The Beneficial Effect of Swimming Training Associated with Quercetin Administration on the Endothelial Nitric Oxide-Dependent Relaxation in the Aorta of Rats with Experimentally Induced Type 1 Diabetes Mellitus
Abstract
1. Introduction
2. Material and Methods
2.1. Drugs and Chemicals
2.2. Equipment
2.3. Procedure
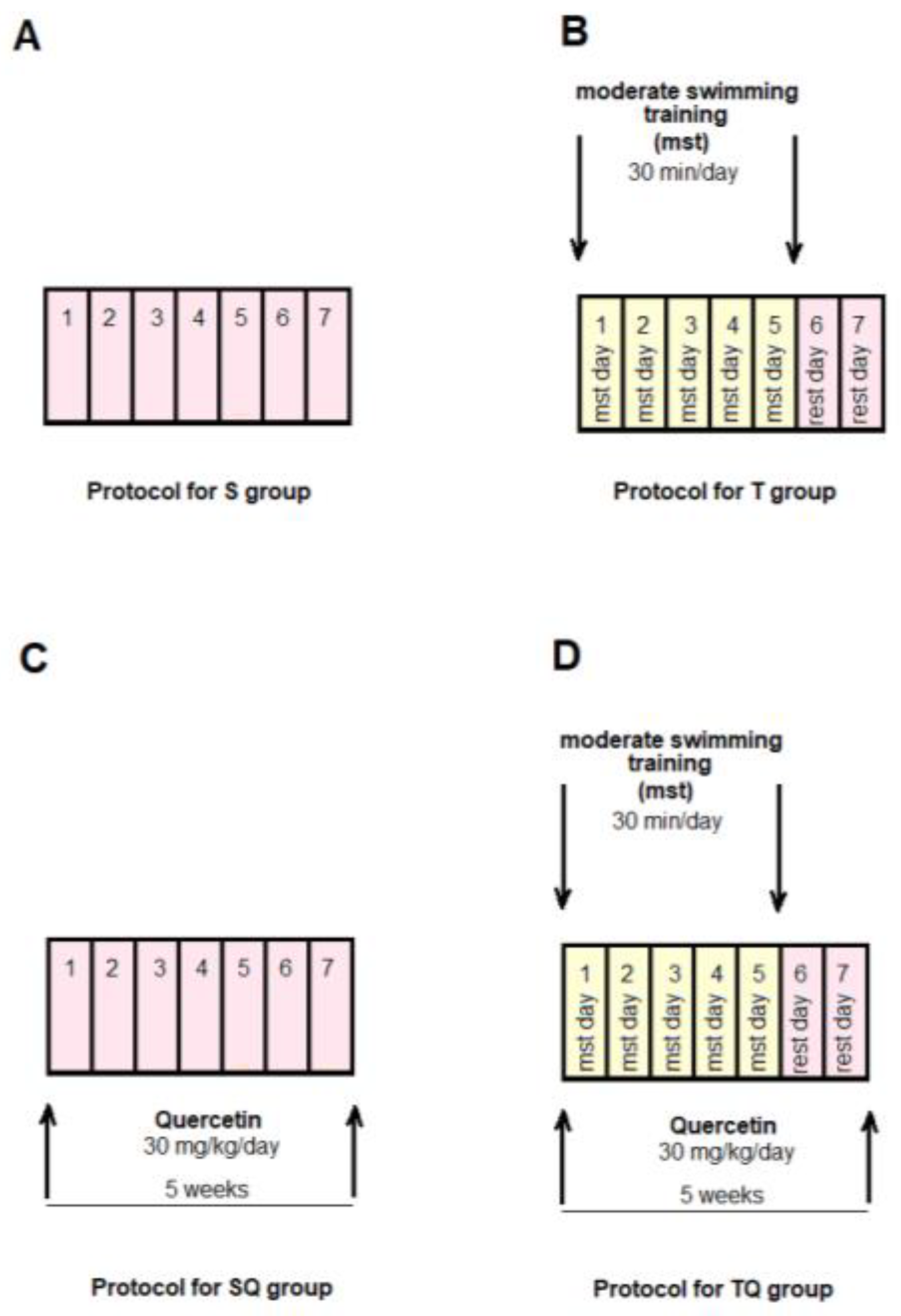
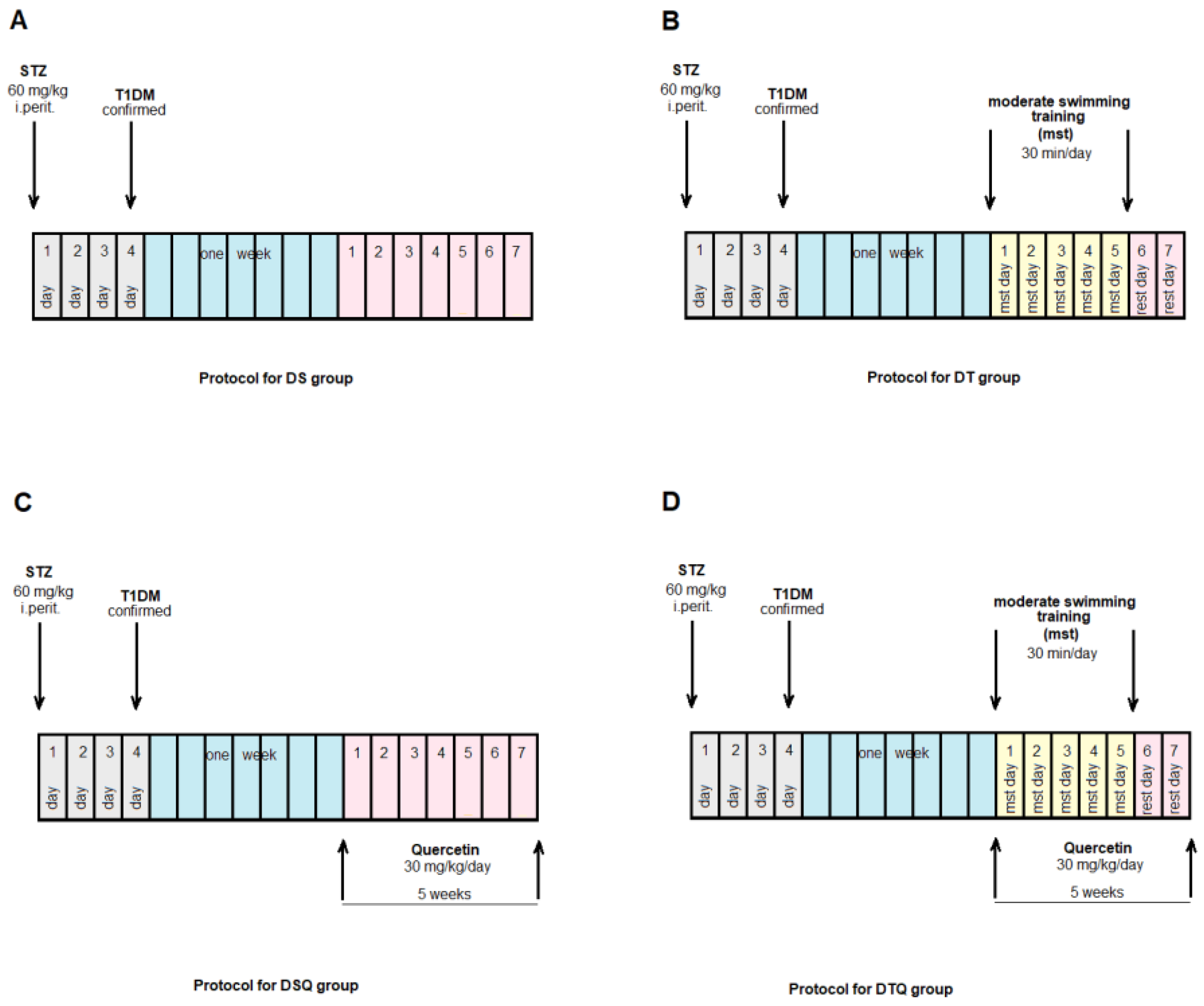
2.3.1. Experimental Protocol
2.3.2. Moderate Swimming Training Protocol
2.3.3. Preparation of Aortic Tissue and Measurement of Isometric Force
2.3.4. Testing Phase: The Evaluation of Phenylephrine (PE), Acetylcholine (Ach), and Sodium Nitroprusside (SNP) Effects on Aorta Rings
2.3.5. Statistical Analysis
3. Results
3.1. Blood Glucose and Animal Body Weights in the Control and Experimental Groups
3.2. Aortic Contractile Responses to Phenylephrine (PE) in Control and Experimental Groups
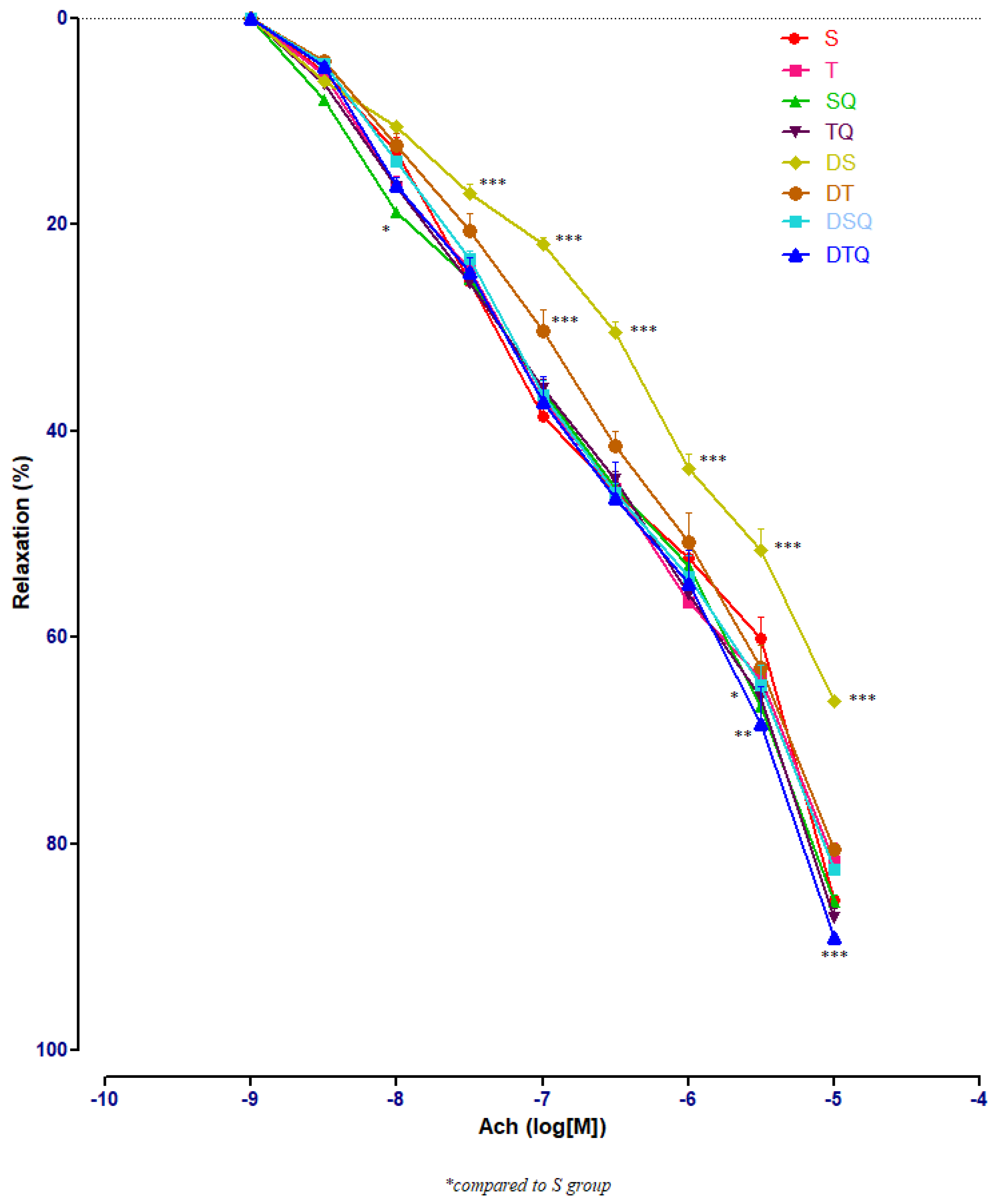
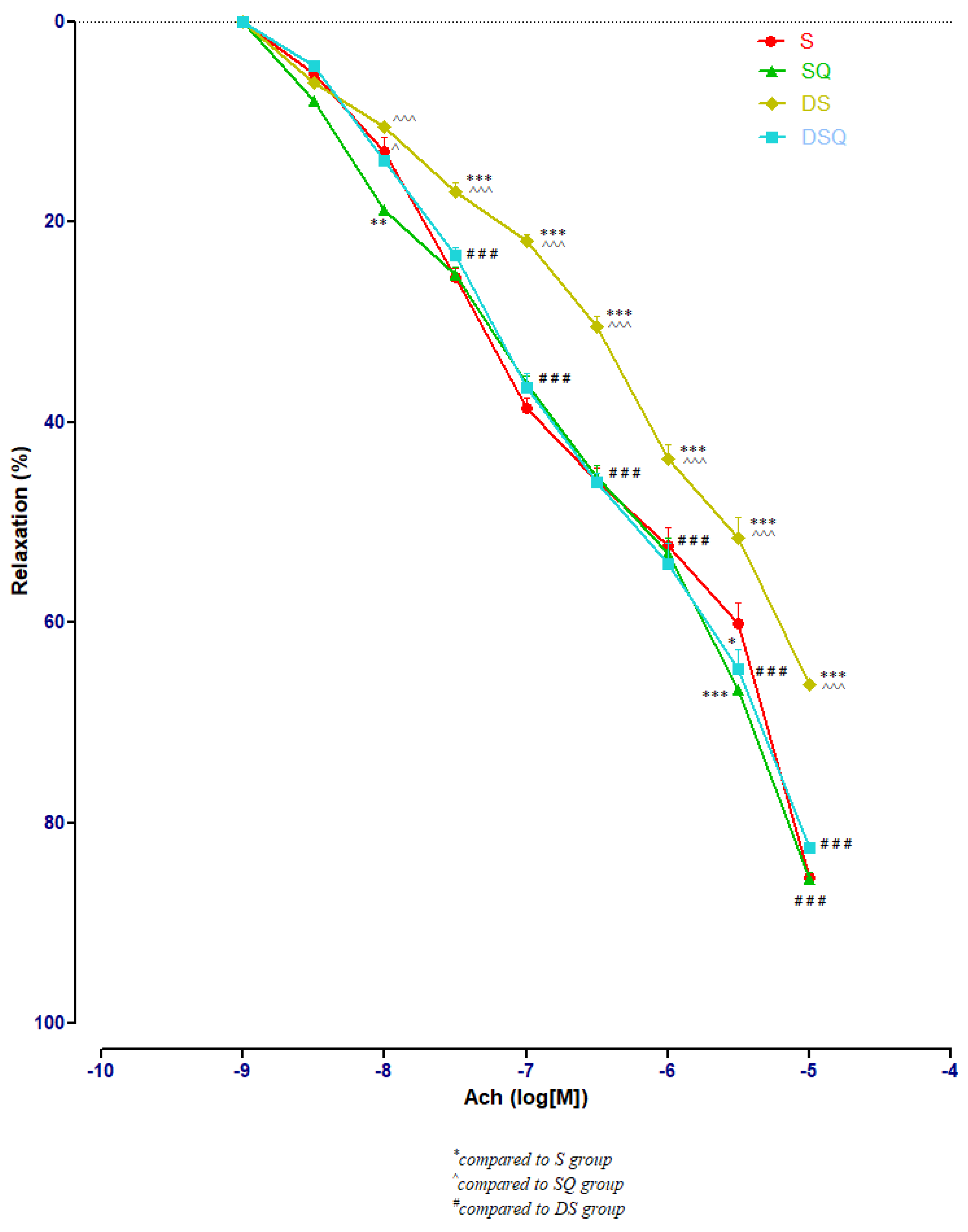
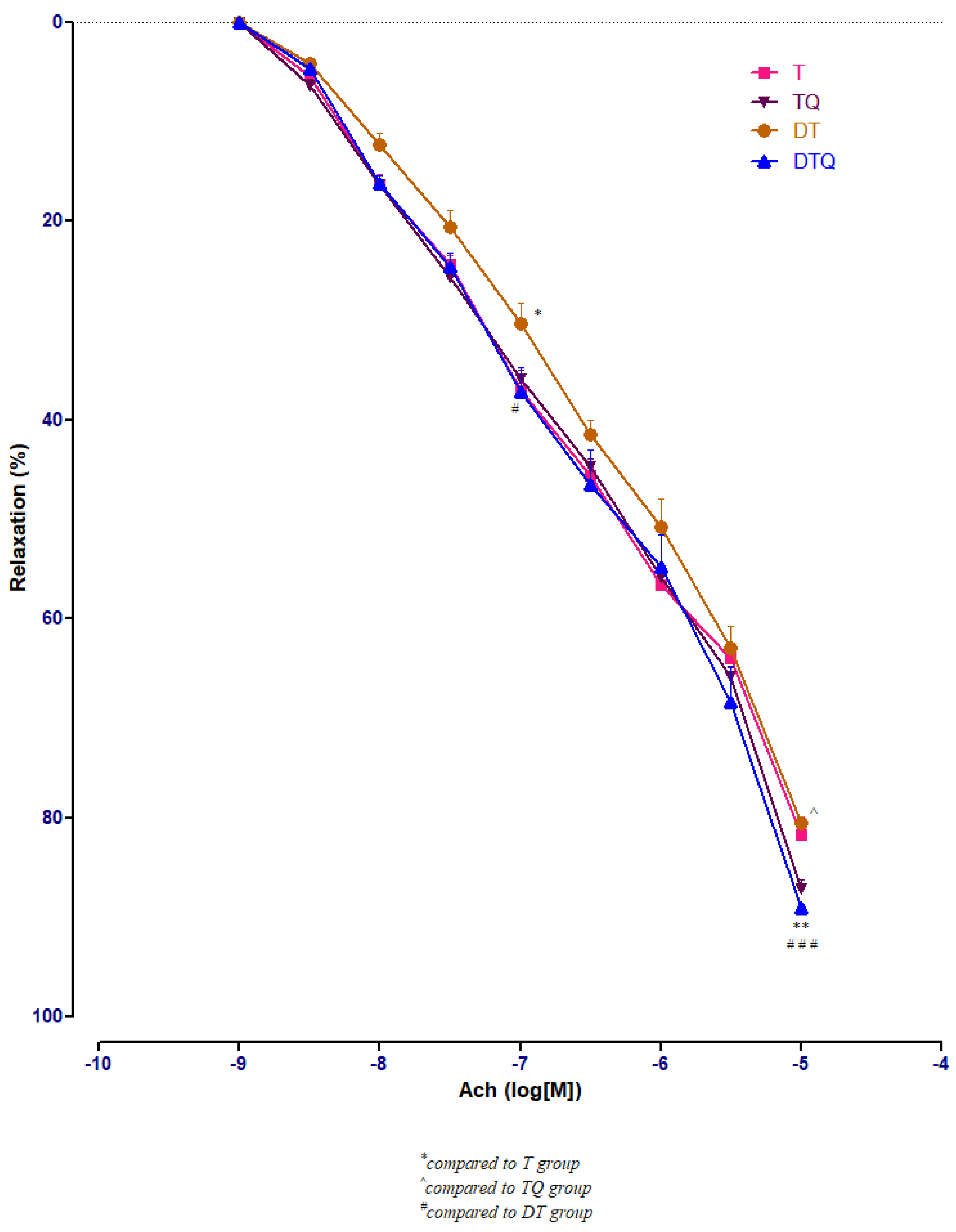
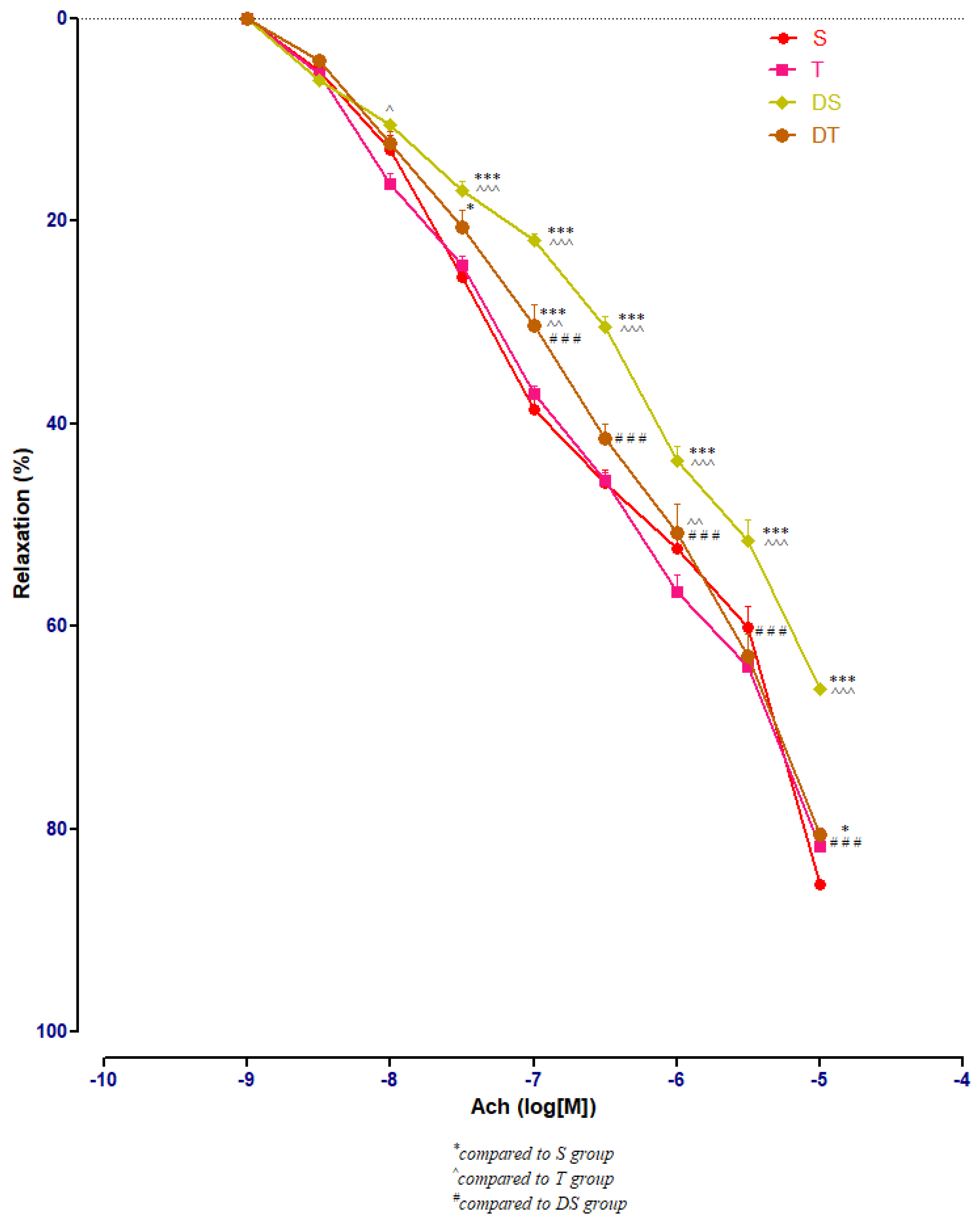
3.3. Aortic Relaxation Responses to Acetylcholine (Ach) in Control and Experimental Groups
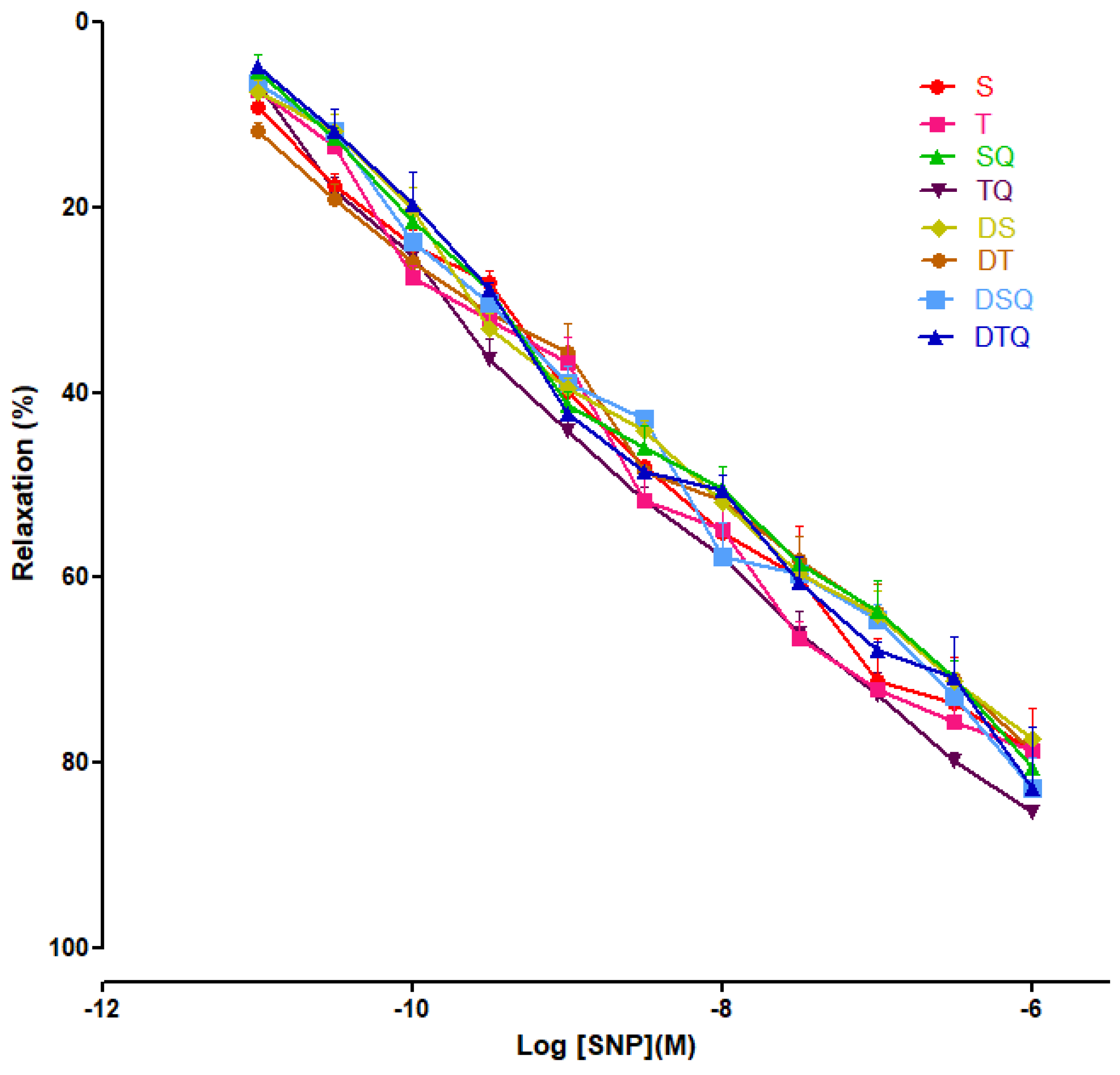
3.4. Aortic Relaxation Responses to Sodium Nitroprusside (SNP) in Control and Experimental Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paneni, F.; Beckman, J.A.; Creager, M.A.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Eur. Heart J. 2013, 34, 2436–2443. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K.; Pandey, K.B.; Abidi, A.B.; Rizvi, S.I. Markers of Oxidative Stress during Diabetes Mellitus. J. Biomarkers 2013, 2013, 378790. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, T.V.; Prioletta, A.; Zuo, P.; Folli, F. Hyperglycemia-induced Oxidative Stress and its Role in Diabetes Mellitus Related Cardiovascular Diseases. Curr. Pharm. Des. 2013, 19, 5695–5703. [Google Scholar] [CrossRef]
- Chis, I.C.; Coseriu, A.; Simedrea, R.; Oros, A.; Nagy, A.L.; Clichici, S. In Vivo Effects of Quercetin in Association with Moderate Exercise Training in Improving Streptozotocin-Induced Aortic Tissue Injuries. Molecules 2015, 20, 21770–21786. [Google Scholar] [CrossRef] [PubMed]
- Chiș, I.C.; Clichici, A.; Simedrea, R.; Moldovan, R.; Lazar, V.L.; Clichici, S.; Oniga, O.; Nastasă, C. The effects of a new chromenyl-methylenethiazolidine-2,4-dione in alleviating oxidative stress in a rat model of streptozotocin induced diabetes. Stud. UBB Chem. 2018, 63, 103–112. [Google Scholar] [CrossRef]
- Chis, I.C.; Socaciu, M.; Moldovan, R.; Clichici, S. Vascular impact of quercetin administration in association with moderate exercise training in experimental type 1 diabetes. Rev. Romana Med. Lab. 2019, 27, 269–279. [Google Scholar] [CrossRef]
- Avogaro, A.; Albiero, M.; Menegazzo, L.; de Kreutzenberg, S.; Fadini, G.P. Endothelial Dysfunction in Diabetes: The role of reparatory mechanisms. Diabetes Care 2011, 34, S285–S290. [Google Scholar] [CrossRef]
- Sena, C.M.; Pereira, A.M.; Seiça, R. Endothelial dysfunction—A major mediator of diabetic vascular disease. Biochim. Biophys. Acta 2013, 1832, 2216–2231. [Google Scholar] [CrossRef]
- Fowler, M.J. Microvascular and Macrovascular Complications of Diabetes. Clin. Diabetes 2008, 26, 77–82. [Google Scholar] [CrossRef]
