1. Introduction
There are numerous terms involved in the labeling of the increased production of ketone bodies. For clarity, in this manuscript, the production of ketone bodies by the liver is referred to as ketogenesis, which is a continuous, naturally occurring state that can vary in magnitude and severity. There are three recognized ketone bodies, acetoacetate (AcAc), β-hydroxybutyrate (β-HB), and acetone. The blood concentrations of each ketone body can be highly variable during different rates of ketogenesis (data provided are for normal vs. elevated conditions, respectively β-HB = 0.05 vs. 23 mmol/L, AcAc = 0.05 vs. 2.0 mmol/L, acetone = 0.05 vs. 11.00 mmol/L) [
1,
2,
3]. The oxidation of ketone bodies is referred to as ketolysis, and the more general reference to elevated ketone bodies involving combined ketogenesis and ketolysis is referred to as ketosis.
The first documented discovery of ketone bodies can be traced back to the latter part of the 19th century when they were discovered in the urine samples of patients in diabetic coma [
4]. Due to the limited scientific technology and understanding of that time, the associated presence of ketone bodies in urine during such dire clinical conditions with high mortality rates set strong foundations for ketone bodies to be viewed as toxic by-products of lipid oxidation. This nefarious status of ketone bodies remained for over 100 years until 1966, when Cahill et al. [
5] first proposed that a benefit of ketosis could be the use of ketone bodies as an alternative to glucose to fuel brain metabolism.
During conditions of prolonged restricted cellular glucose uptake, such as diabetes mellitus or the reduced intake of carbohydrates (severe caloric restriction), the human brain transitions predominantly from glucose-derived ATP production to ketone-body-derived ATP production [
3]. This critical survival process is a consequence of not only restricted cellular glucose uptake or availability but also the human brain’s inability to directly utilize fatty acids as an energy source. While ketosis occurs continuously to a small extent, even during times of fully functioning glucose uptake (plasma ketone levels 0.05–0.5 mmol/L), ketogenesis is dramatically increased during times of glucose restriction or deficiency (plasma ketone levels > 3.0 mmol/L) [
3,
6,
7,
8]. During these conditions, coincident to a decrease in glycolytic flux, is an increased mobilization and transport of fatty acids to the liver to undergo β-oxidation, thereby increasing acetyl-CoA production to such an extent that excess acetyl CoA is converted to ketone bodies to prevent depletion of free CoA [
3,
5]. These ketone bodies can then be transported to the brain and other tissues as an alternative fuel source to glucose for oxidation and ATP production [
7]. While acute, low-level elevations in ketone bodies are not considered dangerous; for example, ketogenic diets are designed specifically to induce small to moderate elevations in the blood concentrations of ketone bodies; severe ketosis can result in conditions, such as diabetic ketoacidosis (DKA), alcoholic ketoacidosis, and starvation ketoacidosis leading to hospitalization, coma, or death [
9].
Prolonged high rates of ketosis are associated with systemic acidosis (currently termed ketoacidosis). As explained by Dreschfeld in 1886 [
4], during this time, the acidosis of severe diabetic ketoacidosis was assumed to be cause–effect; in other words, ketone bodies were thought to be produced as metabolic acids. Such an interpretation pre-dated advances in acid–base chemistry and physiology, similar to the errors of the early understanding of the metabolic biochemistry of the cellular lactic acid production [
10]. Consequently, the early 20th-century mistaken belief of a cellular lactic acid, rather than lactate, production may have reinforced the construct of ketone bodies as metabolic acids and the subsequent traditional metabolic explanation of the condition of ketoacidosis.
Each of β-HB and AcAc is chemically categorized as carboxylic acids due to their carboxylic acid functional groups and associated pKa’s of 4.41 and 3.58, respectively [
11]. However, similar to the production of lactate at physiological pH with a pKa of 3.67 [
11], the organic chemistry reveals that each of these “acids” is produced in their deprotonated, ionic state. Nevertheless, as with the ongoing error of the cellular lactic acid production [
12,
13], the chemical structural classification of ketone bodies (β-HB and AcAc) as acids has further reinforced the view that following their metabolic production, each of β-HB and AcAc will dissociate and release a proton (H
+) within the physiological pH range (6.00–7.60). In extension, if this metabolic condition remains uncorrected, systemic acidosis can ensue.
