Antioxidants as Protection against Reactive Oxidative Stress in Inflammatory Bowel Disease
Abstract
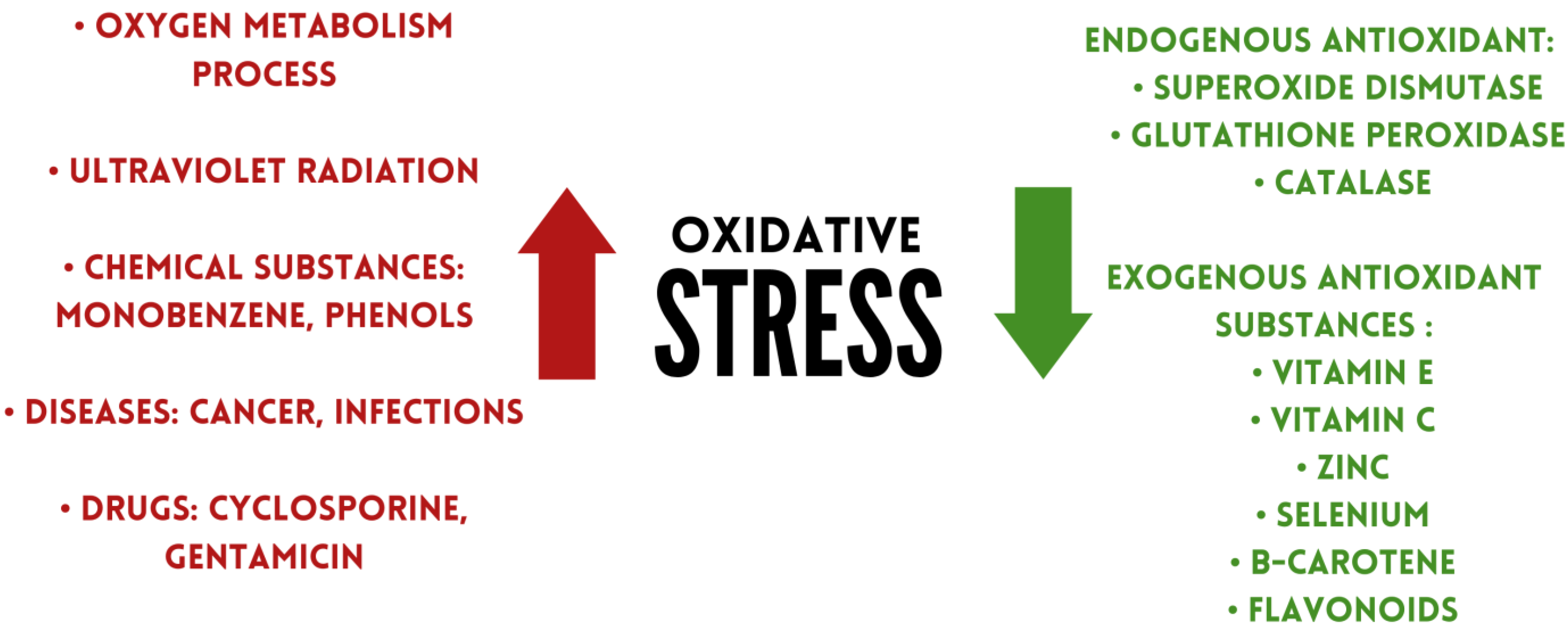
1. Introduction
2. Oxidative Stress and Reactive Oxygen Species
3. Oxidative Stress and Inflammatory Bowel Disease (IBD)
4. Antioxidants
4.1. Exogenous Antioxidant Substances
4.1.1. Vitamin E
4.1.2. Vitamin C
4.1.3. Zinc
4.1.4. Selenium
4.1.5. Betacarotene
4.1.6. Flavonoids
4.2. Endogenous Antioxidant Substances
4.2.1. Superoxide Dismutase
4.2.2. Glutathione Peroxidase (GPX)
4.2.3. Catalase
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dudley, M.; Kojinkov, M.; Baraga, D.; Donnet, X.; Groß, E.; Lantzanaki, S.; de Kwaadsteniet, T.; McArdle, T.; Mossakowska, M.; Perovic, M.; et al. ECCO, EFCCA Patient Guidelines. European Crohn’s and Colitis Organisation. Available online: https://www.efcca.org/sites/default/files/Crohn%C2%B4s%20Disease%20Patient%20Guidelines.pdf (accessed on 24 February 2023).
- Szczeklik, A.; Gajewski, P. Interna Szczeklika 18/19; Medycyna Praktyczna: Cracow, Poland, 2018; pp. 579–590. [Google Scholar]
- Burisch, J.; Jess, T.; Martinato, M.; Lakatos, P.; ECCO-EpiCom. The burden of inflammatory bowel disease in Europe. J. Crohns Colitis 2013, 7, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Windsor, J.; Kaplan, G. