Perturbations of Glutathione and Sphingosine Metabolites in Port Wine Birthmark Patient-Derived Induced Pluripotent Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Preparation
2.2. PWB iPSC Culture and Sample Preparation
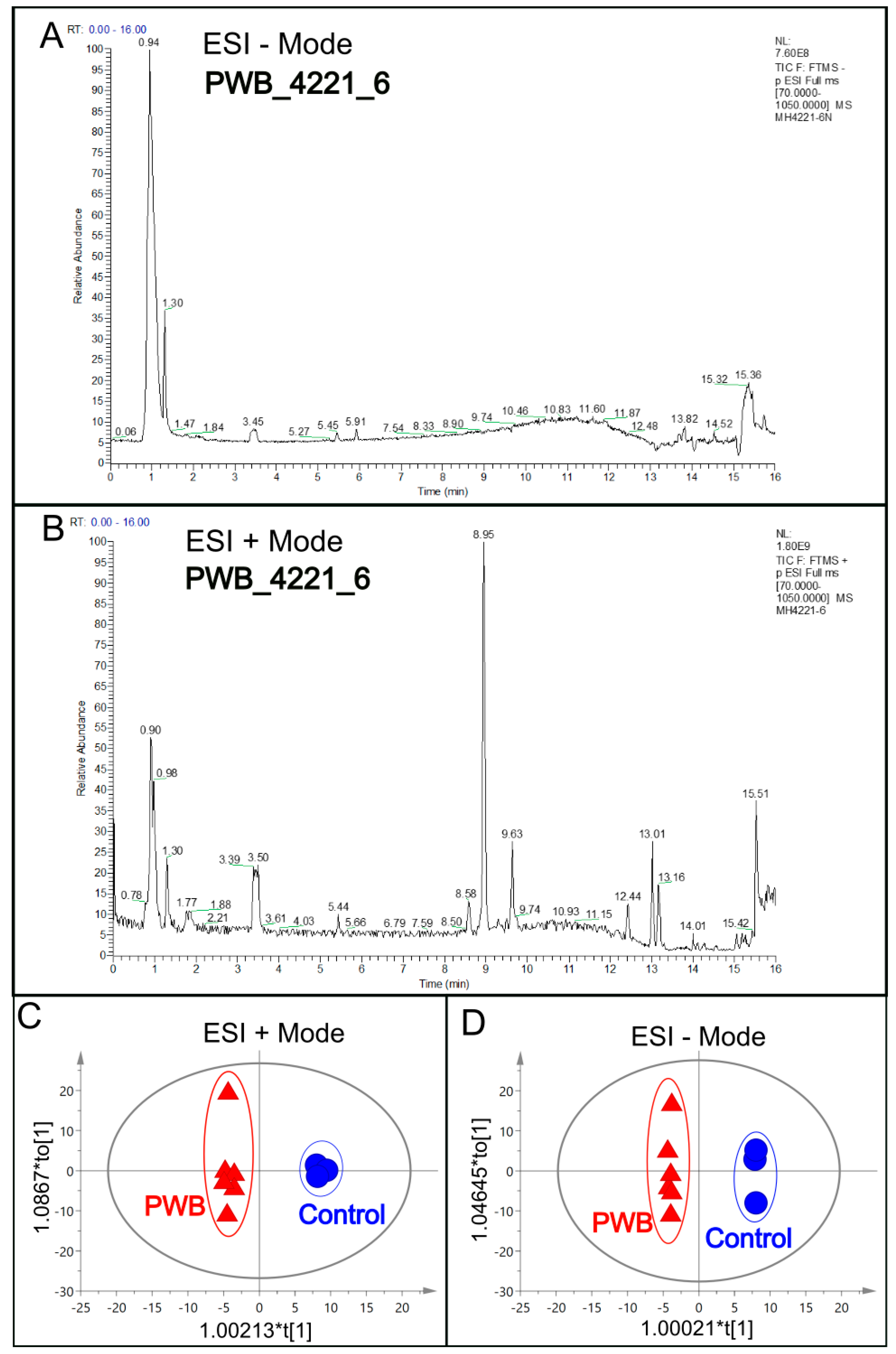
2.3. Metabolomics by LC–MS
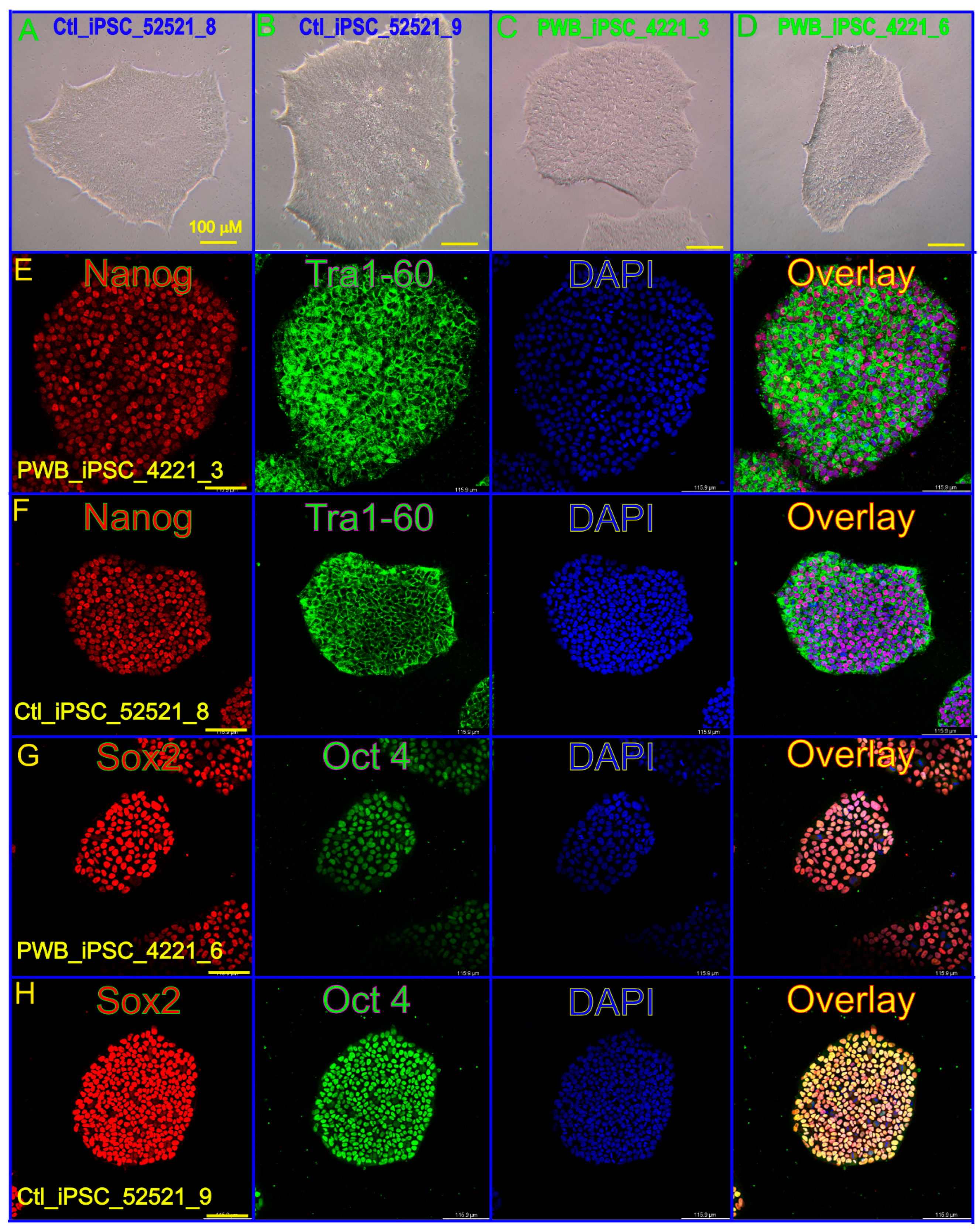
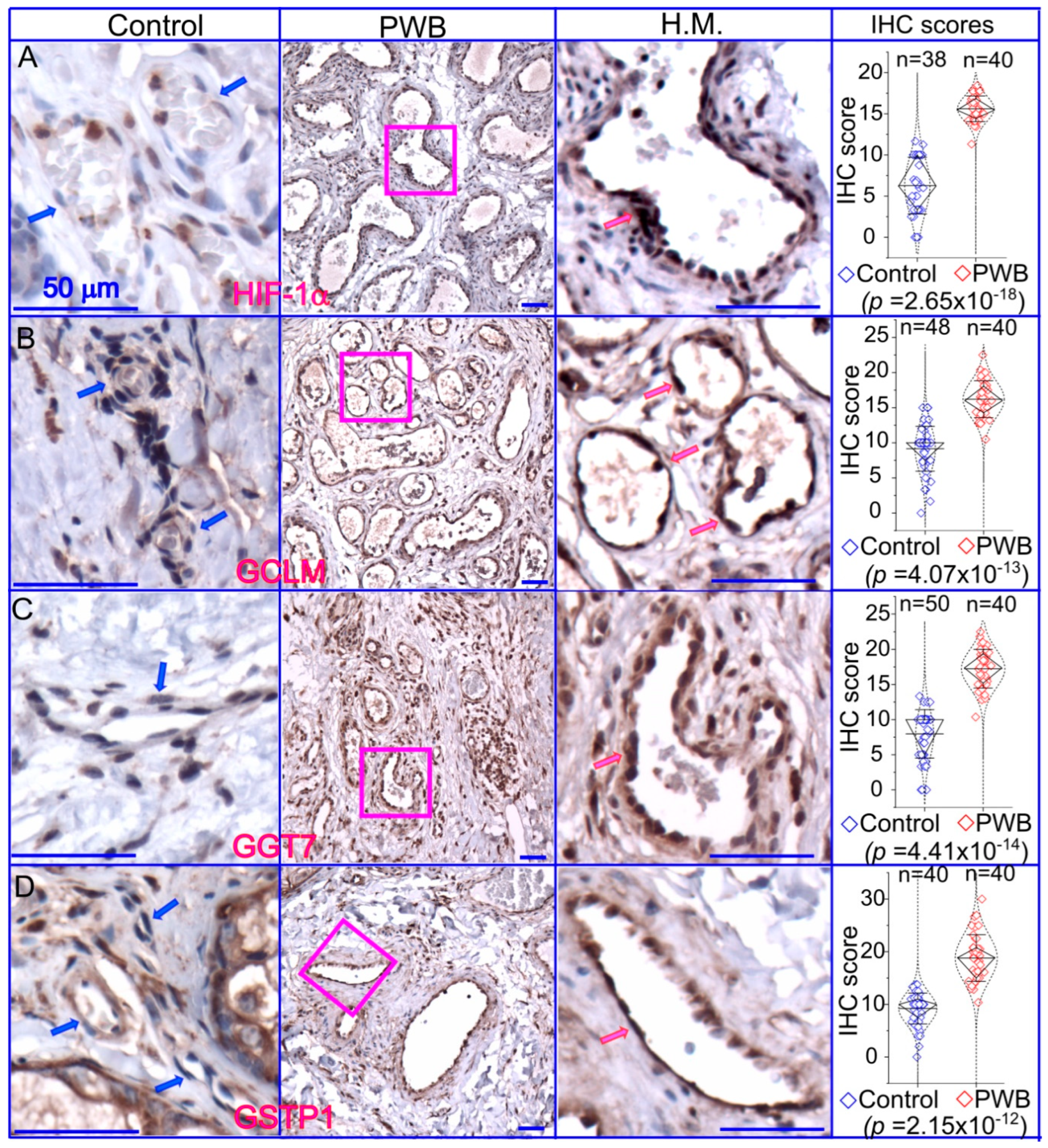
2.4. Immunohistochemistry (IHC)
2.5. Statistical Analysis
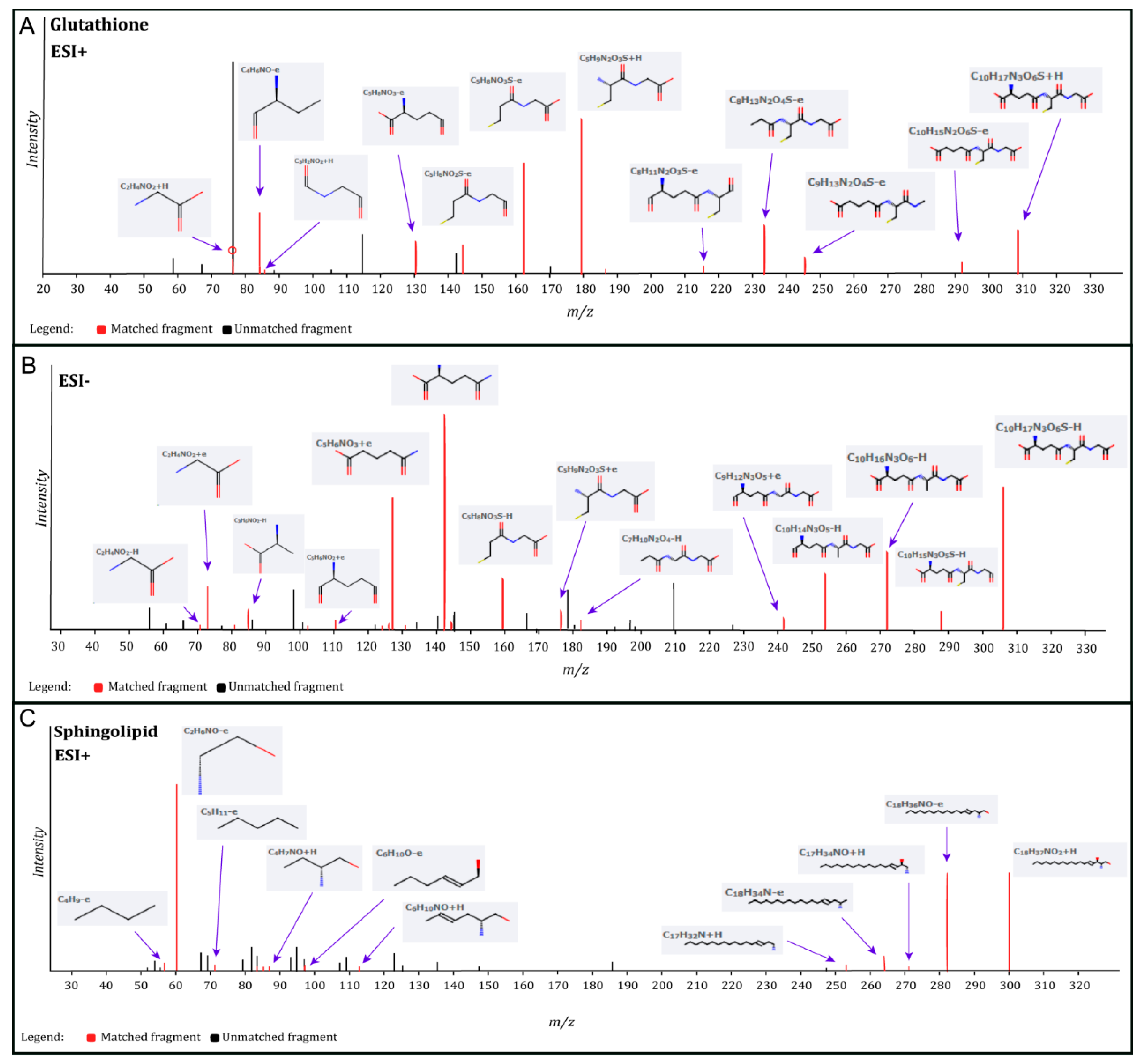
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lever, W.F.; Schaumburg-Lever, G. Histopathology of the Skin; J.B. Lippincott Co.: Philadelphia, PA, USA, 1990. [Google Scholar]
- Barsky, S.H.; Rosen, S.; Geer, D.E.; Noe, J.M. The nature and evolution of port wine stains: A computer-assisted study. J. Investig. Dermatol. 1980, 74, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Goldwyn, R.M. Port wine stain. Plast. Reconstr. Surg. 1980, 66, 647. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Hochman, M.; Mihm, M.C., Jr.; Nelson, J.S.; Tan, W. The pathogenesis of port wine stain and sturge weber syndrome: Complex interactions between genetic alterations and aberrant mapk and pi3k activation. Int. J. Mol. Sci. 2019, 20, 2243. [Google Scholar] [CrossRef] [PubMed]
- ISSVA. Issva Classification of Vascular Anomalies. International Society for the Study of Vascular Anomalies. 2014. Available online: https://www.issva.org/classification (accessed on 21 May 2022).
- Waelchli, R.; Aylett, S.E.; Robinson, K.; Chong, W.K.; Martinez, A.E.; Kinsler, V.A. New vascular classification of port-wine stains: Improving prediction of sturge-weber risk. Br. J. Dermatol. 2014, 171, 861–867. [Google Scholar] [CrossRef]
