Natural Grass Cultivation Management Improves Apple Fruit Quality by Regulating Soil Mineral Nitrogen Content and Carbon–Nitrogen Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites Description
2.2. Experimental Design and Sampling
2.3. Experimental Index Measurements
2.3.1. Levels of SOM, TN, and the Contents Soil NO3−-N and NH4+-N
2.3.2. Photosynthetic Parameter
2.3.3. Levels of Sorbitol, Sucrose, Fructose, and Glucose
2.3.4. Enzyme Activities Determination
2.3.5. 15N and 13C Isotope Analysis
2.3.6. Fruit Quality Indexes Measurement
2.3.7. RNA Isolation and qRT-PCR Analysis
2.4. Data Collection and Statistical Analysis
3. Results
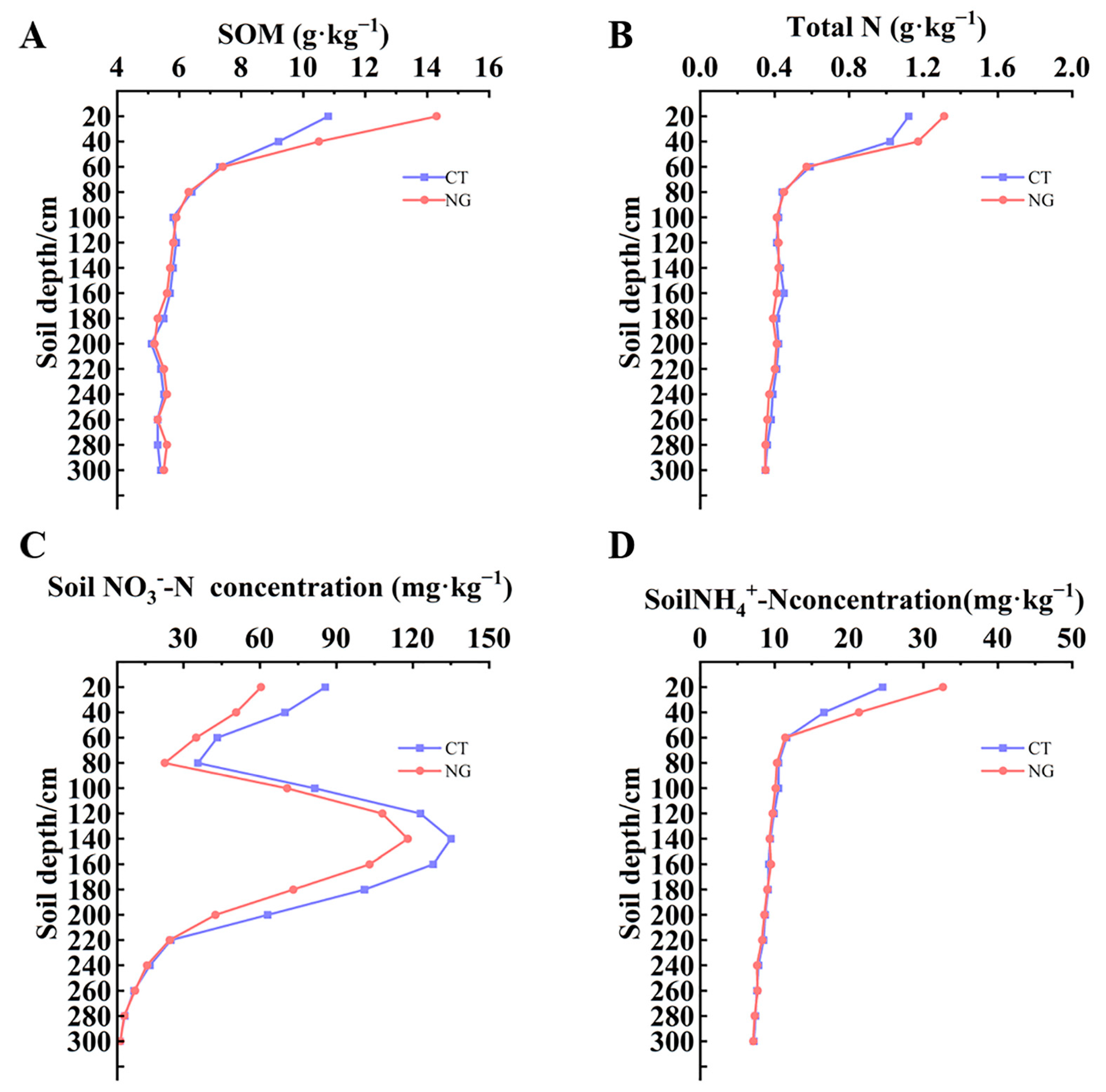
3.1. Level of SOM, TN, and Soil Mineral N (NH4+-N and NO3−-N)
3.1.1. SOM and TN
3.1.2. Soil Mineral N (NH4+-N and NO3−-N)
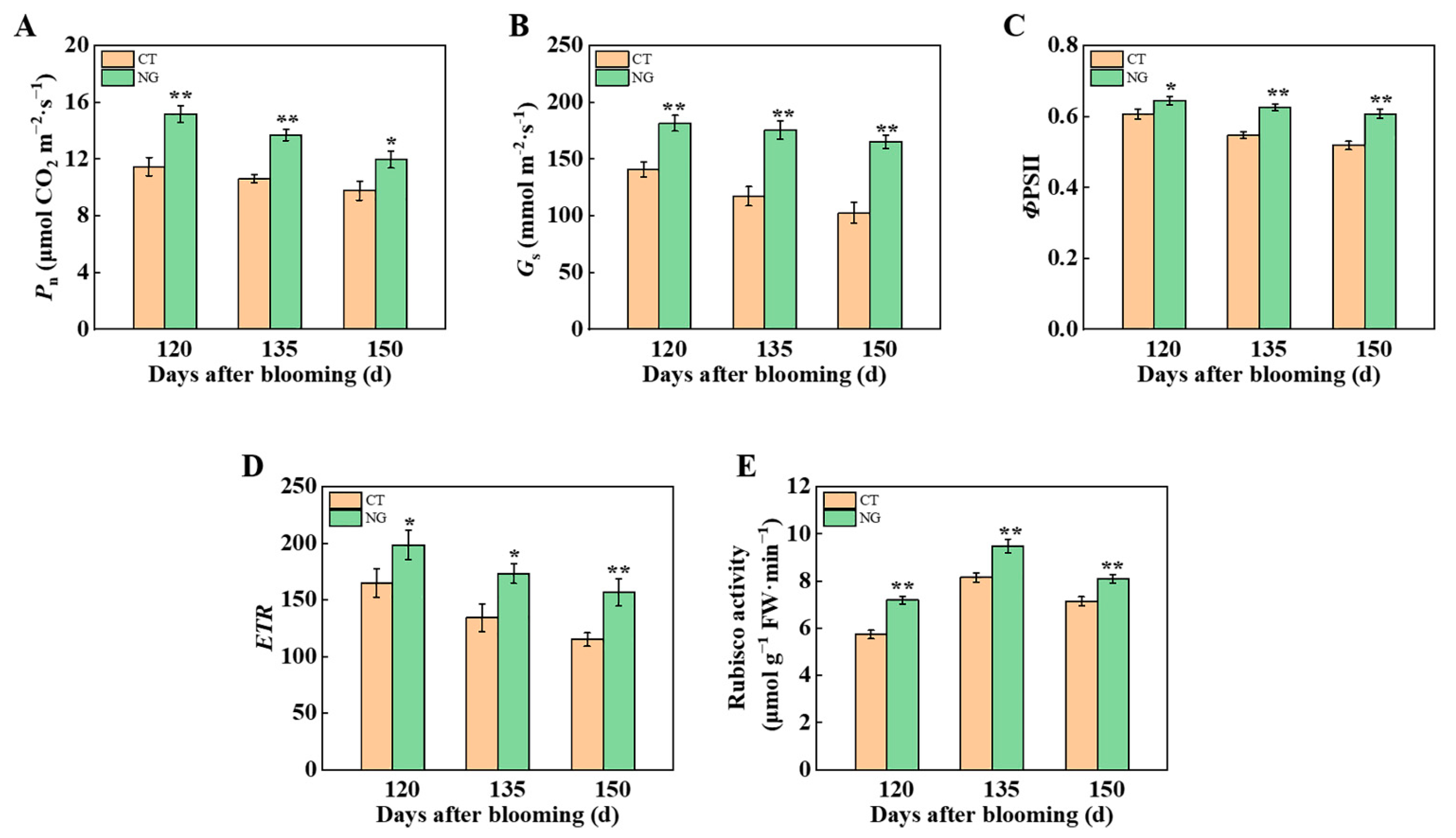
3.2. Plant C–N Nutrition
3.2.1. Photosynthetic Parameter
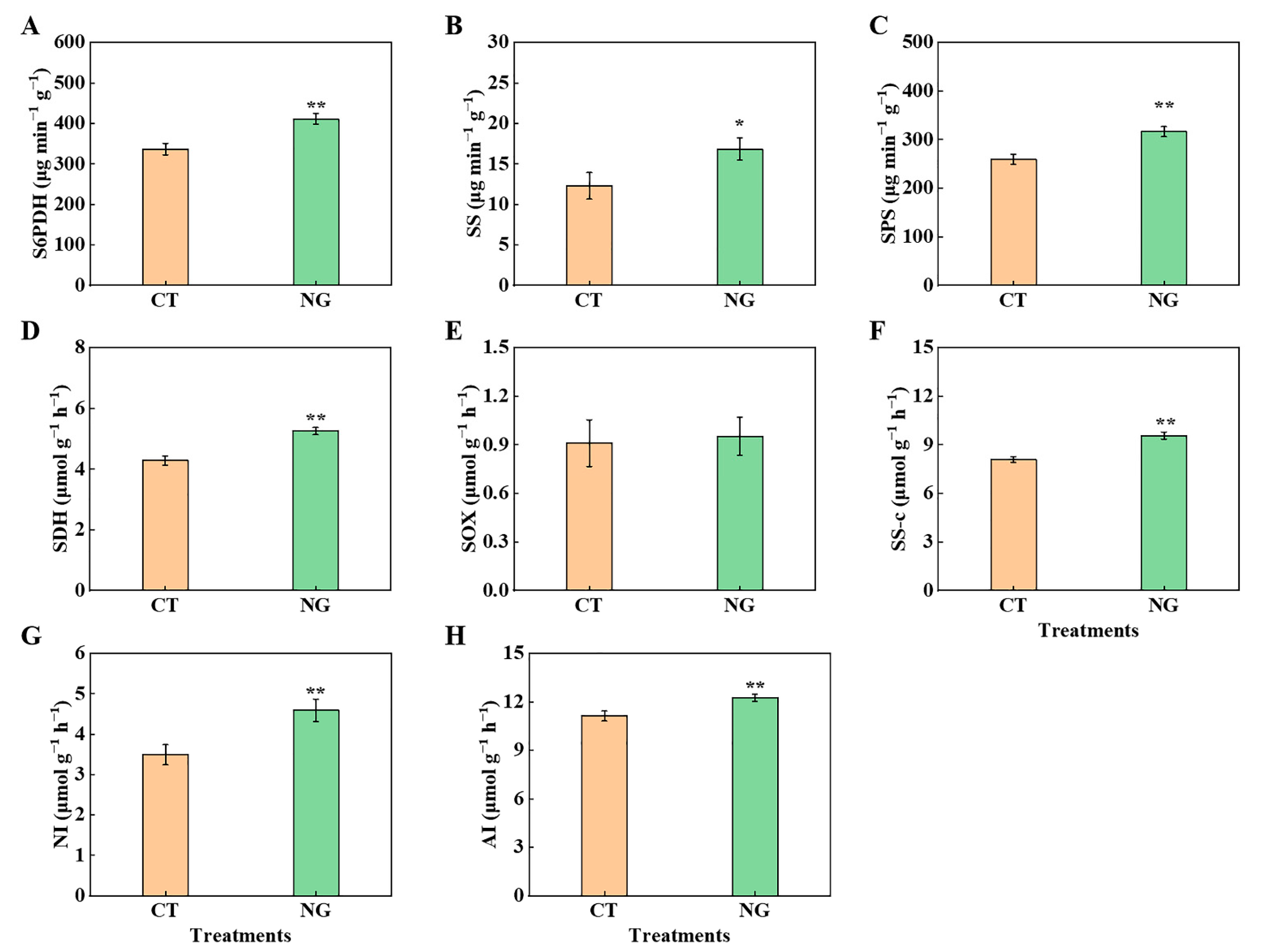
3.2.2. C Metabolism-Related Enzyme Activities in Leaves and Fruits
3.2.3. Concentrations of Sucrose, Sorbitol, Fructose, and Glucose in Leaves and Fruits
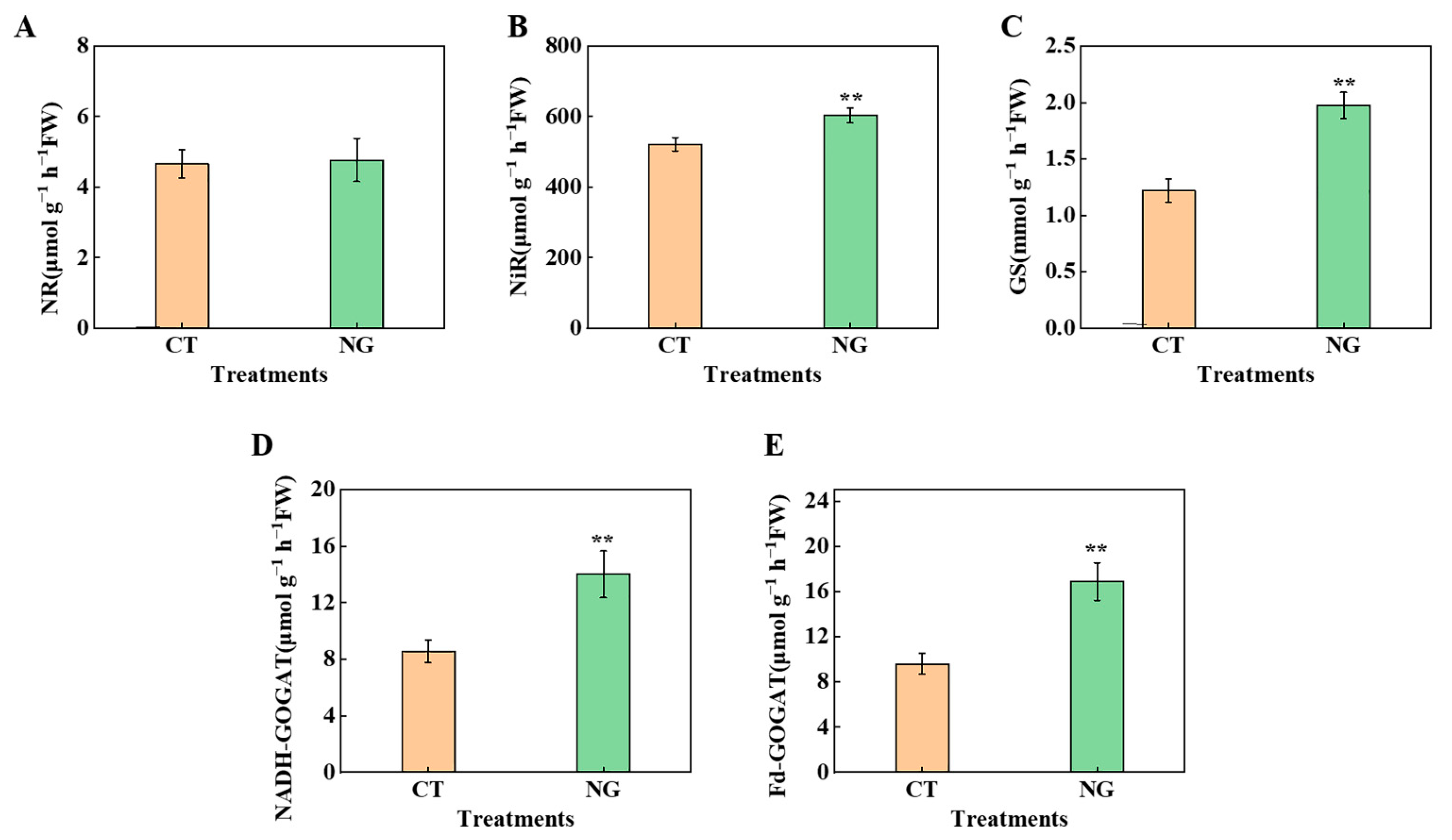
3.2.4. N Metabolism-Related Enzyme Activities in Leaves
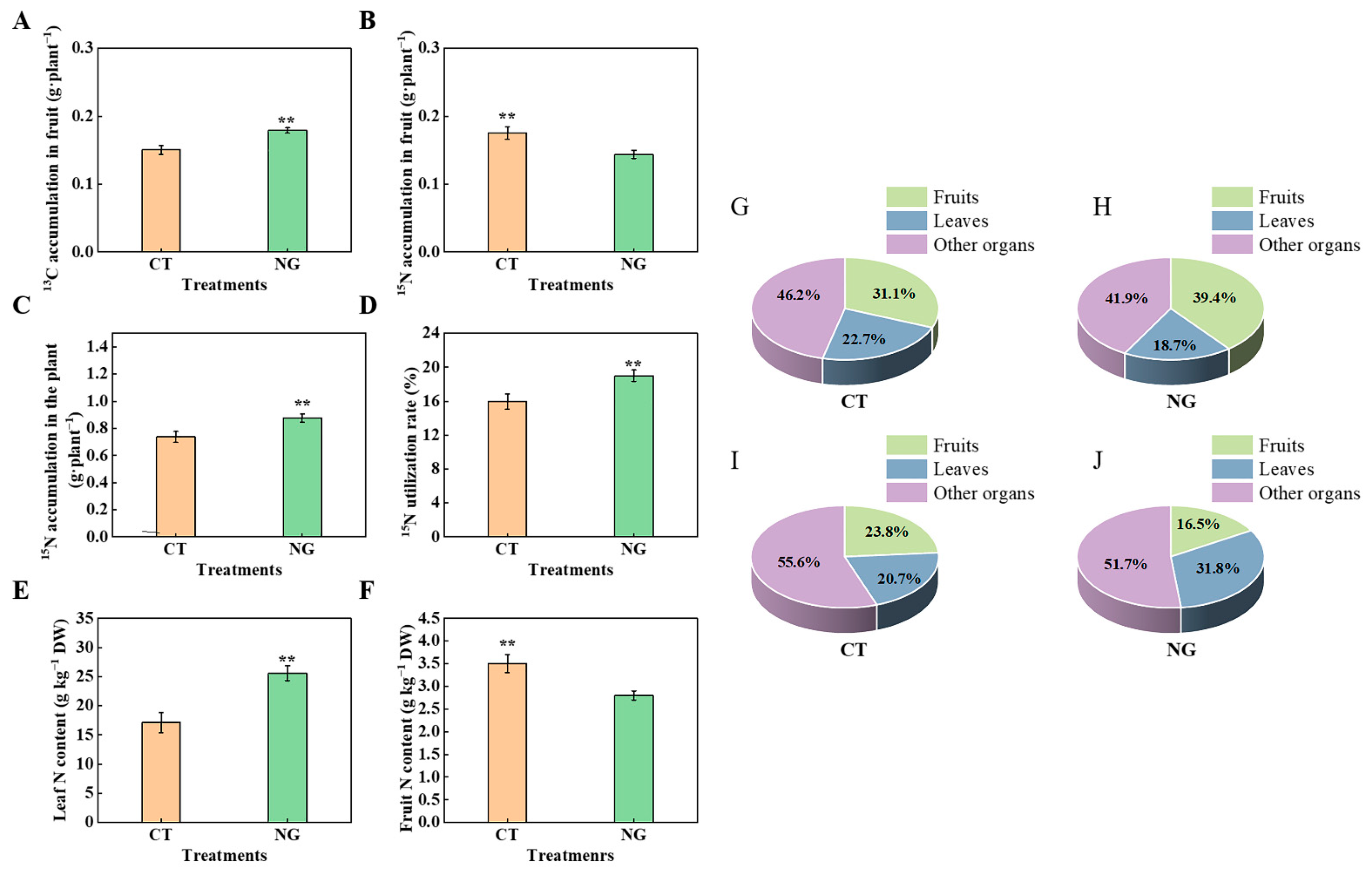
3.2.5. 13C distribution Rate and 13C Accumulation in Organs
3.2.6. 15N Accumulation and 15N Distribution Rate
3.2.7. Gene Expression of Sugar Transporter
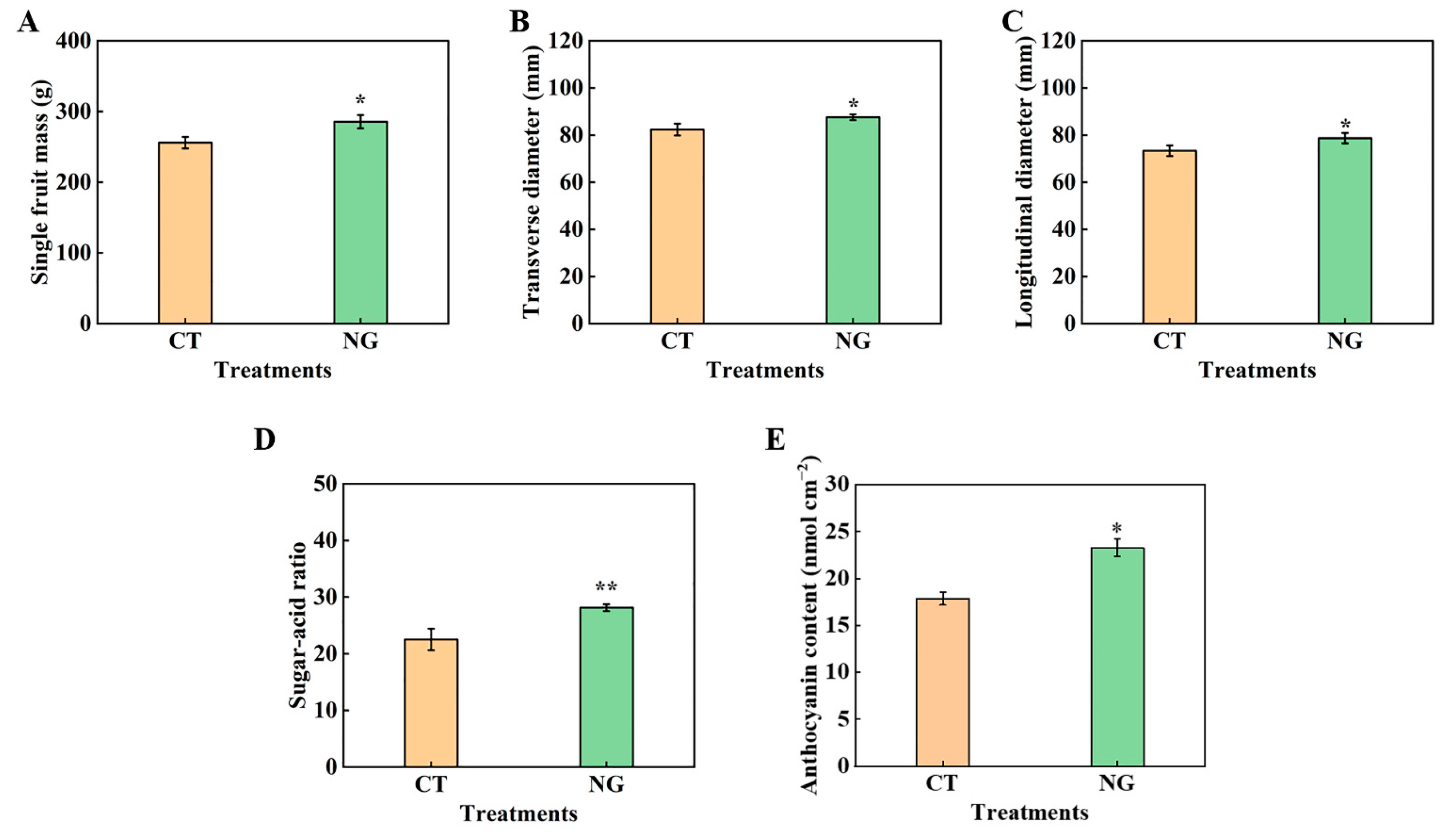
3.3. Fruit Quality
4. Discussion
4.1. Natural Sod Culture Management Alters N Absorption of Trees and Reduces the Risk of Soil Nitrate Leaching
4.2. Natural Sod Culture Management Alters Tree C–N Nutrition and Improves Fruit Quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Lyu, M.; Xu, X.; Liu, C.; Qin, H.; Tian, G. Exogenous sucrose promotes the growth of apple rootstocks under high nitrate supply by modulating carbon and nitrogen metabolism. Plant Physiol. Biochem. 2022, 192, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Qin, H.; Liu, C.; Xing, Y.; Feng, Z.; Xu, X.; Liu, J.; Lyu, M.; Jiang, H.; Zhu, Z.; et al. Magnesium improved fruit quality by regulating photosynthetic nitrogen use efficiency, carbon–nitrogen metabolism, and anthocyanin biosynthesis in ‘Red Fuji’ apple. Front. Plant Sci. 2023, 14, 1136179. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Raba, R. Accumulation of macro- and micronutrients and nitrogen demand-supply relationship of ’Gala’/’Malling 26’ apple trees grown in sand culture. J. Am. Soc. Hortic. Sci. 2009, 134, 3–13. [Google Scholar] [CrossRef]
