The Impacts of Slc19a3 Deletion and Intestinal SLC19A3 Insertion on Thiamine Distribution and Brain Metabolism in the Mouse
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of Human THTR2 Expressing TG Mice
2.2. Quantitative Polymerase Chain Reactions
2.3. Western Blotting
2.4. Thiamine Feeding Study
2.5. In Vivo THTR2 Inhibition Study
2.6. Plasma Thiamine Analysis
2.7. RBC and Tissue Thiamine Vitamer Analysis
2.8. Brain Metabolomics
2.9. Statistics
2.9.1. Group Characterizations
2.9.2. Feeding Study Thiamine Vitamer Analysis
2.9.3. THTR2 Inhibition Study Thiamine Vitamer Analysis
2.9.4. Metabolomics Data Pre-processing
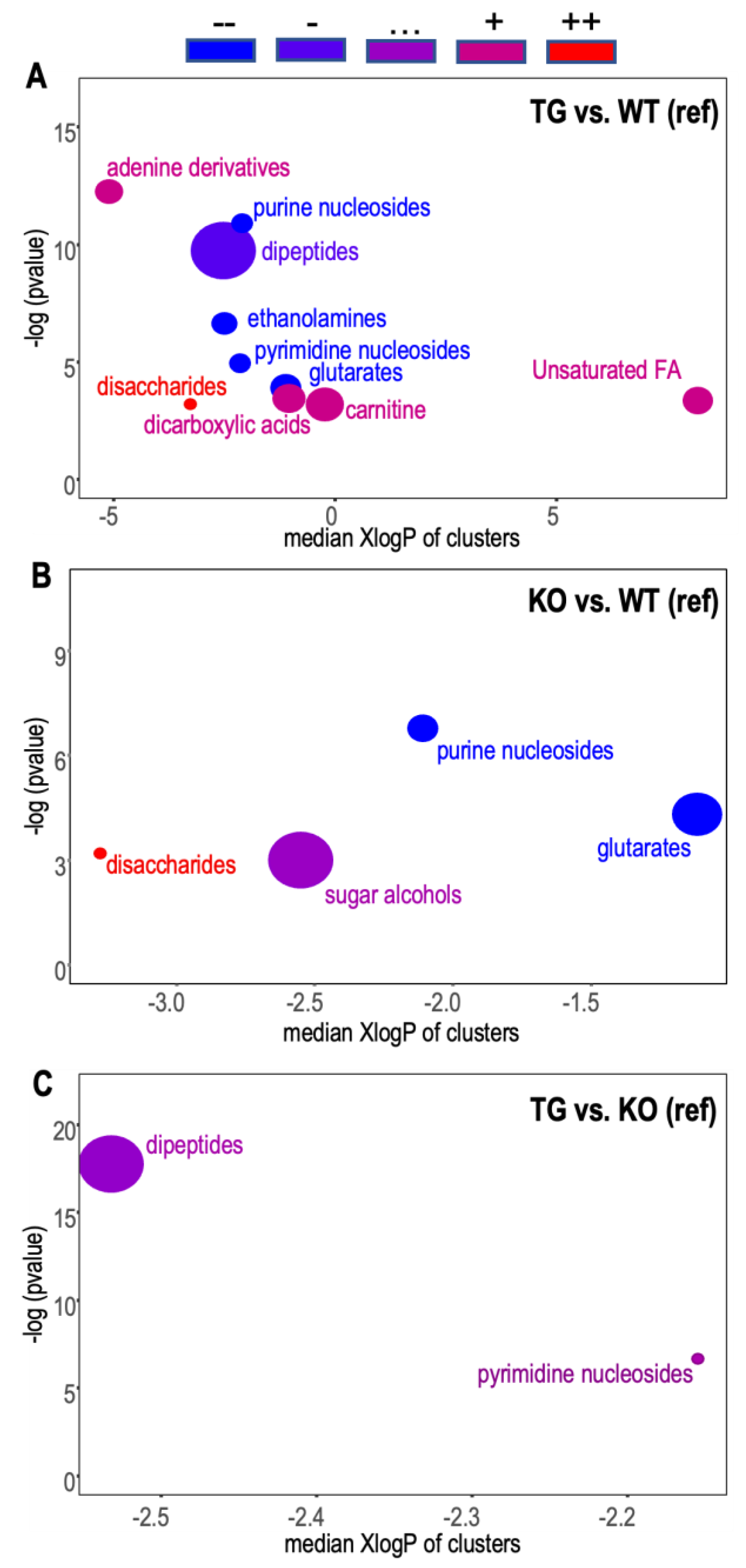
2.9.5. Chemical Enrichment Analysis
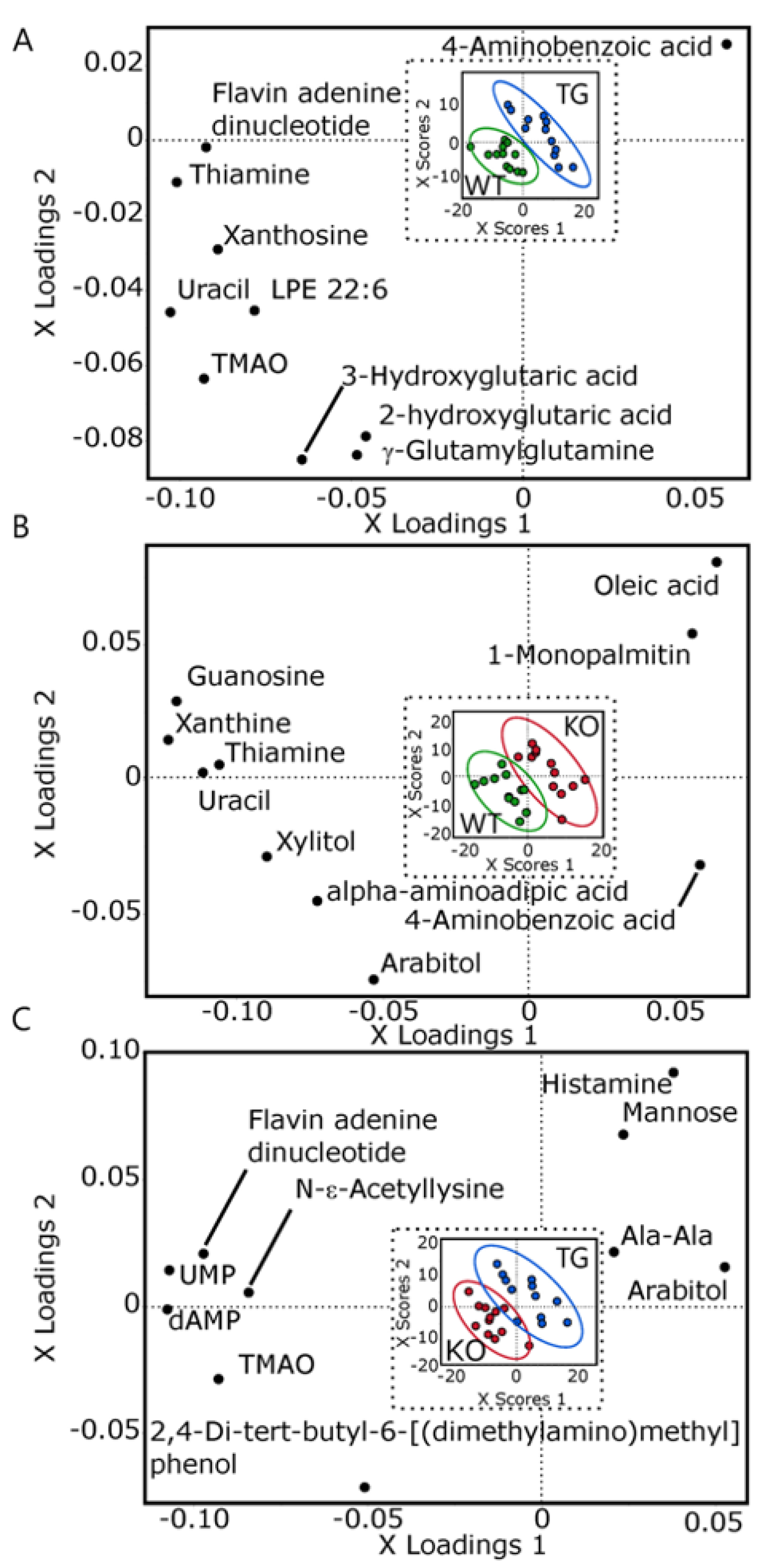
2.9.6. Multivariate Analyses
3. Results
3.1. Expression of Human THTR2, Mouse THTR2, and Mouse THTR1
3.2. Characterization of Groups of Mice
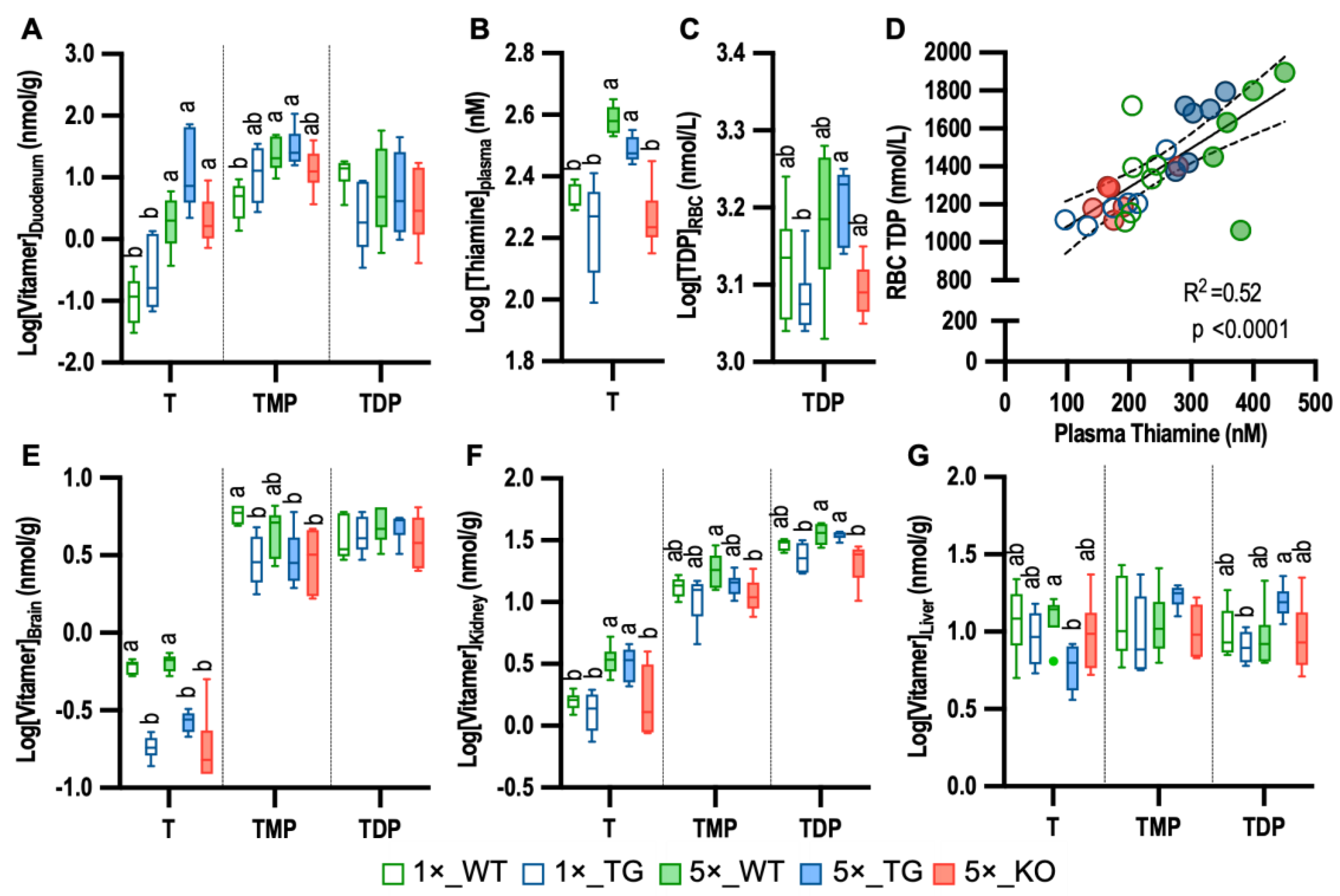
3.3. Impact of THTR2 on Thiamine Distribution
3.4. Impact of Plasma Thiamine, Genotype, and Sex on Tissue Thiamine Levels
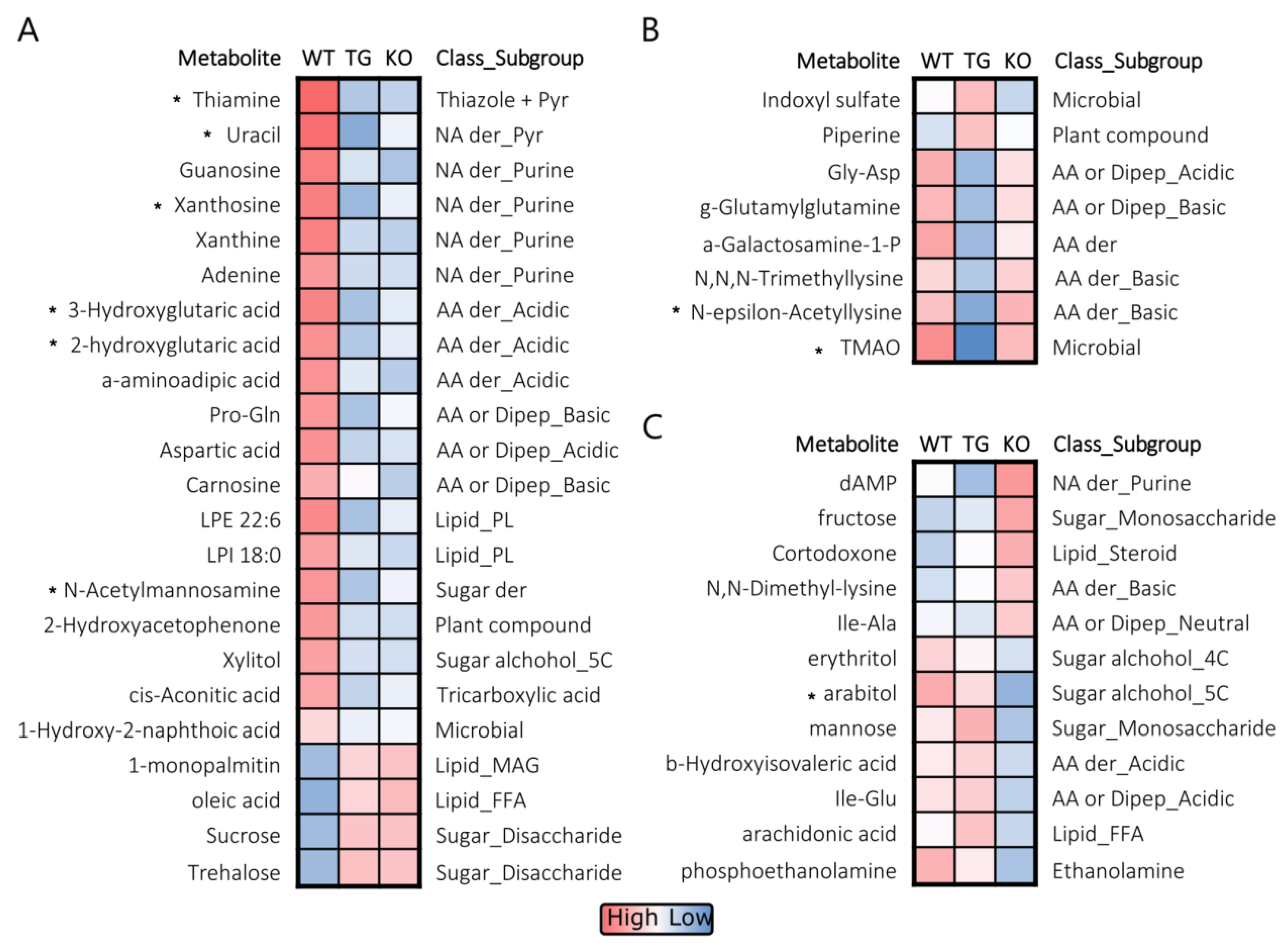
3.5. Brain Metabolomic
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Thiamine Vitamer Assays Performance
References
- Whitfield, K.C.; Bourassa, M.W.; Adamolekun, B.; Bergeron, G.; Bettendorff, L.; Brown, K.H.; Cox, L.; Fattal-Valevski, A.; Fischer, P.R.; Frank, E.L.; et al. Thiamine deficiency disorders: Diagnosis, prevalence, and a roadmap for global control programs. Ann. N. Y. Acad. Sci. 2018, 1430, 3–43. [Google Scholar] [CrossRef]
- Reggiani, C.; Patrini, C.; Rindi, G. Nervous tissue thiamine metabolism in vivo. I. Transport of thiamine and thiamine monophosphate from plasma to different brain regions of the rat. Brain Res. 1984, 293, 319–327. [Google Scholar] [CrossRef]
- Lonsdale, D. A Review of the Biochemistry, Metabolism and Clinical Benefits of Thiamin(e) and Its Derivatives. Evidence-Based Complement. Altern. Med. 2006, 3, 349513. [Google Scholar] [CrossRef] [PubMed]
- Sambon, M.; Pavlova, O.; Alhama-Riba, J.; Wins, P.; Brans, A.; Bettendorff, L. Product inhibition of mammalian thiamine pyrophosphokinase is an important mechanism for maintaining thiamine diphosphate homeostasis. Biochim. et Biophys. Acta (BBA)-Gen. Subj. 2022, 1866. [Google Scholar] [CrossRef] [PubMed]