- Sundaram, B.; Singhal, K.; Sandhir, R. Anti-atherogenic effect of chromium picolinate in streptozotocin-induced experimental diabetes. J. Diabetes 2013, 5, 43–50. [Google Scholar] [CrossRef]
- Zguira, M.S.; Vincent, S.; Le Douairon Lahaye, S.; Malarde, L.; Tabka, Z.; Saïag, B. Intense exercise training is not effective to restore the endothelial NO-dependent relaxation in STZ-diabetic rat aorta. Cardiovasc. Diabetol. 2013, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, C. Vasoprotection by Dietary Supplements and Exercise: Role of TNFαSignaling. Exp. Diabetes Res. 2012, 2012, 972679. [Google Scholar] [CrossRef]
- Lee, S.; Park, Y.; Dellsperger, K.C.; Zhang, C. Exercise training improves endothelial function via adiponectin-dependent and independent pathways in type 2 diabetic mice. Am. J. Physiol. Circ. Physiol. 2011, 301, H306–H314. [Google Scholar] [CrossRef] [PubMed]
- Coskun, O.; Ocakci, A.; Bayraktaroglu, T.; Kanter, M. Exercise Training Prevents and Protects Streptozotocin-Induced Oxidative Stress and beta-Cell Damage in Rat Pancreas. Tohoku J. Exp. Med. 2004, 203, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Heylen, E.; Guerrero, F.; Mansourati, J.; Theron, M.; Thioub, S.; Saïag, B. Effect of training frequency on endothelium-dependent vasorelaxation in rats. Eur. J. Cardiol. Prev. Rehabil. 2008, 15, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Umeno, A.; Horie, M.; Murotomi, K.; Nakajima, Y.; Yoshida, Y. Antioxidative and Antidiabetic Effects of Natural Polyphenols and Isoflavones. Molecules 2016, 21, 708. [Google Scholar] [CrossRef]