- Chen, G.D.; Wang, L.; Fabrice, M.R.; Tian, Y.N.; Qi, K.J.; Chen, Q.; Cao, P.; Wang, P.; Zhang, S.; Wu, J.; et al. Physiological and nutritional responses of pear seedlings to nitrate concentrations. Front. Plant Sci. 2018, 9, 1679. [Google Scholar] [CrossRef]
- Zhu, Z.; Jia, Z.; Peng, L.; Chen, Q.; He, L.; Jiang, Y.; Ge, S. Life cycle assessment of conventional and organic apple production systems in China. J. Clean. Prod. 2018, 201, 156–168. [Google Scholar] [CrossRef]
- Ranadheer, P.; Kona, R.; Sreeharsha, R.V.; Mohan, S.V. Non-lethal nitrate supplementation enhances photosystem ii efficiency in mixotrophic microalgae towards the synthesis of proteins and lipids. Bioresour. Technol. 2019, 283, 373–377. [Google Scholar] [CrossRef]
- Ge, S.F.; Zhu, Z.L.; Peng, L.; Chen, Q.; Jiang, Y.M. Soil nutrient status and leaf nutrient diagnosis in the main apple producing regions in China. Hortic. Plant J. 2018, 4, 89–93. [Google Scholar] [CrossRef]
- Wang, F.; Ge, S.; Xu, X.; Xing, Y.; Du, X.; Zhang, X.; Lv, M.; Liu, J.; Zhu, Z.; Jiang, Y. Multiomics analysis reveals new insights into the apple fruit quality decline under high nitrogen conditions. J. Agric. Food Chem. 2021, 69, 5559–5572. [Google Scholar] [CrossRef]
- Tian, G.; Liu, C.; Xu, X.; Xing, Y.; Liu, J.; Lyu, M.; Feng, Z.; Zhang, X.; Qin, H.; Jiang, H.; et al. Effects of magnesium on nitrate uptake and sorbitol synthesis and translocation in apple seedlings. Plant Physiol. Biochem. 2023, 196, 139–151. [Google Scholar] [CrossRef]
- Yu, B.; Wang, L.; Guan, Q.; Xue, X.; Gao, W.; Nie, P. Exogenous 24-epibrassinolide promoted growth and nitrogen absorption and assimilation efficiency of apple seedlings under salt stress. Front. Plant Sci. 2023, 14, 1178085. [Google Scholar] [CrossRef]
- Vinzent, B.; Fuß, R.; Maidl, F.X.; Hülsbergen, K.J. N2O emissions and nitrogen dynamics of winter rapeseed fertilized with different N forms and a nitrification inhibitor. Agric. Ecosyst. Environ. 2018, 259, 86–97. [Google Scholar] [CrossRef]
- Dessureault-Rompré, J.; Zebarth, B.J.; Georgallas, A.; Burton, D.L.; Grant, C.A.; Drury, C.F. Temperature dependence of soil nitrogen mineralization rate: Comparison of mathematical models, reference temperatures and origin of the soils. Geoderma 2010, 157, 97–108. [Google Scholar] [CrossRef]
- Xing, Y.; Zhu, Z.; Wang, F.; Zhang, X.; Li, B.; Liu, Z.; Wu, X.-X.; Ge, S.-F.; Jiang, Y.-M. Role of calcium as a possible regulator of growth and nitrate nitrogen metabolism in apple dwarf rootstock seedlings. Sci. Hortic. 2021, 276, 109740. [Google Scholar] [CrossRef]
- Wang, F.; Xu, X.X.; Jia, Z.H.; Hou, X.; Chen, Q.; Sha, J.C.; Liu, Z.; Zhu, Z.; Jiang, Y.; Ge, S. Nitrification inhibitor 3,4-dimethylpyrazole phosphate application during the later stage of apple fruit expansion regulates soil mineral nitrogen and tree carbon–nitrogen nutrition, and improves fruit quality. Front. Plant Sci. 2020, 11, 764. [Google Scholar] [CrossRef]
- Wang, F.; Sha, J.C.; Chen, Q.; Xu, X.X.; Zhu, Z.L.; Ge, S.F.; Jiang, Y. Exogenous abscisic acid regulates distribution of 13C and 15N and anthocyanin synthesis in ‘Red fuji’ apple fruit under high nitrogen supply. Front. Plant Sci. 2020, 10, 1738. [Google Scholar] [CrossRef] [PubMed]
- Reguera, M.; Peleg, Z.; Abdel-Tawab, Y.M.; Tumimbang, E.B.; Delatorre, C.A.; Blumwald, E. Stress-induced cytokinin synthesis increases drought tolerance through the coordinated regulation of carbon and nitrogen assimilation in Rice. Plant Physiol. 2013, 163, 1609–1622. [Google Scholar] [CrossRef]
- Sha, J.; Wang, F.; Xu, X.; Chen, Q.; Zhu, Z.; Jiang, Y.; Ge, S. Studies on the translocation characteristics of 13C-photoassimilates to fruit during the fruit development stage in ‘fuji’ apple-sciencedirect. Plant Physiol. Biochem. 2020, 154, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Sha, J.; Ge, S.; Zhu, Z.; Du, X.; Jiang, Y. Paclobutrazol regulates hormone and carbon-nitrogen nutrition of autumn branches, improves fruit quality and enhances storage nutrition in ‘fuji’ apple. Sci. Hortic. 2021, 282, 110022. [Google Scholar] [CrossRef]
- Taguas, E.V.; Vanderlinden, K.; Pedrera-Parrilla, A.; Giraldez, J.V.; Gomez, J.A. Spatial and temporal variability of spontaneous grass cover and its influence on sediment losses in an extensive olive orchard catchment. Catena 2017, 157, 58–66. [Google Scholar] [CrossRef]
- Gómez, J.A.; Guzmán, M.G.; Giráldez, J.V.; Fereres, E. The influence of cover crops and tillage on water and sediment yield, and on nutrient, and organic matter losses in an olive orchard on a sandy loam soil. Soil Tillage Res. 2009, 106, 137–144. [Google Scholar] [CrossRef]
- Ramos, M.E.; Robles, A.B.; Sánchez-Navarro, A.; González-Rebollar, J.L. Soil responses to different management practices in rainfed orchards in semiarid environments. Soil Tillage Res. 2011, 112, 85–91. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, J.; Shi, Z.; Wang, L. Soil aggregates are key factors that regulate erosion-related carbon loss in citrus orchards of southern China: Bare land vs. grass-covered land. Agric. Ecosyst. Environ. 2021, 309, 107254. [Google Scholar] [CrossRef]
- Liang, R.; Wang, L.; Wang, X.; Zhang, J.; Gan, X. Effects of exogenous ALA on leaf photosynthesis, photosynthate transport, and sugar accumulation in Prunus persica L. Forests 2023, 14, 723. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, X.; Sun, Y.; Zhang, J.; Wu, W.; Liao, Y. Mulching practices altered soil bacterial community structure and improved orchard productivity and apple quality after five growing seasons. Sci. Hortic. 2014, 172, 248–257. [Google Scholar] [CrossRef]
- Han, S.; Zhao, J.; Liu, Y.; Xi, L.; Liao, J.; Liu, X.; Su, G. Effects of green manure planting mode on the quality of Korla fragrant pears (Pyrus sinkiangensis Yu). Front. Plant Sci. 2022, 13, 1027595. [Google Scholar] [CrossRef]
- Ren, J.; Li, F.; Yin, C. Orchard grass safeguards development of fruit industry in China. J. Clean. Prod. 2023, 382, 135291. [Google Scholar] [CrossRef]
- Fu, X.; Liu, J.; Huang, W. Effects of natural grass on soil microbiology, nutrient and fruit quality of Nanfeng tangerine yard. Acta Hortic. Sin. 2015, 49, 1404–1414. [Google Scholar]
- Muscas, E.; Cocco, A.; Mercenaro, L.; Cabras, M.; Lentini, A.; Porqueddu, C.; Nieddu, G. Effects of vineyard floor cover crops on grapevine vigor, yield, and fruity quality and the development of the vine mealybug under a Mediterranean climate. Agric. Ecosyst. Environ. 2017, 237, 203–212. [Google Scholar] [CrossRef]
- Tu, A.; Xie, S.; Zheng, H.; Li, H.; Li, Y.; Mo, M. Long-term effects of living grass mulching on soil and water conservation and fruit yield of citrus orchard in south China. Agric. Water Manag. 2021, 252, 106897. [Google Scholar] [CrossRef]
- Qin, S.; Xu, G.; He, J.; Li, L.; Ma, H.; Lyu, D.A. chromosome-scale genome assembly of Malus domestica, a multi-stress resistant apple variety. Genomics 2023, 115, 110627. [Google Scholar] [CrossRef]
- Zhou, T.; Jiao, K.; Qin, S.; Lyu, D. The impact of cover crop shoot decomposition on soil microorganisms in an apple orchard in northeast China. Saudi J. Biol. Sci. 2019, 26, 1936–1942. [Google Scholar] [CrossRef]
- Zhou, E.; Gou, M.; Yu, B.; Sun, C.; He, J.; Qin, S.; Lyu, D. Effects of mowing dominant grasses on root exudation and soil nitrogen cycling in a natural sod culture apple orchard. Plant Soil Environ. 2021, 67, 567–578. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, X.; Wu, Y.; Mao, Z.; Jiang, Y.; Peng, F.; Wang, Z.; Chen, X. Research progress of cover crop in Chinese orchard. Chin. J. Appl. Ecol. 2015, 26, 1892–1900. [Google Scholar]
- Bremner, J.M.; Jenkinson, D.S. Determination of organic carbon soil, II: Effect of carbonized materials. J. Soil Sci. 1960, 11, 403–408. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agrochemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Duan, W.; Shi, Y.; Zhao, J.; Zhang, Y.; Yu, Z. Depth of nitrogen fertiliser placement affects nitrogen accumulation, translocation and nitrate-nitrogen content in soil of rainfed wheat. Int. J. Plant Prod. 2015, 9, 237–256. [Google Scholar]
- Liu, J.R.; Ma, Y.N.; Lv, F.J.; Chen, J.; Zhou, Z.G.; Wang, Y.H.; Abudurezike, A.; Oosterhuis, D.M. Changes of sucrose metabolism in leaf subtending to cotton boll under cool temperature due to late planting. Field Crop Res. 2013, 144, 200–211. [Google Scholar] [CrossRef]
- Ma, Q.J.; Sun, M.H.; Kang, H.; Lu, J.; You, C.X.; Hao, Y.J. A CIPK protein kinase targets sucrose transporter MdSUT2.2 at Ser254 for phosphorylation to enhance salt tolerance. Plant Cell Environ. 2019, 42, 918–930. [Google Scholar] [CrossRef]
- Filip, M.; Vlassa, M.; Coman, V.; Halmagyi, A. Simultaneous determination of glucose, fructose, sucrose and sorbitol in the leaf and fruit peel of different apple cultivars by the HPLC–RI optimized method. Food Chem. 2016, 199, 653–659. [Google Scholar] [CrossRef]
- Berüter, J. Sugar accumulation and changes in the activities of related enzymes during development of the apple fruit. J. Plant Physiol. 1985, 121, 331–341. [Google Scholar] [CrossRef]
- Hu, W.; Jiang, N.; Yang, J.; Meng, Y.; Wang, Y.; Chen, B.; Zhao, W.; Oosterhuis, D.M.; Zhou, Z. Potassium (K) supply affects K accumulation and photosynthetic physiology in two cotton (Gossypium hirsutum L.) cultivars with different K sensitivities. Field Crop. Res. 2016, 196, 51–63. [Google Scholar] [CrossRef]
- Rufly, T.W.; Huber, S.C. Changes in starch formation and activities of sucrose phosphate synthase and cytoplasmic fructose-1,6-biosphatase in response to source-sink alteration. Plant Physiol. 1983, 72, 474–478. [Google Scholar]
- Yamaki, S.; Asakura, T. Stimulation of the uptake of sorbitol into vacuoles from apple fruit flesh by abscisic acid and into protoplasts by indoleacetic acid. Plant Cell Physiol. 1991, 32, 315–318. [Google Scholar] [CrossRef]
- Huber, S.C. Role of sucrose-phosphate synthase in partitioning of carbon in leaves. Plant Physiol. 1983, 71, 818–821. [Google Scholar] [CrossRef]
- Merlo, L.; Passera, C. Changes in carbohydrate and enzyme levels during development of leaves of prunus persica, a sorbitol synthesizing species. Plant Physiol. 1991, 83, 621–626. [Google Scholar] [CrossRef]
- Ding, Y.; Luo, W.; Xu, G. Characterisation of magnesium nutrition and interaction of magnesium and potassium in rice. Ann. Appl. Biol. 2006, 149, 111–123. [Google Scholar] [CrossRef]
- Seith, B.; Setzer, B.; Flaig, H.; Mohr, H. Appearance of nitrate reductase, nitrite reductase and glutamine synthetase in different organs of the scots pine (Pinus sylvestris) seedling as affected by light, nitrate and ammonium. Physiol. Plant. 1994, 91, 419–426. [Google Scholar] [CrossRef]
- Hu, W.; Zhao, W.; Yang, J.; Oosterhuis, D.M.; Loka, D.A.; Zhou, Z.G. Relationship between potassium fertilization and nitrogen metabolism in the leaf subtending the cotton (Gossypium hirsutum L.) boll during the boll development stage. Plant Physiol. Biochem. 2016, 101, 113–123. [Google Scholar] [CrossRef]
- Xu, X.X.; Du, X.; Wang, F.; Sha, J.C.; Chen, Q.; Tian, G.; Zhu, Z.; Ge, S.; Jiang, Y. Effects of potassium Levels on plant growth, accumulation and distribution of carbon, and nitrate metabolism in apple dwarf rootstock seedlings. Front. Plant Sci. 2020, 11, 904. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Liu, L.; Tao, H.; An, Y.; Wang, L. Transcriptomic profiling of apple calli with a focus on the key genes for ALA-induced anthocyanin accumulation. Front. Plant Sci. 2021, 12, 640606. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fu, Y.; Hu, D.; Yu, J.; Liu, H. Effect of green, yellow and purple radiation on biomass, photosynthesis, morphology and soluble sugar content of leafy lettuce via spectral wavebands “knock”out. Sci. Hortic. 2018, 236, 10–17. [Google Scholar] [CrossRef]
- Nie, J.; Li, H.; Li, J.; Wang, K.; Li, Z.; Wu, Y. Studies on taste evaluation indices for fresh apple juice based on cultivars. Acta Hortic. Sin. 2012, 39, 1999–2008. [Google Scholar]
- Li, T.; Wang, Y.; Kamran, M.; Chen, X.; Tan, H.; Long, M. Effects of grass inter-planting on soil nutrients, enzyme activity, and bacterial community diversity in an apple orchard. Front. Plant Sci. 2022, 13, 901143. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diaz, A.; Bienes, R.; Sastre, B.; Novara, A.; Gristina, L.; Cerda, A. Nitrogen losses in vineyards under different types of soil groundcover. A field runoff simulator approach in central Spain. Agric. Ecosyst. Environ. 2017, 236, 256–267. [Google Scholar] [CrossRef]
- Stork, P.R.; Jerie, P.H. Initial studies of the growth, nitrogen sequestering, and de-watering potential of perennial grass selections for use as nitrogen catch crops in orchards. Aust. J. Agric. Res. 2003, 54, 27–37. [Google Scholar] [CrossRef]
- Wang, F.; Ge, S.; Lyu, M.; Liu, J.; Li, M.; Jiang, Y.; Xu, X.; Xing, Y.; Cao, H.; Zhu, Z.; et al. DMPP reduces nitrogen fertilizer application rate, improves fruit quality, and reduces environmental cost of intensive apple production in China. Sci. Total Environ. 2022, 802, 149813. [Google Scholar] [CrossRef]
- Cui, M.; Zeng, L.; Qin, W.; Feng, J. Measures for reducing nitrate leaching in orchards: A review. Environ. Pollut. 2020, 263, 114553. [Google Scholar] [CrossRef]
- Zhu, X.Q.; Zhou, P.; Miao, P.; Wang, H.Y.; Bai, X.L.; Chen, Z.J.; Zhou, J. Nitrogen use and management in orchards and vegetable fields in china: Challenges and solutions. Front. Agric. Sci. Eng. 2022, 9, 386–395. [Google Scholar]
- Peng, L.; Ren, Y.; Ji, M.; Ding, N.; Jiang, H.; Ge, S.; Jiang, Y. Effect of planting herbage on the growth and absorption, utilization of 15N-urea of apple saplings. Acta Hortic. Sin. 2014, 41, 1975–1982. [Google Scholar]
- Haruna, S.I.; Anderson, S.H.; Udawatta, R.P.; Gantzer, C.J.; Phillips, N.C.; Cui, S.; Gao, Y. Improving soil physical properties through the use of cover crops: A review. Agrosyst. Geosci. Environ. 2020, 3, e20105. [Google Scholar] [CrossRef]
- Xiang, Y.Z.; Chang, S.X.; Shen, Y.Y.; Chen, G.; Liu, Y.; Yao, B.; Xue, J.; Li, Y. Grass cover increases soil microbial abundance and diversity and extracellular enzyme activities in orchards: A synthesis across China. Appl. Soil Ecol. 2023, 182, 104720. [Google Scholar] [CrossRef]
- Desnoues, E.; Génard, M.; Baldazzi, V. A kinetic model of sugar metabolism in peach fruit reveals a functional hypothesis of markedly low fructoseto-glucose ratio phenotype. Plant J. 2018, 94, 685–698. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higherplants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.X.; Wang, F.; Xing, Y.; Liu, J.Q.; Lv, M.X.; Meng, H.; Du, X.; Zhu, Z.; Ge, S.; Jiang, Y. Appropriate and constant potassium supply promotes the growth of M9T337 apple rootstocks by regulating endogenous hormones and carbon and nitrogen metabolism. Front. Plant Sci. 2022, 13, 827478. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Fon, M.; Lee, Y.J. The citrus fruit proteome: Insights into citrus fruit metabolism. Planta 2007, 226, 989–1005. [Google Scholar] [CrossRef]
- Falchi, R.; Bonghi, C.; Drincovich, M.F.; Famiani, F.; Lara, M.V.; Walker, R.P.; Vizzotto, G. Sugar metabolism in stone fruit: Source-sink relationships and environmental and agronomical effects. Front. Plant Sci. 2020, 11, 573982. [Google Scholar] [CrossRef]
- Chong, C.; Taper, C.D. Daily variation of sorbitol and related carbohydrates in malus leaves. Can. J. Bot. 1971, 49, 173–177. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, C.X.; Yan, Y.J.; Wang, Y.A.; Li, M.; Chen, M.X.; Qian, J.P.; Yang, X.T.; Cheng, S.H. Zinc sulfate and sugar alcohol zinc sprays at critical stages to improve apple fruit quality. Hortic. Technol. 2013, 23, 490–497. [Google Scholar] [CrossRef]
- Eom, J.S.; Choi, S.B.; Ward, J.M.; Jeon, J.S. The mechanism of phloem loading in rice (Oryza sativa). Mol. Cells 2012, 33, 431–438. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.; Li, M.; Zhou, B.; Zhang, J.; Wei, Q. Correlation analysis between quality characteristics and fruit mineral element contents in ‘Fuji’ apples. Agric. Sci. Technol. 2017, 18, 212–218. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, B.; Wang, L.; Zhang, J.; Lyu, D. Natural Grass Cultivation Management Improves Apple Fruit Quality by Regulating Soil Mineral Nitrogen Content and Carbon–Nitrogen Metabolism. Metabolites 2023, 13, 925. https://doi.org/10.3390/metabo13080925
Yu B, Wang L, Zhang J, Lyu D. Natural Grass Cultivation Management Improves Apple Fruit Quality by Regulating Soil Mineral Nitrogen Content and Carbon–Nitrogen Metabolism. Metabolites. 2023; 13(8):925. https://doi.org/10.3390/metabo13080925
Chicago/Turabian StyleYu, Bo, Lixia Wang, Jiaqi Zhang, and Deguo Lyu. 2023. "Natural Grass Cultivation Management Improves Apple Fruit Quality by Regulating Soil Mineral Nitrogen Content and Carbon–Nitrogen Metabolism" Metabolites 13, no. 8: 925. https://doi.org/10.3390/metabo13080925
APA StyleYu, B., Wang, L., Zhang, J., & Lyu, D. (2023). Natural Grass Cultivation Management Improves Apple Fruit Quality by Regulating Soil Mineral Nitrogen Content and Carbon–Nitrogen Metabolism. Metabolites, 13(8), 925. https://doi.org/10.3390/metabo13080925





