Transcriptome Profiling Based at Different Time Points after Hatching Deepened Our Understanding on Larval Growth and Development of Amphioctopus fangsiao
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and RNA Preparation
2.2. Library Construction and Illumina Sequencing
2.3. De Novo Assembly
2.4. Gene Expression Level Analysis and Function Annotation
2.5. Trend Analysis and Identification of Core Gene
2.6. Functional Enrichment Analyses
2.7. Functional Protein Association Networks Construction
2.8. Quantitative RT-PCR Validation
3. Results
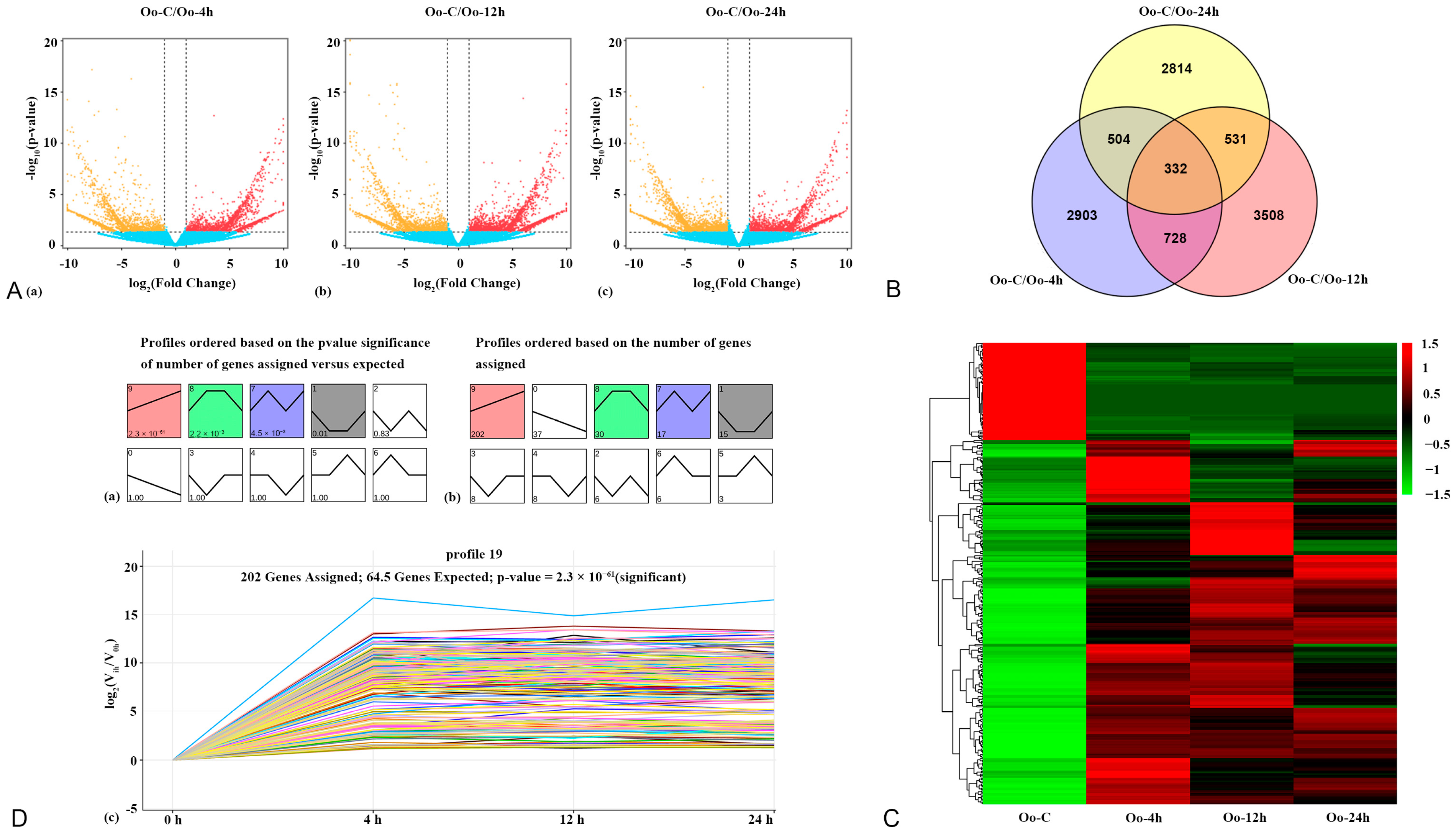
3.1. Distribution and Expression Analysis of DEGs
3.2. Trend Analysis of DEGs
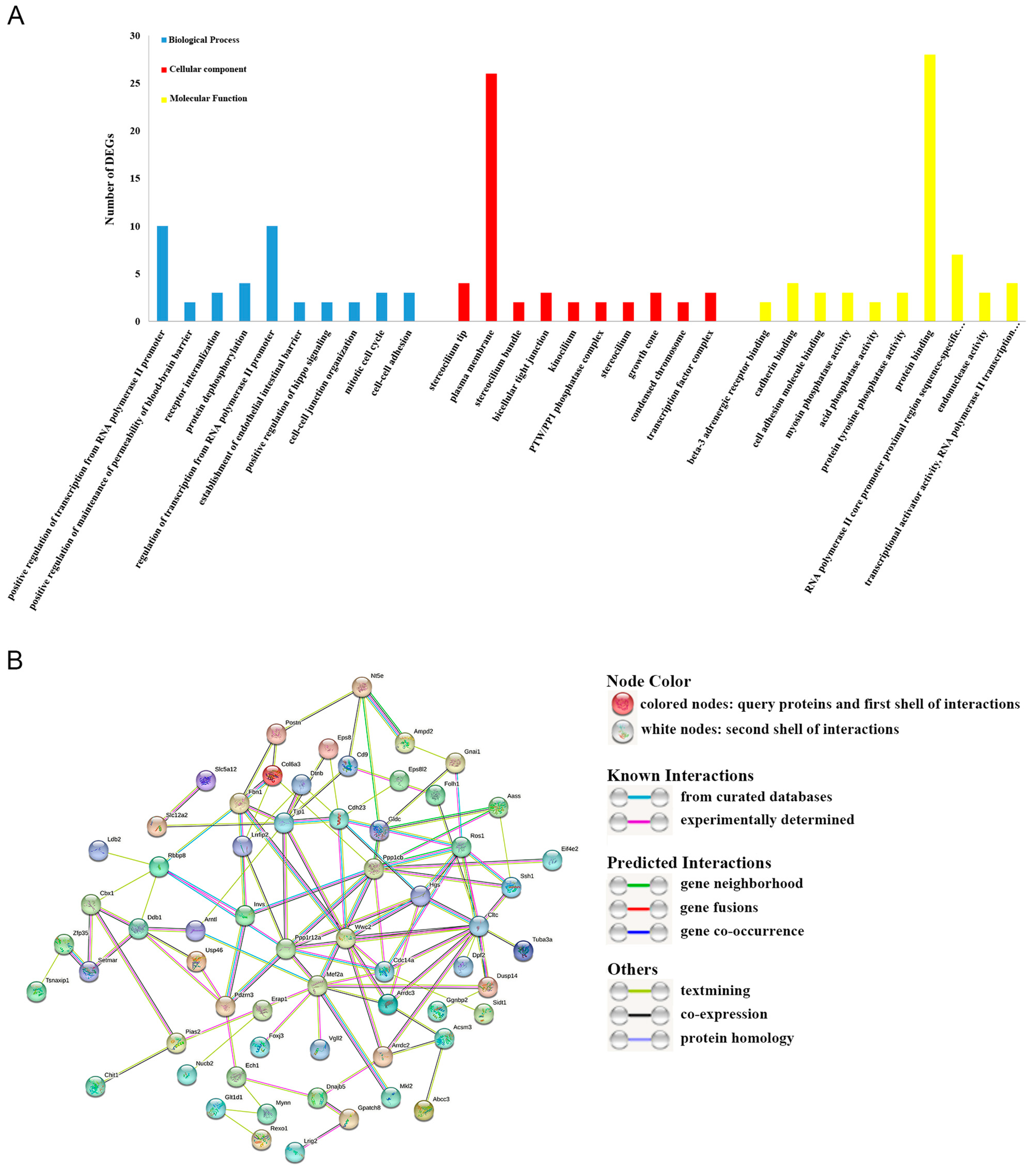
3.3. GO and KEGG Functional Enrichment Analyses
3.4. Construction of the PPI Network
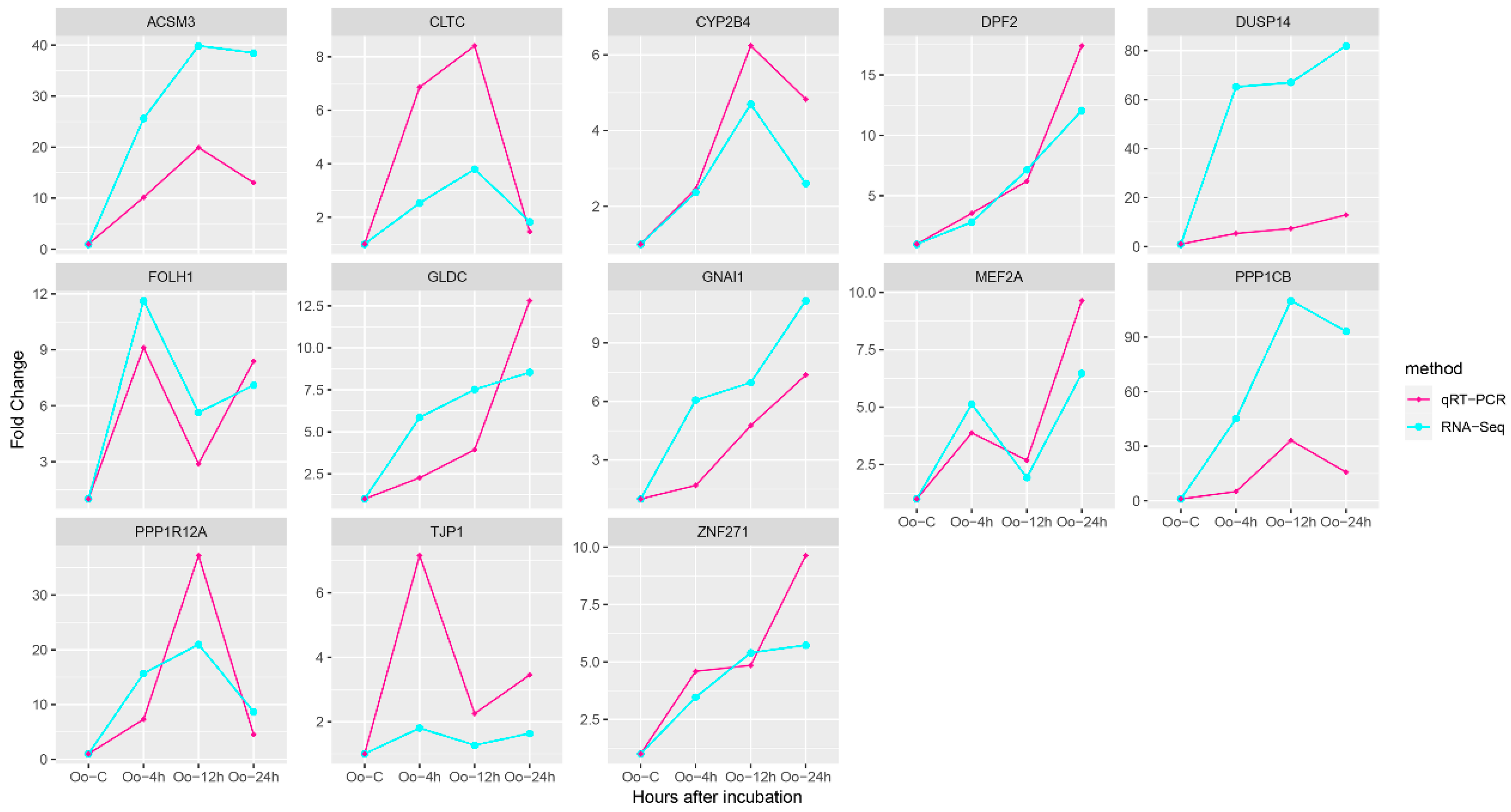
3.5. qRT-PCR Verification
4. Discussion
4.1. DEG Expression Trend Analysis
4.2. Enrichment Analysis of GO Terms KEGG Signaling Pathways
4.3. Speculation of Hub Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cosmo, A.D.; Cristo, C.D. Neuropeptidergic control of the optic gland of Octopus vulgaris: FMRF-amide and GnRH immunoreactivity. J. Comp. Neurol. 2015, 398, 1–12. [Google Scholar] [CrossRef]
- Judkins, H.; Vecchione, M.; Sweeney, M. Cephalopod research across scales: From molecules to ecosystems. Bull. Mar. Sci. 2020, 96, 231–234. [Google Scholar] [CrossRef]
- Schnell, A.K.; Amodio, P.; Boeckle, M.; Clayton, N.S. How intelligent is a cephalopod? Lessons from comparative cognition. Biol. Rev. Camb. Philos. Soc. 2020, 96, 162–178. [Google Scholar] [CrossRef]
- Boletzky, S.V. A contribution to the study of yolk absorption in the cephalopoda. Z. Morph. Tiere. 1975, 80, 229–246. [Google Scholar] [CrossRef]
- Navarro, J.C.; Villanueva, R. The fatty acid composition of Octopus vulgaris paralarvae reared with live and inert food: Deviation from their natural fatty acid profile. Aquaculture 2003, 219, 613–631. [Google Scholar] [CrossRef]
- Wei, X.M.; Xu, J.; Yang, J.M.; Liu, X.Q.; Zhang, R.R.; Wang, W.J.; Yang, J.L. Involvement of a Serpin serine protease inhibitor (OoSerpin) from mollusc Octopus ocellatus in antibacterial response. Fish Shellfish Immun. 2014, 42, 79–87. [Google Scholar] [CrossRef]
- Bao, X.K.; Li, Y.; Zhang, J.B.; Chen, X.P.; Xu, X.H.; Feng, Y.W.; Sun, G.H.; Liu, X.M.; Li, B.; Wang, W.J.; et al. Transcriptome Profiling Based on Different Time Points After Hatching Provides a Core Set of Gene Resource for Understanding Larval Immune Response Mechanisms Against Vibrio anguillarum Infection in Amphioctopus fangsiao. Front. Mar. Sci. 2021, 8, 731517. [Google Scholar] [CrossRef]
- Boletzky, S.V. Recent studies on spawning, embryonic development, and hatching in the cephalopoda. Adv. Mar. Biol. 1989, 25, 85–115. [Google Scholar]
