Urinary 1H NMR Metabolomic Analysis of Prenatal Maternal Stress Due to a Natural Disaster Reveals Metabolic Risk Factors for Non-Communicable Diseases: The QF2011 Queensland Flood Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Post-Flood Recruitment
2.1.2. Assessment of Objective Hardship and Composite Subjective Distress
2.2. Sample Collection and Preparation
2.3. NMR Data Acquisition
2.4. Statistical Analyses
2.5. Metabolite Identification and Pathway Analysis
3. Results
3.1. Maternal Objective Hardship and Subjective Distress Produce Unique Metabolome Profiles
3.2. Maternal Objective Hardship and Subjective Distress Program Protein Synthesis, Energy Metabolism, and Carbohydrate Metabolism
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muldera, E.J.H.; Medinaa, P.G.R.d.; Huizinkb, A.C.; Bergh, B.R.H.V.d.; Buitelaar, J.K.; Visser, G.H.A. Prenatal maternal stress: Effects on pregnancy and the (unborn) child. Early Hum. Dev. 2002, 70, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Van Aalst, M.K. The impacts of climate change on the risk of natural disasters. Disasters 2006, 30, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Lafortune, S.; Laplante, D.P.; Elgbeili, G.; Li, X.; Lebel, S.; Dagenais, C.; King, S. Effect of Natural Disaster-Related Prenatal Maternal Stress on Child Development and Health: A Meta-Analytic Review. Int. J. Environ. Res. Public Health 2021, 18, 8332. [Google Scholar] [CrossRef] [PubMed]
- King, S.; Kildea, S.; Austin, M.-P.; Brunet, A.; Cobham, V.E.; Dawson, P.A.; Harris, M.; Hurrion, E.M.; Laplante, D.P.; McDermott, B.M.; et al. QF2011: A protocol to study the effects of the Queensland flood on pregnant women, their pregnancies, and their children’s early development. BMC Pregnancy Childbirth 2015, 15, 109. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lei, L.; Laplante, D.P.; King, S. Prenatal Maternal Stress and Epigenetics: Review of the Human Research. Curr. Mol. Biol. Rep. 2016, 2, 16–25. [Google Scholar] [CrossRef]
- Cao-Lei, L.; de Rooij, S.R.; King, S.; Matthews, S.G.; Metz, G.A.S.; Roseboom, T.J.; Szyf, M. Prenatal stress and epigenetics. Neurosci. Biobehav. Rev. 2020, 117, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, B.R.H.; van den Heuvel, M.I.; Lahti, M.; Braeken, M.; de Rooij, S.R.; Entringer, S.; Hoyer, D.; Roseboom, T.; Raikkonen, K.; King, S.; et al. Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neurosci. Biobehav. Rev. 2020, 117, 26–64. [Google Scholar] [CrossRef]
- Dancause, K.N.; Laplante, D.P.; Hart, K.J.; O’Hara, M.W.; Elgbeili, G.; Brunet, A.; King, S. Prenatal stress due to a natural disaster predicts adiposity in childhood: The Iowa Flood Study. J. Obes. 2015, 2015, 570541. [Google Scholar] [CrossRef]
- Kroska, E.B.; O’Hara, M.W.; Elgbeili, G.; Hart, K.J.; Laplante, D.P.; Dancause, K.N.; King, S. The impact of maternal flood-related stress and social support on offspring weight in early childhood. Arch. Womens Ment. Health 2018, 21, 225–233. [Google Scholar] [CrossRef]
- Yong Ping, E.; Laplante, D.P.; Elgbeili, G.; Hillerer, K.M.; Brunet, A.; O’Hara, M.W.; King, S. Prenatal maternal stress predicts stress reactivity at 2(1/2) years of age: The Iowa Flood Study. Psychoneuroendocrinology 2015, 56, 62–78. [Google Scholar] [CrossRef]
- Liu, G.T.; Dancause, K.N.; Elgbeili, G.; Laplante, D.P.; King, S. Disaster-related prenatal maternal stress explains increasing amounts of variance in body composition through childhood and adolescence: Project Ice Storm. Environ. Res. 2016, 150, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Laplante, D.P.; Barr, R.G.; Brunet, A.; Galbaud du Fort, G.; Meaney, M.L.; Saucier, J.F.; Zelazo, P.R.; King, S. Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatr. Res. 2004, 56, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V.; Jones, S.L.; Elgbeili, G.; Monnier, P.; Yu, C.; Laplante, D.P.; King, S. Testosterone-cortisol dissociation in children exposed to prenatal maternal stress, and relationship with aggression: Project Ice Storm. Dev. Psychopathol. 2018, 30, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Laplante, D.P.; Hart, K.J.; O’Hara, M.W.; Brunet, A.; King, S. Prenatal maternal stress is associated with toddler cognitive functioning: The Iowa Flood Study. Early Hum. Dev. 2018, 116, 84–92. [Google Scholar] [CrossRef]
- Laplante, D.P.; Brunet, A.; Schmitz, N.; Ciampi, A.; King, S. Project Ice Storm: Prenatal maternal stress affects cognitive and linguistic functioning in 5 1/2-year-old children. J. Am. Acad. Child Adolesc. Psychiatry 2008, 47, 1063–1072. [Google Scholar] [CrossRef]
- Brown, R.W.; Diaz, R.; Robson, A.C.; Kotelevtsev, Y.V.; Mullins, J.J.; Kaufman, M.H.; Seckl, J.R. The ontogeny of 11 beta-hydroxysteroid dehydrogenase type 2 and mineralocorticoid receptor gene expression reveal intricate control of glucocorticoid action in development. Endocrinology 1996, 137, 794–797. [Google Scholar] [CrossRef]
- Brown, R.W.; Chapman, K.E.; Kotelevtsev, Y.; Yau, J.L.; Lindsay, R.S.; Brett, L.; Leckie, C.; Murad, P.; Lyons, V.; Mullins, J.J.; et al. Cloning and production of antisera to human placental 11 beta-hydroxysteroid dehydrogenase type 2. Biochem. J. 1996, 313 Pt 3, 1007–1017. [Google Scholar] [CrossRef]
