Serum-Based Lipid Panels for Diagnosis of Idiopathic Parkinson’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Selection Criteria
2.2. Lipid Extraction from Blood Serum
2.3. Untargeted Lipid Profiling Using Reversed-Phase Liquid Chromatography Coupled with Quadrupole Time-Of-Flight Mass Spectrometry
2.4. Data Processing and Lipid Identification
2.5. Statistical Analysis (Multivariate and Univariate Analyses)
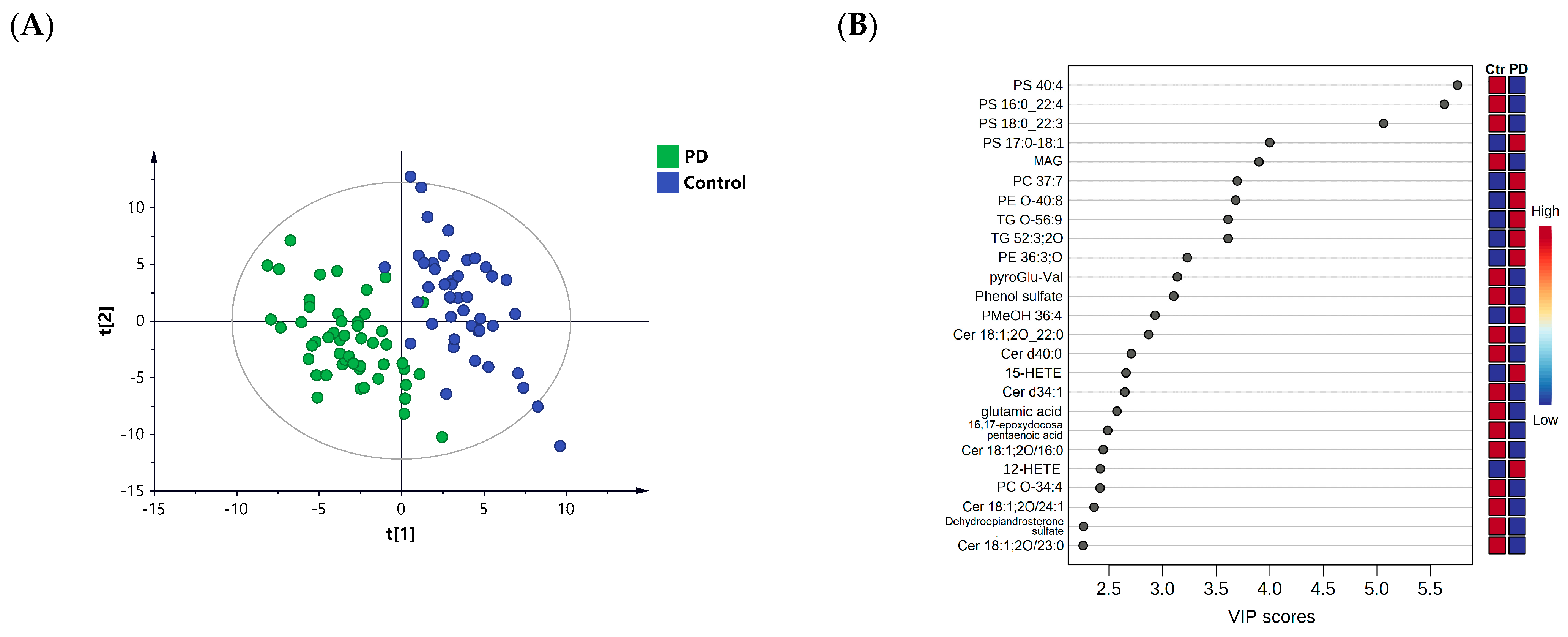
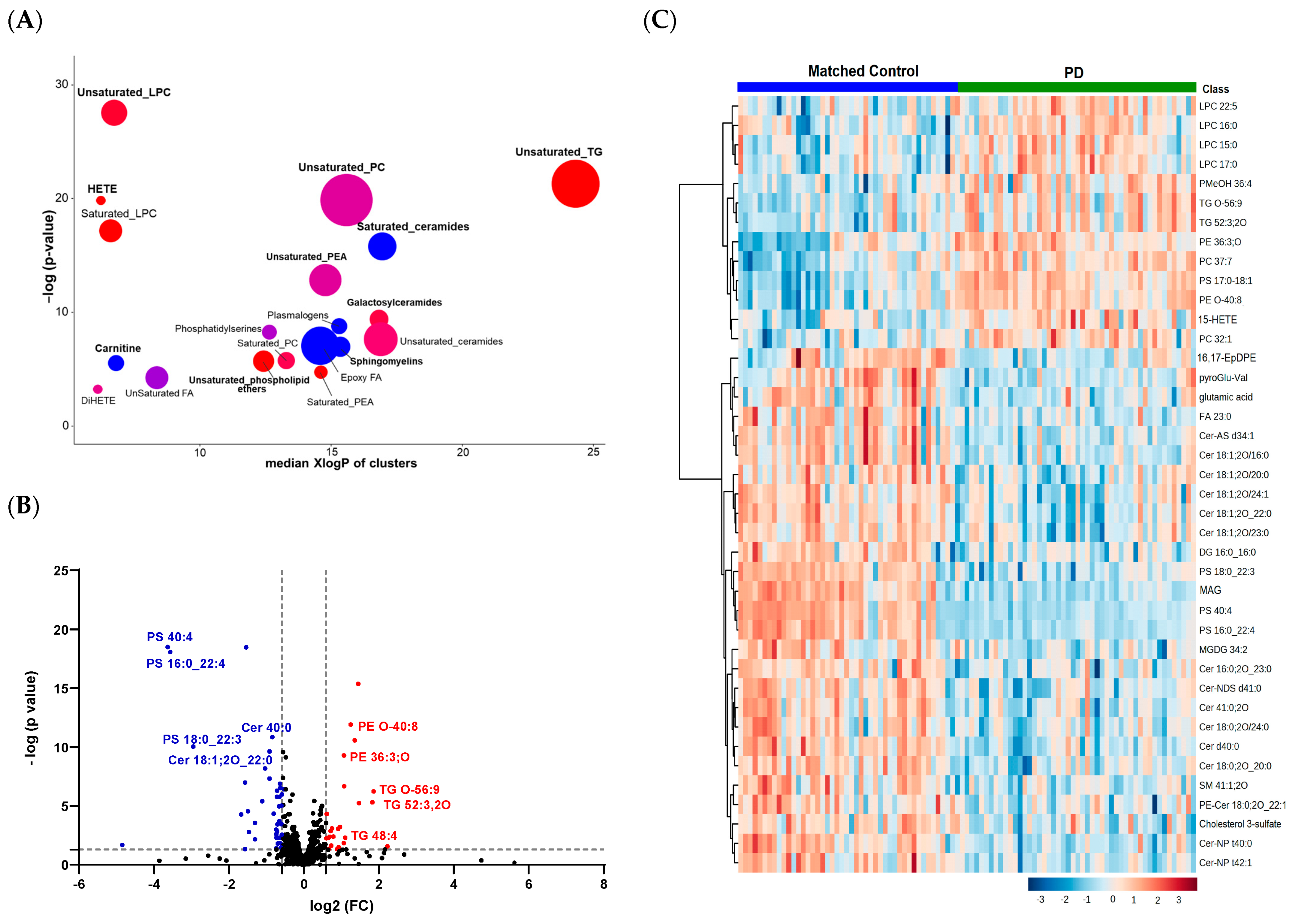
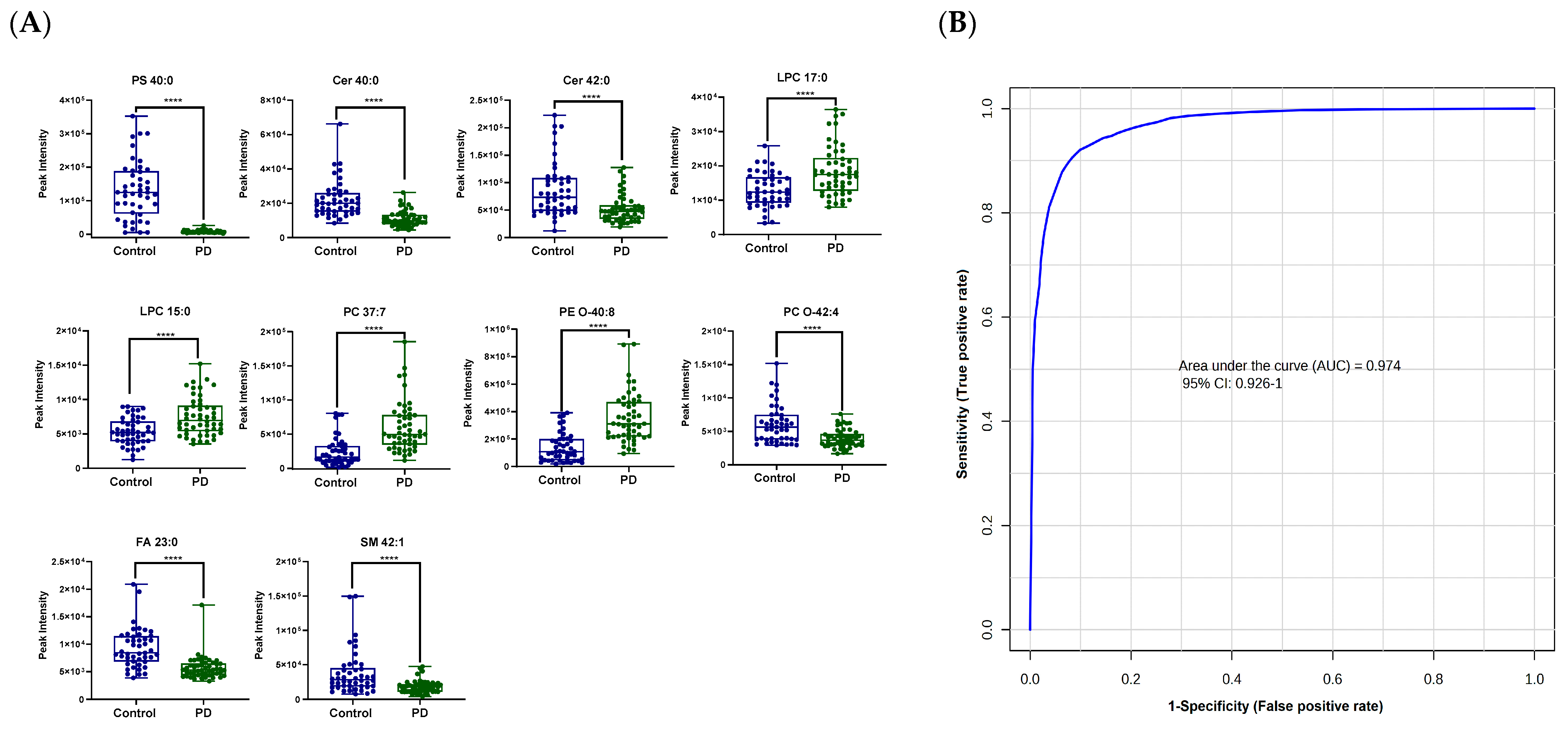
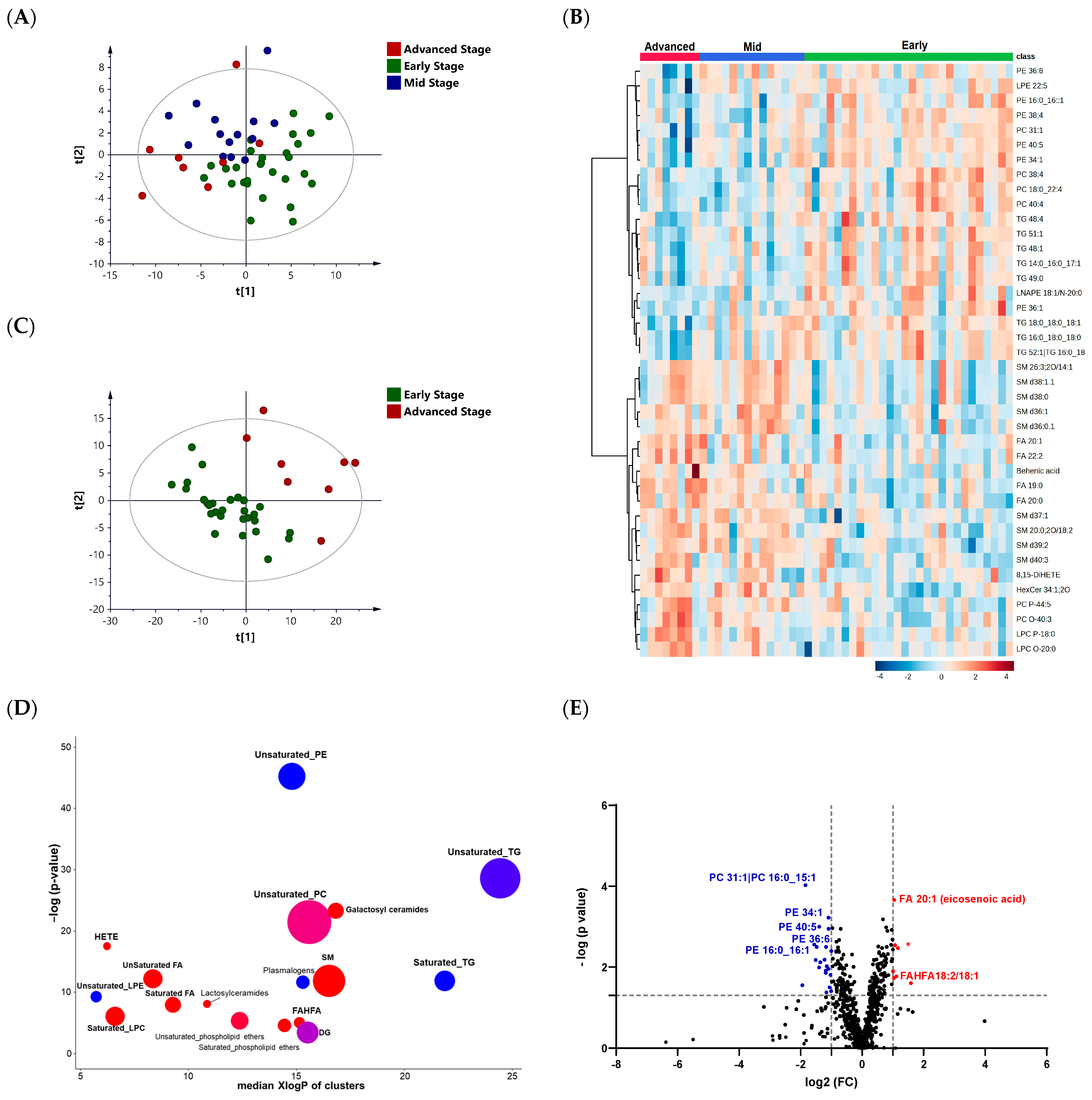
3. Results
3.1. Demographic and Clinical Data of Participants
3.2. Lipid Profiling in Patients with PD Compared to Healthy Controls
3.3. Lipid Profiling of the Three Different Stages of PD: Early, Mid, and Advanced
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease A Review. JAMA J. Am. Med. Assoc. 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Elbaz, A.; Nichols, E.; Abd-Allah, F.; Abdelalim, A.; Adsuar, J.C.; Ansha, M.G.; Brayne, C.; Choi, J.Y.J.; Collado-Mateo, D.; et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef]
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2020, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef]
- Varadi, C. Clinical Features of Parkinson’s Disease: The Evolution of Critical Symptoms. Biology 2020, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Schrag, A.; Horsfall, L.; Walters, K.; Noyce, A.; Petersen, I. Prediagnostic presentations of Parkinson's disease in primary care: A case-control study. Lancet Neurol. 2015, 14, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.; Postuma, R.B.; Adler, C.H.; Bloem, B.R.; Chan, P.; Dubois, B.; Gasser, T.; Goetz, C.G.; Halliday, G.; Joseph, L.; et al. MDS research criteria for prodromal Parkinson’s disease. Mov. Disord. 2015, 30, 1600–1609. [Google Scholar] [CrossRef]