- Ginger, K.W.K.; Vera, C.B.S.; Dineshram, R.; Dennis, C.K.S.; Adela, L.J.; Yu, Z.N.; Thiyagarajan, V. Larval and post-larval stages of Pacific oyster (Crassostrea gigas) are resistant to elevated CO2. PLoS ONE 2013, 8, e64147. [Google Scholar] [CrossRef]
- Kaplan, M.B.; Mooney, T.A.; McCorkle, D.C.; Cohen, A.L. Adverse effects of ocean acidification on early development of squid (Doryteuthis pealeii). PLoS ONE 2013, 8, e63714. [Google Scholar] [CrossRef]
- Bassim, S.; Tanguy, A.; Genard, B.; Moraga, D.; Tremblay, R. Identification of Mytilus edulis genetic regulators during early development. Gene 2014, 551, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.H.; Wang, F.; Xie, S.M.; Sun, F.Y.; Wang, Z.; Peng, M.X.; Li, J.L. Developmental Transcriptome Analysis and Identification of Genes Involved in Larval Metamorphosis of the Razor Clam, Sinonovacula constricta. Mar. Biotechnol. 2016, 18, 168–175. [Google Scholar] [CrossRef]
- Huan, P.; Wang, H.X.; Liu, B.Z. Transcriptomic analysis of the clam Meretrix meretrix on different larval stages. Mar. Biotechnol. 2012, 14, 69–78. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.M.; Liu, J.X.; Zhang, K.; Yu, H.Y.; He, Y.; Wang, X.B.; Qi, J.; Wang, Z.G.; Zhang, Q.Q. Transcriptome profiling based on protein–protein interaction networks provides a core set of genes for understanding blood immune response mechanisms against Edwardsiella tarda infection in Japanese flounder (Paralichthys olivaceus). Dev. Comp. Immunol. 2017, 78, 100–113. [Google Scholar] [CrossRef]
- Ernst, J.; Bar-Joseph, Z. STEM: A tool for the analysis of short time series gene expression data. BMC Bioinform. 2006, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2018, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fan, T.T.; Liu, X.T.; Liu, X.M.; Wang, W.J.; Wang, Q.Q.; You, L.H.; Wang, L.; Wei, X.M.; Yang, J.M. Characterization and functional study on Octopus ocellatus interleukin-17. J. Ocean Univ. China 2019, 18, 1443–1450. [Google Scholar] [CrossRef]
- Xie, H.Y.; Yan, T.T.; Lu, X.X.; Du, Y.Y.; Xu, S.G.; Kong, Y.; Yu, L.J.; Sun, J.; Zhou, L.H.; Ma, J. GLDC mitigated by miR-30e regulates cell proliferation and tumor immune infiltration in TNBC. Front. Immunol. 2022, 13, 1033367. [Google Scholar] [CrossRef]
- Yuan, Y.L.; Sun, L.G.; Wang, X.; Chen, J.X.; Jia, M.N.; Zou, Y.L.; Sa, H.L.; Cai, Y.Y.; Xu, Y.H.; Sun, C.; et al. Identification of a new GLDC gene alternative splicing variant and its protumorigenic roles in lung cancer. Future Oncol. 2019, 15, 4127–4139. [Google Scholar] [CrossRef]
- Zhang, W.C.; Shyh-Chang, N.; Yang, H.; Rai, A.; Umashankar, S.; Ma, S.M.; Soh, B.S.; Sun, L.L.; Tai, B.C.; Nga, M.E.; et al. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell 2012, 148, 259–272. [Google Scholar] [CrossRef]