- Wyrwoll, C.S.; Holmes, M.C.; Seckl, J.R. 11beta-hydroxysteroid dehydrogenases and the brain: From zero to hero, a decade of progress. Front. Neuroendocr. 2011, 32, 265–286. [Google Scholar] [CrossRef]
- Seckl, J.R.; Walker, B.R. Minireview: 11beta-hydroxysteroid dehydrogenase type 1- a tissue-specific amplifier of glucocorticoid action. Endocrinology 2001, 142, 1371–1376. [Google Scholar] [CrossRef]
- Welberg, L.A.; Thrivikraman, K.V.; Plotsky, P.M. Chronic maternal stress inhibits the capacity to up-regulate placental 11beta-hydroxysteroid dehydrogenase type 2 activity. J. Endocrinol. 2005, 186, R7–R12. [Google Scholar] [CrossRef]
- Monk, C.; Lugo-Candelas, C.; Trumpff, C. Prenatal Developmental Origins of Future Psychopathology: Mechanisms and Pathways. Annu. Rev. Clin. Psychol. 2019, 15, 317–344. [Google Scholar] [CrossRef] [PubMed]
- Thayer, Z.M.; Wilson, M.A.; Kim, A.W.; Jaeggi, A.V. Impact of prenatal stress on offspring glucocorticoid levels: A phylogenetic meta-analysis across 14 vertebrate species. Sci. Rep. 2018, 8, 4942. [Google Scholar] [CrossRef] [PubMed]
- Krontira, A.C.; Cruceanu, C.; Binder, E.B. Glucocorticoids as Mediators of Adverse Outcomes of Prenatal Stress. Trends Neurosci. 2020, 43, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Jensen Pena, C.; Monk, C.; Champagne, F.A. Epigenetic effects of prenatal stress on 11beta-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain. PLoS ONE 2012, 7, e39791. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav. Immun. 2005, 19, 296–308. [Google Scholar] [CrossRef]
- Lu, W.T.; Zhao, X.C.; Wang, R.; Li, N.; Song, M.; Wang, L.; Yu, L.L.; Gao, Y.Y.; Wang, Y.M.; An, C.X.; et al. Long-term effects of early stress due to earthquake exposure on depression symptoms in adulthood: A cross-sectional study. Injury 2023, 54, 207–213. [Google Scholar] [CrossRef]
- Guo, C.; He, P.; Song, X.; Zheng, X. Long-term effects of prenatal exposure to earthquake on adult schizophrenia. Br. J. Psychiatry 2019, 215, 730–735. [Google Scholar] [CrossRef]
- Dancause, K.N.; Veru, F.; Andersen, R.E.; Laplante, D.P.; King, S. Prenatal stress due to a natural disaster predicts insulin secretion in adolescence. Early Hum. Dev. 2013, 89, 773–776. [Google Scholar] [CrossRef]
- Paxman, E.J.; Boora, N.S.; Kiss, D.; Laplante, D.P.; King, S.; Montina, T.; Metz, G.A.S. Prenatal Maternal Stress from a Natural Disaster Alters Urinary Metabolomic Profiles in Project Ice Storm Participants. Sci. Rep. 2018, 8, 12932. [Google Scholar] [CrossRef]
- Virk, J.; Li, J.; Vestergaard, M.; Obel, C.; Kristensen, J.K.; Olsen, J. Prenatal exposure to bereavement and type-2 diabetes: A Danish longitudinal population based study. PLoS ONE 2012, 7, e43508. [Google Scholar] [CrossRef]
- St-Pierre, J.; Laplante, D.P.; Elgbeili, G.; Dawson, P.A.; Kildea, S.; King, S.; Vaillancourt, C. Natural disaster-related prenatal maternal stress is associated with alterations in placental glucocorticoid system: The QF2011 Queensland Flood Study. Psychoneuroendocrinology 2018, 94, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Ambeskovic, M.; Laplante, D.P.; Kenney, T.; Elgbeili, G.; Beaumier, P.; Azat, N.; Simcock, G.; Kildea, S.; King, S.; Metz, G.A.S. Elemental analysis of hair provides biomarkers of maternal hardship linked to adverse behavioural outcomes in 4-year-old children: The QF2011 Queensland Flood Study. J. Trace Elem. Med. Biol. 2022, 73, 127036. [Google Scholar] [CrossRef] [PubMed]
- Nugent, B.M.; Bale, T.L. The omniscient placenta: Metabolic and epigenetic regulation of fetal programming. Front. Neuroendocr. 2015, 39, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Kiss, D.; Ambeskovic, M.; Montina, T.; Metz, G.A. Stress transgenerationally programs metabolic pathways linked to altered mental health. Cell Mol. Life Sci. 2016, 73, 4547–4557. [Google Scholar] [CrossRef]
- Joseph, S.; Walejko, J.M.; Zhang, S.; Edison, A.S.; Keller-Wood, M. Maternal hypercortisolemia alters placental metabolism: A multiomics view. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E950–E960. [Google Scholar] [CrossRef]
- Mohammad, S.; Bhattacharjee, J.; Vasanthan, T.; Harris, C.S.; Bainbridge, S.A.; Adamo, K.B. Metabolomics to understand placental biology: Where are we now? Tissue Cell 2021, 73, 101663. [Google Scholar] [CrossRef]
- Bouatra, S.; Aziat, F.; Mandal, R.; Guo, A.C.; Wilson, M.R.; Knox, C.; Bjorndahl, T.C.; Krishnamurthy, R.; Saleem, F.; Liu, P.; et al. The human urine metabolome. PLoS ONE 2013, 8, e73076. [Google Scholar] [CrossRef]
- Costanzo, M.; Caterino, M.; Sotgiu, G.; Ruoppolo, M.; Franconi, F.; Campesi, I. Sex differences in the human metabolome. Biol. Sex Differ. 2022, 13, 30. [Google Scholar] [CrossRef]
- Metz, G.A.; Ng, J.W.; Kovalchuk, I.; Olson, D.M. Ancestral experience as a game changer in stress vulnerability and disease outcomes. Bioessays 2015, 37, 602–611. [Google Scholar] [CrossRef]
- Casas, E.; Vavouri, T. Mechanisms of epigenetic inheritance of variable traits through the germline. Reproduction 2020, 159, R251–R263. [Google Scholar] [CrossRef]