- Hazell, A.S.; Faim, S.; Wertheimer, G.; Silva, V.R.; Marques, C.S. The impact of oxidative stress in thiamine deficiency: A multifactorial targeting issue. Neurochem. Int. 2013, 62, 796–802. [Google Scholar] [CrossRef]
- Butterworth, R.F. Neurotransmitter function in thiamine-deficiency encephalopathy. Neurochem. Int. 1982, 4, 449–464. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- GTEx Consortium. The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science 2015, 348, 648–660. [Google Scholar] [CrossRef]
- Noguchi, S.; Arakawa, T.; Fukuda, S.; Furuno, M.; Hasegawa, A.; Hori, F.; Ishikawa-Kato, S.; Kaida, K.; Kaiho, A.; Kanamori-Katayama, M.; et al. FANTOM5 CAGE profiles of human and mouse samples. Sci. Data 2017, 4, 170112. [Google Scholar] [CrossRef]
- Chen, L.; Shu, Y.; Liang, X.; Chen, E.C.; Yee, S.W.; Zur, A.A.; Li, S.; Xu, L.; Keshari, K.R.; Lin, M.J.; et al. OCT1 is a high-capacity thiamine transporter that regulates hepatic steatosis and is a target of metformin. Proc. Natl. Acad. Sci. USA 2014, 111, 9983–9988. [Google Scholar] [CrossRef]
- Liang, X.; Yee, S.W.; Chien, H.-C.; Chen, E.C.; Luo, Q.; Zou, L.; Piao, M.; Mifune, A.; Chen, L.; Calvert, M.E.; et al. Organic cation transporter 1 (OCT1) modulates multiple cardiometabolic traits through effects on hepatic thiamine content. PLoS Biol. 2018, 16, e2002907. [Google Scholar] [CrossRef]
- Kato, K.; Moriyama, C.; Ito, N.; Zhang, X.; Hachiuma, K.; Hagima, N.; Iwata, K.; Yamaguchi, J.-I.; Maeda, K.; Ito, K.; et al. Involvement of Organic Cation Transporters in the Clearance and Milk Secretion of Thiamine in Mice. Pharm. Res. 2015, 32, 2192–2204. [Google Scholar] [CrossRef]
- Kato, K.; Mori, H.; Kito, T.; Yokochi, M.; Ito, S.; Inoue, K.; Yonezawa, A.; Katsura, T.; Kumagai, Y.; Yuasa, H.; et al. Investigation of Endogenous Compounds for Assessing the Drug Interactions in the Urinary Excretion Involving Multidrug and Toxin Extrusion Proteins. Pharm. Res. 2013, 31, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Rajgopal, A.; Edmondnson, A.; Goldman, I.; Zhao, R. SLC19A3 encodes a second thiamine transporter ThTr2. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2001, 1537, 175–178. [Google Scholar] [CrossRef]