- Sevastre-Berghian, A.C.; Ielciu, I.; Mitre, A.O.; Filip, G.A.; Oniga, I.; Vlase, L.; Benedec, D.; Gheldiu, A.-M.; Toma, V.A.; Mihart, B.; et al. Targeting Oxidative Stress Reduction and Inhibition of HDAC1, MECP2, and NF-kB Pathways in Rats with Experimentally Induced Hyperglycemia by Administration of Thymus marshallianus Willd. Extracts. Front. Pharmacol. 2020, 11, 581470, eCollection 2020. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Chiş, I.C.; Baltaru, D.; Dumitrovici, A.; Coseriu, A.; Radu, B.C.; Moldovan, R.; Mureşan, A. Protective effects of quercetin from oxidative/nitrosative stress under intermittent hypobaric hypoxia exposure in the rat’s heart. Physiol. Int. 2018, 105, 233–246. [Google Scholar] [CrossRef]
- Chiş, I.C.; Mureşan, A.; Oros, A.; Nagy, A.L.; Clichici, S. Protective effects of Quercetin and chronic moderate exercise (training) against oxidative stress in the liver tissue of streptozotocin-induced diabetic rats. Acta Physiol. Hung. 2016, 103, 49–64. [Google Scholar] [CrossRef]
- Alam, M.M.; Meerza, D.; Naseem, I. Protective effect of quercetin on hyperglycemia, oxidative stress and DNA damage in alloxan induced type 2 diabetic mice. Life Sci. 2014, 109, 8–14. [Google Scholar] [CrossRef]
- Pashevin, D.A.; Tumanovska, L.V.; Dosenko, V.E.; Nagibin, V.S.; Gurianova, V.L.; Moibenko, A.A. Antiatherogenic effect of quercetin is mediated by proteasome inhibition in the aorta and circulating leukocytes. Pharmacol. Rep. 2011, 63, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.J.; Symons, J.D.; Jalili, T. Therapeutic Potential of Quercetin to Decrease Blood Pressure: Review of Efficacy and Mechanisms. Adv. Nutr. 2012, 3, 39–46. [Google Scholar] [CrossRef]