Despite advances in acid–base chemistry and computations of the extent of H
+ exchange during chemical reactions [
14,
15,
16,
17], the historically dependent metabolic acid interpretation of ketosis has remained up to the current time. For example, the depictions of both AcAc and β-HB in protonated form from Van Itallie [
18] and Kamel and Halperin [
19] clearly showed that the misinterpretation of the “acidic” nature of ketones has long been connected to the further (mis)understanding and (mis)interpretation of ketoacidosis. Furthermore, current articles on ketosis still claim without proof or reference [
2,
20,
21] that the large hydrogen-ion dissociation of ketones leads to metabolic acidosis. Even prominent modern textbooks on biochemistry [
22] that correctly depict the production of AcAc and β-HB in their ionic forms still state that it is the “acidic” nature of ketones that is the cause of the associated acidosis.
While the mortality rates and treatment of ketoacidosis have significantly improved since the late 19th century, the negatively associated stigma remains for the interpretation that ketone bodies are produced as acids that dissociate at physiological pH, resulting in systemic metabolic acidosis. Occurrences of ketoacidosis are still common in hospital critical care wards, with prevalence in undiagnosed or poorly controlled type I and II diabetes mellitus (diabetic ketoacidosis) [
23], alcoholic ketoacidosis [
24], and starvation ketoacidosis associated with eating disorders [
25]. It is logical and rational to have the view that the source of the underlying acidosis should be known for the most effective preventative treatment methods to be developed. Yet, this is not possible unless the underlying source of the acidosis is understood. This is problematic because the probability of improved treatment options for disease state systemic ketoacidosis in critical care settings would likely benefit greatly if the true metabolic cause of the acidosis is known.
Given the sustained disagreement and differing interpretations of H+ exchange during the cellular production of ketone bodies and the associated metabolic acidosis, the purpose of this research was to (a) present the charge and H+ balanced chemical reactions of ketone body production (ketogenesis), (b) provide the dissociation constants for these reactions and compute the data for the H+ exchange for each ketone body across the physiological pH range (severe cellular metabolic acidosis to systemic alkalosis; pH = 6 to 7.6), (c) perform the above for the oxidation of ketone bodies (ketolysis) that occurs in peripheral tissues (brain and skeletal muscle), and (d) provide recommendations for future research to inform metabolic explanations for the systemic acidosis that coincides with ketosis.
4. Discussion
In much the same way as computational chemistry models contributed fundamental evidence to the understanding of H
+ exchange in skeletal muscle during intense exercise [
10,
14,
15,
16], the current investigation used computational chemistry modeling to provide further insight into ~H
+e during the process of ketosis (ketogenesis, uptake into different tissues, and ketolysis). Traditional interpretations of ketosis involve the view that ketone bodies are produced as fully associated metabolic acids that dissociate upon production, resulting in H
+ release and a gradual H
+ accumulation that leads to systemic acidosis, currently referred to as ketoacidosis [
22]. More recently, these views have been challenged, causing conjecture within the medical and academic communities [
17]. The current debate surrounding ~H
+e during the condition of ketoacidosis primarily revolves around two points of contention. Firstly, the primary source of ~H
+ release/accumulation that leads to clinically significant pH changes during prolonged high rates of ketosis [
17], and secondly, the mechanism of action that is responsible for the ~H
+ release/accumulation [
10,
14,
15,
16].