- Hagen, S.L.; Grey, K.R.; Korta, D.Z.; Kelly, K.M. Quality of life in adults with facial port-wine stains. J. Am. Acad. Dermatol. 2017, 76, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Wang, J.; Zhou, F.; Gao, L.; Rong, Y.; Liu, H.; Sukanthanag, A.; Wang, G.; Mihm, M.C., Jr.; Chen, D.B.; et al. Coexistence of ephb1 and ephrinb2 in port wine stain endothelial progenitor cells contributes to clinicopathological vasculature dilatation. Br. J. Dermatol. 2017, 177, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.; Brasch, H.; Bockett, N.; Patel, J.; Paterson, E.; Davis, P.; Tan, S. Embryonic stem cell-like population in hypertrophic port-wine stain. J. Vasc. Anom. 2021, 2, e006. [Google Scholar] [CrossRef]
- Tan, W.; Nadora, D.M.; Gao, L.; Wang, G.; Mihm, M.C., Jr.; Nelson, J.S. The somatic gnaq mutation (r183q) is primarily located in port wine stain blood vessels. J. Am. Acad. Dermatol. 2016, 74, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Zakka, L.R.; Gao, L.; Wang, J.; Zhou, F.; Selig, M.K.; Sukanthanag, A.; Wang, G.; Mihm, M.C.J.; Nelson, J.S. Pathological alterations involve the entire skin physiological milieu in infantile and early childhood port wine stain. Br. J. Dermatol. 2016, 177, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Savas, J.A.; Ledon, J.A.; Franca, K.; Chacon, A.; Nouri, K. Pulsed dye laser-resistant port-wine stains: Mechanisms of resistance and implications for treatment. Br. J. Dermatol. 2013, 168, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.S.; Jia, W.; Phung, T.L.; Mihm, M.C., Jr. Observations on enhanced port wine stain blanching induced by combined pulsed dye laser and rapamycin administration. Lasers Surg. Med. 2011, 43, 939–942. [Google Scholar] [CrossRef] [PubMed]
