated protein kinase activation. Expert Opin. Investig. Drugs 2012, 21, 1155–1167. [Google Scholar] [CrossRef]
- Bess, E.; Fisslthaler, B.; Frömel, T.; Fleming, I. Nitric oxide-induced activation of the AMP-activated protein kinase α2 subunit attenuates IκB kinase activity and inflammatory responses in endothelial cells. PLoS ONE 2011, 6, e20848. [Google Scholar] [CrossRef]
- Rutherford, C.; Speirs, C.; Williams, J.J.; Ewart, M.A.; Mancini, S.J.; Hawley, S.A.; Delles, C.; Viollet, B.; Costa-Pereira, A.P.; Baillie, G.S.; et al. Phosphorylation of Janus kinase 1 (JAK1) by AMP-activated protein kinase (AMPK) links energy sensing to anti-inflammatory signaling. Sci. Signal. 2016, 9, ra109. [Google Scholar] [CrossRef] [PubMed]
- Mancini, S.J.; White, A.D.; Bijland, S.; Rutherford, C.; Graham, D.; Richter, E.A.; Viollet, B.; Touyz, R.M.; Palmer, T.M.; Salt, I.P. Activation of AMP-activated protein kinase rapidly suppresses multiple pro-inflammatory pathways in adipocytes including IL-1 receptor-associated kinase-4 phosphorylation. Mol. Cell. Endocrinol. 2017, 440, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Lashgari, N.A.; Roudsari, N.M.; Momtaz, S.; Abdolghaffari, A.H. Mammalian target of rapamycin; novel insight for management of inflammatory bowel diseases. World J. Pharmacol. 2022, 11, 1–5. [Google Scholar] [CrossRef]
- Lashgari, N.A.; Roudsari, N.M.; Zadeh, S.S.T.; Momtaz, S.; Abbasifard, M.; Reiner, Ž.; Abdolghaffari, A.H.; Sahebkar, A. Statins block mammalian target of rapamycin pathway: A possible novel therapeutic strategy for inflammatory, malignant and neurodegenerative diseases. Inflammopharmacology 2023, 31, 57–75. [Google Scholar] [CrossRef] [PubMed]
- Lashgari, N.A.; Roudsari, N.M.; Momtaz, S.; Ghanaatian, N.; Kohansal, P.; Farzaei, M.H.; Afshari, K.; Sahebkar, A.; Abdolghaffari, A.H. Targeting mammalian target of rapamycin: Prospects for the treatment of inflammatory bowel diseases. Curr. Med. Chem. 2021, 28, 1605–1624. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhu, M.J. AMP-activated protein kinase: A therapeutic target in intestinal diseases. Open Biol. 2017, 7, 170104. [Google Scholar] [CrossRef]
- Zhenyukh, O.; Civantos, E.; Ruiz-Ortega, M.; Sánchez, M.S.; Vázquez, C.; Peiró, C.; Egido, J.; Mas, S. High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free Radic. Biol. Med. 2017, 104, 165–177. [Google Scholar] [CrossRef]
- Scarneo, S.; Zhang, X.; Wang, Y.; Camacho-Domenech, J.; Ricano, J.; Hughes, P.; Haystead, T.; Nackley, A.G. Transforming Growth Factor-β-Activated Kinase 1 (TAK1) Mediates Chronic Pain and Cytokine Production in Mouse Models of Inflammatory, Neuropathic, and Primary Pain. J. Pain 2023, 24, 1633–1644. [Google Scholar] [CrossRef]
- Calvello, R.; Aresta, A.; Trapani, A.; Zambonin, C.; Cianciulli, A.; Salvatore, R.; Clodoveo, M.L.; Corbo, F.; Franchini, C.; Panaro, M.A. Bovine and soybean milk bioactive compounds: Effects on inflammatory response of human intestinal Caco-2 cells. Food Chem. 2016, 210, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Casper, E. The crosstalk between Nrf2 and NF-κB pathways in coronary artery disease: Can it be regulated by SIRT6? Life Sci. 2023, 330, 122007. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, B.R.E.V.; Makiyama, E.N.; Sampaio, G.R.; Soares-Freitas, R.A.M.; Bonvini, A.; Amaral, A.G.; Bordin, S.; Fock, R.A.; Rogero, M.M. Effects of Branched-Chain Amino Acids on the Inflammatory Response Induced by LPS in Caco-2 Cells. Metabolites 2024, 14, 76. https://doi.org/10.3390/metabo14010076
Garcia BREV, Makiyama EN, Sampaio GR, Soares-Freitas RAM, Bonvini A, Amaral AG, Bordin S, Fock RA, Rogero MM. Effects of Branched-Chain Amino Acids on the Inflammatory Response Induced by LPS in Caco-2 Cells. Metabolites. 2024; 14(1):76. https://doi.org/10.3390/metabo14010076
Chicago/Turabian StyleGarcia, Bruna Ruschel Ewald Vega, Edson Naoto Makiyama, Geni Rodrigues Sampaio, Rosana Aparecida Manólio Soares-Freitas, Andrea Bonvini, Andressa Godoy Amaral, Silvana Bordin, Ricardo Ambrósio Fock, and Marcelo Macedo Rogero. 2024. "Effects of Branched-Chain Amino Acids on the Inflammatory Response Induced by LPS in Caco-2 Cells" Metabolites 14, no. 1: 76. https://doi.org/10.3390/metabo14010076
APA StyleGarcia, B. R. E. V., Makiyama, E. N., Sampaio, G. R., Soares-Freitas, R. A. M., Bonvini, A., Amaral, A. G., Bordin, S., Fock, R. A., & Rogero, M. M. (2024). Effects of Branched-Chain Amino Acids on the Inflammatory Response Induced by LPS in Caco-2 Cells. Metabolites, 14(1), 76. https://doi.org/10.3390/metabo14010076