- Lauwers, E.; Goodchild, R.; Verstreken, P. Membrane Lipids in Presynaptic Function and Disease. Neuron 2016, 90, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Galvagnion, C. The Role of Lipids Interacting with alpha-Synuclein in the Pathogenesis of Parkinson’s Disease. J. Park. Dis. 2017, 7, 433–450. [Google Scholar] [CrossRef]
- Hu, Q.S.; Wang, G.H. Mitochondrial dysfunction in Parkinson’s disease. Transl. Neurodegener. 2016, 5, 14. [Google Scholar] [CrossRef]
- Fanning, S.; Selkoe, D.; Dettmer, U. Vesicle trafficking and lipid metabolism in synucleinopathy. Acta Neuropathol. 2021, 141, 491–510. [Google Scholar] [CrossRef] [PubMed]
- Galper, J.; Dean, N.J.; Pickford, R.; Lewis, S.J.G.; Halliday, G.M.; Kim, W.S.; Dzamko, N. Lipid pathway dysfunction is prevalent in patients with Parkinson’s disease. Brain 2022, 145, 3472–3487. [Google Scholar] [CrossRef]
- Spener, F.; Lagarde, M.; Geloen, A.; Record, M. What is lipidomics? Eur. J. Lipid Sci. Technol. 2003, 105, 481–482. [Google Scholar] [CrossRef]
- Pieragostino, D.; Cicalini, I.; Lanuti, P.; Ercolino, E.; di Ioia, M.; Zucchelli, M.; Zappacosta, R.; Miscia, S.; Marchisio, M.; Sacchetta, P.; et al. Enhanced release of acid sphingomyelinase-enriched exosomes generates a lipidomics signature in CSF of Multiple Sclerosis patients. Sci. Rep. 2018, 8, 3071. [Google Scholar] [CrossRef]
- Liu, Y.; Thalamuthu, A.; Mather, K.A.; Crawford, J.; Ulanova, M.; Wong, M.W.K.; Pickford, R.; Sachdev, P.S.; Braidy, N. Plasma lipidome is dysregulated in Alzheimer’s disease and is associated with disease risk genes. Transl. Psychiatry 2021, 11, 344. [Google Scholar] [CrossRef]
