Human Skin Drug Metabolism: Relationships between Methyl Salicylate Metabolism and Esterase Activities in IVPT Skin Membranes
Abstract
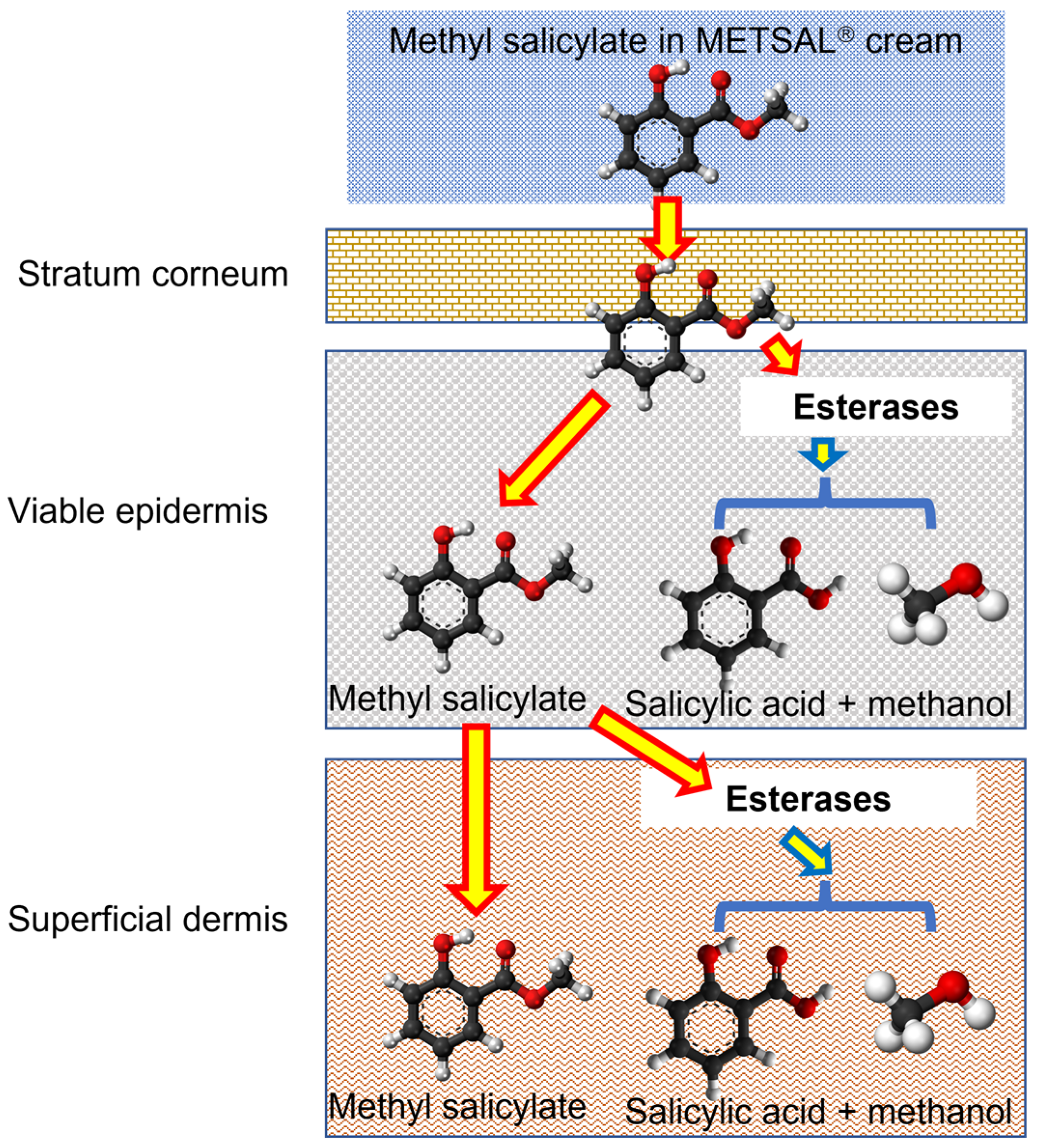
1. Introduction
2. Materials and Methods
2.1. Chemicals
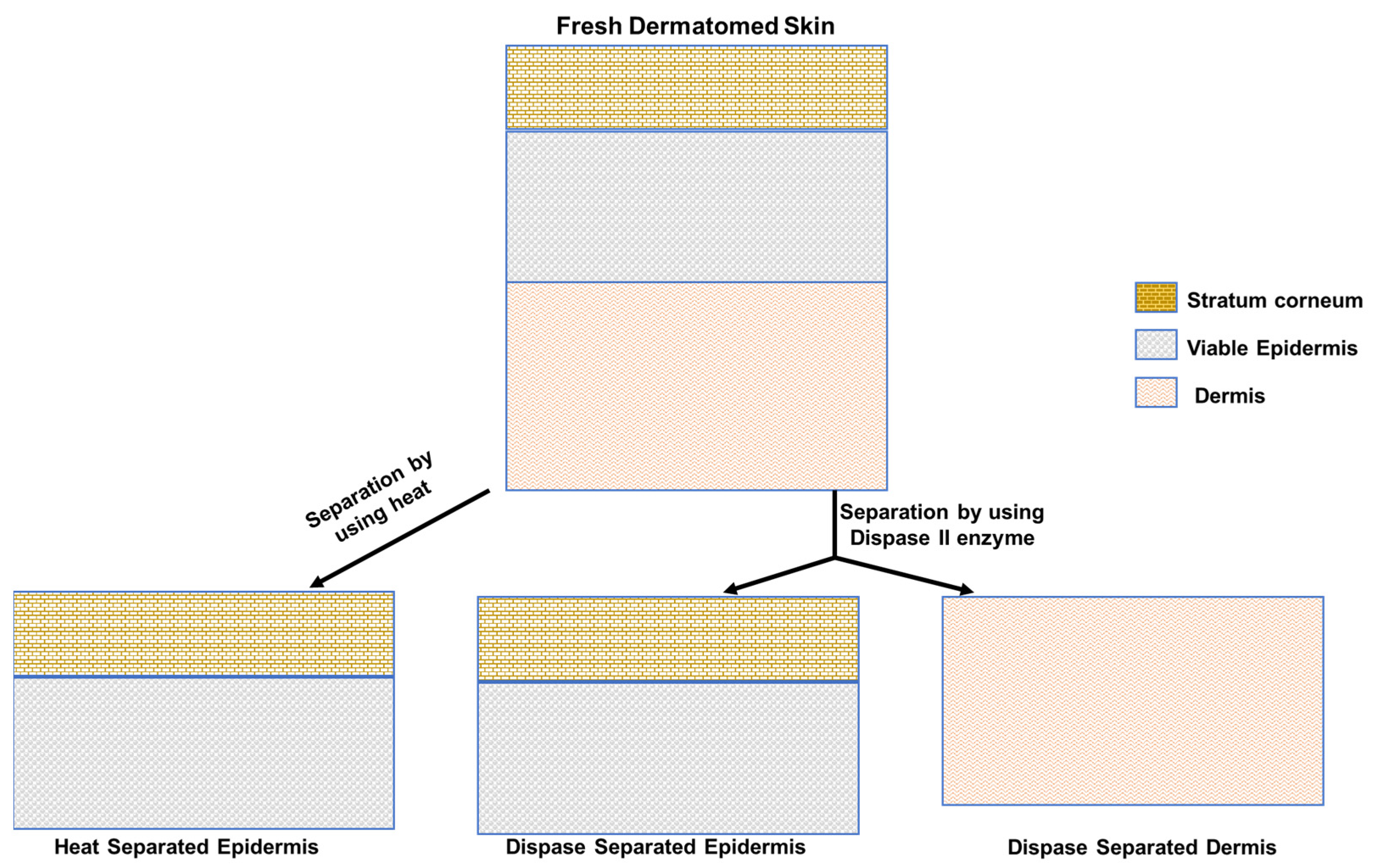
2.2. Membrane Preparation
2.3. In Vitro Skin Permeation of Methyl Salicylate from Metsal™ Cream
2.4. Sample Analysis
2.5. Non-Specific Esterase Staining by α-Naphthyl Acetate
2.6. Data and Statistical Analysis
3. Results
3.1. In Vitro Skin Permeation Testing (IVPT) of Metsal™ Cream
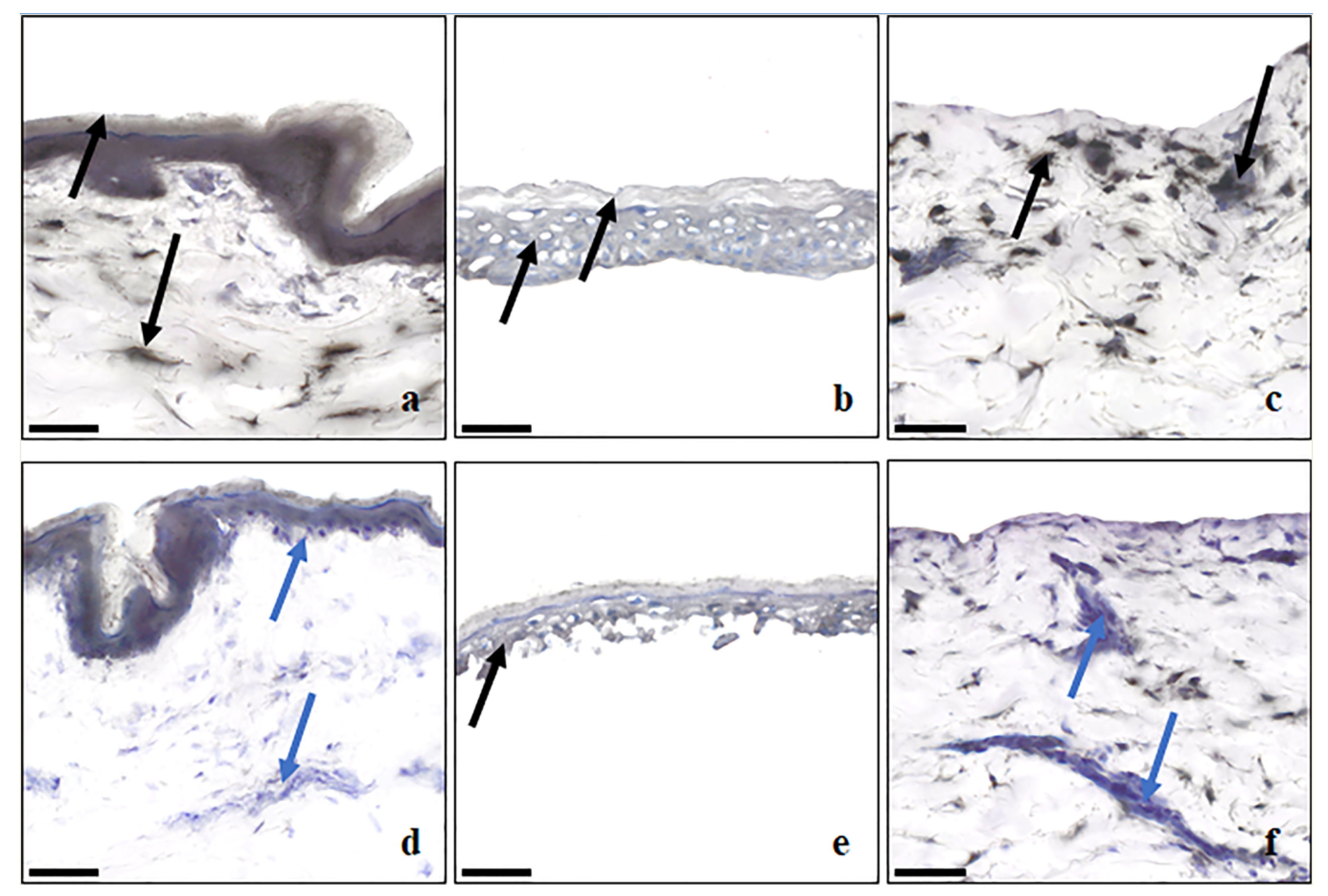
3.2. Non-Specific Esterase Staining by α-Napthyl Acetate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Cheruvu, H.S.; Anissimov, Y.G.; van der Hoek, J.; Tsakalozou, E.; Ni, Z.; Ghosh, P.; Grice, J.E.; Roberts, M.S. Percutaneous absorption of steroids from finite doses: Predicting urinary excretion from in vitro skin permeation testing. Int. J. Pharm. 2022, 625, 122095. [Google Scholar] [CrossRef]
- Liu, X.; Anissimov, Y.G.; Grice, J.E.; Cheruvu, H.S.; Ghosh, P.; Raney, S.G.; Maibach, H.I.; Roberts, M.S. Relating transdermal delivery plasma pharmacokinetics with in vitro permeation test (IVPT) findings using diffusion and compartment-in-series models. J. Control. Release 2021, 334, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Cross, S.E.; Anderson, C.; Roberts, M.S. Topical penetration of commercial salicylate esters and salts using human isolated skin and clinical microdialysis studies. Br. J. Clin. Pharmacol. 1998, 46, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Luu-The, V.; Duche, D.; Ferraris, C.; Meunier, J.R.; Leclaire, J.; Labrie, F. Expression profiles of phases 1 and 2 metabolizing enzymes in human skin and the reconstructed skin models Episkin and full thickness model from Episkin. J. Steroid Biochem. Mol. Biol. 2009, 116, 178–186. [Google Scholar] [CrossRef]
- Zhang, Q.; Grice, J.E.; Wang, G.; Roberts, M.S. Cutaneous metabolism in transdermal drug delivery. Curr. Drug Metab. 2009, 10, 227–235. [Google Scholar] [CrossRef]
- Roberts, M.S.; Cheruvu, H.S.; Mangion, S.E.; Alinaghi, A.; Benson, H.A.E.; Mohammed, Y.; Holmes, A.; van der Hoek, J.; Pastore, M.; Grice, J.E. Topical drug delivery: History, percutaneous absorption, and product development. Adv. Drug Deliv. Rev. 2021, 177, 113929. [Google Scholar] [CrossRef]
- Ahmad, N.; Mukhtar, H. Cytochrome p450: A target for drug development for skin diseases. J. Investig. Dermatol. 2004, 123, 417–425. [Google Scholar] [CrossRef]