- Blaze, J.; Roth, T.L. Evidence from clinical and animal model studies of the long-term and transgenerational impact of stress on DNA methylation. Semin. Cell Dev. Biol. 2015, 43, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Azar, N.; Booij, L. DNA methylation as a mediator in the association between prenatal maternal stress and child mental health outcomes: Current state of knowledge. J. Affect. Disord. 2022, 319, 142–163. [Google Scholar] [CrossRef] [PubMed]
- Tracy, S.K.; Hartz, D.; Hall, B.; Allen, J.; Forti, A.; Lainchbury, A.; White, J.; Welsh, A.; Tracy, M.; Kildea, S. A randomised controlled trial of caseload midwifery care: M@NGO (Midwives @ New Group practice Options). BMC Pregnancy Childbirth 2011, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Tracy, S.K.; Hartz, D.L.; Tracy, M.B.; Allen, J.; Forti, A.; Hall, B.; White, J.; Lainchbury, A.; Stapleton, H.; Beckmann, M.; et al. Caseload midwifery care versus standard maternity care for women of any risk: M@NGO, a randomised controlled trial. Lancet 2013, 382, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Simcock, G.; Laplante, D.P.; Elgbeili, G.; Kildea, S.; Cobham, V.; Stapleton, H.; King, S. Infant Neurodevelopment is Affected by Prenatal Maternal Stress: The QF2011 Queensland Flood Study. Infancy 2017, 22, 282–302. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Laplante, D.P.; Elgbeili, G.; Brunet, A.; Simcock, G.; Kildea, S.; King, S. Coping during Pregnancy Following Exposure to a Natural Disaster: The QF2011 Queensland Flood Study. J. Affect. Disord. 2020, 273, 341–349. [Google Scholar] [CrossRef]
- Weiss, D.S.; Marmar, C.R. The Impact of Event Scale-Revised. In Assessing Psychological Trauma and PTSD: A Practitioner’s Handbook; Wilson, J.P., Keane, T.M., Eds.; The Guilford Press: New York, NY, USA, 1997; pp. 399–411. [Google Scholar]
- Brunet, A.; Weiss, D.S.; Metzler, T.J.; Best, S.R.; Neylan, T.C.; Rogers, C.; Fagan, J.; Marmar, C.R. The Peritraumatic Distress Inventory: A proposed measure of PTSD criterion A2. Am. J. Psychiatry 2001, 158, 1480–1485. [Google Scholar] [CrossRef]
- Marmar, C.R.; Weiss, D.S.; Metzler, T.J. The Peritraumatic Dissociative Experiences Questionnaire. In Assessing Psychological Trauma and PTSD; The Guilford Press: New York, NY, USA, 1997; pp. 412–428. [Google Scholar]
- Brock, R.L.; O’Hara, M.W.; Hart, K.J.; McCabe-Beane, J.E.; Williamson, J.A.; Brunet, A.; Laplante, D.P.; Yu, C.; King, S. Peritraumatic Distress Mediates the Effect of Severity of Disaster Exposure on Perinatal Depression: The Iowa Flood Study. J. Trauma Stress 2015, 28, 515–522. [Google Scholar] [CrossRef]
- Boudou, M.; Sejourne, N.; Chabrol, H. Childbirth pain, perinatal dissociation and perinatal distress as predictors of posttraumatic stress symptoms. Gynecol. Obs. Fertil. 2007, 35, 1136–1142. [Google Scholar] [CrossRef]
- Veselkov, K.A.; Lindon, J.C.; Ebbels, T.M.; Crockford, D.; Volynkin, V.V.; Holmes, E.; Davies, D.B.; Nicholson, J.K. Recursive segment-wise peak alignment of biological (1)h NMR spectra for improved metabolic biomarker recovery. Anal. Chem. 2009, 81, 56–66. [Google Scholar] [CrossRef]
- Anderson, P.E.; Mahle, D.A.; Doom, T.E.; Reo, N.V.; DelRaso, N.J.; Raymer, M.L. Dynamic adaptive binning: An improved quantification technique for NMR spectroscopic data. Metabolomics 2010, 7, 179–190. [Google Scholar] [CrossRef]
- Craig, A.; Cloarec, O.; Holmes, E.; Nicholson, J.K.; Lindon, J.C. Scaling and normalization effects in NMR spectroscopic metabonomic data sets. Anal. Chem. 2006, 78, 2262–2267. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, A.M.; Romick-Rosendale, L.E.; Kennedy, M.A. Statistical significance analysis of nuclear magnetic resonance-based metabonomics data. Anal. Biochem. 2010, 401, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.-H.; Liang, F.; Deng, B.-C.; Lai, G.-B.; Vicente Gonçalves, C.M.; Lu, H.-M.; Yan, J.; Huang, X.; Yi, L.-Z.; Liang, Y.-Z. Informative metabolites identification by variable importance analysis based on random variable combination. Metabolomics 2015, 11, 1539–1551. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. Multivariate Analysis in Metabolomics. Curr. Metab. 2013, 1, 92–107. [Google Scholar] [CrossRef]
- Szymanska, E.; Saccenti, E.; Smilde, A.K.; Westerhuis, J.A. Double-check: Validation of diagnostic statistics for PLS-DA models in metabolomics studies. Metabolomics 2012, 8, 3–16. [Google Scholar] [CrossRef]
- Westerhuis, J.A.; Hoefsloot, H.C.J.; Smit, S.; Vis, D.J.; Smilde, A.K.; van Velzen, E.J.J.; van Duijnhoven, J.P.M.; van Dorsten, F.A. Assessment of PLSDA cross validation. Metabolomics 2008, 4, 81–89. [Google Scholar] [CrossRef]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Eisner, R.; Young, N.; Gautam, B.; Hau, D.D.; Psychogios, N.; Dong, E.; Bouatra, S.; et al. HMDB: A knowledgebase for the human metabolome. Nucleic Acids Res. 2009, 37, D603–D610. [Google Scholar] [CrossRef]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0--The Human Metabolome Database in 2013. Nucleic Acids Res. 2013, 41, D801–D807. [Google Scholar] [CrossRef]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The Human Metabolome Database. Nucleic Acids Res. 2007, 35, D521–D526. [Google Scholar] [CrossRef]