- Pizarro, C.; Esteban-Diez, I.; Espinosa, M.; Rodriguez-Royo, F.; Gonzalez-Saiz, J.M. An NMR-based lipidomic approach to identify Parkinson’s disease-stage specific lipoprotein-lipid signatures in plasma. Analyst 2019, 144, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiu, V.; Tiberi, M.; Matteocci, A.; Fazio, F.; Siffeti, H.; Saracini, S.; Mercuri, N.B.; Sancesario, G. Lipidomics of Bioactive Lipids in Alzheimer’s and Parkinson’s Diseases: Where Are We? Int. J. Mol. Sci. 2022, 23, 6235. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Irigoyen, J.; Cartas-Cejudo, P.; Iruarrizaga-Lejarreta, M.; Santamaria, E. Alteration in the Cerebrospinal Fluid Lipidome in Parkinson’s Disease: A Post-Mortem Pilot Study. Biomedicines 2021, 9, 491. [Google Scholar] [CrossRef]
- Hacker, M.L.; DeLong, M.R.; Turchan, M.; Heusinkveld, L.E.; Ostrem, J.L.; Molinari, A.L.; Currie, A.D.; Konrad, P.E.; Davis, T.L.; Phibbs, F.T.; et al. Effects of deep brain stimulation on rest tremor progression in early stage Parkinson disease. Neurology 2018, 91, E463–E471. [Google Scholar] [CrossRef]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression, and mortality. Neurology 1967, 17, 427, reprinted in Neurology 2001, 57, S11–S26. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Chong, I.G.; Jun, C.H. Performance of some variable selection methods when multicollinearity is present. Chemom. Intell. Lab. Syst. 2005, 78, 103–112. [Google Scholar] [CrossRef]
- Banerjee, P.; Ghosh, S.; Dutta, M.; Subramani, E.; Khalpada, J.; RoyChoudhury, S.; Chakravarty, B.; Chaudhury, K. Identification of Key Contributory Factors Responsible for Vascular Dysfunction in Idiopathic Recurrent Spontaneous Miscarriage. PLoS ONE 2013, 8, e80940. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef] [PubMed]
