Abstract
Mutagenesis is a highly efficient tool for establishing genetic variation and is widely used for genetic enhancement in various plants. The key benefit of mutation breeding is the prospect of enhancing one or several characteristics of a variety without altering the genetic background. In this study, we exposed the seeds of Salvia officinalis to four concentrations of hydrazine hydrate (HZ), i.e., (0%, 0.1%, 0.2%, and 0.3%) for 6 h. The contents of terpenoid compounds in the S. officinalis plantlets driven from the HZ-treated seeds were determined by GC-MS, which resulted in the identification of a total of 340 phytochemical compounds; 163 (87.48%), 145 (84.49%), 65 (97.45%), and 62 (98.32%), from the four concentrations of HZ (0%, 0.1%, 0.2%, and 0.3%), respectively. Furthermore, we used the qRT-PCR system to disclose the “transcriptional control” for twelve TPS genes related to terpenoid and terpene biosynthesis, namely, SoGPS, SoMYRS, SoNEOD, SoCINS, SoSABS, SoLINS, SoFPPS, SoHUMS, SoTPS6, SoSQUS, SoGGPS, and SoGA2. Altogether, results are likely to ensure some positive relationship between the concentrations of the chemical mutagen HZ used for treating the seeds, the type and amount of the produced terpenes, and the expression of their corresponding genes.
1. Introduction
Plant improvement has been the cornerstone of the ever-growing human population’s food security for many years. Despite the availability of large germplasm collections, crop development still depends on effective genetic diversity evaluation [1,2,3]. Furthermore, genetic diversity is essential for crop improvement and climate adaptation, especially in plants with low genetic diversity that are more susceptible to stresses [4,5,6,7,8,9]. Genetic variation is greatly influenced by the degree of DNA damage and the cell’s capacity to repair it [10]. According to estimates made by McCulloch and Kunkel (2008) [11], each day during the normal replication mechanism, each cell experiences between 1000 and 1,000,000 molecular damages. Unrepaired DNA damage has the ability to lead to mutations in somatic or germline cells, which can change both the genotype and phenotype of the cell by impairing protein synthesis’s transcription and translation processes [12]. Alkylating agents, such as hydrazine hydrate, ethyl methane sulphonate (EMS), ethyleneimides, alkyl methane sulphonates, sulphur mustards, methyl methane sulfonate, epoxides, and alkyl nitrosoureas, can be utilized as chemical mutagens [13,14]. In this context, mutagenesis is a highly efficient tool for establishing genetic variation, and it has been widely used for genetic enhancement in many different plants, including; cauliflower (Brassica oleracea) [15], dianthus (Dianthus caryophyllus) [16], chickpea (Cicerarietinum L.) [17], barley (Hordeumvulgare L.) [18], Arabidopsis (Arabidopsis thaliana) [19], Malaysian rice and Korean commercial rice (Oryza sativa) [20,21], and sweet corn (Zea mays) [22].
Salvia officinalis, an important annual medicinal herb of the Lamiaceae family, is extensively cultivated in Europe, the Middle East, Mediterranean areas, Northern Africa, and North Sinai in Egypt. The active pharmaceutical ingredients of salvia species mainly include (1,8-cineole, sabinene, limonene, a-terpineole, ocimene, myrcene, a- and b-pinene, and caryophyllene) [23,24]. As one of the most important popular Egyptian medicinal plants, S. officinalis has been used to treat various diseases because of its antioxidant, choleretic, antihypertension, antitumor, antiulcer, anticancer, antimicrobial, anti-thrombosis, antibacterial, antitumorigenic, anti-inflammatory, and anticoagulant properties [25,26]. Terpenoids or isoprenoids are considered one of the biggest secondary metabolites compounds with various structures and sizes [23,24,27,28]. On the other hand, thousands of terpene and terpenoid compounds, such as hemiterpenes, oxygenated monoterpenes, monoterpene hydrocarbons, sesquiterpene hydrocarbons, oxygenated sesquiterpenes, diterpenes, non-iso-prenoid, and triterpene compounds are derived from mevalonate and non-mevalonate pathways [23,24,29,30,31,32]. Due to terpene compounds being involved in the synthesis of various pharmaceutical ingredients, more attention has been paid to them in different salvia species, such as; Salvia santolinifolia, S. isensis, S. hydrangea, S. epidermidis, S. mirzayanii, S. fruticosa, S. tomentosa, S. officinalis, S. chloroleuca, S. guaranitica, S. lavandulifolia, S. przewalskii, S. japonica, S. macrochlamys, S. allagospadonopsis, S. recognita, S. lavandulaefolia, S. lanigeraPoir., S. glabrescens, S. aureus, S. euphratica, S. tuxtlensis, S. eremophila, S. sclaria, Salvia staminea, Salvia virgata, S. nipponica, and Salvia verbenaca [23,31,32,33].
In this study, we inspected the effect of different concentrations of HZ on the expression levels of various terpene biosynthesis genes and determined the biological effect of HZ on terpene and terpenoid production. These results suggested that each concentration of HZ has various effects on the expression level of every terpene synthases gene and terpene production in S. officinalis plants. The results of our investigation identifieda method of determining the suitable concentration from chemical mutagenesisand analyzed the biological effects of chemical mutagens, which will facilitate the use of chemical mutagens for the improvement of the salvia plant through mutation breeding.
2. Materials and Methods
2.1. Plant Material and Growth Conditions
Seeds of S. officinalis were pre-soaked in distilled water for 12 h, then soaked in four different concentrations (0%, 0.1%, 0.2%, and 0.3%) of HZ solution for 6 h by placing seeds in 10 mm × 100 mm petri plates (about 60 seeds per plate in a single layer), as described by [34,35,36]. The seeds from each treatment were then washed with distilled water three times to remove the traces of the HZ solution. The S. officinalis seeds were then surface sterilized with 75% (v/v) ethanol for 1:30 min and then in 2.5% (v/v) sodium hypochlorite solution for 12 min, thoroughly washed three times with sterilized distilled water, and sown in solid Murashige and Skoog (MS) [37] with pH 5.8 medium containing 30 g L−1 sucrose and 2.5 g L−1 phytagel. The seeds were incubated in the dark for three days then grown at 23 °C under a photoperiod of 8 h dark and 16 h light (110 µmol m−2s−1) in a controlled growth chamber until the plantlets were grown for 6 weeks. After sowing, the percentage of surviving seedlings (survival rate, SR) was investigated.
2.2. RNA Extraction and cDNA Library Preparation
The total RNAs from the three biological plantlets replicates from each S. officinalis line (three plantlets from each treatment) were extracted using the plant TRIzol Reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s protocol. RNA purity was analyzed using a Nano-Photometer® spectrophotometer (IMPLEN, CA, USA), and the quality was examined on 1.4% agarose gels as described previously [23,24,31,38,39]. For quantitative RT-PCR, the first strand of complementary DNA (cDNA) was synthesized from 1 µg total RNA, which was previously treated with DNase I (Takara), using an M-Malva Reverse Transcriptase (RNase H) kit, according to the manufacturer’s protocol. The second strand of cDNA was synthesized in the presence of DNA Polymerase I and Rnase-H, as described previously [23,24,31,38,39].