- van Raath, M.I.; Chohan, S.; Wolkerstorfer, A.; van der Horst, C.; Storm, G.; Heger, M. Port wine stain treatment outcomes have not improved over the past three decades. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Gao, C.; Hochman, M.; Kravitz, J.; Chen, E.; Friedman, H.; Wenceslau, C.; Chen, D.; Wang, Y.; Nelson, J.S.; et al. Supporting materials: Endothelial cells differentiated from patient dermal fibroblast-derived induced pluripotent stem cells resemble vascular malformations of Port Wine Birthmark. bioRxiv 2023. [Google Scholar] [CrossRef]
- Tan, W.; Chernova, M.; Gao, L.; Sun, V.; Liu, H.; Jia, W.; Langer, S.; Wang, G.; Mihm, M.C., Jr.; Nelson, J.S. Sustained activation of c-jun n-terminal and extracellular signal-regulated kinases in port-wine stain blood vessels. J. Am. Acad. Dermatol. 2014, 71, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Rice, S.J.; Wang, J.; Gao, L.; Tsai, J.; Anvari, R.T.; Zhou, F.; Liu, X.; Wang, G.; Tang, Y.; et al. Membrane trafficking and exocytosis are upregulated in port wine stain blood vessels. Histol. Histopathol. 2018, 34, 18051. [Google Scholar] [CrossRef]
- Igarashi, Y. Functional roles of sphingosine, sphingosine 1-phosphate, and methylsphingosines: In regard to membrane sphingolipid signaling pathways. J. Biochem. 1997, 122, 1080–1087. [Google Scholar] [CrossRef]
- Woodcock, J. Sphingosine and ceramide signalling in apoptosis. IUBMB Life 2006, 58, 462–466. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Gulbins, E.; Grassme, H. The anti-infectious role of sphingosine in microbial diseases. Cells 2021, 10, 1105. [Google Scholar] [CrossRef] [PubMed]