- Reidling, J.C.; Lambrecht, N.; Kassir, M.; Said, H.M. Impaired Intestinal Vitamin B1 (Thiamin) Uptake in Thiamin Transporter-2–Deficient Mice. Gastroenterology 2010, 138, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Ortigoza-Escobar, J.D.; Molero-Luis, M.; Arias, A.; Martí-Sánchez, L.; Rodriguez-Pombo, P.; Artuch, R.; Pérez-Dueñas, B. Treatment of genetic defects of thiamine transport and metabolism. Expert Rev. Neurother. 2016, 16, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Tabarki, B.; Alfadhel, M. SLC19A3 Gene Defects Sorting the Phenotype and Acronyms: Review. Neuropediatrics 2017, 49, 083–092. [Google Scholar] [CrossRef]
- Said, H.M.; Balamurugan, K.; Subramanian, V.S.; Marchant, J.S. Expression and functional contribution of hTHTR-2 in thiamin absorption in human intestine. Am. J. Physiol. Liver Physiol. 2004, 286, G491–G498. [Google Scholar] [CrossRef]
- Zhang, K.; Huentelman, M.J.; Rao, F.; Sun, E.I.; Corneveaux, J.J.; Schork, A.J.; Wei, Z.; Waalen, J.; Miramontes-Gonzalez, J.P.; Hightower, C.M.; et al. Genetic Implication of a Novel Thiamine Transporter in Human Hypertension. J. Am. Coll. Cardiol. 2014, 63, 1542–1555. [Google Scholar] [CrossRef]
- Zhao, R.; Gao, F.; Goldman, I.D. Reduced folate carrier transports thiamine monophosphate: An alternative route for thiamine delivery into mammalian cells. Am. J. Physiol. Physiol. 2002, 282, C1512–C1517. [Google Scholar] [CrossRef]
- Dang, Y.; Zhou, D.; Du, X.; Zhao, H.; Lee, C.-H.; Yang, J.; Wang, Y.; Qin, C.; Guo, Z.; Zhang, Z. Molecular mechanism of substrate recognition by folate transporter SLC19A1. Cell Discov. 2022, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fang, B.; Hu, X.; Guo, R.; Guo, J.; Fang, K.; Ni, J.; Li, W.; Qian, S.; Hao, C. Identification and functional analysis of novel SLC25A19 variants causing thiamine metabolism dysfunction syndrome 4. Orphanet J. Rare Dis. 2021, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lindhurst, M.J.; Fiermonte, G.; Song, S.; Struys, E.; De Leonardis, F.; Schwartzberg, P.L.; Chen, A.; Castegna, A.; Verhoeven, N.; Mathews, C.K.; et al. Knockout of Slc25a19 causes mitochondrial thiamine pyrophosphate depletion, embryonic lethality, CNS malformations, and anemia. Proc. Natl. Acad. Sci. USA 2006, 103, 15927–15932. [Google Scholar] [CrossRef] [PubMed]
- Nabokina, S.M.; Inoue, K.; Subramanian, V.S.; Valle, J.E.; Yuasa, H.; Said, H.M. Molecular Identification and Functional Characterization of the Human Colonic Thiamine Pyrophosphate Transporter. J. Biol. Chem. 2014, 289, 4405–4416. [Google Scholar] [CrossRef] [PubMed]