2.3. Metabolite Extraction from S. officinalis Plantlet after Treatment with Different Concentrationsof HZ
For extraction and analysis of terpenoid compounds from the non-treated (control) and treated S. officinalis plantlets under different concentrations from hydrazine hydrate, we followed the procedures described by Ali et al., 2017, 2018, 2021, 2022, 2022, 2022 [23,24,31,38,39]. In short, the plantlets from each S. officinalis line (three plantlets from each treatment) were collected. Then all collected samples from non-treated (control) and treated S. officinalis plantlets were homogenized in liquid nitrogen and the powder was directly soaked in n-hexane in 60 mL bottles. Then, the bottles were incubated with shaking at 36 °C and 210 rpm for 73 h. Afterward, the solvent was purified using a centrifuge at 5050 rpm for 9 min at 5 °C to remove plant debris. The extract was concentrated and transferred to fresh 1.5 mL crimp neck vial amber glass screw-top vials. The terpenoid content in the extract solution was determined by gas chromatography–mass spectrometer (GC-MS: Shimadzu model GCMS-QP2010 Ultra (Tokyo, Japan) system). Three libraries: NIST Library (2014 edition), Volatile Organic Compounds (VOC) Analysis S/W software, and Wiley GC/MS Library (10th Edition), were used to identify the terpenoid constituents by parallel comparison of terpenoid recorded mass spectra with the data that stored in these previous Libraries [23,24,31,38,39]. All of the experiments were performed simultaneously three times under the same conditions for each isolation technique, with a total GC running time of80 min.
2.4. Quantitative Real-Time PCR (qRT-PCR) Analysis
Quantitative real-time PCR was performed using an IQTM5 Multicolor Real-Time PCR Detection System (Bio-Rad, Agitech, New Cairo Cairo, Egypt) as described previously by Ali et al., 2017 [23], with SYBR Green I Mix-Master (Roche Diagnostics Ltd., Lewes, UK) following the manufacturer’s instructions, with a total reaction volume of 20 µL, andgene-specific primers for SoActin as, a reference gene, and the other twelve genes involved in the biosynthesis of terpenes: SoGPS (geranyldiphosphatesynthase), SoMYRS (myrcene/ocimene synthase), SoNEOD ((+)-neomenthol dehydrogenase), SoCINS (1,8-cineole synthase), SoSABS ((+)-sabinene synthase), SoLINS ((3S)-linalool synthase), SoFPPS (farnesyl pyrophosphate synthase), SoHUMS (a-humulene/b-caryophyllene synthase), SoTPS6 ((−)-germacrene D synthase), SoSQUS (squalenemonooxygenase), SoGGPS (geranylgeranyl pyrophosphate synthase) and SoGA2 (gibberellin 2-oxidase) from S. officinalis. The primers for these previous genes were designed using the primer designing tools of IDTdna (http://www.idtdna.com/scitools/Applications/RealTimePCR/ (accessed on 25 December 2022); primer sequences are listed in (Supplementary Table S1). The quantitative RT-PCR conditions were set as standard conditions: 97 °C for 3:30 min, 36 cycles of amplification (94 °C for 12 s, 58 °C, or 59 °C, or 60 °C for 30 s, and 72 °C for 22 s), and a final extension at 66 °C for 1 min, then to 65 °C for 5 s, and 95 °C for 5 s). The values are means ± SE of the three replicates normalized using SoActin as a reference gene. The relative expression levels were calculated by comparing the cycle thresholds (CTs) of the target genes with that of the reference gene SoActin using the 2−ΔΔCt method [23,24,31,38,39]. The sizes of amplification products were 150–161 bp. The quantified data were analyzed using Bio-Rad IQTM 5 Multicolor Real-Time Manager software. Finally, the relative expression levels of SoGPS, SoMYRS, SoNEOD, SoCINS, SoSABS, SoLINS, SoFPPS, SoHUMS, SoTPS6, SoSQUS, SoGGPS, and SoGA2 genes were detected.