- Barupal, D.K.; Fiehn, O. Chemical Similarity Enrichment Analysis (ChemRICH) as alternative to biochemical pathway mapping for metabolomic datasets. Sci. Rep. 2017, 7, 14567. [Google Scholar] [CrossRef]
- Alecu, I.; Bennett, S.A.L. Dysregulated Lipid Metabolism and Its Role in alpha-Synucleinopathy in Parkinson’s Disease. Front. Neurosci. 2019, 13, 328. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Soler, M.; Cordobilla, B.; Morato, X.; Fernandez-Duenas, V.; Domingo, J.C.; Ciruela, F. Triglyceride Form of Docosahexaenoic Acid Mediates Neuroprotection in Experimental Parkinsonism. Front. Neurosci. 2018, 12, 604. [Google Scholar] [CrossRef]
- Zhang, M.M.; Chen, H.M.; Liu, G.L.; Wang, X.M.; Wang, Z.; Feng, T.; Zhang, Y.M. Lower serum triglyceride levels linked to more severe motor performance in Parkinson’s disease. Neurol. Sci. 2022, 43, 5343–5353. [Google Scholar] [CrossRef]
- Huang, X.X.; Ng, S.Y.E.; Chia, N.S.Y.; Acharyya, S.; Setiawan, F.; Lu, Z.H.; Tan, Y.J.; Ng, E.; Wen, M.C.; Ng, A.S.L.; et al. Higher serum triglyceride levels are associated with Parkinson’s disease mild cognitive impairment. Mov. Disord. 2018, 33, 1970–1971. [Google Scholar] [CrossRef]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The Role of Lipids in Parkinson’s Disease. Cells 2019, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Huang, B.X.; Spector, A.A. Phosphatidylserine in the brain: Metabolism and function. Prog. Lipid Res. 2014, 56, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Zhang, X.; Wang, L.J.; Yang, C.D. High Performance Liquid Chromatography-Mass Spectrometry (LC-MS) Based Quantitative Lipidomics Study of Ganglioside-NANA-3 Plasma to Establish Its Association with Parkinson’s Disease Patients. Med. Sci. Monit. 2017, 23, 5345–5353. [Google Scholar] [CrossRef]