- Nabokina, S.M.; Subramanian, V.S.; Valle, J.E.; Said, H.M.; Anandam, K.Y.; Srinivasan, P.; Sabui, S.; Kapadia, R. Adaptive regulation of human intestinal thiamine uptake by extracellular substrate level: A role for THTR-2 transcriptional regulation. Am. J. Physiol. Liver Physiol. 2013, 305, G593–G599. [Google Scholar] [CrossRef]
- Oishi, K.; Hofmann, S.; Diaz, G.A.; Brown, T.; Manwani, D.; Ng, L.; Young, R.; Vlassara, H.; Ioannou, Y.A.; Forrest, D.; et al. Targeted disruption of Slc19a2, the gene encoding the high-affinity thiamin transporter Thtr-1, causes diabetes mellitus, sensorineural deafness and megaloblastosis in mice. Hum. Mol. Genet. 2002, 11, 2951–2960. [Google Scholar] [CrossRef]
- Habeb, A.M.; International Neonatal Diabetes Consortium; Flanagan, S.E.; Zulali, M.A.; Abdullah, M.A.; Pomahačová, R.; Boyadzhiev, V.; Colindres, L.E.; Godoy, G.V.; Vasanthi, T.; et al. Pharmacogenomics in diabetes: Outcomes of thiamine therapy in TRMA syndrome. Diabetologia 2018, 61, 1027–1036. [Google Scholar] [CrossRef]
- Liang, X.; Chien, H.-C.; Yee, S.W.; Giacomini, M.M.; Chen, E.C.; Piao, M.; Hao, J.; Twelves, J.; Lepist, E.-I.; Ray, A.S.; et al. Metformin Is a Substrate and Inhibitor of the Human Thiamine Transporter, THTR-2 (SLC19A3). Mol. Pharm. 2015, 12, 4301–4310. [Google Scholar] [CrossRef]
- Giacomini, M.M.; Hao, J.; Liang, X.; Chandrasekhar, J.; Twelves, J.; Whitney, J.A.; Lepist, E.-I.; Ray, A.S. Interaction of 2,4-Diaminopyrimidine–Containing Drugs Including Fedratinib and Trimethoprim with Thiamine Transporters. Drug Metab. Dispos. 2016, 45, 76–85. [Google Scholar] [CrossRef]
- Vora, B.; Green, E.A.; Khuri, N.; Ballgren, F.; Sirota, M.; Giacomini, K.M. Drug–nutrient interactions: Discovering prescription drug inhibitors of the thiamine transporter ThTR-2 (SLC19A3). Am. J. Clin. Nutr. 2019, 111, 110–121. [Google Scholar] [CrossRef]