- Salim, S. Oxidative stress: A potential link between emotional wellbeing and immune response. Curr. Opin. Pharm. 2016, 29, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Dowell, J.; Elser, B.A.; Schroeder, R.E.; Stevens, H.E. Cellular stress mechanisms of prenatal maternal stress: Heat shock factors and oxidative stress. Neurosci. Lett. 2019, 709, 134368. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T. Conversion of psychological stress into cellular stress response: Roles of the sigma-1 receptor in the process. Psychiatry Clin. Neurosci. 2015, 69, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Eick, S.M.; Barrett, E.S.; van ‘t Erve, T.J.; Nguyen, R.H.N.; Bush, N.R.; Milne, G.; Swan, S.H.; Ferguson, K.K. Association between prenatal psychological stress and oxidative stress during pregnancy. Paediatr. Perinat. Epidemiol. 2018, 32, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Wittenbecher, C.; Guasch-Ferre, M.; Haslam, D.E.; Dennis, C.; Li, J.; Bhupathiraju, S.N.; Lee, C.H.; Qi, Q.; Liang, L.; Eliassen, A.H.; et al. Changes in metabolomics profiles over ten years and subsequent risk of developing type 2 diabetes: Results from the Nurses’ Health Study. EBioMedicine 2022, 75, 103799. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Li, X.; Deng, X.; Kong, Y.; Wang, W.; Zhou, Y. Machine learning of plasma metabolome identifies biomarker panels for metabolic syndrome: Findings from the China Suboptimal Health Cohort. Cardiovasc. Diabetol. 2022, 21, 288. [Google Scholar] [CrossRef]
- Chen, M.; Yang, Z.; Gan, H.; Wang, Y.; Li, C.; Gao, Y. Investigation into potential mechanisms of metabolic syndrome by integrative analysis of metabolomics and proteomics. PLoS ONE 2022, 17, e0270593. [Google Scholar] [CrossRef]
- Hernandez-Baixauli, J.; Quesada-Vázquez, S.; Mariné-Casadó, R.; Gil Cardoso, K.; Caimari, A.; Del Bas, J.M.; Escoté, X.; Baselga-Escudero, L. Detection of Early Disease Risk Factors Associated with Metabolic Syndrome: A New Era with the NMR Metabolomics Assessment. Nutrients 2020, 12, 806. [Google Scholar] [CrossRef]
- Pompella, A.; Visvikis, A.; Paolicchi, A.; De Tata, V.; Casini, A.F. The changing faces of glutathione, a cellular protagonist. Biochem. Pharmacol. 2003, 66, 1499–1503. [Google Scholar] [CrossRef]
- Ripps, H.; Shen, W. Review: Taurine: A “very essential” amino acid. Mol. Vis. 2012, 18, 2673–2686. [Google Scholar]
- Hanna, P.E.; Anders, M.W. The mercapturic acid pathway. Crit. Rev. Toxicol. 2019, 49, 819–929. [Google Scholar] [CrossRef] [PubMed]
- Waterfield, C.J.; Turton, J.A.; Scales, M.D.; Timbrell, J.A. Taurine, a possible urinary marker of liver damage: A study of taurine excretion in carbon tetrachloride-treated rats. Arch. Toxicol. 1991, 65, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Connor, S.C.; Hansen, M.K.; Corner, A.; Smith, R.F.; Ryan, T.E. Integration of metabolomics and transcriptomics data to aid biomarker discovery in type 2 diabetes. Mol. Biosyst. 2010, 6, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Martin-Lorenzo, M.; Zubiri, I.; Maroto, A.S.; Gonzalez-Calero, L.; Posada-Ayala, M.; de la Cuesta, F.; Mourino-Alvarez, L.; Lopez-Almodovar, L.F.; Calvo-Bonacho, E.; Ruilope, L.M.; et al. KLK1 and ZG16B proteins and arginine-proline metabolism identified as novel targets to monitor atherosclerosis, acute coronary syndrome and recovery. Metabolomics 2015, 11, 1056–1067. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Qiu, X.; Wang, R.; Wang, D. (1)H NMR-based metabolomics study of the dynamic effect of Xue-Fu-Zhu-Yu capsules on coronary heart disease rats induced by high-fat diet, coronary artery ligation. J. Pharm. Biomed. Anal. 2021, 195, 113869. [Google Scholar] [CrossRef] [PubMed]
- Oudit, G.Y.; Trivieri, M.G.; Khaper, N.; Husain, T.; Wilson, G.J.; Liu, P.; Sole, M.J.; Backx, P.H. Taurine supplementation reduces oxidative stress and improves cardiovascular function in an iron-overload murine model. Circulation 2004, 109, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, P.I.; Gardner-Stephen, D.A.; Miners, J.O. UDP-Glucuronosyltransferases*. In Comprehensive Toxicology; McQueen, C.A., Ed.; Elsevier: Oxford, UK, 2010; pp. 413–434. [Google Scholar]
- Sanchez, R.I.; Kauffman, F.C. Regulation of Xenobiotic Metabolism in the Liver. In Comprehensive Toxicology; McQueen, C.A., Ed.; Elsevier: Oxford, UK, 2010; pp. 109–128. [Google Scholar]
- Testa, B.; Clement, B. Biotransformation Reactions and their Enzymes. In The Practice of Medicinal Chemistry; Wermuth, C.G., Aldous, D., Raboisson, P., Rognan, D., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 561–584. [Google Scholar]
- Kander, M.C.; Cui, Y.; Liu, Z. Gender difference in oxidative stress: A new look at the mechanisms for cardiovascular diseases. J. Cell. Mol. Med. 2017, 21, 1024–1032. [Google Scholar] [CrossRef]
- Buss, I.H.; Senthilmohan, R.; Darlow, B.A.; Mogridge, N.; Kettle, A.J.; Winterbourn, C.C. 3-Chlorotyrosine as a marker of protein damage by myeloperoxidase in tracheal aspirates from preterm infants: Association with adverse respiratory outcome. Pediatr. Res. 2003, 53, 455–462. [Google Scholar] [CrossRef]