- Bucks, D.A. Skin structure and metabolism: Relevance to the design of cutaneous therapeutics. Pharm. Res. 1984, 1, 148–153. [Google Scholar] [CrossRef]
- Kazem, S.; Linssen, E.C.; Gibbs, S. Skin metabolism phase I and phase II enzymes in native and reconstructed human skin: A short review. Drug Discov. Today 2019, 24, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Pyo, S.M.; Maibach, H.I. Skin Metabolism: Relevance of Skin Enzymes for Rational Drug Design. Skin Pharmacol. Physiol. 2019, 32, 283–294. [Google Scholar] [CrossRef]
- Lau, W.M.; Ng, K.W.; Sakenyte, K.; Heard, C.M. Distribution of esterase activity in porcine ear skin, and the effects of freezing and heat separation. Int. J. Pharm. 2012, 433, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Stinchcomb, A.L.; Swaan, P.W.; Ekabo, O.; Harris, K.K.; Browe, J.; Hammell, D.C.; Cooperman, T.A.; Pearsall, M. Straight-chain naltrexone ester prodrugs: Diffusion and concurrent esterase biotransformation in human skin. J. Pharm. Sci. 2002, 91, 2571–2578. [Google Scholar] [CrossRef] [PubMed]
- Sugibayashi, K.; Hayashi, T.; Morimoto, Y. Simultaneous transport and metabolism of ethyl nicotinate in hairless rat skin after its topical application: The effect of enzyme distribution in skin. J. Control. Release 1999, 62, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Tang-Liu, D.D.; Matsumoto, R.M.; Usansky, J.I. Clinical pharmacokinetics and drug metabolism of tazarotene: A novel topical treatment for acne and psoriasis. Clin. Pharmacokinet. 1999, 37, 273–287. [Google Scholar] [CrossRef]
- Batz, F.M.; Klipper, W.; Korting, H.C.; Henkler, F.; Landsiedel, R.; Luch, A.; von Fritschen, U.; Weindl, G.; Schafer-Korting, M. Esterase activity in excised and reconstructed human skin—Biotransformation of prednicarbate and the model dye fluorescein diacetate. Eur. J. Pharm. Biopharm. 2013, 84, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Sadgrove, M.; Marson, L.; Jay, M. Biotransformation Capacity of Carboxylesterase in Skin and Keratinocytes for the Penta-Ethyl Ester Prodrug of DTPA. Drug Metab. Dispos. 2016, 44, 1313–1318. [Google Scholar] [CrossRef]
- Boehnlein, J.; Sakr, A.; Lichtin, J.L.; Bronaugh, R.L. Characterization of esterase and alcohol dehydrogenase activity in skin. Metabolism of retinyl palmitate to retinol (vitamin A) during percutaneous absorption. Pharm. Res. 1994, 11, 1155–1159. [Google Scholar] [CrossRef]
- Amr, S.; Brown, M.B.; Martin, G.P.; Forbes, B. Activation of clindamycin phosphate by human skin. J. Appl. Microbiol. 2001, 90, 550–554. [Google Scholar] [CrossRef]