- Mullally, A.; Hood, J.; Harrison, C.; Mesa, R. Fedratinib in myelofibrosis. Blood Adv. 2020, 4, 1792–1800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, Y.; Diamond, S.; Boer, J.; Harris, J.J.; Li, Y.; Rupar, M.; Behshad, E.; Gardiner, C.; Collier, P.; et al. The Janus Kinase 2 Inhibitor Fedratinib Inhibits Thiamine Uptake: A Putative Mechanism for the Onset of Wernicke’s Encephalopathy. Drug Metab. Dispos. 2014, 42, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A. Fedratinib: First Approval. Drugs 2019, 79, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Ittner, L.M.; Götz, J. Pronuclear injection for the production of transgenic mice. Nat. Protoc. 2007, 2, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Rutlin, M.; Rastelli, D.; Kuo, W.; Estep, J.; Louis, A.; Riccomagno, M.; Turner, J.; Rao, M. The Villin1 Gene Promoter Drives Cre Recombinase Expression in Extraintestinal Tissues. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 864–867.e5. [Google Scholar] [CrossRef]
- Verstraete, J.; Strobbe, S.; Van Der Straeten, D.; Stove, C.P. The First Comprehensive LC–MS/MS Method Allowing Dissection of the Thiamine Pathway in Plants. Anal. Chem. 2020, 92, 4073–4081. [Google Scholar] [CrossRef]
- Ding, J.; Ji, J.; Rabow, Z.; Shen, T.; Folz, J.; Brydges, C.R.; Fan, S.; Lu, X.; Mehta, S.; Showalter, M.R.; et al. A metabolome atlas of the aging mouse brain. Nat. Commun. 2021, 12, 6021. [Google Scholar] [CrossRef]
- Skogerson, K.; Wohlgemuth, G.; Barupal, D.K.; Fiehn, O. The volatile compound BinBase mass spectral database. BMC Bioinform. 2011, 12, 321. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- DeFelice, B.C.; Mehta, S.S.; Samra, S.; Čajka, T.; Wancewicz, B.; Fahrmann, J.F.; Fiehn, O. Mass Spectral Feature List Optimizer (MS-FLO): A Tool To Minimize False Positive Peak Reports in Untargeted Liquid Chromatography–Mass Spectroscopy (LC-MS) Data Processing. Anal. Chem. 2017, 89, 3250–3255. [Google Scholar] [CrossRef]
- Barupal, D.K.; Fiehn, O. Chemical Similarity Enrichment Analysis (ChemRICH) as alternative to biochemical pathway mapping for metabolomic datasets. Sci. Rep. 2017, 7, 14567. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.N.; Ziegler, A. Ranger: A Fast Implementation of Random Forests for High Dimensional Data in C++ and R. J. Stat. Softw. 2017, 77, 1–17. [Google Scholar] [CrossRef]
- Strobl, C.; Boulesteix, A.-L.; Zeileis, A.; Hothorn, T. Bias in random forest variable importance measures: Illustrations, sources and a solution. BMC Bioinform. 2007, 8, 25. [Google Scholar] [CrossRef]
- Probst, P.; Wright, M.N.; Boulesteix, A.L. Hyperparameters and tuning strategies for random forest. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2019, 9, e1301. [Google Scholar] [CrossRef]
- Chen, R.; Jiang, X.; Sun, D.; Han, G.; Wang, F.; Ye, M.; Wang, L.; Zou, H. Glycoproteomics Analysis of Human Liver Tissue by Combination of Multiple Enzyme Digestion and Hydrazide Chemistry. J. Proteome Res. 2009, 8, 651–661. [Google Scholar] [CrossRef]
- Kleinert, P.; Kuster, T.; Arnold, D.; Jaeken, J.; Heizmann, C.W.; Troxler, H. Effect of glycosylation on the protein pattern in 2-D-gel electrophoresis. Proteomics 2007, 7, 15–22. [Google Scholar] [CrossRef]
- Suzuki, K.; Yamada, K.; Fukuhara, Y.; Tsuji, A.; Shibata, K.; Wakamatsu, N. High-dose thiamine prevents brain lesions and prolongs survival of Slc19a3-deficient mice. PLoS ONE 2017, 12, e0180279. [Google Scholar] [CrossRef]
- Stanley, W.C.; Recchia, F.A.; Lopaschuk, G.D. Myocardial Substrate Metabolism in the Normal and Failing Heart. Physiol. Rev. 2005, 85, 1093–1129. [Google Scholar] [CrossRef]
- Gangolf, M.; Czerniecki, J.; Radermecker, M.; Detry, O.; Nisolle, M.; Jouan, C.; Martin, D.; Chantraine, F.; Lakaye, B.; Wins, P.; et al. Thiamine Status in Humans and Content of Phosphorylated Thiamine Derivatives in Biopsies and Cultured Cells. PLoS ONE 2010, 5, e13616. [Google Scholar] [CrossRef]
- Ott, M.; Werneke, U. Wernicke’s encephalopathy—From basic science to clinical practice. Part 1: Understanding the role of thiamine. Ther. Adv. Psychopharmacol. 2020, 10. [Google Scholar] [CrossRef]
- Butterworth, R.F. Effects of thiamine deficiency on brain metabolism: Implications for the pathogenesis of the wernicke-korsakoff syndrome. Alcohol Alcohol. 1989, 24, 271–279. [Google Scholar] [CrossRef]
- Kevelam, S.H.; Bugiani, M.; Salomons, G.S.; Feigenbaum, A.; Blaser, S.; Prasad, C.; Häberle, J.; Barić, I.; Bakker, I.M.C.; Postma, N.L.; et al. Exome sequencing reveals mutated SLC19A3 in patients with an early-infantile, lethal encephalopathy. Brain 2013, 136, 1534–1543. [Google Scholar] [CrossRef]
- Vora, B.; Wen, A.; Yee, S.W.; Trinh, K.; Azimi, M.; Green, E.A.E.; Sirota, M.; Greenberg, A.S.; Newman, J.W.; Giacomini, K.M. The Effect of Trimethoprim on Thiamine Absorption: A Transporter-Mediated Drug-Nutrient Interaction. Clin. Pharmacol. Ther. 2023. [Google Scholar] [CrossRef]