- Lin, J.; Lee, J.H.J.; Paramasivam, K.; Pathak, E.; Wang, Z.X.; Pramono, Z.A.D.; Lim, B.; Wee, K.B.; Surana, U. Induced-Decay of Glycine Decarboxylase Transcripts as an Anticancer Therapeutic Strategy for Non-Small-Cell Lung Carcinoma. Mol. Ther. Nucleic Acids 2017, 9, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Mukha, D.; Fokra, M.; Feldman, A.; Sarvin, B.; Sarvin, N.; Nevo-Dinur, K.; Besser, E.; Hallo, E.; Aizenshtein, E.; Schug, Z.T.; et al. Glycine decarboxylase maintains mitochondrial protein lipoylation to support tumor growth. Cell Metab. 2022, 34, 775–782. [Google Scholar] [CrossRef]
- Wang, D.W.; Wu, L.W.; Cao, Y.; Yang, L.; Liu, W.; Xiao-Qiang, E.; Ji, G.R.; Bi, Z.G. A novel mechanism of mTORC1-mediated serine/glycine metabolism in osteosarcoma development. Cell. Signal. 2016, 29, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Lountos, G.T.; Tropea, J.E.; Cherry, S.; Waugh, D.S. Overproduction, purification and structure determination of human dual-specificity phosphatase 14. Acta Crystallogr. D Biol. Crystallogr. 2009, 65, 1013–1020. [Google Scholar] [CrossRef]
- Owens, D.M.; Keyse, S.M. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene 2007, 26, 3203–3213. [Google Scholar] [CrossRef]
- Wang, S.Y.; Yan, Z.Z.; Yang, X.; An, S.M.; Zhang, K.; Qi, Y.; Zheng, J.L.; Ji, Y.X.; Wang, P.X.; Fang, C.; et al. Hepatocyte DUSP14 maintains metabolic homeostasis and suppresses inflammation in the liver. Hepatology 2018, 67, 1320–1338. [Google Scholar] [CrossRef]
- Xie, L.F.; Wang, P.X.; Zhang, P.; Zhang, X.J.; Zhao, G.N.; Wang, A.B.; Guo, J.H.; Zhu, X.Y.; Zhang, Q.; Li, H.L. DKK3 expression in hepatocytes defines susceptibility to liver steatosis and obesity. J. Hepatol. 2016, 65, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Z.; Mao, W.Z.; Fang, C.; Tian, S.; Zhu, X.Y.; Yang, L.; Huang, Z.; Li, H.L. Dusp14 protects against hepatic ischaemia-reperfusion injury via Tak1 suppression. J. Hepatol. 2017, 8278, 32275–32284. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, C.; Bian, C.B.; Tempel, W.; Crombet, L.; MacKenzie, F.; Min, J.R.; Liu, Z.L.; Qi, C. Crystal structure of the Cys2His2-type zinc finger domain of human DPF2. Biochem. Biophys. Res. Commun. 2011, 413, 58–61. [Google Scholar] [CrossRef]
- Gaertner, B.; Zeitlinger, J. RNA polymerase II pausing during development. Development 2014, 141, 1179–1183. [Google Scholar] [CrossRef]
- Core, L.; Adelman, K. Promoter-proximal pausing of RNA polymerase II: A nexus of gene regulation. Genes Dev. 2019, 33, 960–982. [Google Scholar] [CrossRef] [PubMed]
- Grünberg, S.; Hahn, S. Structural insights into transcription initiation by RNA polymerase II. Trends Biochem. Sci. 2013, 38, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.Y.; Kaplan, C.D. Relationships of RNA polymerase II genetic interactors to transcription start site usage defects and growth in Saccharomyces cerevisiae. G3 Genes Genomes Genet. 2014, 5, 21–33. [Google Scholar] [CrossRef]