3. Results
3.1. Identification of Terpenoid Compounds from S. officinalis Plantlets under Different Concentrationsof HZ by GC-MS
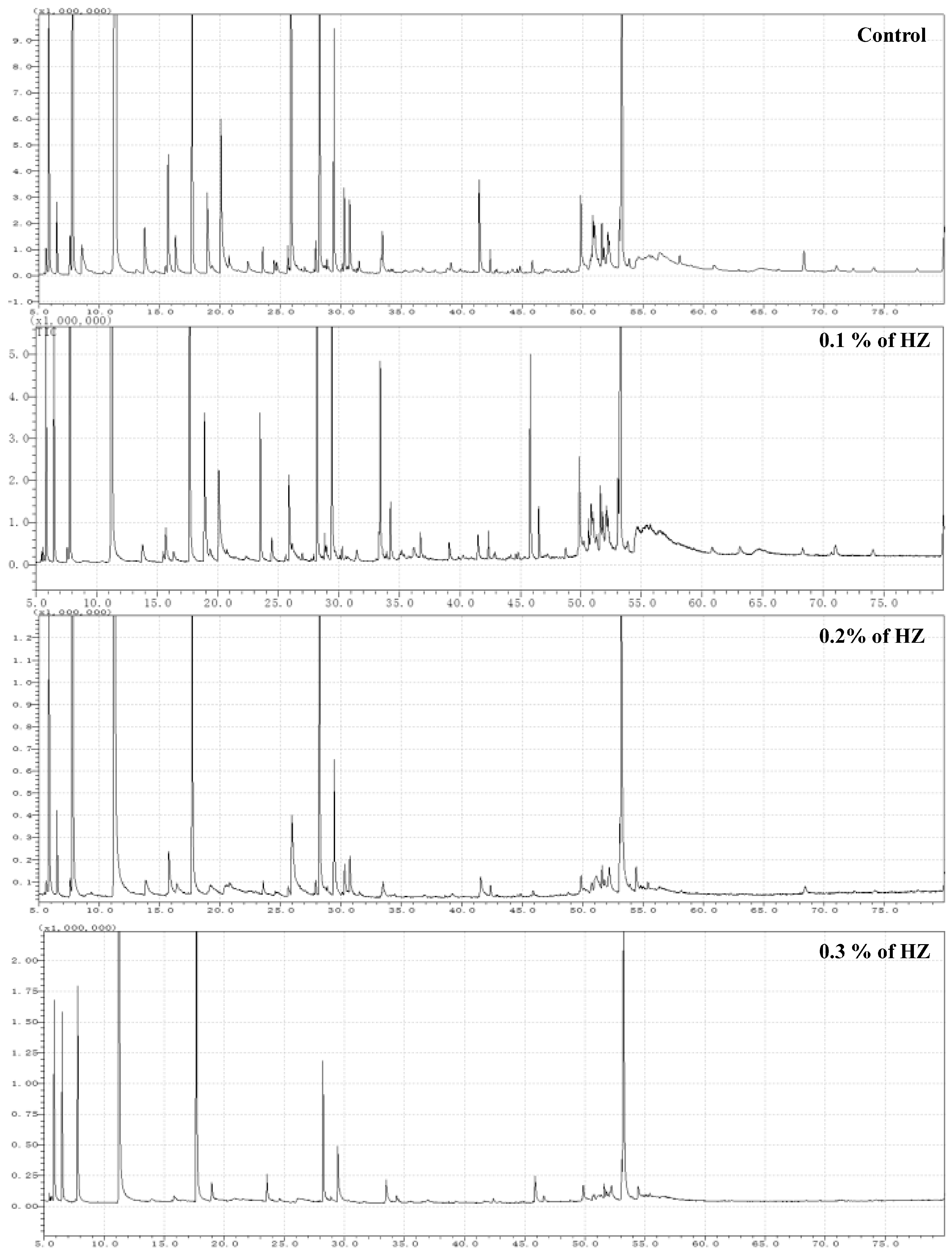
To study the effect of HZ on the percentage of survived seedlings, after 6 weeks from plantlets growth, the SR rate was investigated, and we found an inverse relationship between the HZ concentrations and seedlings’ survival rate, which means the SR dramatically decreased with the increase in HZ concentration, and the percentage of SR rate at different concentrations (0%, 0.1%, 0.2%, and 0.3%) of HZ were 51 seedlings (85%), 42 (70%), 33 (55%), and 18 (30%), respectively. Then, the contents of terpenoid compounds in S. officinalis plantlets after being treated with different concentrations of HZ were determined by GC-MS, and the results are shown in Figure 1 and Table 1. S. officinalis plantlets, after being treated with different concentrations of HZ, produced various levels of mono-, sesquit-, dit-and triterpenes when compared with the control treatment. The numbers of obtained terpenoid and phytochemical compounds from S. officinalis plantlets at different concentrations (0%, 0.1%, 0.2%, and 0.3%) of HZ were 163 (87.48%), 145 (84.49%), 65 (97.45%), and 62 (98.32%), respectively. From the GC-MS analysis, we identified 274 phytochemical compounds using n-hexane extracts from the four S. officinalis plantlets extracts at different concentrations (0.0%, 0.1%, 0.2%, and 0.3%) of HZ. In S. officinalis plantlet extract at 0.0% (control), the monoterpene compounds were shown as the main group (63.3%), followed by the group of sesquiterpene compounds (22.4%) and diterpene compounds (1.78%). At 0.1%, the monoterpene compounds were shown as the main group (52.88%), followed by the group of sesquiterpene compounds (18.13%), then by the group of diterpene compounds (13.41%) and one triterpene compound (0.07%). Monoterpene forms the main group of compounds (78.4%) found in the extract of S. officinalis plantlet at 0.2% concentration, followed by the sesquiterpene group (13.27%), diterpenes group (5.78%). Finally, at 0.3% concentration, the monoterpenes compounds were shown as the main group (74.57%), followed by the diterpenes group (14.44%) and sesquiterpene group (9.31%), as shown in Figure 1 and Table 1.
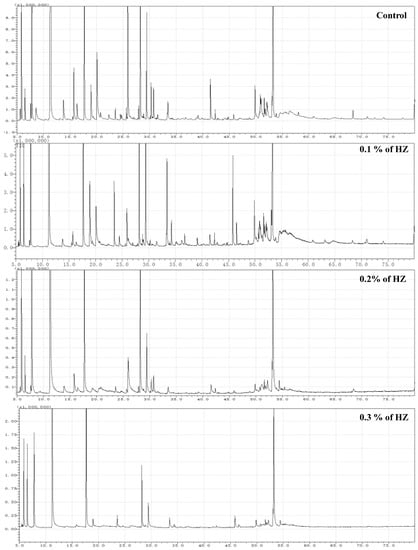
Figure 1.
Typical GC-MS mass spectragraphs for terpenoids from S. officinalis plantlet under the effect of different concentrations (0%, 0.1%, 0.2%, and 0.3%) of HZ.

Table 1.
The major chemical composition of the essential oils of S. officinalis under the effect of different concentrationsof HZ.
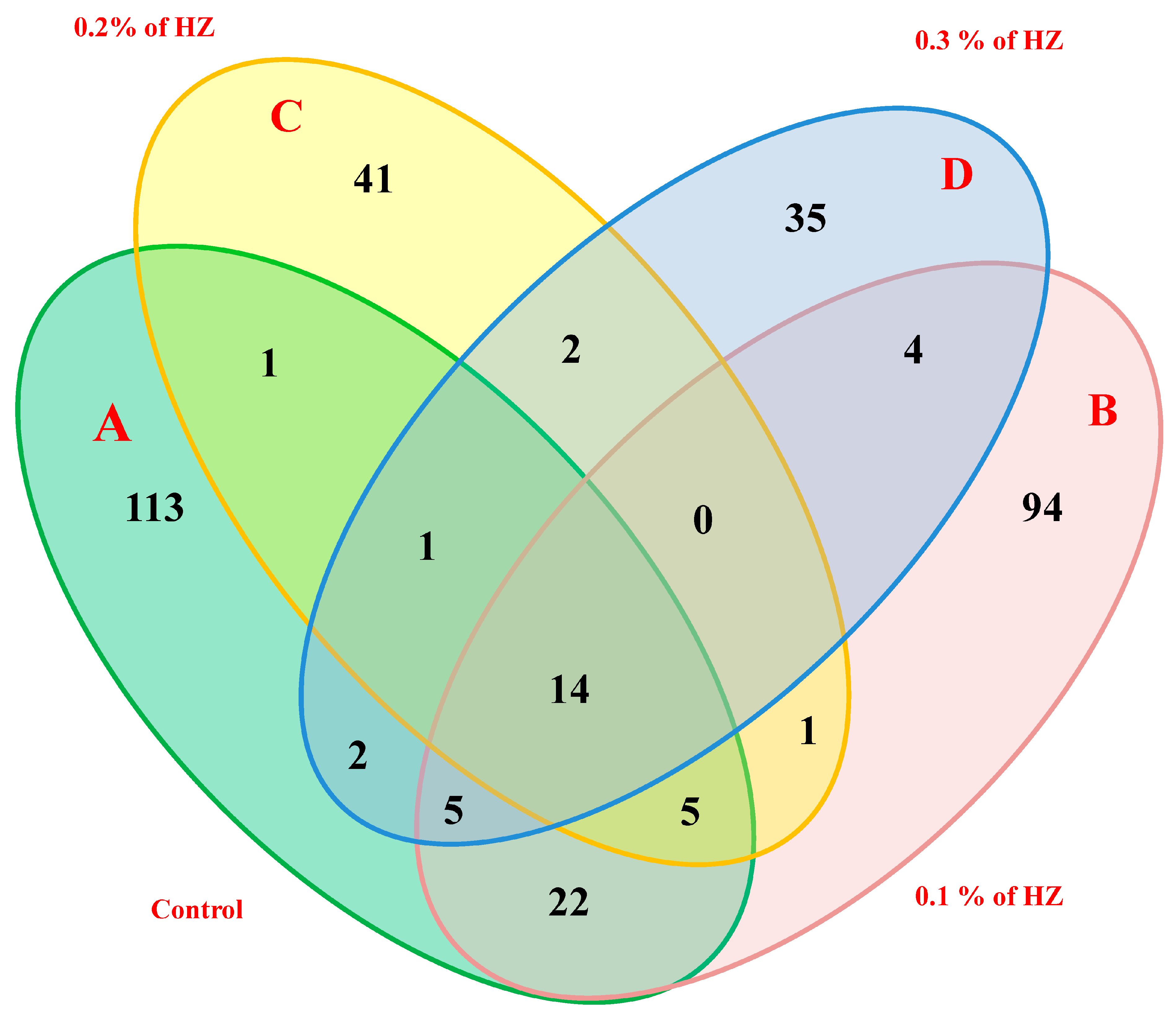
Moreover, the four hexane extracts from the different concentrations (0%, 0.1%, 0.2%, and 0.3%) of HZ have unique, common, and major compounds. For example, the extracts at 0.0% (control) of essential oils (A) had 113 unique compounds, 22 common compounds shared with the extract at 0.1%, 1 common compound shared with the extract at 0.2%, 2 common compounds shared with the extract at 0.3%, and 14 common compounds shared among all 4 HZ concentrations. Furthermore, the extracts at 0.1% concentration (B) contained 94 unique compounds, 1 common compound shared with the extract at 0.2%, and 4 common compounds shared with the extract at 0.3%. In addition, the extracts at 0.2% concentration (C) contained 41 unique, and 2 common compounds shared with the extract at 0.3%. On the other hand, extract at 0.3% (D) contained 35 unique compounds, as reported in (Figure 2).