- Pan, X.; Gong, N.; Zhao, J.; Yu, Z.; Gu, F.; Chen, J.; Sun, X.; Zhao, L.; Yu, M.; Xu, Z.; et al. Powerful beneficial effects of benfotiamine on cognitive impairment and -amyloid deposition in amyloid precursor protein/presenilin-1 transgenic mice. Brain 2010, 133, 1342–1351. [Google Scholar] [CrossRef]
- Sambon, M.; Gorlova, A.; Demelenne, A.; Alhama-Riba, J.; Coumans, B.; Lakaye, B.; Wins, P.; Fillet, M.; Anthony, D.C.; Strekalova, T.; et al. Dibenzoylthiamine Has Powerful Antioxidant and Anti-Inflammatory Properties in Cultured Cells and in Mouse Models of Stress and Neurodegeneration. Biomedicines 2020, 8, 361. [Google Scholar] [CrossRef]
- Bettendorff, L.; Mastrogiacomo, F.; Kish, S.J.; Grisar, T. Thiamine, Thiamine Phosphates, and Their Metabolizing Enzymes in Human Brain. J. Neurochem. 2002, 66, 250–258. [Google Scholar] [CrossRef]
- Bettendorff, L. Update on Thiamine Triphosphorylated Derivatives and Metabolizing Enzymatic Complexes. Biomolecules 2021, 11, 1645. [Google Scholar] [CrossRef] [PubMed]
- Mkrtchyan, G.; Aleshin, V.; Parkhomenko, Y.; Kaehne, T.; Di Salvo, M.L.; Parroni, A.; Contestabile, R.; Vovk, A.; Bettendorff, L.; Bunik, V. Molecular mechanisms of the non-coenzyme action of thiamin in brain: Biochemical, structural and pathway analysis. Sci. Rep. 2015, 5, srep12583. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.J.; de Aguiar Vallim, T.Q.; Wang, Z.; Shih, D.M.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-Oxide, a Metabolite Associated with Atherosclerosis, Exhibits Complex Genetic and Dietary Regulation. Cell Metab. 2013, 17, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Schänzer, A.; Döring, B.; Ondrouschek, M.; Goos, S.; Garvalov, B.K.; Geyer, J.; Acker, T.; Neubauer, B.; Hahn, A. Stress-Induced Upregulation of SLC19A3 is Impaired in Biotin-Thiamine-Responsive Basal Ganglia Disease. Brain Pathol. 2014, 24, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.J.; Tuerkova, A.; Römer, S.; Wenzel, C.; Seitz, T.; Gaedcke, J.; Oswald, S.; Brockmöller, J.; Zdrazil, B.; Tzvetkov, M.V. Differences in Metformin and Thiamine Uptake between Human and Mouse Organic Cation Transporter 1: Structural Determinants and Potential Consequences for Intrahepatic Concentrations. Drug Metab. Dispos. 