- Jeong, S.-M.; Kang, M.-J.; Choi, H.-N.; Kim, J.-H.; Kim, J.I. Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice. Nutr. Res. Pract. 2012, 6, 201–207. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, M.-J.; Choi, H.-N.; Jeong, S.-M.; Lee, Y.-M.; Kim, J.I. Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus. Nutr. Res. Pract. 2011, 5, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.F.; Hassan, N.A.; El-Bassossy, H.M.; Fahmy, A. Quercetin Protects against Diabetes-Induced Exaggerated Vasoconstriction in Rats: Effect on Low Grade Inflammation. PLoS ONE 2013, 8, e63784. [Google Scholar] [CrossRef] [PubMed]
- Chis, I.C.; Ungureanu, M.I.; Marton, A.; Simedrea, R.; Muresan, A.; Postescu, I.-D.; Decea, N. Antioxidant effects of a grape seed extract in a rat model of diabetes mellitus. Diabetes Vasc. Dis. Res. 2009, 6, 200–204. [Google Scholar] [CrossRef]
- Chis, I.C.; Clichici, A.; Nagy, A.L.; Oros, A.; Catoi, C.; Clichici, S. Quercetin in association with moderate exercise training attenuates injuries induced by experimental diabetes in sciatic nerves. J. Physiol. Pharmacol. 2017, 68, 877–886. [Google Scholar]
- Pyun, S.B.; Kwon, H.K.; Uhm, C.S. Effect of exercise on reinnervating soleus muscle after sciatic nerve injury in rats. J. Korean Acad. Rehabil. Med. 1999, 23, 1063–1075. [Google Scholar]