Our results from the computational chemistry of ~H
+e for all substrates and products indicate that meaningful net ~H
+ release occurs during ketosis. Of the four reactions of β-HB and acetone production and the three reactions of AcAc production during ketogenesis, calculations show that H
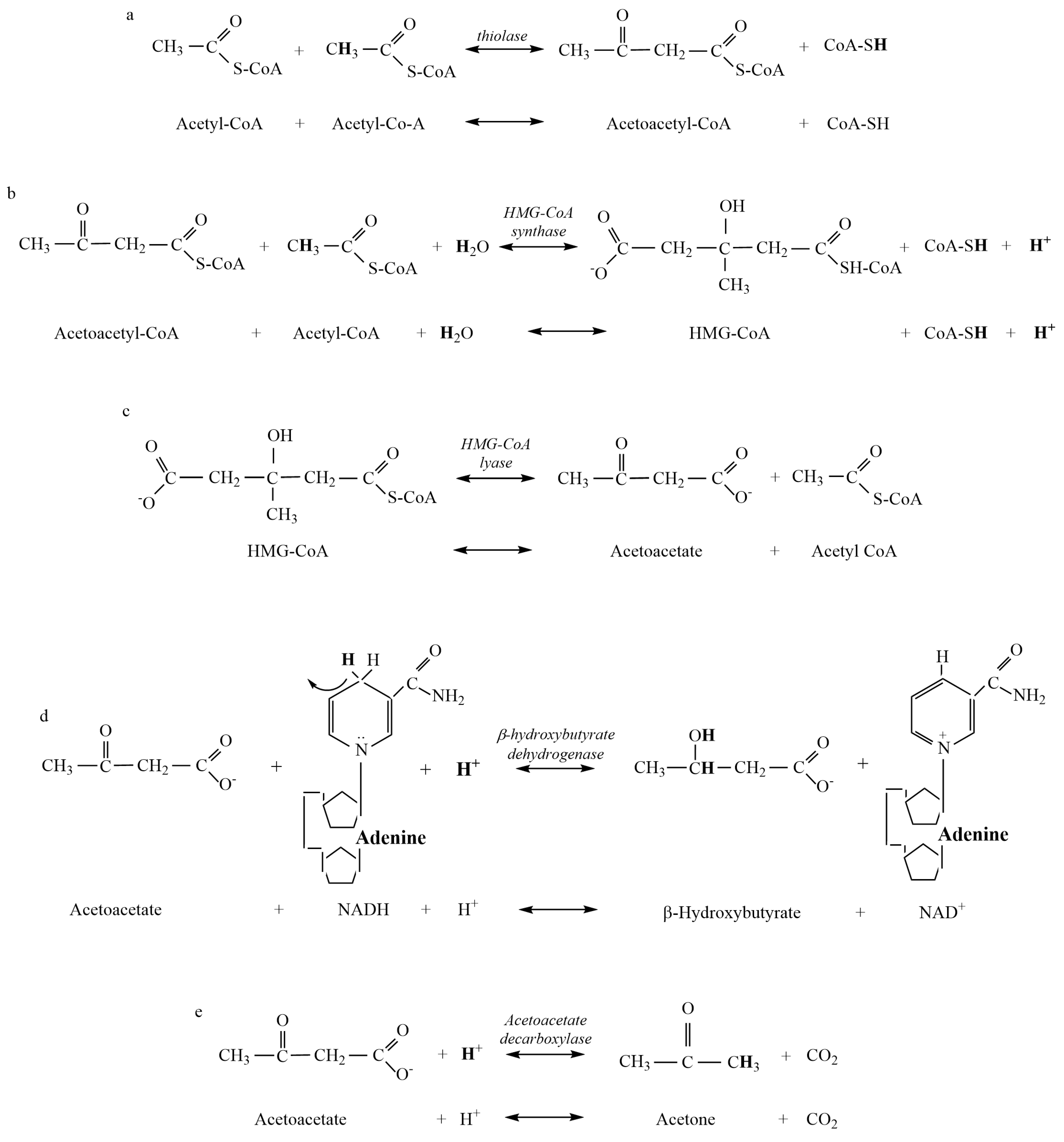
+ release occurs during the HMG-CoA Synthase reaction (
Figure 1b). Added H
+ release also occurs during ketolysis via the reversal of the β-HB Dehydrogenase reaction (
Figure 2a). Such ~H
+ release is opposed by ~H
+ uptake of the β-HB Dehydrogenase and AcAc Decarboxylase reactions of ketogenesis. All remaining reactions are negligible in their contribution to ~H
+e (
Figure 1 and
Figure 2). These calculations indicate that a meaningful increase in rates of ketosis (such as during times of starvation or poorly controlled diabetes) could theoretically contribute to an increase in H
+ release and subsequent systemic acidosis, currently termed ketoacidosis. However, it must be stated that meaningful increases in rates of ketosis are also accompanied by many additional alterations of cellular metabolism spanning multiple tissues. These alterations include but are not limited to, increased rates of lipolysis, changes in plasma ketone ratios, changes in uptake and oxidation rates of ketone bodies in multiple tissues, and alterations to glycolysis and β-oxidation pathways. These alterations have previously been documented in the early works of Cahill [
5] and more recently explored by Green and Bishop [
17]. Consequently, the results of this research only address our stated purpose, which was to evaluate the reactions of ketogenesis and ketolysis to discern their roles as a source of the ~H
+e during ketosis.