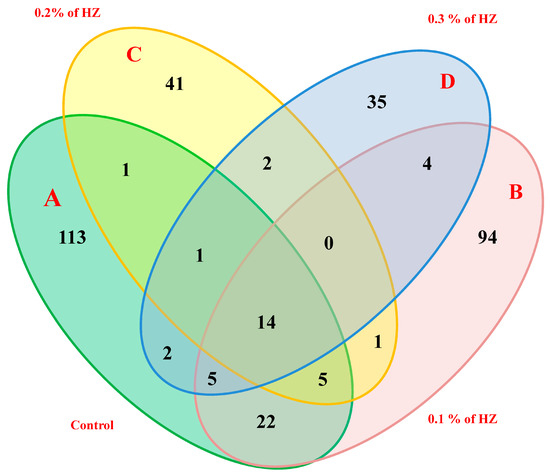
Figure 2.
Four-way Venn diagram to show the number of unique and common compounds in the essential oil extracts from S. officinalis plantlet under the effect of different concentrations of HZ such as; control (A), 0.1% (B), 0.2% (C), and 0.3% (D).
Regarding the major terpenoid compounds, 1,8-cineole (15.63%) was the major compound in the extracts from S. officinalis plantlet at 0.0% concentration, followed by (E)-β-caryophyllene (9.80%), humulene (5.83%), camphor (4.88%), l-2-camphanol e (2.64%), and caryophyllene oxide (1.74%). Whereas the essential oil extract at 0.1% concentration was characterized by 1,8-cineole (35.83%), followed by (E)-β-caryophyllene (9.63%), camphor (8.18%), sugiol (7.76%), α-pinene (3.87%), camphor (2.70%), and humulene (2.46%).
Moreover, 1,8-cineole (47.96%) was the major compound in the extracts from S. officinalis plantlet at 0.2% concentration, followed by (E)-β-caryophyllene (8.69%), camphor (6.77%), podocarpa-8,11,13-trien-7-one,12-hydroxy-13-isopropyl (3.89%), α-pinene (3.81%), and 1,4,7,-cycloundecatriene, 1,5,9,9-tetramethyl-,Z,Z,Z (2.50%). In addition, 1,8-cineole (32.32%) was characterized as the major compound in the extracts from S. officinalis plantlet at 0.3% concentration, followed by camphor (9.75%), podocarpa-8,11,13-trien-7-one,12-hydroxy-13-isopropyl (6.81%), (E)-β-caryophyllene (6.26%), (+)-camphene (6.02%), β-pinene (3.55%), humulene (4.91%), caryophyllene oxide (1.16%), and sugiol (1.06%) (Table 1). On the other hand, we found fourteen common compounds shared among all four extracts, such as (α-thujene, α-pinene, sabinen, β-pinene, 1,8-cineole, p-menth-8-en-1-ol, stereoisomer, camphor, bornyl acetate, (E)-β-caryophyllene, (+)-germacrene D, caryophyllene oxide, trans-biformene, estra-1,3,5(10)-trien-16-one, 3-((trimethylsilyl)oxy), and ferruginol (Table 1)
When the alignment of the terpenoid composition of the four S. officinalis plantlets extracts at different concentrations of HZ, we deduced that some common terpenoid compounds exist at different levels within the four extracts. Therefore, we propose that different concentrations of HZ have a major effect on the kind and level of terpenoid composition in their extract. An important query has been prompted by these data: How does the accumulation of the terpenoid composition in the S. officinalis plantlets change depending on the HZ concentration? Before beginning our research, it was difficult to address this question because there was less information at the molecular genetics level regarding the effect of different concentrations of HZ on the terpenoid biosynthetic in S. officinalis plantlets.
3.2. Overexpressing Terpenoid and Terpene Biosynthesis Genes under the Effect of Different Concentrations of HZ
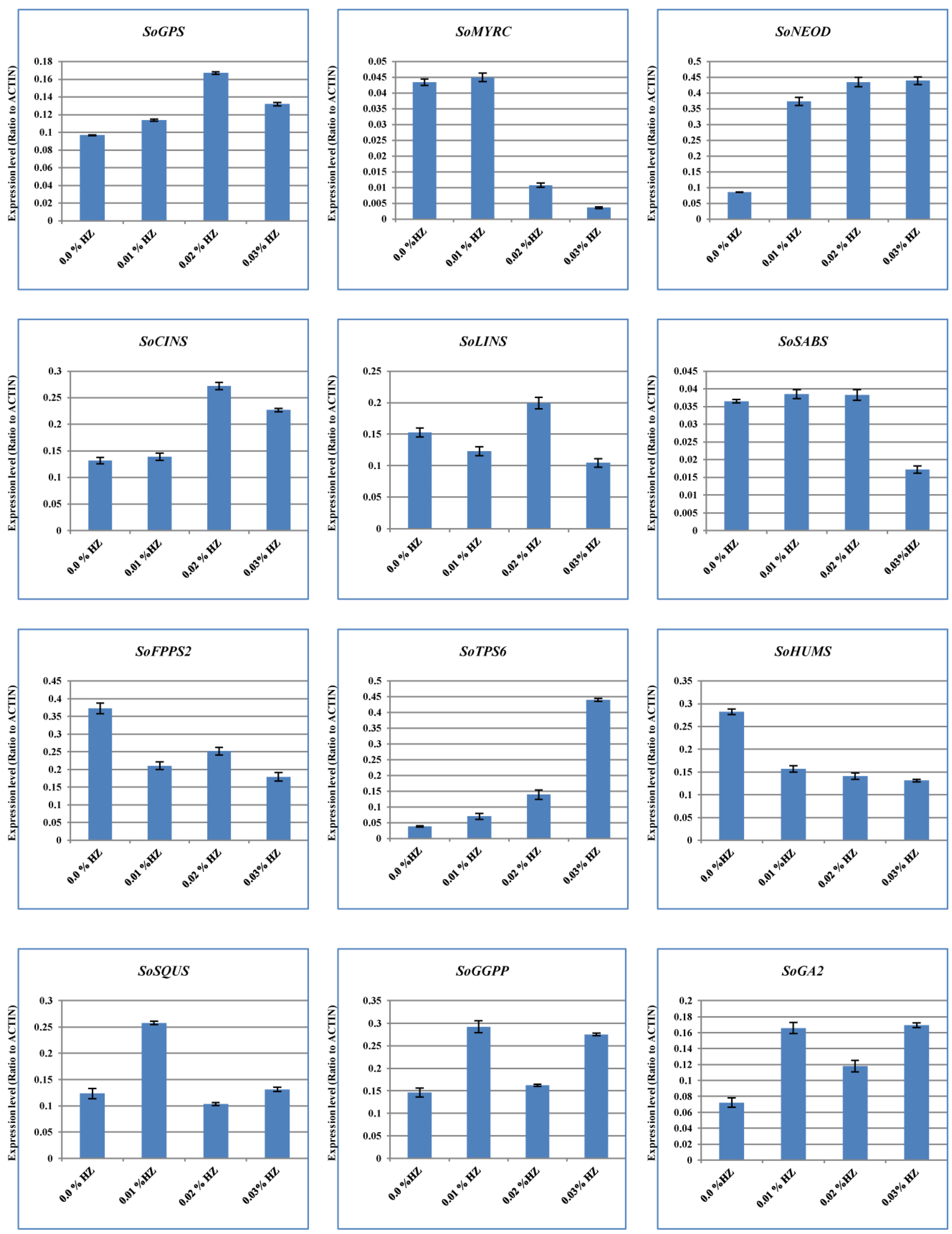
To unveil the effects of different concentrations of HZ on various terpenoid and terpene genes expression, we used the qRT-PCR system to measure the level of expression. The results showed that the expression patterns of our candidate genes at different concentrations of HZ (e.g., 0.1%, 0.2%, and 0.3%) were detected, and their expression profiles were compared with the control (0.0% HZ) (Figure 3). For example, the expression levels of SoFPPS and SoHUMS were the highest under the effect of HZ at 0.0% concentration. Moreover, the highest expression levels for SoMYRS, SoSABS, SoSQUS, and SoGGPS were observed at a concentration of 0.1% of HZ. On the other hand, the expression of SoGPS, SoCINS, and SoLINS were the highest in HZ at a concentration of 0.2%. Furthermore, the highest expression levels for SoNEOD, SoTPS6, and SoGA2 were detected in a concentration of 0.3% HZ (Figure 3). Therefore, different concentrations of HZ may have an impact on the level of gene expression for genes involved in terpenoid and terpene biosynthesis, according to differences in expression patterns for these genes.