- Mita, H.; Higashi, N.; Taniguchi, M.; Higashi, A.; Kawagishi, Y.; Akiyama, K. Urinary 3-bromotyrosine and 3-chlorotyrosine concentrations in asthmatic patients: Lack of increase in 3-bromotyrosine concentration in urine and plasma proteins in aspirin-induced asthma after intravenous aspirin challenge. Clin. Exp. Allergy 2004, 34, 931–938. [Google Scholar] [CrossRef]
- MacPherson, J.C.; Comhair, S.A.; Erzurum, S.C.; Klein, D.F.; Lipscomb, M.F.; Kavuru, M.S.; Samoszuk, M.K.; Hazen, S.L. Eosinophils are a major source of nitric oxide-derived oxidants in severe asthma: Characterization of pathways available to eosinophils for generating reactive nitrogen species. J. Immunol. 2001, 166, 5763–5772. [Google Scholar] [CrossRef]
- Hazen, S.L.; Heinecke, J.W. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J. Clin. Investig. 1997, 99, 2075–2081. [Google Scholar] [CrossRef]
- Rosa, M.J.; Lee, A.G.; Wright, R.J. Evidence establishing a link between prenatal and early-life stress and asthma development. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.L.; Miller, G.E.; Brehm, J.M.; Celedon, J.C. Stress and asthma: Novel insights on genetic, epigenetic, and immunologic mechanisms. J. Allergy Clin. Immunol. 2014, 134, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Toskala, E.; Kennedy, D.W. Asthma risk factors. Int. Forum Allergy Rhinol. 2015, 5 (Suppl. S1), S11–S16. [Google Scholar] [CrossRef] [PubMed]
- Ambeskovic, M.; Ilnytskyy, Y.; Kiss, D.; Currie, C.; Montina, T.; Kovalchuk, I.; Metz, G.A.S. Ancestral stress programs sex-specific biological aging trajectories and non-communicable disease risk. Aging 2020, 12, 3828–3847. [Google Scholar] [CrossRef] [PubMed]
- Poplawski, J.; Radmilovic, A.; Montina, T.D.; Metz, G.A.S. Cardiorenal metabolic biomarkers link early life stress to risk of non-communicable diseases and adverse mental health outcomes. Sci. Rep. 2020, 10, 13295. [Google Scholar] [CrossRef]
- Scott, H.D.; Buchan, M.; Chadwick, C.; Field, C.J.; Letourneau, N.; Montina, T.; Leung, B.M.Y.; Metz, G.A.S. Metabolic dysfunction in pregnancy: Fingerprinting the maternal metabolome using proton nuclear magnetic resonance spectroscopy. Endocrinol. Diabetes Metab. 2021, 4, e00201. [Google Scholar] [CrossRef]
- Alves, A.; Bassot, A.; Bulteau, A.L.; Pirola, L.; Morio, B. Glycine Metabolism and Its Alterations in Obesity and Metabolic Diseases. Nutrients 2019, 11, 1356. [Google Scholar] [CrossRef]
- Messana, I.; Forni, F.; Ferrari, F.; Rossi, C.; Giardina, B.; Zuppi, C. Proton nuclear magnetic resonance spectral profiles of urine in type II diabetic patients. Clin. Chem. 1998, 44, 1529–1534. [Google Scholar] [CrossRef]
- Salek, R.M.; Maguire, M.L.; Bentley, E.; Rubtsov, D.V.; Hough, T.; Cheeseman, M.; Nunez, D.; Sweatman, B.C.; Haselden, J.N.; Cox, R.D.; et al. A metabolomic comparison of urinary changes in type 2 diabetes in mouse, rat, and human. Physiol. Genom. 2007, 29, 99–108. [Google Scholar] [CrossRef]
- Xie, B.; Waters, M.J.; Schirra, H.J. Investigating potential mechanisms of obesity by metabolomics. J. Biomed. Biotechnol. 2012, 2012, 805683. [Google Scholar] [CrossRef] [PubMed]
- Yousri, N.A.; Mook-Kanamori, D.O.; Selim, M.M.; Takiddin, A.H.; Al-Homsi, H.; Al-Mahmoud, K.A.; Karoly, E.D.; Krumsiek, J.; Do, K.T.; Neumaier, U.; et al. A systems view of type 2 diabetes-associated metabolic perturbations in saliva, blood and urine at different timescales of glycaemic control. Diabetologia 2015, 58, 1855–1867. [Google Scholar] [CrossRef] [PubMed]
- Fonteh, A.N.; Harrington, R.J.; Tsai, A.; Liao, P.; Harrington, M.G. Free amino acid and dipeptide changes in the body fluids from Alzheimer’s disease subjects. Amino Acids 2007, 32, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.M.; Mostafa, H.; Arif, N.H.; Abdul Kader, M.A.S.; Kah Hay, Y. Metabolomics profiling and pathway analysis of human plasma and urine reveal further insights into the multifactorial nature of coronary artery disease. Clin. Chim. Acta 2019, 493, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Bird, R.P. Chapter Four—The Emerging Role of Vitamin B6 in Inflammation and Carcinogenesis. In Advances in Food and Nutrition Research; Eskin, N.A.M., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 83, pp. 151–194. [Google Scholar]
- Hellmann, H.; Mooney, S. Vitamin B6: A molecule for human health? Molecules 2010, 15, 442–459. [Google Scholar] [CrossRef]
- Smith, A.D.; Refsum, H.; Bottiglieri, T.; Fenech, M.; Hooshmand, B.; McCaddon, A.; Miller, J.W.; Rosenberg, I.H.; Obeid, R. Homocysteine and Dementia: An International Consensus Statement. J. Alzheimers Dis. 2018, 62, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y. Seizures caused by pyridoxine (vitamin B6) deficiency in adults: A case report and literature review. Intractable Rare Dis. Res. 2014, 3, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M.; McCann, A.; Midttun, O.; Ulvik, A. Inflammation, vitamin B6 and related pathways. Mol. Asp. Med. 2017, 53, 10–27. [Google Scholar] [CrossRef]
- Posada-Ayala, M.; Zubiri, I.; Martin-Lorenzo, M.; Sanz-Maroto, A.; Molero, D.; Gonzalez-Calero, L.; Fernandez-Fernandez, B.; de la Cuesta, F.; Laborde, C.M.; Barderas, M.G.; et al. Identification of a urine metabolomic signature in patients with advanced-stage chronic kidney disease. Kidney Int. 2014, 85, 103–111. [Google Scholar] [CrossRef]