- Xicoy, H.; Brouwers, J.F.; Kalnytska, O.; Wieringa, B.; Martens, G.J.M. Lipid Analysis of the 6-Hydroxydopamine-Treated SH-SY5Y Cell Model for Parkinson’s Disease. Mol. Neurobiol. 2020, 57, 848–859. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Choi, H.; Chevalier, A.; Hogan, D.; Akgoc, Z.; Schneider, J.S. Sex-Related Abnormalities in Substantia Nigra Lipids in Parkinson’s Disease. ASN Neuro 2018, 10, 1759091418781889. [Google Scholar] [CrossRef]
- Farmer, K.; Smith, C.A.; Hayley, S.; Smith, J. Major Alterations of Phosphatidylcholine and Lysophosphotidylcholine Lipids in the Substantia Nigra Using an Early Stage Model of Parkinson’s Disease. Int. J. Mol. Sci. 2015, 16, 18865–18877. [Google Scholar] [CrossRef]
- Hung, N.D.; Sok, D.E.; Kim, M.R. Prevention of 1-palmitoyl lysophosphatidylcholine-induced inflammation by polyunsaturated acyl lysophosphatidylcholine. Inflamm. Res. 2012, 61, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Law, S.H.; Chan, M.L.; Marathe, G.K.; Parveen, F.; Chen, C.H.; Ke, L.Y. An Updated Review of Lysophosphatidylcholine Metabolism in Human Diseases. Int. J. Mol. Sci. 2019, 20, 1149. [Google Scholar] [CrossRef] [PubMed]
- Hollie, N.I.; Cash, J.G.; Matlib, A.; Wortman, M.; Basford, J.E.; Abplanalp, W.; Hui, D.Y. Micromolar changes in lysophosphatidylcholine concentration cause minor effects on mitochondrial permeability but major alterations in function. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 888–895. [Google Scholar] [CrossRef]
- Lee, E.S.Y.; Chen, H.; Charlton, C.G.; Soliman, K.F.A. The role of phospholipid methylation in 1-methyl-4-phenyl-pyridinium ion (MPP+)-induced neurotoxicity in PC12 cells. Neurotoxicology 2005, 26, 945–957. [Google Scholar] [CrossRef]