- Hegre, S.A.; Samdal, H.; Klima, A.; Stovner, E.B.; Nørsett, K.G.; Liabakk, N.B.; Olsen, L.C.; Chawla, K.; Aas, P.A.; Sætrom, P. Joint changes in RNA, RNA polymerase II, and promoter activity through the cell cycle identify non-coding RNAs involved in proliferation. Sci. Rep. 2021, 11, 18952. [Google Scholar] [CrossRef]
- Schröder, S.; Herker, E.; Itzen, F.; He, D.; Thomas, S.; Gilchrist, D.A.; Kaehlcke, K.; Cho, S.; Pollard, K.S.; Capra, J.A.; et al. Acetylation of RNA polymerase II regulates growth-factor-induced gene transcription in mammalian cells. Mol. Cell 2013, 52, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Gilman, A.G. G proteins: Transducers of receptor-generated signals. Annu. Rev. Biochem. 1987, 56, 615–649. [Google Scholar] [CrossRef]
- Yao, J.; Liang, L.H.; Zhang, Y.; Ding, J.; Tian, Q.; Li, J.J.; He, X.H. GNAI1 Suppresses Tumor Cell Migration and Invasion and is Post-Transcriptionally Regulated by Mir-320a/c/d in Hepatocellular Carcinoma. Cancer Biol. Med. 2012, 9, 234–241. [Google Scholar]
- Muir, A.M.; Gardner, J.F.; van Jaarsveld, R.H.; de Lange, I.M.; van der Smagt, J.J.; Wilson, G.N.; Dubbs, H.; Goldberg, E.M.; Zitano, L.; Bupp, C.; et al. Variants in GNAI1 cause a syndrome associated with variable features including developmental delay, seizures, and hypotonia. Genet. Med. 2021, 23, 881–887. [Google Scholar] [CrossRef]
- Solis, G.P.; Kozhanova, T.V.; Koval, A.; Zhilina, S.S.; Mescheryakova, T.I.; Abramov, A.A.; Ishmuratov, E.V.; Bolshakova, E.S.; Osipova, K.V.; Ayvazyan, S.O.; et al. Pediatric Encephalopathy: Clinical, Biochemical and Cellular Insights into the Role of Gln52 of GNAO1 and GNAI1 for the Dominant Disease. Cells 2021, 10, 2749. [Google Scholar] [CrossRef]
- Kong, F.; Li, Z.; Parks, W.M.; Dumbauld, D.W.; García, A.J.; Mould, A.P.; Humphries, M.J.; Zhu, C. Cyclic mechanical reinforcement of integrin-ligand interactions. Mol. Cell 2013, 49, 1060–1068. [Google Scholar] [CrossRef]
- Pinheiro, D.; Bellaïche, Y. Mechanical Force-Driven Adherens Junction Remodeling and Epithelial Dynamics. Dev. Cell 2018, 47, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Reglero-Real, N.; Colom, B.; Bodkin, J.V.; Nourshargh, S. Endothelial Cell Junctional Adhesion Molecules: Role and Regulation of Expression in Inflammation. Arter. Thromb. Vasc. Biol. 2016, 36, 2048–2057. [Google Scholar] [CrossRef] [PubMed]
- Schepis, A.; Sepich, D.; Nelson, W.T. αE-catenin regulates cell-cell adhesion and membrane blebbing during zebrafish epiboly. Development 2012, 139, 537–546. [Google Scholar] [CrossRef][Green Version]