- Bailly, J.; Crettaz, M.; Schifflers, M.H.; Marty, J.P. In vitro metabolism by human skin and fibroblasts of retinol, retinal and retinoic acid. Exp. Dermatol. 1998, 7, 27–34. [Google Scholar] [CrossRef]
- Morin, A.; Simard, M.; Rioux, G.; Grenier, A.; Morin, S.; Pouliot, R. Application of an In Vitro Psoriatic Skin Model to Study Cutaneous Metabolization of Tazarotene. Processes 2019, 7, 871. [Google Scholar] [CrossRef]
- Roberts, M.S.; Favretto, W.A.; Meyer, A.; Reckmann, M.; Wongseelashote, T. Topical bioavailability of methyl salicylate. Aust. N. Z. J. Med. 1982, 12, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Bernauer, U.; Bodin, L.; Chaudhry, Q.; Coenraad, P.J.; Dusinska, M.; Ezendam, J.; Gaffet, E.; Galli, C.L.; Granum, B.B.; Panteri, E. Opinion on Methyl Salicylate (Methyl 2- Hydroxybenzoate)-Submission I-SCCS/1633/21-Preliminary Opinion. 2021. Available online: https://health.ec.europa.eu/system/files/2022-08/sccs_o_255.pdf (accessed on 16 July 2022).
- Kligman, A.M.; Christophers, E. Preparation of isolated sheets of human stratum corneum. Arch. Dermatol. 1963, 88, 702–705. [Google Scholar] [CrossRef]
- Jynto. Ball-and-Stick Model of the Methyl Salicylate Molecule. Available online: https://commons.wikimedia.org/wiki/File:Methyl_salicylate_3D_ball.png (accessed on 5 July 2023).