- Lobasso, S.; Tanzarella, P.; Vergara, D.; Maffia, M.; Cocco, T.; Corcelli, A. Lipid profiling of parkin-mutant human skin fibroblasts. J. Cell. Physiol. 2017, 232, 3540–3551. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Wang, C.; Zhao, N.; Li, W.X.; Yang, Z.F.; Liu, X.X.; Le, W.D.; Zhang, X.Z. Potential biomarkers of Parkinson’s disease revealed by plasma metabolic profiling. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1081, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.X.; Zhang, S.Y.; Liou, L.C.; Ren, Q.; Zhang, Z.J.; Caldwell, G.A.; Caldwell, K.A.; Witt, S.N. Phosphatidylethanolamine deficiency disrupts alpha-synuclein homeostasis in yeast and worm models of Parkinson disease. Proc. Natl. Acad. Sci. USA 2014, 111, E3976–E3985. [Google Scholar] [CrossRef]
- Abbott, S.K.; Li, H.Y.; Munoz, S.S.; Knoch, B.; Batterham, M.; Murphy, K.E.; Halliday, G.M.; Garner, B. Altered ceramide acyl chain length and ceramide synthase gene expression in Parkinson’s disease. Mov. Disord. 2014, 29, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Seet, R.C.S.; Lee, C.Y.J.; Lim, E.C.H.; Tan, J.J.H.; Quek, A.M.L.; Chong, W.L.; Looi, W.F.; Huang, S.H.; Wang, H.S.; Chan, Y.H.; et al. Oxidative damage in Parkinson disease: Measurement using accurate biomarkers. Free Radic. Biol. Med. 2010, 48, 560–566. [Google Scholar] [CrossRef]
- Nahm, F.S. Receiver operating characteristic curve: Overview and practical use for clinicians. Korean J. Anesthesiol. 2022, 75, 25–36. [Google Scholar] [CrossRef] [PubMed]

| Demographic and Clinical Characteristics | PD (n = 50) | Control (n = 45) | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p-Value b | |