- Terahara, K.; Takahashi, K.G.; Mori, K. Pacific oyster hemocytes undergo apoptosis following cell-adhesion mediated by integrin-like molecules. Comp. Biochem. Phys. A 2005, 141, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.H.; Meng, Z.P.; Chen, R.; Guan, K.L. The Hippo Pathway: Biology and Pathophysiology. Annu. Rev. Biochem. 2019, 88, 577–604. [Google Scholar] [CrossRef] [PubMed]
- Masliantsev, K.; Karayan-Tapon, L.; Guichet, P.O. Hippo Signaling Pathway in Gliomas. Cells 2021, 10, 184. [Google Scholar] [CrossRef]
- Sahu, M.R.; Mondal, A.C. Neuronal Hippo signaling: From development to diseases. Dev. Neurobiol. 2021, 81, 92–109. [Google Scholar] [CrossRef]
- Wu, Z.M.; Guan, K.L. Hippo Signaling in Embryogenesis and Development. Trends Biochem. Sci. 2021, 46, 51–63. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Ma, X.L.; Hu, H. The Influence of Cell Cycle Regulation on Chemotherapy. Int. J. Mol. Sci. 2021, 22, 6923. [Google Scholar] [CrossRef]
- Swanhart, L.; Kupsco, J.; Duronio, R.J. Developmental control of growth and cell cycle progression in Drosophila. Methods Mol. Biol. 2005, 296, 69–94. [Google Scholar]
- Barbieri, E.; Fiore, P.P.D.; Sigismund, S. Endocytic control of signaling at the plasma membrane. Curr. Opin. Cell Biol. 2016, 39, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Hinze, C.; Boucrot, E. Endocytosis in proliferating, quiescent and terminally differentiated cells. J. Cell Sci. 2018, 131, 216804. [Google Scholar] [CrossRef] [PubMed]
- Blume-Jensen, P.; Hunter, T. Oncogenic kinase signalling. Nature 2001, 411, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.; Pujol, M.G.; Zhu, Z.; Smythe, E. Interplay of Endocytosis and Growth Factor Receptor Signalling. Prog. Mol. Subcell. Biol. 2018, 57, 181–202. [Google Scholar]
- Gopal, R.; Selvarasu, K.; Pandian, P.P.; Ganesan, K. Integrative transcriptome analysis of liver cancer profiles identifies upstream regulators and clinical significance of ACSM3 gene expression. Cell. Oncol. 2017, 40, 219–233. [Google Scholar] [CrossRef]
- Ruan, H.Y.; Yang, C.; Tao, X.M.; He, J.; Wang, T.; Wang, H.; Wang, C.; Jin, G.Z.; Jin, H.J.; Qin, W.X. Downregulation of ACSM3 promotes metastasis and predicts poor prognosis in hepatocellular carcinoma. Am. J. Cancer Res. 2017, 7, 543–553. [Google Scholar]
- Rengaraj, D.; Lee, B.R.; Jang, H.J.; Kim, Y.M.; Han, J.Y. Comparative metabolic pathway analysis with special reference to nucleotide metabolism-related genes in chicken primordial germ cells. Theriogenology 2012, 79, 28–39. [Google Scholar] [CrossRef]
- Perera, R.; Sono, M.; Kinloch, R.; Zhang, H.; Tarasev, M.; Im, S.C.; Waskell, L.; Dawson, J.H. Stabilization and spectroscopic characterization of the dioxygen complex of wild-type cytochrome P4502B4 (CYP2B4) and its distal side E301Q, T302A and proximal side F429H mutants at subzero temperatures. Biochim. Biophys. Acta 2011, 1814, 69–75. [Google Scholar] [CrossRef][Green Version]