While the results of this research support the acidic nature of ketosis, the mechanism by which this net H
+ release occurs does not support traditional views. As previously stated, ~H
+ release occurs during the HMG-CoA Synthase reaction of ketogenesis (
Figure 1b) and the β-HB Dehydrogenase reaction of ketolysis (
Figure 2a). In reference to traditional views, this indicates that meaningful ~H
+ release occurs prior to the creation of any ketone body during ketogenesis and during the catabolism (ketolysis) of β-HB. As such, the traditional theory of the “acidic nature” of ketone bodies causing systemic acidosis is not plausible. For traditionalists who would argue that the metabolic intermediates of ketone body production during ketogenesis, rather than the ketone bodies themselves, are acidic in nature, calculations show that no reactions of ketogenesis result in pH-dependent H
+ dissociation of acidic functional groups (
Figure 1).
The H
+ release that occurs during the HMG-CoA Synthase reaction of ketogenesis occurs when water is covalently modified to release an H
+ after the addition of a hydroxyl group to HMG-CoA (hydrolysis) (
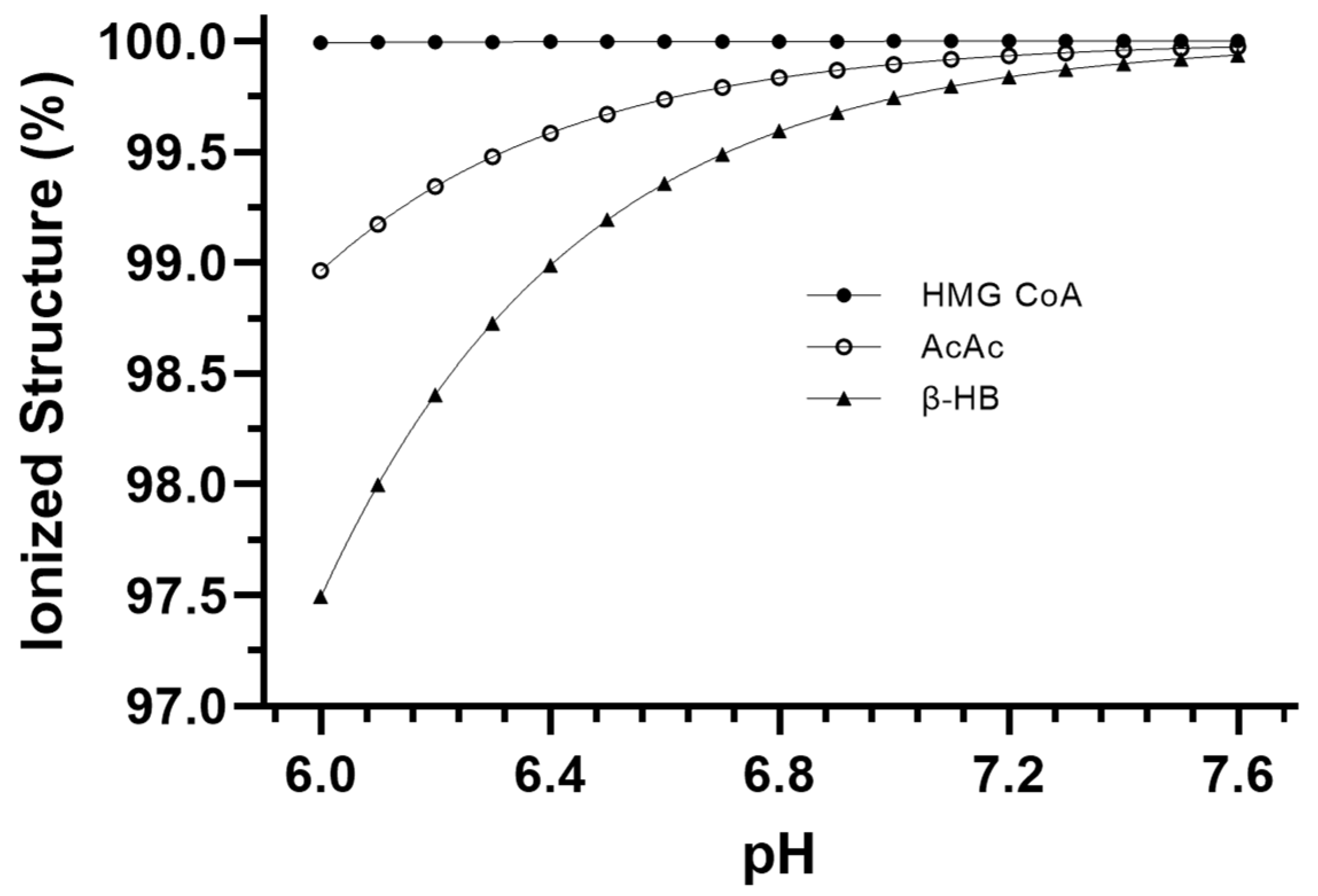
Figure 1b). Our calculations show that such additions occur via covalent modification and not pH-dependent ionization, which, therefore, explains the near-constant profile of ~H
+e shown in
Figure 3. For example, compare this result to that of the metabolites and reactions of glycolysis, as documented by Robergs [
14]. In a similar manner, and a fundamental finding of this research, metabolic H
+ release occurs via other chemical processes and not through the generation of organic acids. This understanding is also relevant during ketolysis for the ~H
+ release for the reversal of the β-HB Dehydrogenase reaction causing the conversion of β-HB to AcAc (
Figure 2a). Significant H