- Teixeira de Lemos, E.; Pinto, R.; Oliveira, J.; Garrido, P.; Sereno, J.; Mascarenhas-Melo, F.; Páscoa-Pinheiro, J.; Teixeira, F.; Reis, F. Differential Effects of Acute (Extenuating) and Chronic (Training) Exercise on Inflammation and Oxidative Stress Status in an Animal Model of Type 2 Diabetes Mellitus. Mediat. Inflamm. 2011, 2011, 253061. [Google Scholar] [CrossRef]
- Gajdosík, A.; Gajdosíková, A.; Stefek, M.; Navarová, J.; Hozová, R. Streptozotocin-induced experimental diabetes in male Wistar rats. Gen. Physiol. Biophys. 1999, 18, 54–62. [Google Scholar] [PubMed]
- Prabakaran, D.; Ashokkumar, N. Protective effect of esculetin on hyperglycemia-mediated oxidative damage in the hepatic and renal tissues of experimental diabetic rats. Biochimie 2013, 95, 366–373. [Google Scholar] [CrossRef] [PubMed]
- De Vriese, A.S.; Verbeurenm, T.J.; Van de Voorde, J.; Lameire, N.H.; Vanhoutte, P.M. Endothelial dysfunction in diabetes. Br. J. Pharmacol. 2000, 130, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Meza, C.A.; La Favor, J.D.; Kim, D.-H.; Hickner, R.C. Endothelial Dysfunction: Is There a Hyperglycemia-Induced Imbalance of NOX and NOS? Int. J. Mol. Sci. 2019, 20, 3775. [Google Scholar] [CrossRef]
- Ji, B.; Yuan, K.; Li, J.; Ku, B.J.; Leung, P.S.; He, W. Protocatechualdehyde restores endothelial dysfunction in streptozotocin-induced diabetic rats. Ann. Transl. Med. 2021, 9, 711. [Google Scholar] [CrossRef]
- Chellian, J.; Mak, K.-K.; Chellappan, D.K.; Krishnappa, P.; Pichika, M.R. Quercetin and metformin synergistically reverse endothelial dysfunction in the isolated aorta of streptozotocin-nicotinamide- induced diabetic rats. Sci. Rep. 2022, 12, 21393. [Google Scholar] [CrossRef]