- Barnaba, C.; Sahoo, B.R.; Ravula, T.; Medina-Meza, I.G.; Im, S.C.; Anantharamaiah, G.M.; Waskell, L.; Ramamoorthy, A. Cytochrome-P450-Induced Ordering of Microsomal Membranes Modulates Affinity for Drugs. Angew. Chem. Int. Ed. Engl. 2018, 57, 3391–3395. [Google Scholar] [CrossRef]
- Reed, J.R.; dela Cruz, A.L.; Lomnicki, S.M.; Backes, W.L. Inhibition of cytochrome P450 2B4 by environmentally persistent free radical-containing particulate matter. Biochem. Pharmacol. 2015, 95, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Shafizadeh, T.B.; Halsted, C.H. gamma-Glutamyl hydrolase, not glutamate carboxypeptidase II, hydrolyzes dietary folate in rat small intestine. J. Nutr. 2007, 137, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Šilhavý, J.; Krijt, J.; Sokolová, J.; Zídek, V.; Mlejnek, P.; Šimáková, M.; Škop, V.; Trnovská, J.; Oliyarnyk, O.; Marková, I.; et al. Dissecting the role of Folr1 and Folh1 genes in the pathogenesis of metabolic syndrome in spontaneously hypertensive rats. Physiol. Res. 2018, 67, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Latomanski, E.A.; Newton, H.J. Interaction between autophagic vesicles and the Coxiella-containing vacuole requires CLTC (clathrin heavy chain). Autophagy 2018, 14, 1710–1725. [Google Scholar] [CrossRef]
- Lu, A.; Zhou, C.J.; Wang, D.H.; Han, Z.; Kong, X.W.; Ma, Y.Z.; Yun, Z.Z.; Liang, C.G. Cytoskeleton-associated protein 5 and clathrin heavy chain binding regulates spindle assembly in mouse oocytes. Oncotarget 2017, 8, 17491–17503. [Google Scholar] [CrossRef]
- Vassilopoulos, S.; Gentil, C.; Laine, J.; Buclez, P.O.; Franck, A.; Ferry, A.; Précigout, G.; Roth, R.; Heuser, J.E.; Brodsky, F.M.; et al. Actin scaffolding by clathrin heavy chain is required for skeletal muscle sarcomere organization. J. Cell Biol. 2014, 205, 377–393. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, L.; Zhou, H.X.; Liu, L.; Lu, A.; Li, G.P.; Schatten, H.; Liang, C.G. Clathrin heavy chain 1 is required for spindle assembly and chromosome congression in mouse oocytes. Microsc. Microanal. 2013, 19, 1364–1373. [Google Scholar] [CrossRef]
- Chen, X.Y.; Liu, G.L.; Zhang, W.; Zhang, J.N.; Yan, Y.G.; Dong, W.Q.; Liang, E.S.; Zhang, Y.; Zhang, M.X. Inhibition of MEF2A prevents hyperglycemia-induced extracellular matrix accumulation by blocking Akt and TGF-β1/Smad activation in cardiac fibroblasts. Int. J. Biochem. Cell Biol. 2015, 69, 52–61. [Google Scholar] [CrossRef]
- Wang, Y.N.; Mei, C.G.; Su, X.T.; Wang, H.B.; Yang, W.C.; Zan, L.S. MEF2A Regulates the MEG3-DIO3 miRNA Mega Cluster-Targeted PP2A Signaling in Bovine Skeletal Myoblast Differentiation. Int. J. Mol. Sci. 2019, 20, 2748. [Google Scholar] [CrossRef]