- Mills, B. Ball-and-Stick Model of the Salicylic Acid Molecule. Available online: https://commons.wikimedia.org/wiki/File:Salicylic-acid-from-xtal-2006-3D-balls.png (accessed on 5 July 2023).
- Benjah-bmm27. Methanol-3D-Balls.png. Available online: https://commons.wikimedia.org/wiki/File:Methanol-3D-balls.png (accessed on 5 July 2023).
- Yousef, S.; Mohammed, Y.; Namjoshi, S.; Grice, J.; Sakran, W.; Roberts, M. Mechanistic Evaluation of Hydration Effects on the Human Epidermal Permeation of Salicylate Esters. AAPS J. 2017, 19, 180–190. [Google Scholar] [CrossRef]
- Bajza, A.; Kocsis, D.; Berezvai, O.; Laki, A.J.; Lukacs, B.; Imre, T.; Ivan, K.; Szabo, P.; Erdo, F. Verification of P-Glycoprotein Function at the Dermal Barrier in Diffusion Cells and Dynamic “Skin-On-A-Chip” Microfluidic Device. Pharmaceutics 2020, 12, 804. [Google Scholar] [CrossRef]
- Schafer-Korting, M.; Bock, U.; Gamer, A.; Haberland, A.; Haltner-Ukomadu, E.; Kaca, M.; Kamp, H.; Kietzmann, M.; Korting, H.C.; Krachter, H.U.; et al. Reconstructed human epidermis for skin absorption testing: Results of the German prevalidation study. Altern. Lab. Anim. 2006, 34, 283–294. [Google Scholar] [CrossRef]
- Neupane, R.; Boddu, S.H.S.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to Biological Skin in Permeation Studies: Current Trends and Possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef]
- Risueno, I.; Valencia, L.; Jorcano, J.L.; Velasco, D. Skin-on-a-chip models: General overview and future perspectives. APL Bioeng. 2021, 5, 030901. [Google Scholar] [CrossRef] [PubMed]
- Montagna, W. Histology and cytochemistry of human skin. IX. The distribution of non-specific esterases. J. Biophys. Biochem. Cytol. 1955, 1, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Muller, B.; Kasper, M.; Surber, C.; Imanidis, G. Permeation, metabolism and site of action concentration of nicotinic acid derivatives in human skin. Correlation with topical pharmacological effect. Eur. J. Pharm. Sci. 2003, 20, 181–195. [Google Scholar] [CrossRef]
- Tokudome, Y.; Katayanagi, M.; Hashimoto, F. Esterase Activity and Intracellular Localization in Reconstructed Human Epidermal Cultured Skin Models. Ann. Dermatol. 2015, 27, 269–274. [Google Scholar] [CrossRef]
- Kitano, Y.; Okada, N. Separation of the epidermal sheet by dispase. Br. J. Dermatol. 1983, 108, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Sano, K.; Yoshizato, K.; Shioya, N.; Sasaki, K. Comparative studies on methods of isolating rat epidermal cells. Ann. Plast. Surg. 1985, 14, 258–266. [Google Scholar] [CrossRef]
- Wei, J.C.J.; Haridass, I.N.; Crichton, M.L.; Mohammed, Y.H.; Meliga, S.C.; Sanchez, W.Y.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S.; Kendall, M.A.F. Space- and time-resolved investigation on diffusion kinetics of human skin following macromolecule delivery by microneedle arrays. Sci. Rep. 2018, 8, 17759. [Google Scholar] [CrossRef] [PubMed]
- Okano, J.; Kojima, H.; Katagi, M.; Nakae, Y.; Terashima, T.; Nakagawa, T.; Kurakane, T.; Okamoto, N.; Morohashi, K.; Maegawa, H.; et al. Epidermis-dermis junction as a novel location for bone marrow-derived cells to reside in response to ionizing radiation. Biochem. Biophys. Res. Commun. 2015, 461, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Sigma-Aldrich. D4693 Dispase® II. Available online: https://www.sigmaaldrich.com/AU/en/product/sigma/d4693 (accessed on 22 June 2023).