- Alkaabi, J.; Sharma, C.; Yasin, J.; Afandi, B.; Beshyah, S.A.; Almazrouei, R.; Alkaabi, A.; Al Hamad, S.; Ahmed, L.A.; Beiram, R.; et al. Relationship between lipid profile, inflammatory and endothelial dysfunction biomarkers, and type 1 diabetes mellitus: A case-control study. Am. J. Transl. Res. 2022, 14, 4838–4847. [Google Scholar]
- McDonald, M.W.; Olver, T.D.; Dotzert, M.S.; Jurrissen, T.J.; Noble, E.G.; Padilla, J.; Melling, C.J. Aerobic exercise training improves insulin-induced vasorelaxation in a vessel-specific manner in rats with insulin-treated experimental diabetes. Diabetes Vasc. Dis. Res. 2019, 16, 77–86. [Google Scholar] [CrossRef]
- Shi, G.-J.; Li, Y.; Cao, Q.-H.; Wu, H.-X.; Tang, X.-Y.; Gao, X.-H.; Yu, J.-Q.; Chen, Z.; Yang, Y. In vitro and in vivo evidence that quercetin protects against diabetes and its complications: A systematic review of the literature. Biomed. Pharmacother. 2019, 109, 1085–1099. [Google Scholar] [CrossRef]

| Parameters | S | T | SQ | TQ | DS | DT | DSQ | DTQ |
|---|---|---|---|---|---|---|---|---|
| Initial FBG (mg/dL) | 69.9 ± 2.23 | 68.4 ± 1.84 | 67.6.0 ± 4.6 | 67.4 ± 3.37 | 573.1 ± 45.35 aaa | 542.8 ± 62.04 aaa | 535.5 ± 35.63 aaa | 523.0 ± 38.7 aaa |
| Final FBG (mg/dL) | 72.0 ± 3.2 | 70.0 ± 2.6 | 69.8 ± 3.6 | 70.3 ± 2.75 | 583.1 ± 31.24 aaa | 477.4 ± 44.2 b | 380.5 ± 25.2 bbb | 293.0 ± 38.0 bbb |
| Initial body weight (g) | 271.5 ± 14.6 | 270.0 ± 16.9 | 281.4 ± 13.7 | 288.9 ± 12.9 | 273.5 ± 18.9 | 282.5 ± 18.49 | 279.8 ± 15.27 | 280.4 ± 18.3 |