- Wang, R.; Reddy, P.H. Role of Glutamate and NMDA Receptors in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, T.; Sun, L.; Zhao, Z.; Qi, X.; Zhou, K.; Cao, Y.; Wang, X.; Qiu, Y.; Su, M.; et al. Potential metabolite markers of schizophrenia. Mol. Psychiatry 2013, 18, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Cui, Y.; An, Z.; Yang, Q.; Zou, X.; Yu, N. Attenuated glutamate induced ROS production by antioxidative compounds in neural cell lines. RSC Adv. 2019, 9, 34735–34743. [Google Scholar] [CrossRef] [PubMed]
- Spate, U.; Schulze, P.C. Proinflammatory cytokines and skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 265–269. [Google Scholar] [CrossRef]
- Zoico, E.; Roubenoff, R. The role of cytokines in regulating protein metabolism and muscle function. Nutr. Rev. 2002, 60, 39–51. [Google Scholar] [CrossRef] [PubMed]
- van Hall, G. Cytokines: Muscle protein and amino acid metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Veru, F.; Dancause, K.; Laplante, D.P.; King, S.; Luheshi, G. Prenatal maternal stress predicts reductions in CD4+ lymphocytes, increases in innate-derived cytokines, and a Th2 shift in adolescents: Project Ice Storm. Physiol. Behav. 2015, 144, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Weiner, I.D.; Verlander, J.W. Recent advances in understanding renal ammonia metabolism and transport. Curr. Opin. Nephrol. Hypertens. 2016, 25, 436–443. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Khare, P.; Nagar, H.K.; Raghuwanshi, N.; Srivastava, R. Hydroxyproline: A Potential Biochemical Marker and Its Role in the Pathogenesis of Different Diseases. Curr. Protein Pept. Sci. 2016, 17, 596–602. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, J.; Li, S.; Edwards, J.L. Amine metabolomics of hyperglycemic endothelial cells using capillary LC-MS with isobaric tagging. J. Proteome Res. 2011, 10, 5242–5250. [Google Scholar] [CrossRef]
- Wijekoon, E.P.; Skinner, C.; Brosnan, M.E.; Brosnan, J.T. Amino acid metabolism in the Zucker diabetic fatty rat: Effects of insulin resistance and of type 2 diabetes. Can. J. Physiol. Pharm. 2004, 82, 506–514. [Google Scholar] [CrossRef]
- Lin, H.; Levison, B.S.; Buffa, J.A.; Huang, Y.; Fu, X.; Wang, Z.; Gogonea, V.; DiDonato, J.A.; Hazen, S.L. Myeloperoxidase-mediated protein lysine oxidation generates 2-aminoadipic acid and lysine nitrile in vivo. Free. Radic. Biol. Med. 2017, 104, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.G.; O’Neill, E.M.; Pollitt, R.J. Alpha-aminoadipic aciduria: Chemical and enzymatic studies. J. Inherit. Metab. Dis. 1980, 2, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Park, S.G.; Schimmel, P.; Kim, S. Aminoacyl tRNA synthetases and their connections to disease. Proc. Natl. Acad. Sci. USA 2008, 105, 11043–11049. [Google Scholar] [CrossRef] [PubMed]
- Dancause, K.N.; Laplante, D.P.; Fraser, S.; Brunet, A.; Ciampi, A.; Schmitz, N.; King, S. Prenatal exposure to a natural disaster increases risk for obesity in 5(1/2)-year-old children. Pediatr. Res. 2012, 71, 126–131. [Google Scholar] [CrossRef]
- Lipner, E.; Murphy, S.K.; Ellman, L.M. Prenatal Maternal Stress and the Cascade of Risk to Schizophrenia Spectrum Disorders in Offspring. Curr. Psychiatry Rep. 2019, 21, 99. [Google Scholar] [CrossRef]
- Beydoun, H.; Saftlas, A.F. Physical and mental health outcomes of prenatal maternal stress in human and animal studies: A review of recent evidence. Paediatr. Perinat. Epidemiol. 2008, 22, 438–466. [Google Scholar] [CrossRef]
- Ronald, A.; Pennell, C.E.; Whitehouse, A.J. Prenatal Maternal Stress Associated with ADHD and Autistic Traits in early Childhood. Front. Psychol. 2010, 1, 223. [Google Scholar] [CrossRef]
- Chen, J.J.; Zhou, C.J.; Zheng, P.; Cheng, K.; Wang, H.Y.; Li, J.; Zeng, L.; Xie, P. Differential urinary metabolites related with the severity of major depressive disorder. Behav. Brain Res. 2017, 332, 280–287. [Google Scholar] [CrossRef]
- Liang, Y.; Xiao, Z.; Ke, X.; Yao, P.; Chen, Y.; Lin, L.; Lu, J. Urinary Metabonomic Profiling Discriminates Between Children with Autism and Their Healthy Siblings. Med. Sci. Monit 2020, 26, e926634. [Google Scholar] [CrossRef]
- Mavel, S.; Nadal-Desbarats, L.; Blasco, H.; Bonnet-Brilhault, F.; Barthelemy, C.; Montigny, F.; Sarda, P.; Laumonnier, F.; Vourc’h, P.; Andres, C.R.; et al. 1H-13C NMR-based urine metabolic profiling in autism spectrum disorders. Talanta 2013, 114, 95–102. [Google Scholar] [CrossRef]
- Tian, J.S.; Peng, G.J.; Gao, X.X.; Zhou, Y.Z.; Xing, J.; Qin, X.M.; Du, G.H. Dynamic analysis of the endogenous metabolites in depressed patients treated with TCM formula Xiaoyaosan using urinary (1)H NMR-based metabolomics. J. Ethnopharmacol. 2014, 158 Pt A, 1–10. [Google Scholar] [CrossRef]
- Cai, H.L.; Li, H.D.; Yan, X.Z.; Sun, B.; Zhang, Q.; Yan, M.; Zhang, W.Y.; Jiang, P.; Zhu, R.H.; Liu, Y.P.; et al. Metabolomic analysis of biochemical changes in the plasma and urine of first-episode neuroleptic-naive schizophrenia patients after treatment with risperidone. J. Proteome Res. 2012, 11, 4338–4350. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Rammal, H.; Soulimani, R. Oxidative stress and anxiety: Relationship and cellular pathways. Oxid Med. Cell Longev. 2009, 2, 63–67. [Google Scholar] [CrossRef]