2020, 48, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.; Matthaei, J.; Blome, F.; Schwab, M.; Tzvetkov, M.V.; Brockmöller, J. Variability and Heritability of Thiamine Pharmacokinetics with Focus on OCT1 Effects on Membrane Transport and Pharmacokinetics in Humans. Clin. Pharmacol. Ther. 2019, 107, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Jonker, J.W.; Wagenaar, E.; van Eijl, S.; Schinkel, A.H. Deficiency in the Organic Cation Transporters 1 and 2 (Oct1/Oct2 [Slc22a1/Slc22a2]) in Mice Abolishes Renal Secretion of Organic Cations. Mol. Cell. Biol. 2003, 23, 7902–7908. [Google Scholar] [CrossRef] [PubMed]
| Tissue | Vitamer | β Coefficient a | ||
|---|---|---|---|---|
| Genotype [TG] | Plasma Thiamine | Sex [F] | ||
| RBC | TDP | 0.007 ** | ||
| Duodenum | Thiamine | 0.488 *** | 0.009 **** | |
| TMP | 0.702 ** | 0.012 *** | ||
| TDP | ||||
| Brain | Thiamine | −0.55 **** | −0.109 * | |
| TMP | −1.04 *** | |||
| TDP | 0.006 * | |||
| Kidney | Thiamine | 0.002 **** | ||
| TMP | 0.001 ** | |||
| TDP | 0.059 **** | 1.883 * | ||
| Liver | Thiamine | −0.108 ** | ||
| TMP | ||||
| TDP | ||||
| Heart | Thiamine | |||
| TMP | ||||
| TDP | −4.05 * | −0.039 * | ||
| Fat | Thiamine | 0.002 ** | ||
| TMP | ||||
| TDP | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, A.; Zhu, Y.; Yee, S.W.; Park, B.I.; Giacomini, K.M.; Greenberg, A.S.; Newman, J.W. The Impacts of Slc19a3 Deletion and Intestinal SLC19A3 Insertion on Thiamine Distribution and Brain Metabolism in the Mouse. Metabolites 2023, 13, 885. https://doi.org/10.3390/metabo13080885
Wen A, Zhu Y, Yee SW, Park BI, Giacomini KM, Greenberg AS, Newman JW. The Impacts of Slc19a3 Deletion and Intestinal SLC19A3 Insertion on Thiamine Distribution and Brain Metabolism in the Mouse. Metabolites. 2023; 13(8):885. https://doi.org/10.3390/metabo13080885
Chicago/Turabian StyleWen, Anita, Ying Zhu, Sook Wah Yee, Brian I. Park, Kathleen M. Giacomini, Andrew S. Greenberg, and John W. Newman. 2023. "The Impacts of Slc19a3 Deletion and Intestinal SLC19A3 Insertion on Thiamine Distribution and Brain Metabolism in the Mouse" Metabolites 13, no. 8: 885. https://doi.org/10.3390/metabo13080885
APA StyleWen, A., Zhu, Y., Yee, S. W., Park, B. I., Giacomini, K. M., Greenberg, A. S., & Newman, J. W. (2023). The Impacts of Slc19a3 Deletion and Intestinal SLC19A3 Insertion on Thiamine Distribution and Brain Metabolism in the Mouse. Metabolites, 13(8), 885. https://doi.org/10.3390/metabo13080885