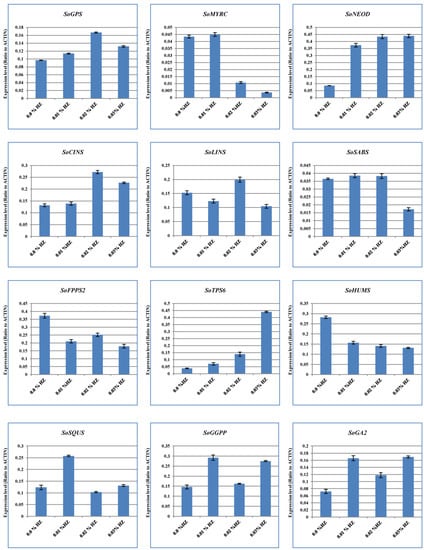
Figure 3.
Quantitative RT-PCR validation of expression of terpene synthase genes selected from S. officinalis plantlet under the effect of different concentrations of HZ (0%, 0.1%, 0.2%, and 0.3%). Total RNAs were extracted from the previous concentrations, and the expression of SoGPS, SoMYRS, SoNEOD, SoCINS, SoSABS, SoLINS, SoFPPS, SoHUMS, SoTPS6, SoSQUS, SoGGPS, and SoGA2 genes were analyzed using quantitative real-time. SoACTIN was used as the internal reference. The values are means ± SE of three biological replicates.
4. Discussion
4.1. Validation of the Relationship between the Type and Amount of Terpenoid and Gene Expression under Different Concentrations of HZ
We used the qRT-PCR system to reveal the “transcriptional control”, which represents the natural link between the “number of mRNA copies” and “the number of copies of enzyme”, which follow the end-product quantity, in order to ascertain the relationship between the type and amount of terpenoid that was produced under the effect of different concentrations of HZ and gene expression. Therefore, the expression patterns of our twelve candidate genes with various expression levels were detected, and their quantity expression profiles were compared with our GC-MS analysis data. Therefore, according to the gene’s expression levels and the findings of our GC-MS analysis, we found that the SoGPS gene showed the highest expression levels at 0.2% concentration, followed by 0.3%, 0.1%, and 0.0% of HZ, and these qRT-PCR results are in line with our GC-MS analysis data, which indicate that the main group of monoterpene compounds was observed at 0.2% concentration, followed by 0.3%, 0.0%, and 0.1% of HZ. Moreover, the highest expression levels for the SoFPPS2 gene were observed at a concentration of 0.0% of HZ, followed by 0.2%, 0.1%, and 0.3%. In this context, the most abundant sesquiterpene compounds group was detected at a concentration of 0.0% of HZ, followed by 0.1%, 0.2%, and 0.3%.
Additionally, we discovered a favorable link between the gene expression levels of 1, 8-cineole synthase at various concentrations of HZ. For instance, the higher of the 1, 8-cineole synthase gene product and expression level presented at 0.2% concentration, followed by 0.3%, 0.1%, and 0.0%of HZ. In addition, we found an engagement between the (+)-germacrene-D product and germacrene-D-synthase (SoTPS-6):the highest product and gene expression were discovered at a concentration of 0.3% of HZ, followed by 0.2%, 0.1%, and 0.0%. Likewise, there is a linkage detected between the (E)-β-caryophyllene, gamma-caryophyllene, humulene, caryophyllene oxide, and 1,2-humulene epoxide as a product and the expression of humulene synthase (SoHUM) gene at different concentrations of HZ. Our findings concur with those of other researchers and our earlier research [23,24,31,38,39,40,41,42,43,44,45,46,47], which discovered and discussed a connection between gene expression and the end product, which gives the impression that the production of the terpene compounds under study can be controlled by the gene transcription process.