- Xiong, Y.J.; Wang, L.; Jiang, W.Y.; Pang, L.H.; Liu, W.H.; Li, A.Q.; Zhong, Y.; Ou, W.C.; Liu, B.R.; Liu, S.M. MEF2A alters the proliferation, inflammation-related gene expression profiles and its silencing induces cellular senescence in human coronary endothelial cells. BMC Mol. Biol. 2019, 20, 8. [Google Scholar] [CrossRef]
- Xiao, Q.; Gan, Y.Q.; Li, Y.M.; Fan, L.L.; Liu, J.Q.; Lu, P.Y.; Liu, J.X.; Chen, A.A.; Shu, G.; Yin, G. MEF2A transcriptionally upregulates the expression of ZEB2 and CTNNB1 in colorectal cancer to promote tumor progression. Oncogene 2021, 40, 3364–3377. [Google Scholar] [CrossRef]
- Mai, H.M.; Xie, H.S.; Hou, J.; Chen, H.T.; Zhou, B.; Hou, J.L.; Jiang, D.K. A Genetic Variant of PPP1CB Influences Risk of Hepatitis B Virus-Related Hepatocellular Carcinoma in Han Chinese: A Pathway Based Analysis. J. Hepatocell. Carcinoma 2021, 8, 1055–1064. [Google Scholar] [CrossRef]
- Kim, J.; Ahn, D.; Chung, S.J. Chebulinic Acid Suppresses Adipogenesis in 3T3-L1 Preadipocytes by Inhibiting PPP1CB Activity. Int. J. Mol. Sci. 2022, 23, 865. [Google Scholar] [CrossRef] [PubMed]
- Korrodi-Gregório, L.; Esteves, S.L.; Fardilha, M. Protein phosphatase 1 catalytic isoforms: Specificity toward interacting proteins. Transl. Res. 2014, 164, 366–391. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.L.; Min, J.K.; Roh, K.M.; Kim, W.K.; Han, B.S.; Bae, K.H.; Lee, S.C.; Chung, S.J.; Kang, H.J. Phosphoprotein phosphatase 1CB (PPP1CB), a novel adipogenic activator, promotes 3T3-L1 adipogenesis. Biochem. Biophys. Res. Commun. 2015, 467, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Alessi, D.; MacDougall, L.K.; Sola, M.M.; Ikebe, M.; Cohen, P. The control of protein phosphatase-1 by targetting subunits. The major myosin phosphatase in avian smooth muscle is a novel form of protein phosphatase-1. Eur. J. Biochem. 1992, 210, 1023–1035. [Google Scholar] [CrossRef]
- Printen, J.A.; Brady, M.J.; Saltiel, A.R. PTG, a protein phosphatase 1-binding protein with a role in glycogen metabolism. Science 1997, 275, 1475–1478. [Google Scholar] [CrossRef]
- Hughes, J.J.; Alkhunaizi, E.; Kruszka, P.; Pyle, L.C.; Grange, D.K.; Berger, S.I.; Payne, K.K.; Masser-Frye, D.; Hu, T.; Christie, M.R.; et al. Loss-of-Function Variants in PPP1R12A: From Isolated Sex Reversal to Holoprosencephaly Spectrum and Urogenital Malformations. Am. J. Hum. Genet. 2020, 106, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.H.; Liu, R.; Liang, Y.K.; Zhou, R.; Dai, Q.S.; Han, Z.D.; Jiang, M.Y.; Zhuo, Y.J.; Zhang, Y.X.; Feng, Y.F.; et al. Identification and Validation of a PPP1R12A-Related Five-Gene Signature Associated with Metabolism to Predict the Prognosis of Patients with Prostate Cancer. Front. Genet. 2021, 12, 703210. [Google Scholar] [CrossRef]
- Lee, E.Y.; Yu, J.Y.; Paek, A.R.; Lee, S.H.; Jang, H.; Cho, S.Y.; Kim, J.H.; Kang, H.G.; Yun, T.; Oh, S.E.; et al. Targeting TJP1 attenuates cell–cell aggregation and modulates chemosensitivity against doxorubicin in leiomyosarcoma. J. Mol. Med. 2020, 98, 761–773. [Google Scholar] [CrossRef]