- Zou, Y.; Maibach, H.I. Dermal-epidermal separation methods: Research implications. Arch. Dermatol. Res. 2018, 310, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Roberts, M.S. Skin permeability and local tissue concentrations of nonsteroidal anti-inflammatory drugs after topical application. J. Pharmacol. Exp. Ther. 1994, 268, 144–151. [Google Scholar]
- Bronaugh, R.L. Methods for in vitro skin metabolism studies. Toxicol. Mech. Methods 1995, 5, 275–281. [Google Scholar] [CrossRef]
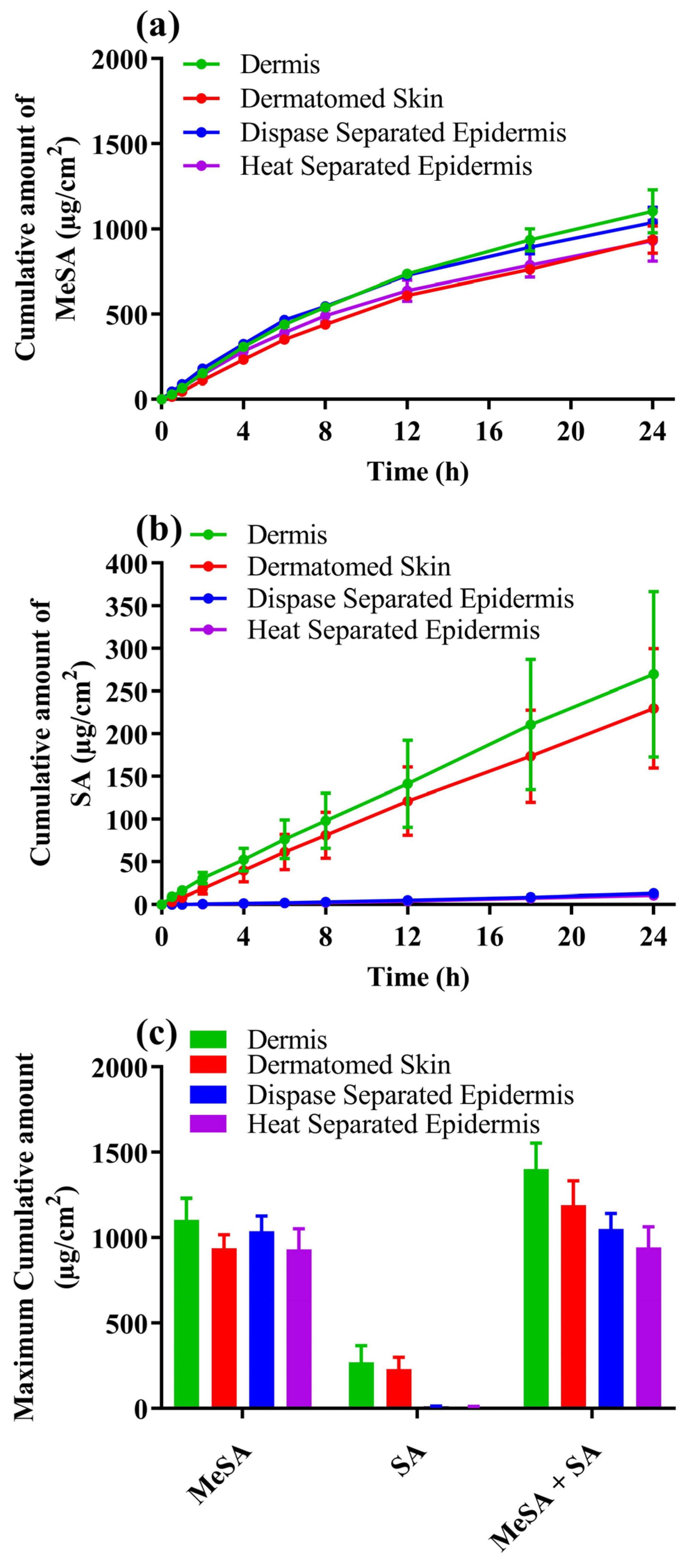
| Membrane Type | Applied Methyl Salicylate (mg/cm2) | % As Methyl Salicylate | % as Salicylic Acid | % as Total Actives | Ratio (SA/MeSA) |
|---|---|---|---|---|---|
| Dermis | 25.47 ± 1.36 | 4.44 ± 0.68 | 1.16 ± 0.31 | 5.61 ± 0.74 | 0.26 |
| Dermatomed skin | 26.3 ± 0.75 | 3.59 ± 0.35 a | 0.97 ± 0.25 | 4.56 ± 0.56 b | 0.27 c |
| Dispase-separated epidermis | 27.2 ± 1.28 | 3.88 ± 0.46 a | 0.05 ± 0.004 * | 3.93 ± 0.47 b | 0.013 # |
| Heat-separated epidermis | 28.21 ± 2.23 | 2.88 ± 0.79 a | 0.03 ± 0.01 * | 3.86 ± 0.8 b | 0.010 # |
| Membrane Type | Location of Esterase Enzymes |
|---|---|
| Dermatomed Skin | Prescence of esterase staining indicated by black and grey color staining uniform across the epidermal regions and pockets of staining areas across dermis. |
| Dispase-Separated Epidermis | Prescence of esterase staining indicated by uniform grey color staining across SC and VE parts of the membrane. |
| Dermis (remained after dispase-separated epidermis) | Prescence of esterase staining indicated by pockets of black color staining distributed throughout dermis and more towards the dermal epidermal junction. |