On the other hand, we discovered that some genes (e.g., SoMYRS, SoSABS, SoSQUS, SoNEOD, SoLINS, and SoGA2) fluctuate in their gene expression level, and also, some terpene compounds (e.g., camphene, geranylisobutyrate, cajeputol, Cis-β-terpineo, thujan-3-one, thujone, l-2-camphanol, ledene, elemene, labda-8(20),14-dien-13-ol, (13R)-, and labda-8(20),14-dien-13-ol, (13R)-) were not detected or were detected in quantities that are not commensurate with the expression levels of their genes under the influence of some concentrations of the HZ. There are numerous explanations for this. First, the expression of some terpene synthase genes may be controlled by the cell’s circadian rhythm. Secondly, the fact that there is a positive correlation between transcript levels and terpene emission which suggests that changes in transcript level are an important determinant of terpene production, even though changes in transcript levels might not directly affect protein levels or enzyme activities due to potential posttranscriptional, post-translational, or enzyme-regulatory mechanisms. Third, the reason why some terpene compounds do not appear may be due to the different rates of protein synthesis, protein modifications, protein degradation, and protein proteolytic turnover. Additionally, by converting some compounds to other compounds through oxidation or glycosylation of monoterpene olefins and sequestration [23,24,48,49]
4.2. Assessment of the Effects of Hydrazine Hydrate on the Terpene Genes Expression and Terpenoid Production
Hydrazine hydrate (N2H4 × H2O) is an important inorganic compound, which is mainly used in agrochemicals as a foaming agent [50]. Hydrazine hydrate is considered as one of the alkylating agents (e.g., ethyl methane sulphonate (EMS), ethyleneimides, alkyl methane sulphonates, sulfur mustards, methyl methane sulfonate, epoxides, and alkyl nitrosoureas), which can be used as chemical mutagens [13,14]. Chemical mutagens have been widely used to alter plants’ genetic makeup in various ways, such as: changing the chemistry of the base pairs; nucleotides, and confusing the DNA replication machinery; stripping DNA nucleotides from the essential modifications; in sertion or deletion of some extra base pairs through a round of DNA replication; and two DNA nucleotides cross-linking together, introducing a single base pair (SNPs) [51]. Moreover, the chemical mutagens are usually high in induction and more applicable in the in-vitro compared to physical and radiation approaches [52,53,54,55]. Cells generally attempt to fix these mutations via the cell cycle checkpoints by nucleotide excision repair (NER) and base excision repair (BER) for the eradication of damaged bases and the repair of nucleotides, respectively [52]. Furthermore, in some cases, the disruption of the DNA nucleotide mutation repair mechanism could create mutations in the genetic makeup, which can alter the gene expression and encoding protein and create genetic variability for increased crop productivity through crop improvements, such as that seen in Mung bean (Phaseolus aureus Roxb.) [51,56]; for example, EMS, which belongs to the alkylating agents, and is used as a chemical mutagen in plants. EMS has the ability to induce GC → AT transitions in the genomic DNA, which results in mutant proteins that perform alternate roles to those of the normal protein [51]. Additionally, it has been demonstrated that the application of alkylating agents such as EMS and HZ is a practical and efficient way to develop distinctive gene pools in plants [34,35,36,51,56]. Therefore, we can assume that the alkylating compounds are the ones that are used the most commonly use dto cause point mutations, a sort of genetic modification in which only one nucleotide base from an organism’s DNA or RNA sequence is changed, added, or removed [57]. According to the type of point mutation, these changes can have a variety of effects on protein function, composition, and synthesis, ranging from positive effects (synonymous mutations) to negative effects (nonsynonymous mutations) [58,59]. In this context, these mutations can lead to different effects on the differential protein expression levels, such as removing or adding stop codon, which causes the translated protein to be abnormally extended or shortened, change in chemical and physical properties of the amino acids, protein may lose its function and may exhibit a new function or become activated [60,61]. For example, in Yokoyama et al.’s 2022, 2021 study on the stability of mutations in aromatic amino acid (AAAs) compounds from A. thaliana seeds, that were mutagenized using EMS [62,63], they isolated a total of 351 suppressor of tyra2 (sota) mutants that lacked one of two TyrA genes that were associated with Tyr biosynthesis—used as a common substrate for the shikimate pathway [62]. This kind of mutant showed an increase in AAAs compared with other amino acids at F1 and F2 of the plant population with dominant or semidominant characteristics, and was accompanied by an increase in net CO2 assimilation and flux through the shikimate pathway [62,63,64,65]. These results provide genetic evidence that the induction of point mutations using chemical mutagens has the ability to enhance plant metabolic levels in a dominant fashion.
5. Conclusions
The application of chemical mutagens on crops is an easy and effective method for the improvement of various agronomic traits. Seeds from many different plants have been widely used in investigations as the initial materials to produce plant mutations via chemical mutagenesis. There have been fewer studies about the effect of chemical mutagens on medicinal plants because it is difficult to determine the biological and molecular genetic effects of a chemical mutagen on the seeds and plantlets of these plants. This investigation was carried out in order to comprehend the potential impacts of HZ on S. officinalis terpenoids and terpene synthesis genes. The goal of the study was to shed light on the effects of four HZ concentrations on the expression levels of the genes involved in terpene synthesis, as well as terpene and terpenoid biosynthesis. The acquired results showed that varied HZ concentrations considerably raised the percentage of most monoterpenes, sesquiterpenes, and diterpenes while increasing the expression levels of twelve terpene genes. The findings of our study open the door to additional research on the use of chemical mutagens to improve a variety of features in medicinal and aromatic plants without changing their genetic makeup. This study might be widely applicable toother salvia species or other genera that principally belong to the Lamiaceae family plants.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/metabo13070807/s1, Table S1: List of S. officinalis genes and primer pairs used for qRT-PCR.
Author Contributions
M.A. conceived and designed the study; M.A., D.B.E.D., A.M.A., H.A.A., D.A., H.A.-A. and F.A.S. performed the experiments, A.M.A. performed the plant tissue culture experiment, and M.A. wrote the draft paper and GC-MS data analyses. M.A., D.B.E.D., A.M.A., H.A.A., D.A., H.A.-A. and F.A.S. reviewed the final draft of the manuscript. All authors discussed the results and commented on the manuscript, and participated in the analysis of the data. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data supporting the findings are available and found in the supplementary data.