- Hornor, G. Resilience. J. Pediatr. Health Care 2017, 31, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Osorio, C.; Probert, T.; Jones, E.; Young, A.H.; Robbins, I. Adapting to Stress: Understanding the Neurobiology of Resilience. Behav. Med. 2017, 43, 307–322. [Google Scholar] [CrossRef] [PubMed]
- McCreary, J.K.; Metz, G.A.S. Environmental enrichment as an intervention for adverse health outcomes of prenatal stress. Environ. Epigenetics 2016, 2, dvw013. [Google Scholar] [CrossRef]
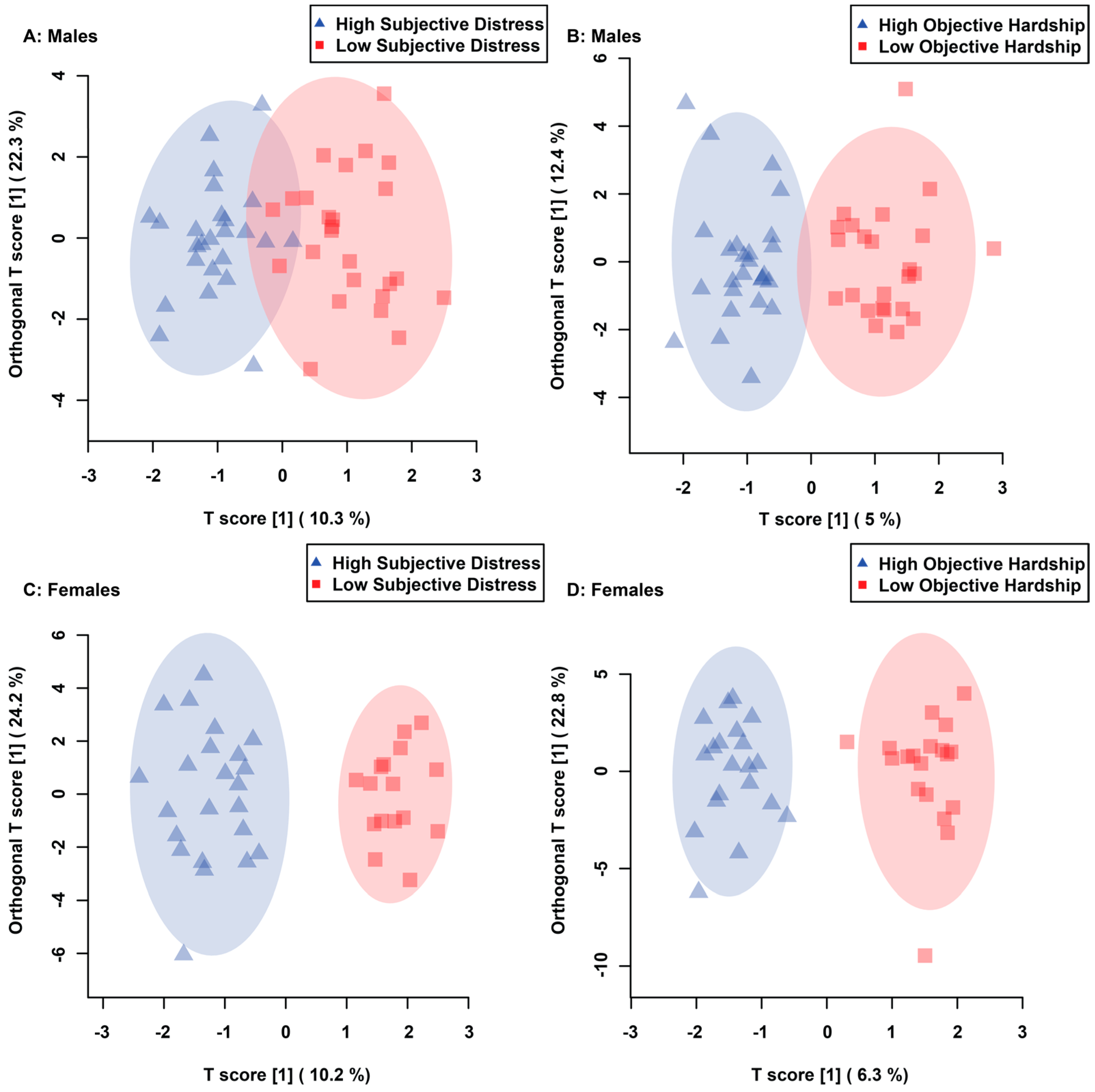
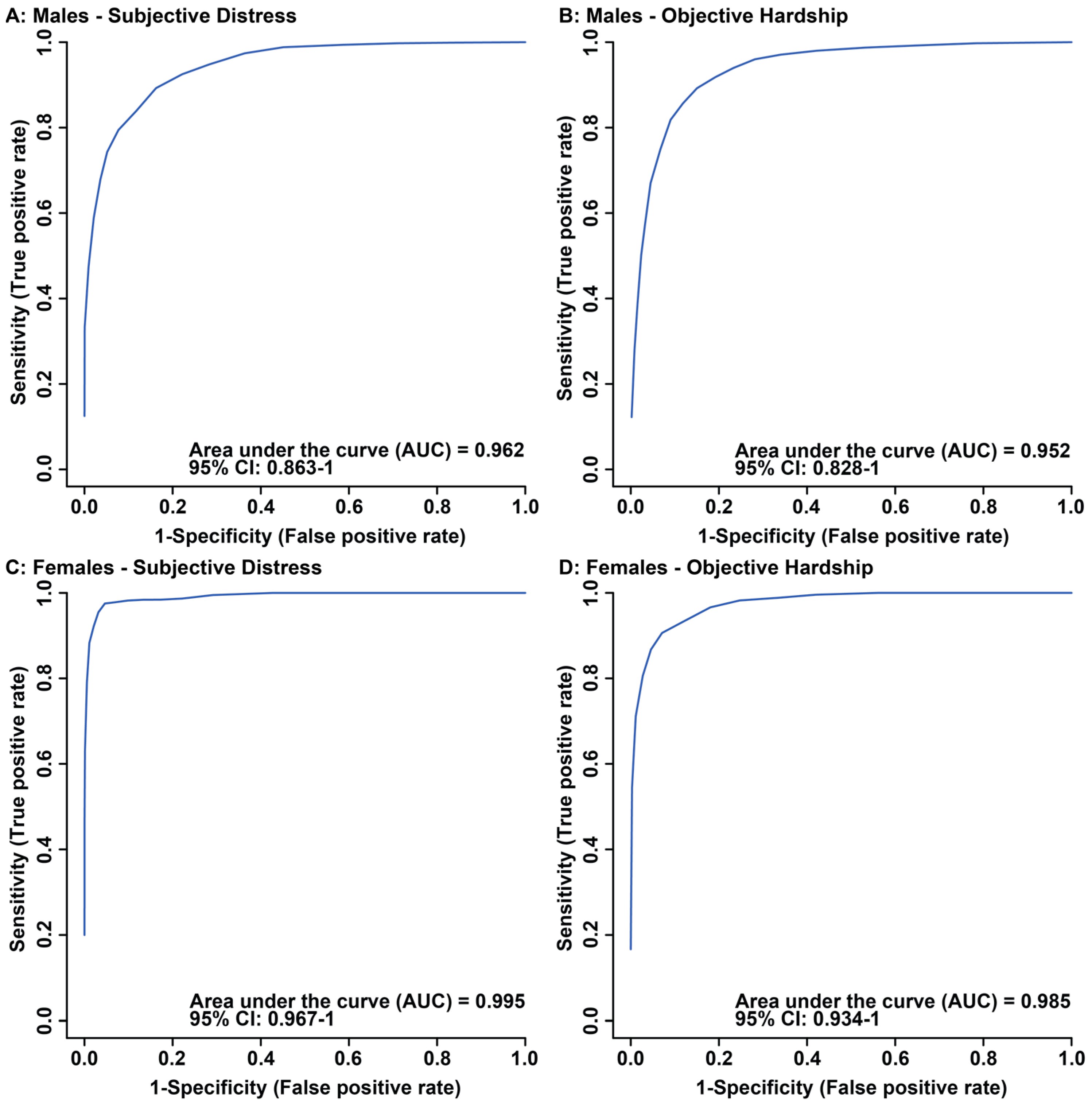
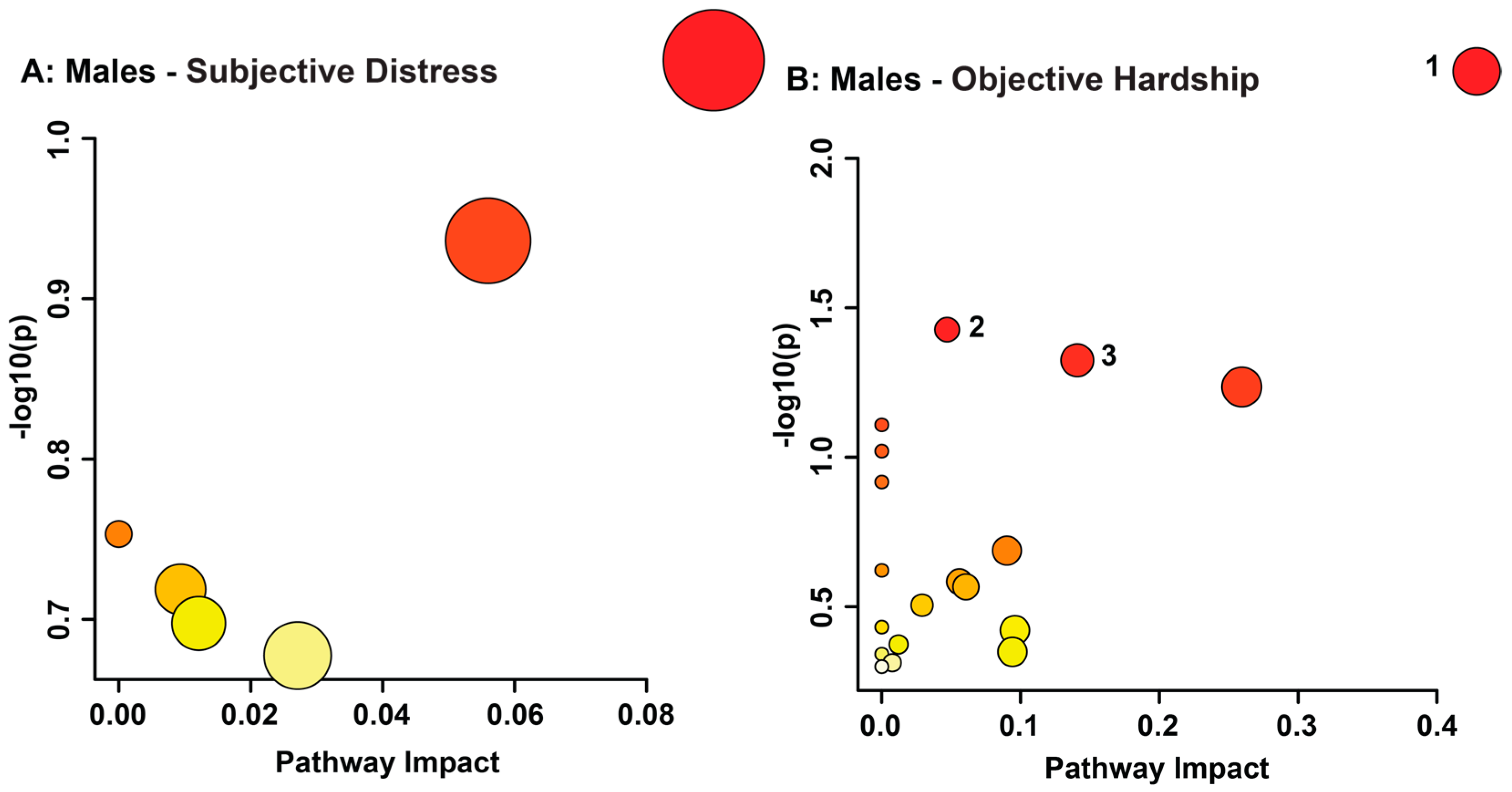
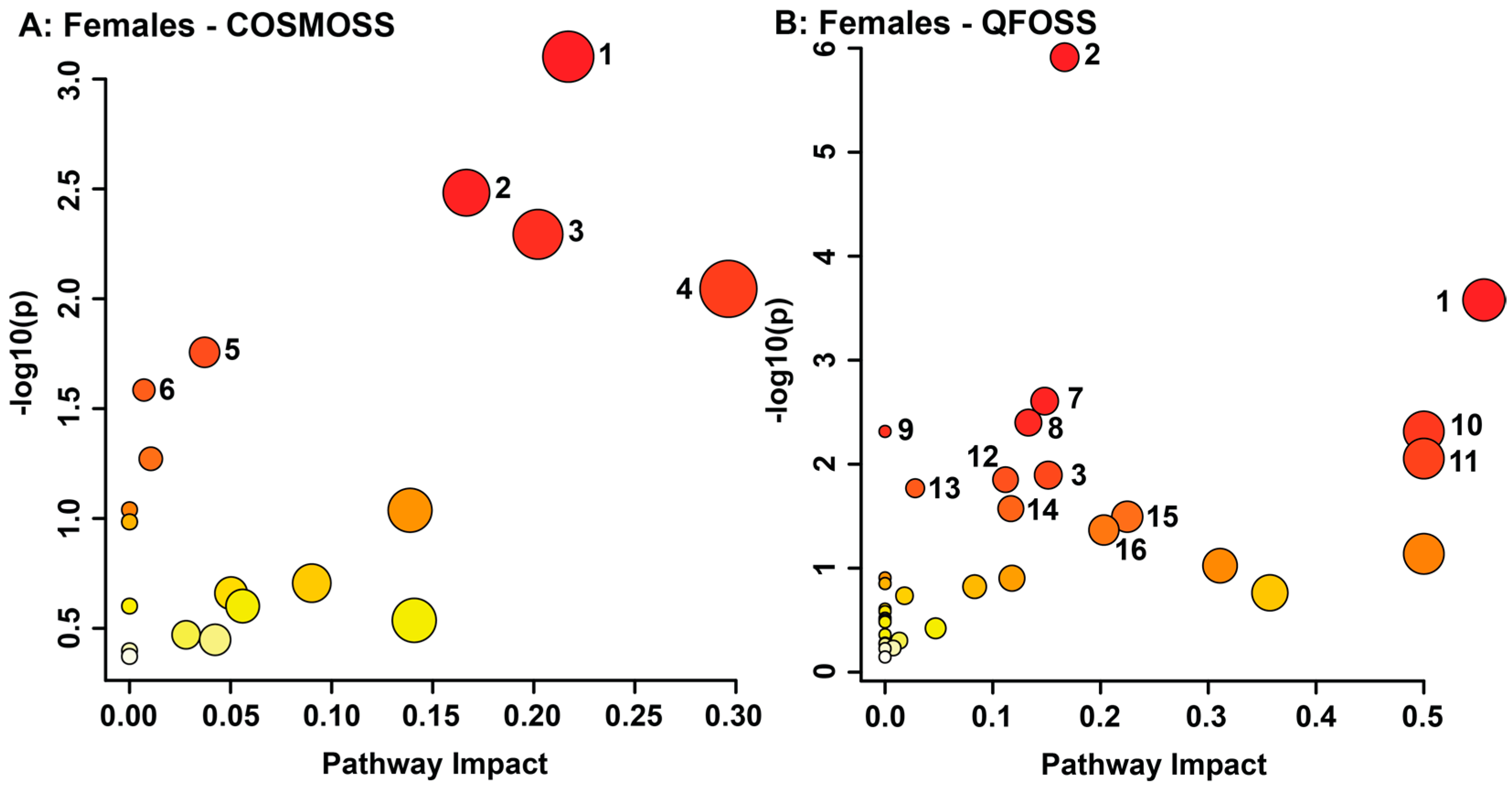
| Group | Metabolite | NMR Chemical Shift Range of Bin (ppm) | VIAVC | Mann-Whitney U Test | Regulation |
|---|---|---|---|---|---|
| Male Subjective Distress (COSMOSS) | 3-Hydroxyisovalerate | 1.281–1.27 | 5.98 × 10−122 | Not sig. | Up |
| Indole-3-lactate.1 | 7.285–7.263 | 3.56 × 10−82 | Not sig. | Up | |
| Creatine.1 | 3.947–3.929 | 1.32 × 10−65 | 1.04 × 10−2 | Down | |
| Glycylproline | 3.929–3.916 | 3.92 × 10−53 | Not sig. | Down | |
| Methylguanidine | 2.875–2.86 | 6.83 × 10−48 | Not sig. | Down | |
| Citramalic acid | 2.75–2.733 | 5.51 × 10−37 | Not sig. | Down | |
| Creatine.2 | 3.045–3.03 | 2.19 × 10−36 | 2.57 × 10−2 | Down | |
| Carnosine | 2.671–2.662 | 3.09 × 10−30 | Not sig. | Up | |
| Carnitine | 3.227–3.215 | 1.20 × 10−29 | Not sig. | Down | |
| 3-Chlorotyrosine | 7.129–7.116 | 3.25 × 10−27 | Not sig. | Down | |
| 3-Methylhistamine | 3.723–3.712 | 1.40 × 10−23 | 2.17 × 10−3 | Up | |
| Indole-3-lactate.2 | 4.383–4.33 | 2.10 × 10−23 | 2.04 × 10−3 | Up | |
| (S)-3-Hydroxyisobutyric acid | 1.116–1.105 | 2.98 × 10−23 | Not sig. | Down | |
| O-Phosphocholine | 3.241–3.227 | 4.17 × 10−19 | 4.45 × 10−4 | Down | |
| Male Objective Hardship (QFOSS) | Xanthurenate | 7.105–7.095 | 1.41 × 10−83 | Not sig. | Up |
| Cysteine | 3.011–2.996 | 2.01 × 10−65 | Not sig. | Down | |
| 3-Aminoisobutyrate.1 | 3.118–3.101 | 3.02 × 10−48 | Not sig. | Down | |
| Creatine.1 | 3.947–3.929 | 5.98 × 10−47 | Not sig. | Down | |
| Creatinine.1 | 3.066–3.045 | 1.06 × 10−41 | Not sig. | Down | |
| Erythritol, Glycylproline | 3.643–3.604 | 1.78 × 10−35 | Not sig. | Up | |
| Gluconate.1 | 4.053–4.03 | 5.96 × 10−34 | 8.03 × 10−3 | Up | |
| Dimethyl sulfone | 3.164–3.156 | 2.37 × 10−33 | Not sig. | Up | |
| Acetate | 1.932–1.919 | 3.58 × 10−30 | Not sig. | Up | |
| Creatine.2 | 3.045–3.03 | 5.73 × 10−28 | Not sig. | Down | |
| 5-Hydroxylysine.1 | 1.963–1.953 | 1.11 × 10−24 | Not sig. | Up | |
| Creatinine.2 | 4.081–4.053 | 9.63 × 10−24 | Not sig. | Down | |
| 2-Methylbutyroylcarnitine.1 | 0.8867–0.8768 | 5.24 × 10−23 | Not sig. | Down | |
| 5-Hydroxylysine.2, Glutathione | 2.963–2.942 | 3.40 × 10−22 | Not sig. | Up | |
| Carnosine.1 | 2.702–2.684 | 2.02 × 10−19 | Not sig. | Up | |
| 5-Hydroxylysine.3 | 3.172–3.164 | 7.56 × 10−19 | Not sig. | Up | |
| 3-Aminoisobutyrate.2 | 1.196–1.185 | 1.49 × 10−18 | Not sig. | Up | |
| 4-Pyridoxic acid | 2.358–2.339 | 7.62 × 10−17 | Not sig. | Up | |
| Vanylglycol | 3.375–3.366 | 1.19 × 10−15 | Not sig. | Up | |
| Gluconate.2 | 4.157–4.134 | 1.66 × 10−15 | 4.05 × 10−2 | Up | |
| Carnosine.2 | 2.671–2.662 | 2.03 × 10−15 | Not sig. | Up | |
| 2-Aminoadipate | 2.26–2.247 | 2.10 × 10−15 | Not sig. | Up | |
| Ribose | 4.134–4.106 | 3.55 × 10−14 | Not sig. | Up | |
| Allantoin | 5.401–5.381 | 3.92 × 10−14 | Not sig. | Down | |
| Taurine.1 | 3.448–3.437 | 5.86 × 10−14 | Not sig. | Up | |
| Taurine.2 | 3.426–3.418 | 6.32 × 10−14 | Not sig. | Up | |
| Citramalic acid | 2.75–2.733 | 1.32 × 10−13 | Not sig. | Up | |
| 2-Methylbutyroylcarnitine.2 | 0.8768–0.866 | 1.82 × 10−12 | Not Sig. | Down | |
| 3-Phenyllactate | 7.333–7.316 | 3.02 × 10−12 | 4.45 × 10−2 | Down | |
| 3-indoxylsulfate | 7.513–7.495 | 2.10 × 10−10 | Not sig. | Up | |
| Cysteine-S-sulfate | 3.524–3.511 | 1.01 × 10−09 | Not sig. | Down | |
| 3,4,5-Trimethoxycinnamic acid, Kynurenine | 6.815–6.776 | 1.39 × 10−09 | Not sig. | Down |
| Group | Metabolite | NMR Chemical Shift Range of Bin (ppm) | VIAVC | Mann-Whitney U Test | Regulation |
|---|---|---|---|---|---|
| Female Subjective Distress (COSMOSS) | Proline.1 | 3.409–3.4 | 1.56 × 10−99 | Not sig. | Up |
| Lactose.1 | 4.699–4.676 | 2.63 × 10−80 | 1.45 × 10−3 | Down | |
| Epinephrine | 2.797–2.779 | 2.41 × 10−77 | Not sig. | Down | |
| 5-Aminolevulinic acid | 2.806–2.797 | 6.22 × 10−77 | 1.35 × 10−2 | Down | |
| Cysteine, Serine | 3.966–3.947 | 1.68 × 10−69 | 2.50 × 10−2 | Down | |
| Galactose | 5.29–5.276 | 2.05 × 10−50 | 1.77 × 10−3 | Down | |
| 4-Hydroxyproline | 3.498–3.489 | 1.74 × 10−49 | 2.89 × 10−2 | Down | |
| Lactose.2 | 5.261–5.239 | 1.94 × 10−24 | 1.85 × 10−2 | Down | |
| Tyramine, Carnosine | 3.252–3.241 | 2.47 × 10−23 | 3.11 × 10−2 | Up | |
| Sucrose | 3.489–3.482 | 1.24 × 10−22 | Not sig. | Down | |
| Cystathionine | 2.208–2.198 | 8.34 × 10−22 | Not sig. | Up | |
| 2-Aminoadipate.1 | 2.26–2.247 | 5.47 × 10−21 | Not sig. | Up | |