- Tommasino, C.; Marconi, M.; Ciarlo, L.; Matarrese, P.; Malorni, W. Autophagic flux and autophagosome morphogenesis require the participation of sphingolipids. Apoptosis 2015, 20, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Merrill, A.H.; Obeid, L.M.; Hannun, Y.A. Effects of sphingosine and other sphingolipids on protein kinase c. Methods Enzymol. 2000, 312, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Tang, C.; Wu, J.X.; Ji, B.W.; Gong, B.M.; Wu, X.H.; Wang, X. Mass spectrometry detects sphingolipid metabolites for discovery of new strategy for cancer therapy from the aspect of programmed cell death. Metabolites 2023, 13, 867. [Google Scholar] [CrossRef] [PubMed]
- Matwiejuk, M.; Mysliwiec, H.; Chabowski, A.; Flisiak, I. The role of sphingolipids in the pathogenesis of psoriasis. Metabolites 2022, 12, 1171. [Google Scholar] [CrossRef]
- Cappuccio, G.; Donti, T.; Pinelli, M.; Bernardo, P.; Bravaccio, C.; Elsea, S.H.; Brunetti-Pierri, N. Sphingolipid metabolism perturbations in rett syndrome. Metabolites 2019, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Hakomori, S. Bifunctional role of glycosphingolipids. Modulators for transmembrane signaling and mediators for cellular interactions. J. Biol. Chem. 1990, 265, 18713–18716. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Bell, R.M. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science 1989, 243, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Gao, L.; Tan, W.; Guo, W.; Zhao, T.; Nelson, J.S.; Wang, G. Activation of pkcα and pi3k kinases in hypertrophic and nodular port wine stain lesions. Am. J. Dermatopathol. 2017, 39, 747–752. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Simon, M.C. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, P.G.; Ferrington, D.A.; Kannan, R. Glutathione metabolism and the novel role of mitochondrial gsh in retinal degeneration. Antioxidants 2021, 10, 661. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.T.; Kaufmann, Y.C.; Luo, S.; Todorova, V.; Klimberg, V.S. Effect of glutamine on glutathione, igf-i, and tgf-beta 1. J. Surg. Res. 2003, 111, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Reeds, P.J.; Burrin, D.G.; Stoll, B.; Jahoor, F.; Wykes, L.; Henry, J.; Frazer, M.E. Enteral glutamate is the preferential source for mucosal glutathione synthesis in fed piglets. Am. J. Physiol. 1997, 273, E408–E415. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic. Biol. Med. 1999, 27, 922–935. [Google Scholar] [CrossRef]
- Estrela, J.M.; Ortega, A.; Obrador, E. Glutathione in cancer biology and therapy. Crit. Rev. Clin. Lab. Sci. 2006, 43, 143–181. [Google Scholar] [CrossRef] [PubMed]
- Lien, E.C.; Lyssiotis, C.A.; Juvekar, A.; Hu, H.; Asara, J.M.; Cantley, L.C.; Toker, A. Glutathione biosynthesis is a metabolic vulnerability in pi(3)k/akt-driven breast cancer. Nat. Cell Biol. 2016, 18, 572–578. [Google Scholar] [CrossRef]
- Manchel, A.; Mahadevan, R.; Bataller, R.; Hoek, J.B.; Vadigepalli, R. Genome-scale metabolic modeling reveals sequential dysregulation of glutathione metabolism in livers from patients with alcoholic hepatitis. Metabolites 2022, 12, 1157. [Google Scholar] [CrossRef] [PubMed]
- Kyo, M.; Zhu, Z.; Nanishi, M.; Shibata, R.; Ooka, T.; Freishtat, R.J.; Mansbach, J.M.; Camargo, C.A., Jr.; Hasegawa, K. Association of nasopharyngeal and serum glutathione metabolism with bronchiolitis severity and asthma risk: A prospective multicenter cohort study. Metabolites 2022, 12, 674. [Google Scholar] [CrossRef]