+ release does not occur via acid dissociation but instead by the oxidation–reduction nature of the reaction. If we are to continue accepting the narrative of traditional views by which the “acidic nature” of ketones is the cause of ketoacidosis while clearly demonstrating that H
+ release is a result of covalent modifications, then for consistency, these same views must be applied across the biological spectrum to all organic reactions. However, if these views are applied across the biological spectrum, it becomes clear that their application is severely inappropriate. For example, substrates, such as ATP, would need to be classified as a metabolic acid, as hydrolysis of ATP during the reaction ATPase produces ADP + Pi and releases an H
+. This is the same covalent modification reaction (hydrolysis) as during the HMG-CoA Synthase reaction of ketogenesis, yet the traditionalist interpretation classifies ketone bodies as acids but not ATP. Depending on the differences between the dissociation constants of substrates vs. products, added minor H
+ exchange can occur via pH-dependent association and/or dissociation, though once again, note that for ketosis, there is minimal pH-dependent dissociation or association.
Since their discovery over 100 years ago, the acidic nature of ketone bodies has been continually reinforced up to the present time [
1,
2,
22]. In stark contrast to historical and traditional views stating ketone bodies are responsible for the acidosis associated with severe ketosis, H
+ exchange computations of β-HB (
Table 2) show that β-HB is a net H
+ consumer during ketogenesis. This finding is a glaring reality check for the present time, where the understanding, interpretation, and research inquiry into ketone bodies and the process of ketosis has been in error. Computations of ~H
+e show that AcAc is the only ketone body in which the production results in net (across all reactions leading to production) H
+ release. In other words, both β-HB and acetone production buffer against ~H release (
Figure 1,
Table 2).