| Chlorogenate | 5.357–5.352 | 1.11 × 10−16 | 2.15 × 10−2 | Down | |
| 5-Methoxytryptamine | 6.972–6.964 | 4.92 × 10−16 | 4.12 × 10−2 | Up | |
| Homovanillic acid | 6.776–6.739 | 2.43 × 10−15 | Not sig. | Up | |
| 3-Chlorotyrosine | 6.984–6.972 | 4.27 × 10−14 | Not sig. | Up | |
| 3-Nitrotyrosine | 7.028–7.02 | 1.27 × 10−13 | 4.41 × 10−2 | Up | |
| Indole-3-lactate | 7.775–7.762 | 2.25 × 10−13 | Not sig. | Up | |
| Proline.2 | 3.4–3.393 | 2.44 × 10−11 | Not sig. | Up | |
| Pantothenate | 0.9397–0.9273 | 8.19 × 10−11 | Not sig. | Down | |
| Isobutyrate | 1.085–1.075 | 1.50 × 10−8 | Not sig. | Down | |
| 2-Hydroxy-3-methylvalerate | 0.8528–0.8431 | 3.12 × 10−8 | Not sig. | Down | |
| Isoleucine | 1.012–1.003 | 3.29 × 10−8 | Not sig. | Up | |
| 2-Aminoadipate.2 | 1.695–1.653 | 1.08 × 10−6 | Not sig. | Up | |
| Pyroglutamate | 2.391–2.385 | 1.14 × 10−6 | Not sig. | Up | |
| Female Objective Hardship (QFOSS) | Epinephrine | 2.797–2.779 | 2.33 × 10−61 | Not sig. | Down |
| Cysteine, Serine.1 | 3.966–3.947 | 8.49 × 10−53 | 7.29 × 10−3 | Down | |
| Glycine | 3.586–3.564 | 3.61 × 10−52 | Not sig. | Up | |
| Serine.2 | 3.869–3.863 | 4.24 × 10−52 | Not sig. | Down | |
| (S)-3-Hydroxybutyric acid | 1.254–1.245 | 1.55 × 10−49 | Not sig. | Up | |
| Glutamine.1 | 2.163–2.152 | 1.11 × 10−41 | 3.89 × 10−2 | Up | |
| Serine.3 | 4.002–3.994 | 8.30 × 10−39 | Not sig. | Down | |
| Cysteine-S-sulfate | 3.536–3.524 | 9.29 × 10−37 | Not sig. | Down | |
| 3-Chlorotyrosine | 7.129–7.116 | 8.67 × 10−32 | Not sig. | Up | |
| Isobutyrate.1 | 1.085–1.075 | 2.59 × 10−30 | Not sig. | Down | |
| Erythritol.1 | 3.801–3.784 | 1.06 × 10−29 | 4.45 × 10−2 | Down | |
| Theophylline | 7.949–7.937 | 1.65 × 10−29 | Not sig. | Up | |
| 4-Hydroxyproline | 3.498–3.489 | 3.55 × 10−29 | Not sig. | Down | |
| Glucuronate | 4.66–4.649 | 6.10 × 10−27 | Not sig. | Up | |
| Erythritol.2, Lactose.1 | 3.643–3.604 | 5.15 × 10−23 | Not sig. | Down | |
| 3-Phenyllactate | 2.887–2.875 | 8.49 × 10−22 | Not sig. | Up | |
| Isobutyrate.2 | 1.075–1.065 | 7.82 × 10−19 | Not sig. | Down | |
| Glutamine.2 | 2.474–2.465 | 4.10 × 10−18 | Not sig. | Up | |
| Lactose.2 | 5.261–5.239 | 1.81 × 10−17 | Not sig. | Down | |
| Isoleucine | 1.036–1.012 | 1.02 × 10−16 | Not sig. | Up | |
| Fucose | 1.265–1.254 | 3.11 × 10−16 | Not sig. | Down | |
| Phenylalanine | 4.03–4.021 | 5.43 × 10−13 | 3.32 × 10−3 | Down | |
| Symmetric dimethylarginine | 2.85–2.817 | 2.32 × 10−12 | Not sig. | Down | |
| Saccharopine.1 | 2.425–2.417 | 1.14 × 10−11 | Not sig. | Down | |
| Sucrose | 3.489–3.482 | 1.32 × 10−10 | Not sig. | Down | |
| Maltose | 5.407–5.401 | 4.81 × 10−10 | Not sig. | Down | |
| Saccharopine.2 | 2.417–2.404 | 2.44 × 10−9 | Not sig. | Down | |
| Proline | 3.418–3.409 | 6.91 × 10−9 | Not sig. | Up | |
| Ethanolamine | 3.146–3.14 | 9.59 × 10−9 | Not sig. | Up | |
| Glutamate | 2.329–2.319 | 1.12 × 10−8 | Not sig. | Up | |
| UDP-glucose | 4.397–4.383 | 4.25 × 10−8 | 3.39 × 10−2 | Down | |
| 5-Aminolevulinic acid | 2.806–2.797 | 7.56 × 10−8 | Not sig. | Down | |
| Sarcosine | 2.768–2.75 | 8.93 × 10−8 | Not sig. | Down | |
| 3,4-Dihydroxybenzeneacetic acid | 6.739–6.715 | 2.07 × 10−7 | Not sig. | Up | |
| Unidentified Metabolite @ 8.65 ppm | 8.663–8.646 | 2.85 × 10−6 | Not sig. | Down | |
| Malonate | 3.133–3.118 | 3.07 × 10−6 | Not sig. | Down | |
| Unidentified Metabolite @ 0.55 ppm | 0.5574–0.535 | 3.29 × 10−6 | Not sig. | Up | |
| N-Acetylglutamine | 2.036–2.03 | 1.32 × 10−5 | Not sig. | Up | |
| Arabinose | 4.527–4.52 | 1.76 × 10−5 | Not sig. | Up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heynen, J.P.; McHugh, R.R.; Boora, N.S.; Simcock, G.; Kildea, S.; Austin, M.-P.; Laplante, D.P.; King, S.; Montina, T.; Metz, G.A.S. Urinary 1H NMR Metabolomic Analysis of Prenatal Maternal Stress Due to a Natural Disaster Reveals Metabolic Risk Factors for Non-Communicable Diseases: The QF2011 Queensland Flood Study. Metabolites 2023, 13, 579. https://doi.org/10.3390/metabo13040579
Heynen JP, McHugh RR, Boora NS, Simcock G, Kildea S, Austin M-P, Laplante DP, King S, Montina T, Metz GAS. Urinary 1H NMR Metabolomic Analysis of Prenatal Maternal Stress Due to a Natural Disaster Reveals Metabolic Risk Factors for Non-Communicable Diseases: The QF2011 Queensland Flood Study. Metabolites. 2023; 13(4):579. https://doi.org/10.3390/metabo13040579
Chicago/Turabian StyleHeynen, Joshua P., Rebecca R. McHugh, Naveenjyote S. Boora, Gabrielle Simcock, Sue Kildea, Marie-Paule Austin, David P. Laplante, Suzanne King, Tony Montina, and Gerlinde A. S. Metz. 2023. "Urinary 1H NMR Metabolomic Analysis of Prenatal Maternal Stress Due to a Natural Disaster Reveals Metabolic Risk Factors for Non-Communicable Diseases: The QF2011 Queensland Flood Study" Metabolites 13, no. 4: 579. https://doi.org/10.3390/metabo13040579
APA StyleHeynen, J. P., McHugh, R. R., Boora, N. S., Simcock, G., Kildea, S., Austin, M.-P., Laplante, D. P., King, S., Montina, T., & Metz, G. A. S. (2023). Urinary 1H NMR Metabolomic Analysis of Prenatal Maternal Stress Due to a Natural Disaster Reveals Metabolic Risk Factors for Non-Communicable Diseases: The QF2011 Queensland Flood Study. Metabolites, 13(4), 579. https://doi.org/10.3390/metabo13040579