| Frozen full thickness skin | Decrease in esterase activity because of freezing indicated by appearance of blue to violet color because of hematoxylin staining in the epidermis regions and more regions in dermis. |
| Heat-Separated epidermis | Decrease in esterase activity because of heat application indicated by appearance of blue to violet color because of hematoxylin staining in the epidermis regions and more regions in dermis. |
| Dermis (remained after HSE separation) | Decrease in esterase activity because of heat application indicated by appearance of blue to violet color because of hematoxylin staining throughout dermis and more towards the dermal epidermal junction. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Telaprolu, K.C.; Grice, J.E.; Mohammed, Y.H.; Roberts, M.S. Human Skin Drug Metabolism: Relationships between Methyl Salicylate Metabolism and Esterase Activities in IVPT Skin Membranes. Metabolites 2023, 13, 934. https://doi.org/10.3390/metabo13080934
Telaprolu KC, Grice JE, Mohammed YH, Roberts MS. Human Skin Drug Metabolism: Relationships between Methyl Salicylate Metabolism and Esterase Activities in IVPT Skin Membranes. Metabolites. 2023; 13(8):934. https://doi.org/10.3390/metabo13080934
Chicago/Turabian StyleTelaprolu, Krishna C., Jeffrey E. Grice, Yousuf H. Mohammed, and Michael S. Roberts. 2023. "Human Skin Drug Metabolism: Relationships between Methyl Salicylate Metabolism and Esterase Activities in IVPT Skin Membranes" Metabolites 13, no. 8: 934. https://doi.org/10.3390/metabo13080934
APA StyleTelaprolu, K. C., Grice, J. E., Mohammed, Y. H., & Roberts, M. S. (2023). Human Skin Drug Metabolism: Relationships between Methyl Salicylate Metabolism and Esterase Activities in IVPT Skin Membranes. Metabolites, 13(8), 934. https://doi.org/10.3390/metabo13080934