Similar to the increasing presence of lactate during anaerobic glycolysis, β-HB is an H
+ consumer/buffer against acidosis that can also be used as a substrate for ATP production. It is also important to re-emphasize that H
+ consumption during the conversion of AcAc to β-HB is not a result of acid association. It is the result of the oxidation–reduction reaction where NADH is oxidized to NAD
+, and AcAc is reduced to β-HB. This would make physiological sense for why the ratio of β-HB to AcAc in the blood increases from 1:1 to 10:1 [
30] during severe ketosis, presumably in compensation for the significant net H
+ release when AcAc is not converted to β-HB (
Table 4). This finding is not only significant in the further understanding of acid–base chemistry for organic reactions but may, in fact, have tangible benefits in the treatment of ketoacidosis. Currently, increasing concentrations of plasma ketone bodies are considered a concern for acid–base balance; however, a more appropriate focus may be on the plasma concentration of AcAc, as it is when AcAc is not converted to β-HB or acetone that a larger net ~H
+ release would occur during ketogenesis.
It is commonly noted in research papers, textbooks, and by medical professionals that the “fruity” breath of individuals during ketoacidosis is caused by excessive blood acetone and is a key indicator of the condition. Yet, acetone’s influence on acid–base balance during ketoacidosis is often neglected and under-reported, which is likely due to its historical perception as a harmful by-product of ketosis due to its volatile chemistry and expulsion from the body via urine and exhaled air [
29,
30,
31]. Structurally, acetone is neither an acid nor a base, which may further explain why it has been underrepresented, as it does not fit the historical narrative that the acidic nature of ketone bodies causes ketoacidosis. In contrast, the influence of acetone is an important consideration for acid–base balance during ketosis, as the ~H
+e coefficients during the conversion of AcAc to acetone show meaningful ~H
+ consumption (
Figure 2,
Table 4). It has been previously shown that during starvation ketoacidosis, blood concentrations of acetone can be similar [
2] or in excess of AcAc [
29,
30,
31]. Our results have used this data to reveal how the production of acetone during ketosis plays a large role in reducing the net ~H
+ release and, as such, is an extremely important H
+ consumer during high rates of ketosis (
Table 4). Nevertheless, the role of the metabolic removal of acetone is difficult to estimate at this time due to limited evidence for the chemical reactions involved and the exact fraction of the acetone pool that is cleared in this manner [
29,
31].
When trying to elucidate the importance of ketosis to systemic acid–base balance, it is important to consider the systematic changes in ~H
+e that may occur when transitioning from glucose-derived ATP production to ketone-derived ATP production. Ketone bodies replace the utilization of glucose for cellular ATP production, so it is an important consideration to determine the net H
+ release when comparing the catabolism of both substrates. In simple terms, would ketolysis contribute more, less, or the same amount of H
+ to the system than that of the process it replaces (glycolysis)? Previous research of the H
+ exchange during glycolysis from Robergs [
10] indicates that the H
+ exchange from glycogenolysis/glycolysis would be greater than or equal to that of ketolysis, which, of course, leaves the evidence of a severe systemic blood acidosis during ketosis to require further open-minded research inquiry and explanation.
Limitations
It is openly acknowledged that the calculations and factors used in the current investigation are not exhaustive in their findings, and further research is required to account for additional factors during ketosis. These include but are not limited to, competing cations during all phases of ketosis, the varying pH levels of different bodily compartments during ketolysis in addition to the brain and skeletal muscle, systemic changes in pH during the development of ketoacidosis (i.e., the role of the MCTs in metabolite movement in and out of cells, increased rates of lipolysis, decreased glycolytic flux), changes in the ratio of ketone bodies and the varying uptake rates of ketone bodies from different tissues through the entire development of ketoacidosis. It is also important to note that tissue concentrations of the ketone bodies are unlikely to be true to the cellular production rates, especially when considering the simultaneous functioning of ketogenesis and ketolysis. Similarly, the complications surrounding the uncertain metabolic clearance of acetone require future measurements and clarification, and it would be pertinent to replicate these procedures using in vitro preparations of metabolic pathways.
The data presented here are specifically for the H+ exchange coefficients for the chemical reactions of ketogenesis and ketolysis, which directly address the purpose of this research, which was to test whether the production of ketone bodies directly causes ~H+ release, and, if so, for what reason. Further research is needed that adds the reactions involved in the metabolic perturbations that accompany ketosis.