Acknowledgments
This work is under the activity of the Maryout Research Station, Genetic Resources Department, Desert Research Center (DRC), Egypt. Thanks and appreciation to Mahmoud El-Habbak at the Department of Economics and Mohamed El-Habbak at the Department of Plant Pathology, Benha University, for polishing and proofreading our manuscript. Special thanks are given to Ahmed Ali at the Department of Plant Agricultural, Faculty of Agriculture Science, Al-Azhar University, Assiut, Egypt, for their valuable support. We also owe thanks to Mohamed Hamdy Amar and Wael Moussa at the Desert Research Center (DRC) for constructive comments and help. We also acknowledge Ali Abd El-hammed, Azza Mohammed Mutawa, and Samah Elsayed Ali for their constructive comments and help.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Pathirana, R. Plant mutation breeding in agriculture. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2011, 6, 107–126. [Google Scholar] [CrossRef]
- Shu, Q.Y.; Forster, B.P.; Nakagawa, H.; Nakagawa, H. (Eds.) Plant Mutation Breeding and Biotechnology; CABI: Wallingford, UK, 2012. [Google Scholar]
- Oladosu, Y.; Rafii, M.Y.; Abdullah, N.; Hussin, G.; Ramli, A.; Rahim, H.A.; Miah, G.; Usman, M. Principle and application of plant mutagenesis in crop improvement: A review. Biotechnol. Biotechnol. Equip. 2016, 30, 1–6. [Google Scholar] [CrossRef]
- Kostov, K.; Batchvarova, R.; Slavov, S. Application of chemical mutagenesis to increase the resistance of tomato to Orobancheramosa L. Bulg. J. Agric. Sci. 2007, 13, 505–513. [Google Scholar]
- Ragvendra, T.; Suresh, B.G.; Mishra, V.K.; Ashutosh, K.; Ashok, K. Genetic variability and character association in direct seeded upland rice (Oryza sativa). Environ. Ecol. 2011, 29, 2132–2135. [Google Scholar]
- Sumanth, V.; Suresh, B.G.; Ram, B.J.; Srujana, G. Estimation of genetic variability, heritability and genetic advance for grain yield components in rice (Oryza sativa L.). J. Pharmacogn. Phytochem. 2017, 6, 1437–1439. [Google Scholar]
- Kharkwa, M.C. A Brief History of Plant Mutagenesis Plant Mutation Breeding and Biotechnology; Shu, Q.Y., Forster, B.P., Nakagawa, H., Eds.; Food and Agriculture Organization of the United Nations: Vienna, Austria, 2012; pp. 21–30. [Google Scholar]
- El-Degwy, I.S. Mutation induced genetic variability in rice (Oryza sativa L.). Int. J. Agric. Crop Sci. 2013, 5, 2789–2794. [Google Scholar]
- Dewi, A.K.; Dwimahyani, I. Application of induced mutation technique to improve genetic variability of Indonesian traditional rice varieties. IOP Conf. Ser. Earth Environ. Sci. 2020, 482, 012016. [Google Scholar] [CrossRef]
- Manova, V.; Gruszka, D. DNA damage and repair in plants—From models to crops. Front. Plant Sci. 2015, 6, 885. [Google Scholar] [CrossRef]
- McCulloch, S.D.; Kunkel, T.A. The fidelity of DNA synthesis by eukaryotic replicative and translesion synthesis polymerases. Cell Res. 2008, 18, 148–161. [Google Scholar] [CrossRef]
- Polyn, S.; Willems, A.; De Veylder, L. Cell cycle entry, maintenance, and exit during plant development. Curr. Opin. Plant Biol. 2015, 23, 1–7. [Google Scholar] [CrossRef]
- Auerbach, C.; Robson, J.M. Chemical Production of Mutations. Nature 1984, 157, 302. [Google Scholar] [CrossRef] [PubMed]
- Till, B.J.; Cooper, J.; Tai, T.H.; Colowit, P.; Greene, E.A.; Henikoff, S.; Comai, L. Discovery of chemically induced mutations in rice by TILLING. BMC Plant Biol. 2007, 7, 19. [Google Scholar] [CrossRef]
- Ke, C.; Guan, W.; Bu, S.; Li, X.; Deng, Y.; Wei, Z.; Wu, W.; Zheng, Y. Determination of absorption dose in chemical mutagenesis in plants. PLoS ONE 2019, 14, e0210596. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, R.; Tah, J. Chemical mutagenic action on seed germination and related agro-metrical traits in M1 Dianthus generation. Curr. Bot. 2011, 2, 19–23. [Google Scholar]
- Wani, A.A. Mutagenic effectiveness and efficiency of Gamma rays, Ethyl Methane Sulphonate and their combination treatments in Chickpea (Cicer arietinum L.). Asian J. Plant Sci. 2009, 8, 318–321. [Google Scholar] [CrossRef]
- Caldwell, D.G.; McCallum, N.; Shaw, P.; Muehlbauer, G.; Marshall, D.F.; Waugh, R. A structured mutant population for forward and reverse genetics in Barley (Hordeum vulgare L.). Plant J. 2004, 40, 143–150. [Google Scholar] [CrossRef]
- Jander, G.; Baerson, S.R.; Hudak, J.A.; Gonzalez, K.A.; Gruys, K.J.; Last, R.L. Ethylmethanesulfonate saturation mutagenesis in Arabidopsis to determine frequency of herbicide resistance. Plant Physiol. 2003, 131, 139–146. [Google Scholar] [CrossRef]
- Talebi, A.B.; Shahrokhifar, B. Ethyl methane sulphonate (EMS) induced mutagenesis in malaysian rice (cv. MR219) for lethal dose determination. Am. J. Plant Sci. 2012, 3, 1661–1665. [Google Scholar] [CrossRef]
- Lee, D.K.; Kim, Y.S.; Kim, J.K. Determination of the optimal condition for ethylmethanesulfonate-mediated mutagenesis in a Korean commercial rice, Japonica cv. Dongjin. Appl. Biol. Chem. 2017, 60, 241–247. [Google Scholar] [CrossRef]
- Rajapandian, P.; Dhanam, S. Utilization of physical and chemical mutagenesis on germination studies of sweet corn Zea mays (L.). Int. J. Res. Bot. 2017, 7, 1–5. [Google Scholar]
- Ali, M.; Li, P.; She, G.; Chen, D.; Wan, X.; Zhao, J. Transcriptome and metabolite analyses reveal the complex metabolic genes involved in volatile terpenoid biosynthesis in garden sage (Salvia officinalis). Sci. Rep. 2017, 7, 16074. [Google Scholar] [CrossRef]
- Ali, M.; Hussain, R.M.; Rehman, N.U.; She, G.; Li, P.; Wan, X.; Guo, L.; Zhao, J. De novo transcriptome sequencing and metabolite profiling analyses reveal the complex metabolic genes involved in the terpenoid biosynthesis in Blue Anise Sage (Salvia guaranitica L.). DNA Res. 2018, 25, 597–617. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.P.; Wang, Z.Z.; Tian, W.; Dong, Z.M.; Spencer, D.F. Generation and analysis of expressed sequence tags from the medicinal plant Salvia miltiorrhiza. Life Sci. 2010, 53, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Atsuko, T.; Hiroshi, O. Phylogenetic relationships among subgenera, species, and varieties of Japanese Salvia L. (Lamiaceae). J. Plant Res. 2011, 124, 245–252. [Google Scholar]
- Liu, J.; Huang, F.; Wang, X.; Zhang, M.; Zheng, R.; Wang, J.; Yu, D. Genome-wide analysis of terpene synthases in soybean: Functional characterization of GmTPS3. Gene 2014, 544, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Srividya, N.; Davis, E.M.; Croteau, R.B.; Lange, B.M. Functional analysis of (4S)-limonene synthase mutants reveals determinants of catalytic outcome in a model monoterpene synthase. Proc. Natl. Acad. Sci. USA 2015, 112, 3332–3337. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Wang, H.; Hu, X.; Sun, Z.; Han, C. The Pharmacological Properties of Salvia Essential Oils. J. Appl. Pharm. Sci. 2013, 3, 122–127. [Google Scholar] [CrossRef]
- Halfmann, C.; Gu, L.; Gibbons, W.; Zhou, R. Genetically engineering cyanobacteria to convert CO₂, water, and light into the long-chain hydrocarbon farnesene. Appl. Microbiol. Biotechnol. 2014, 98, 9869–9877. [Google Scholar] [CrossRef]
- Ali, M.; Miao, L.; Hou, Q.; Darwish, D.B.; Alrdahe, S.S.; Ali, A.; Benedito, V.A.; Tadege, M.; Wang, X.; Zhao, J. Overexpression of Terpenoid Biosynthesis Genes From Garden Sage (Salvia officinalis) Modulates Rhizobia Interaction and Nodulation in Soybean. Front Plant Sci. 2021, 12, 783269. [Google Scholar] [CrossRef]