- Gupta, R.K.; Patel, A.K.; Shah, N.; Chaudhary, A.K.; Jha, U.K.; Yadav, U.C.; Gupta, P.K.; Pakuwal, U. Oxidative stress and antioxidants in disease and cancer: A review. Asian Pac. J. Cancer Prev. 2014, 15, 4405–4409. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noe, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ros damage and regulating ros signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Venneti, S.; Nagrath, D. Glutaminolysis: A hallmark of cancer metabolism. Annu. Rev. Biomed. Eng. 2017, 19, 163–194. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Aspects Med. 2009, 30, 42–59. [Google Scholar] [CrossRef]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.Z.; Chen, C.; Zeng, Z.; Yang, H.; Oh, J.; Chen, L.; Lu, S.C. Mechanism and significance of increased glutathione level in human hepatocellular carcinoma and liver regeneration. FASEB J. 2001, 15, 19–21. [Google Scholar] [CrossRef]
- Hakimi, A.A.; Reznik, E.; Lee, C.H.; Creighton, C.J.; Brannon, A.R.; Luna, A.; Aksoy, B.A.; Liu, E.M.; Shen, R.; Lee, W.; et al. An integrated metabolic atlas of clear cell renal cell carcinoma. Cancer Cell 2016, 29, 104–116. [Google Scholar] [CrossRef]
- Hofbauer, S.L.; Stangl, K.I.; de Martino, M.; Lucca, I.; Haitel, A.; Shariat, S.F.; Klatte, T. Pretherapeutic gamma-glutamyltransferase is an independent prognostic factor for patients with renal cell carcinoma. Br. J. Cancer 2014, 111, 1526–1531. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Sharma, M.; Gupta, P.K. Generation of ros in cells on exposure to cw and pulsed near-infrared laser tweezers. Photochem. Photobiol. Sci. 2006, 5, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Shoge, M.; Oda, E.; Yamamoto, Y.; Giddings, J.C.; Kashiwagi, S.; Suematsu, M.; Yamamoto, J. The free-radical scavenger, edaravone, augments no release from vascular cells and platelets after laser-induced, acute endothelial injury in vivo. Platelets 2006, 17, 201–206. [Google Scholar] [CrossRef]
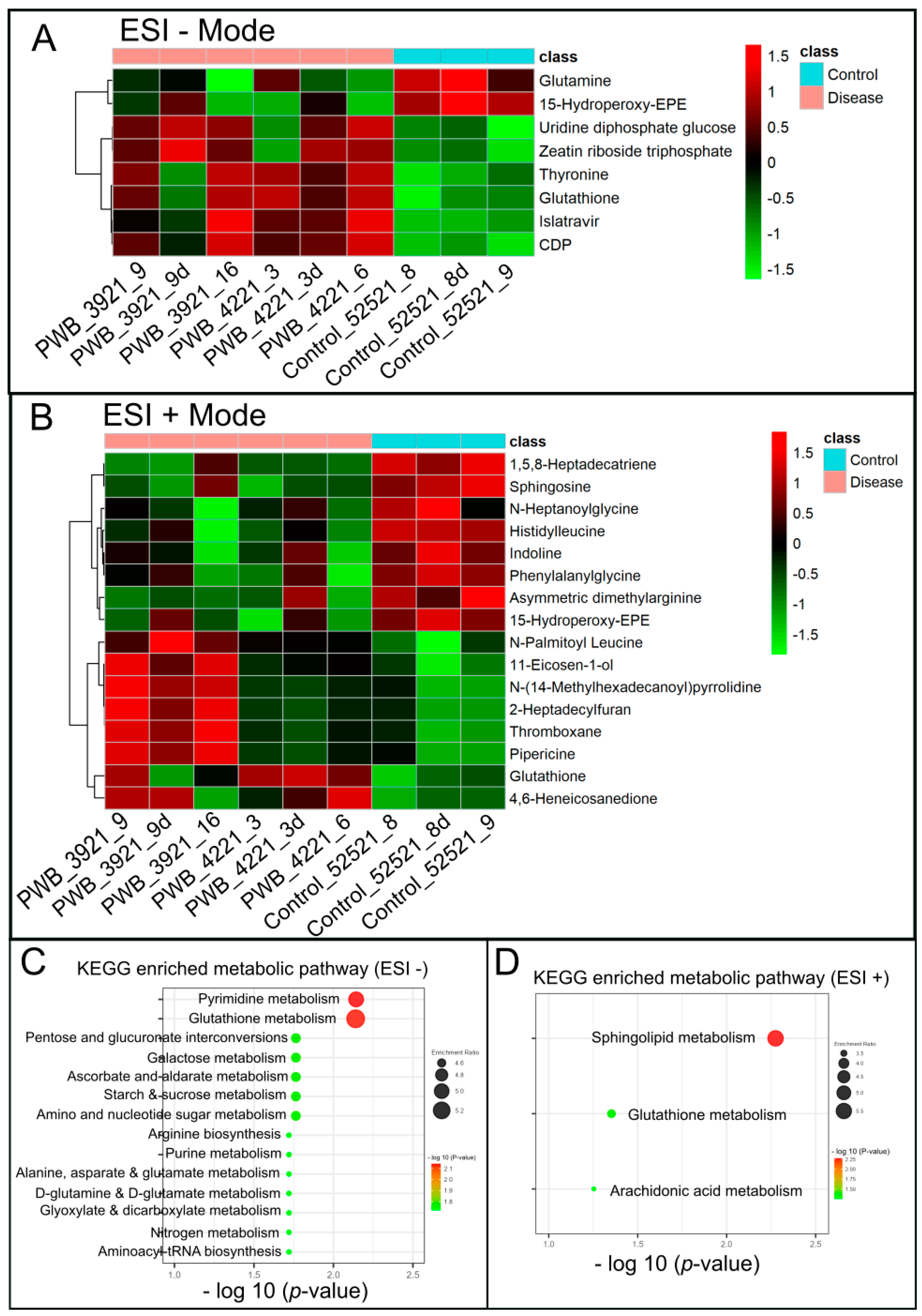
| ESI − Mode | ESI + Mode | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Metabolite | HMDB_ID | VIP | Log2(FC) | p Value | Metabolite | HMDB_ID | VIP | Log2(FC) | p Value |
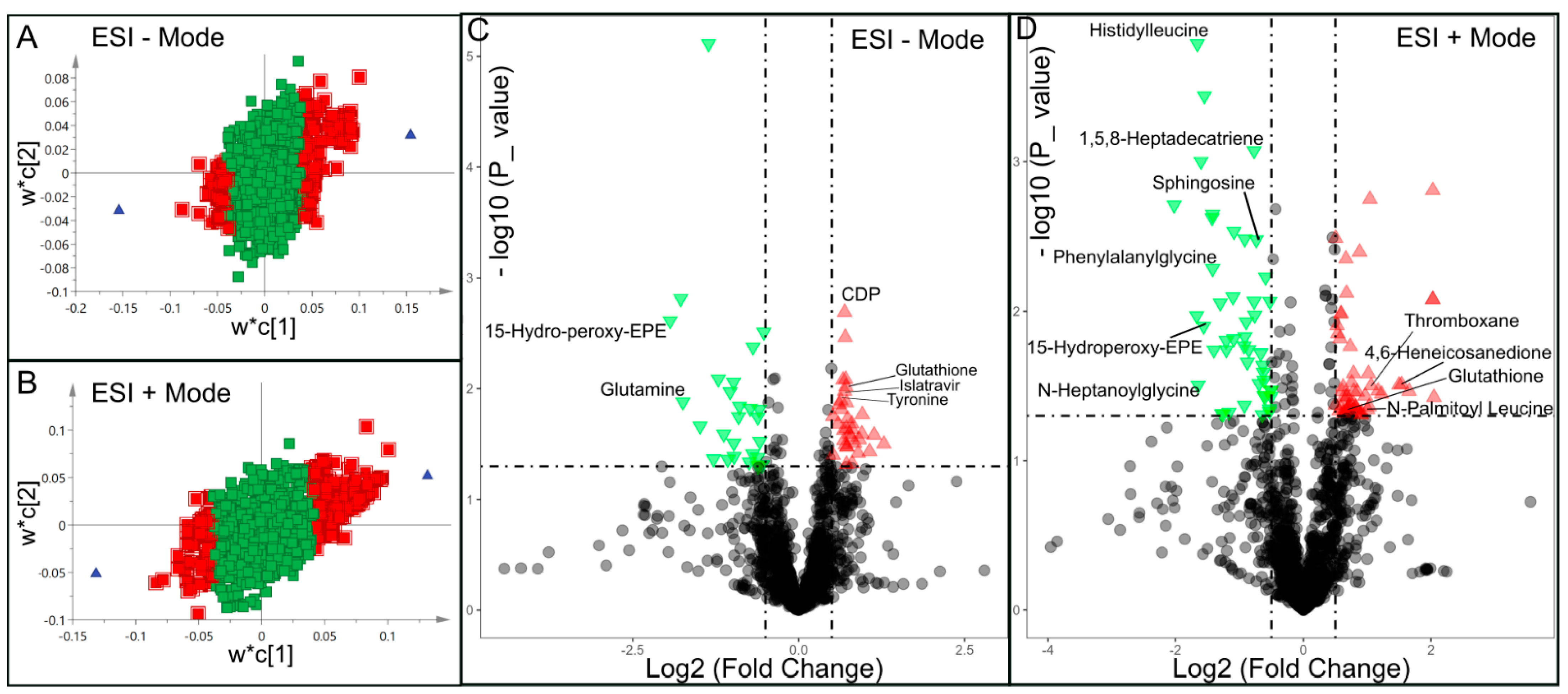
| Glutamine | HMDB000641 | 2.914 | −1.742 | 0.013 | Indoline | HMDB253472 | 1.891 | −0.876 | 0.022 |
| 15-Hydro-peroxy-EPE | HMDB062295 | 3.286 | −1.937 | 0.002 | Asymmetric dimethylarginine | HMDB001539 | 1.921 | −0.916 | 0.015 |
| Phenylalanylglycine | HMDB028995 | 2.554 | −1.419 | 0.005 | |||||
| N-Heptanoylglycine | HMDB013010 | 2.446 | −1.648 | 0.031 | |||||
| 15-Hydroperoxy-EPE | HMDB062295 | 2.675 | −1.561 | 0.012 | |||||
| 1,5,8-Heptadecatriene | HMDB041082 | 1.868 | −0.770 | 0.001 | |||||
| Histidylleucine | HMDB028889 | 2.820 | −1.660 | <0.001 | |||||
| Sphingosine | HMDB000252 | 1.767 | −0.736 | 0.003 | |||||
| Thyronine | HMDB000667 | 1.733 | 0.635 | 0.013 | 2-Heptadecylfuran | HMDB033608 | 1.562 | 0.802 | 0.045 |
| Glutathione | HMDB000125 | 1.867 | 0.710 | 0.009 | Glutathione | HMDB000125 | 1.549 | 0.677 | 0.046 |
| Islatravir | HMDB253596 | 1.870 | 0.691 | 0.010 | Thromboxane | HMDB003208 | 1.774 | 0.969 | 0.037 |
| CDP | HMDB001546 | 1.953 | 0.691 | 0.002 | 11-Eicosen-1-ol | HMDB034933 | 1.817 | 0.822 | 0.044 |