| Age (years) | 64.2 | 13.3 | 59.4 | 10.4 | 0.06 |
| Gender (F/M) a | 19/31 | NA | 23/22 | NA | 0.20 |
| BMI (kg/m2) | 28.3 | 5.2 | 29.9 | 4.4 | 0.10 |
| Systolic blood pressure (mmHg) | 131.6 | 16.4 | NA | NA | |
| Diastolic blood pressure (mmHg) | 84.2 | 6.8 | NA | NA | |
| Duration of PD (years) | 9.8 | 7.2 | NA | NA | |
| Total cholesterol (mmol/L) | 5.18 | 0.53 | NA | NA | |
| HDL cholesterol (mmol/L) | 1.28 | 0.25 | NA | NA | |
| LDL cholesterol (mmol/L) | 3.45 | 0.90 | NA | NA | |
| Demographic and Clinical Characteristics | PD—Early (n = 28) | PD—Mid (n = 14) | PD—Advanced (n = 8) | p-Value b |
|---|---|---|---|---|
| Age (years) | 63.9 ± 12.1 | 68.7 ± 13.3 | 57.5 ± 15.8 | 0.07 |
| Gender (F/M) a | 10/18 | 6/8 | 3/5 | 0.12 |
| BMI (kg/m2) | 28.2 ± 6.4 | 28.5 ± 2.8 | 28.3 ± 3.4 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahabiyeh, L.A.; Nimer, R.M.; Rashed, M.; Wells, J.D.; Fiehn, O. Serum-Based Lipid Panels for Diagnosis of Idiopathic Parkinson’s Disease. Metabolites 2023, 13, 990. https://doi.org/10.3390/metabo13090990
Dahabiyeh LA, Nimer RM, Rashed M, Wells JD, Fiehn O. Serum-Based Lipid Panels for Diagnosis of Idiopathic Parkinson’s Disease. Metabolites. 2023; 13(9):990. https://doi.org/10.3390/metabo13090990
Chicago/Turabian StyleDahabiyeh, Lina A., Refat M. Nimer, Maha Rashed, Jeremiah D. Wells, and Oliver Fiehn. 2023. "Serum-Based Lipid Panels for Diagnosis of Idiopathic Parkinson’s Disease" Metabolites 13, no. 9: 990. https://doi.org/10.3390/metabo13090990
APA StyleDahabiyeh, L. A., Nimer, R. M., Rashed, M., Wells, J. D., & Fiehn, O. (2023). Serum-Based Lipid Panels for Diagnosis of Idiopathic Parkinson’s Disease. Metabolites, 13(9), 990. https://doi.org/10.3390/metabo13090990