- Ali, M.; Alshehri, D.; Alkhaibari, A.M.; Elhalem, N.A.; Darwish, D.B.E. Cloning and Characterization of 1,8-Cineole Synthase (SgCINS) Gene From the Leaves of Salvia guaranitica Plant. Front Plant Sci. 2022, 13, 869432. [Google Scholar] [CrossRef]
- Laura, P.; Barbara, R.; Barberini, S. Molecular cloning of SoHPPR encoding a hydroxyphenylpyruvate reductase, and its expression in cell suspension cultures of Salvia officinalis. Plant Cell Tissue Organ Cult. 2013, 114, 131–138. [Google Scholar]
- Khan, I.A. Mutation studies in mung bean (Phaseolus aureus Roxb.). VI estemates of Genetic variability. Bot. Bull. Acad. Sin. 1983, 24, 121–128. [Google Scholar]
- Khan, I.A. Mutations induced by gamma-irradiation, ethyl methane sulfonate and hydrazine hydrate in mung bean (Phaseolus aureus Roxb.). Bot. Bull. Acad. Sin. 1984, 25, 103–110. [Google Scholar]
- Khan, I.A. Quantitative variation induced by gamma rays, ethyl methane sulphonate, and hydrazine hydrate in mung bean (Phaseolus aureus Roxb.). Can. J. Genet. Cytol. 1984, 26, 492–496. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Plant Physiol. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Ali, M.; Nishawy, E.; Ramadan, W.A.; Ewas, M.; Rizk, M.S.; Sief-Eldein, A.G.M.; El-Zayat, M.A.S.; Hassan, A.H.M.; Guo, M.; Hu, G.W.; et al. Molecular characterization of a Novel NAD+-dependent farnesol dehydrogenase SoFLDH gene involved in sesquiterpenoid synthases from Salvia officinalis. PLoS ONE 2022, 17, e0269045. [Google Scholar] [CrossRef]
- Ali, M.; Miao, L.; Soudy, F.A.; Darwish, D.B.E.; Alrdahe, S.S.; Alshehri, D.; Benedito, V.A.; Tadege, M.; Wang, X.; Zhao, J. Overexpression of Terpenoid Biosynthesis Genes Modifies Root Growth and Nodulation in Soybean (Glycine max). Cells 2022, 11, 2622. [Google Scholar] [CrossRef]
- Dudareva, N.; Cseke, L.; Blanc, V.M.; Pichersky, E. Evolution of floral scent in Clarkia: Novel patterns of S-linalool synthase gene expression in the Clarkia breweri flower. Plant Cell 1996, 8, 1137–1148. [Google Scholar]
- McConkey, M.E.; Gershenzon, J.; Croteau, R.B. Developmental regulation of monoterpene biosynthesis in the glandular trichomes of peppermint. Plant Physiol. 2000, 122, 215–224. [Google Scholar] [CrossRef]
- Mahmoud, S.S.; Croteau, R.B. Menthofuran regulates essential oil biosynthesis in peppermint by controlling a downstream monoterpene reductase. Proc. Natl. Acad. Sci. USA 2003, 100, 14481–14486. [Google Scholar] [CrossRef]
- Mahmoud, S.S.; Williams, M.; Croteau, R. Cosuppression oflimonene-3-hydroxylase in peppermint promotes accumulation of limonene in the essential oil. Phytochemistry 2004, 65, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Kapteyn, J.; Gang, D.R. A systems biology investigation of the MEP/terpenoid and shikimate/phenylpropanoid pathways points to multiple levels of metabolic control in sweet basil glandular trichomes. Plant J. 2008, 54, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.; Boecklemann, A.; Woronuk, G.N.; Sarker, L.; Mahmoud, S.S. A genomics resource for investigating regulation of essential oil production in Lavandula angustifolia. Planta 2010, 231, 835–845. [Google Scholar] [CrossRef]
- Schmiderer, C.; Grausgruber-Gröger, S.; Grassi, P.; Steinborn, R.; Novak, J. Influence of gibberellin and daminozide on the expression of terpene synthases in common sage (Salvia officinalis). J. Plant Physiol. 2010, 167, 779–786. [Google Scholar] [CrossRef]
- Kampranis, S.; Ioannidis, D.; Purvis, A.; Mahrez, W.; Ninga, E.; Katerelos, N.A.; Anssour, S.; Dunwell, J.M.; Degenhardt, J.; Makris, A.M.; et al. Rational conversion of substrate and product specificity in a Salvia monoterpene synthase: Structural insights into the evolution of terpene synthase function. Plant Cell. 2007, 19, 1994–2005. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Sangita, K.; Piyush, P.; Gopal, M.; Gitanjali, Y. Structural and biochemical perspectives in plant isoprenoid biosynthesis. Phytochem. Rev. 2013, 12, 255–291. [Google Scholar]
- Tsubakizaki, S.; Takada, M.; Gotou, H.; Mawatari, K.; Ishihara, N.; Kai, R. Alternatives to Hydrazine in Water Treatment at Thermal Power Plants. Mitsubishi Heavy Ind. Tech. Rev. 2009, 6, 43–47. [Google Scholar]
- SaeedAwan, F.; Sadia, B.; Altaf, J.; Habib, M.; Hameed, K.; Hussain, S. Genetic Variability through Induced Mutation. In Genetic Variation; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Richa Kumar, R.; Tyagi, M.B.; Sinha, R.P. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucleic Acids 2010, 2010, 592980. [Google Scholar] [CrossRef]
- Acquaah, G. Principles of Plant Genetics and Breeding, 3rd ed.; Wiley-Blackwell: Chichester, UK, 2006; ISBN 978-1-119-62632-9. [Google Scholar]
- Wu, J.L.; Wu, C.; Lei, C.; Baraoidan, M.; Bordeos, A.; Madamba, M.R.; Ramos-Pamplona, M.; Mauleon, R.; Portugal, A.; Ulat, V.J.; et al. Chemical-and irradiation-induced mutants of indica rice IR64 for forward and reverse genetics. Plant Mol. Biol. 2005, 59, 85–97. [Google Scholar] [CrossRef]
- Viana, V.E.; Pegoraro, C.; Busanello, C.; de Oliveira, A.C. Mutagenesis in rice: The basis for breeding a new super plant. Front. Plant Sci. 2019, 10, 1326. [Google Scholar] [CrossRef] [PubMed]
- Ralston, A. Environmental mutagens, cell signalling and DNA repair. Nat. Educ. 2008, 1, 114. [Google Scholar]
- Li, M.; Goncearenco, A.; Panchenko, A.R. Annotating Mutational Effects on Proteins and Protein Interactions: Designing Novel and Revisiting Existing Protocols. Proteom. Methods Protoc. 2017, 1550, 235–260. [Google Scholar]
- Forner, J.; Kleinschmidt, D.; Meyer, E.H.; Fischer, A.; Morbitzer, R.; Lahaye, T.; Schöttler, M.A.; Bock, R. Targeted introduction of heritable point mutations into the plant mitochondrial genome. Nat. Plants 2022, 8, 245–256. [Google Scholar] [CrossRef]
- Lin, M.T.; Orr, D.J.; Worrall, D.; Parry, M.A.J.; Carmo-Silva, E.; Hanson, M.R. A procedure to introduce point mutations into the Rubisco large subunit gene in wild-type plants. Plant J. 2021, 106, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Ikenoue, T.; Hikiba, Y.; Kanai, F.; Aragaki, J.; Tanaka, Y.; Imamura, J.; Imamura, T.; Ohta, M.; Ijichi, H.; Tateishi, K.; et al. Different effects of point mutations within the B-Raf glycine-rich loop in colorectal tumors on mitogen-activated protein/extracellular signal-regulated kinase kinase/extracellular signal-regulated kinase and nuclear factor κB pathway and cellular transformation. Cancer Res. 2004, 64, 3428–3435. [Google Scholar]
- Yokoyama, R.; de Oliveira, M.V.V.; Takeda-Kimura, Y.; Ishihara, H.; Alseekh, S.; Arrivault, S.; Kukshal, V.; Jez, J.M.; Stitt, M.; Fernie, A.R.; et al. Point mutations that boost aromatic amino acid production and CO2 assimilation in plants. Sci. Adv. 2022, 8, eabo3416. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, R.; de Oliveira, M.V.V.; Kleven, B.; Maeda, H.A. The entry reaction of the plant shikimate pathway is subjected to highly complex metabolite-mediated regulation. Plant Cell 2021, 33, 671–696. [Google Scholar] [CrossRef]
- Westfall, C.S.; Xu, A.; Jez, J.M. Structural evolution of differential amino acid effector regulation in plant chorismite mutases. J. Biol. Chem. 2014, 289, 28619–28628. [Google Scholar] [CrossRef]
- Schenck, C.A.; Chen, S.; Siehl, D.L.; Maeda, H.A. Non-plastidic, tyrosine-insensitive prephenate dehydrogenases from legumes. Nat. Chem. Biol. 2015, 11, 52–57. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).