- Lee, S.H.; Paek, A.R.; Yoon, K.; Kim, S.H.; Lee, S.Y.; You, H.J. Tight junction protein 1 is regulated by transforming growth factor-β and contributes to cell motility in NSCLC cells. BMB Rep. 2015, 48, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Balda, M.S.; Matter, K. Tight junctions and the regulation of gene expression. Biochim. Biophys. Acta 2009, 1788, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Bauer, H.; Zweimueller-Mayer, J.; Steinbacher, P.; Lametschwandtner, A.; Bauer, H.C. The dual role of zonula occludens (ZO) proteins. J. Biomed. Biotechnol. 2010, 2010, 402593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.N.; Feng, T.; Spicer, L.J. The role of tight junction proteins in ovarian follicular development and ovarian cancer. Reproduction 2018, 155, 183–198. [Google Scholar] [CrossRef]
| Gene Name (Abbreviation) | Gene Name (Official Full Name) | Number of Protein–Protein Interactions |
|---|---|---|
| CLTC | clathrin heavy chain | 12 |
| MEF2A | myocyte enhancer factor 2A | 10 |
| PPP1CB | protein phosphatase 1 catalytic subunit beta | 10 |
| PPP1R12A | protein phosphatase 1 regulatory subunit 12A | 9 |
| TJP1 | tight junction protein 1 | 9 |
| ROS1 | ROS proto-oncogene 1, receptor tyrosine kinase | 8 |
| WWC2 | WW and C2 domain containing 2 | 8 |
| CDC14A | cell division cycle 14A | 7 |
| CDH23 | cadherin related 23 | 7 |
| DDB1 | damage specific DNA binding protein 1 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Bao, X.; Liu, X.; Wang, W.; Yang, J.; Zhu, X.; Wang, S. Transcriptome Profiling Based at Different Time Points after Hatching Deepened Our Understanding on Larval Growth and Development of Amphioctopus fangsiao. Metabolites 2023, 13, 927. https://doi.org/10.3390/metabo13080927
Li Z, Bao X, Liu X, Wang W, Yang J, Zhu X, Wang S. Transcriptome Profiling Based at Different Time Points after Hatching Deepened Our Understanding on Larval Growth and Development of Amphioctopus fangsiao. Metabolites. 2023; 13(8):927. https://doi.org/10.3390/metabo13080927
Chicago/Turabian StyleLi, Zan, Xiaokai Bao, Xiumei Liu, Weijun Wang, Jianmin Yang, Xibo Zhu, and Shuhai Wang. 2023. "Transcriptome Profiling Based at Different Time Points after Hatching Deepened Our Understanding on Larval Growth and Development of Amphioctopus fangsiao" Metabolites 13, no. 8: 927. https://doi.org/10.3390/metabo13080927
APA StyleLi, Z., Bao, X., Liu, X., Wang, W., Yang, J., Zhu, X., & Wang, S. (2023). Transcriptome Profiling Based at Different Time Points after Hatching Deepened Our Understanding on Larval Growth and Development of Amphioctopus fangsiao. Metabolites, 13(8), 927. https://doi.org/10.3390/metabo13080927