| Final body weight (g) | 277.5 ± 13.9 | 265.5 ± 12.9 | 286.4 ± 11.8 | 290.0 ± 11.67 | 238.5 ± 10.34 a | 270.9 ± 12.80 | 274.2 ± 11.03 | 276.4 ± 13.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chis, I.-C.; Micu, C.-M.; Toader, A.; Moldovan, R.; Lele, L.; Clichici, S.; Mitrea, D.-R. The Beneficial Effect of Swimming Training Associated with Quercetin Administration on the Endothelial Nitric Oxide-Dependent Relaxation in the Aorta of Rats with Experimentally Induced Type 1 Diabetes Mellitus. Metabolites 2023, 13, 586. https://doi.org/10.3390/metabo13050586
Chis I-C, Micu C-M, Toader A, Moldovan R, Lele L, Clichici S, Mitrea D-R. The Beneficial Effect of Swimming Training Associated with Quercetin Administration on the Endothelial Nitric Oxide-Dependent Relaxation in the Aorta of Rats with Experimentally Induced Type 1 Diabetes Mellitus. Metabolites. 2023; 13(5):586. https://doi.org/10.3390/metabo13050586
Chicago/Turabian StyleChis, Irina-Camelia, Carmen-Maria Micu, Alina Toader, Remus Moldovan, Laura Lele, Simona Clichici, and Daniela-Rodica Mitrea. 2023. "The Beneficial Effect of Swimming Training Associated with Quercetin Administration on the Endothelial Nitric Oxide-Dependent Relaxation in the Aorta of Rats with Experimentally Induced Type 1 Diabetes Mellitus" Metabolites 13, no. 5: 586. https://doi.org/10.3390/metabo13050586
APA StyleChis, I.-C., Micu, C.-M., Toader, A., Moldovan, R., Lele, L., Clichici, S., & Mitrea, D.-R. (2023). The Beneficial Effect of Swimming Training Associated with Quercetin Administration on the Endothelial Nitric Oxide-Dependent Relaxation in the Aorta of Rats with Experimentally Induced Type 1 Diabetes Mellitus. Metabolites, 13(5), 586. https://doi.org/10.3390/metabo13050586