| Uridine diphosphate glucose | HMDB000286 | 1.558 | 0.5164 | 0.018 | N-(14-Methylhexadecanoyl)pyrrolidine | HMDB034373 | 1.803 | 1.030 | 0.043 |
| Zeatin riboside triphosphate | HMDB304509 | 1.645 | 0.594 | 0.021 | Pipericine | HMDB031678 | 1.686 | 0.908 | 0.049 |
| 4,6-Heneicosanedione | HMDB035571 | 2.106 | 1.538 | 0.031 | |||||
| N-Palmitoyl Leucine | HMDB241928 | 1.537 | 0.629 | 0.048 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.; Kravitz, J.; Gao, C.; Hochman, M.L.; Meng, D.; Chen, D.; Wang, Y.; Jegga, A.G.; Nelson, J.S.; Tan, W. Perturbations of Glutathione and Sphingosine Metabolites in Port Wine Birthmark Patient-Derived Induced Pluripotent Stem Cells. Metabolites 2023, 13, 983. https://doi.org/10.3390/metabo13090983
Nguyen V, Kravitz J, Gao C, Hochman ML, Meng D, Chen D, Wang Y, Jegga AG, Nelson JS, Tan W. Perturbations of Glutathione and Sphingosine Metabolites in Port Wine Birthmark Patient-Derived Induced Pluripotent Stem Cells. Metabolites. 2023; 13(9):983. https://doi.org/10.3390/metabo13090983
Chicago/Turabian StyleNguyen, Vi, Jacob Kravitz, Chao Gao, Marcelo L. Hochman, Dehao Meng, Dongbao Chen, Yunguan Wang, Anil G. Jegga, J Stuart Nelson, and Wenbin Tan. 2023. "Perturbations of Glutathione and Sphingosine Metabolites in Port Wine Birthmark Patient-Derived Induced Pluripotent Stem Cells" Metabolites 13, no. 9: 983. https://doi.org/10.3390/metabo13090983
APA StyleNguyen, V., Kravitz, J., Gao, C., Hochman, M. L., Meng, D., Chen, D., Wang, Y., Jegga, A. G., Nelson, J. S., & Tan, W. (2023). Perturbations of Glutathione and Sphingosine Metabolites in Port Wine Birthmark Patient-Derived Induced Pluripotent Stem Cells. Metabolites, 13(9), 983. https://doi.org/10.3390/metabo13090983









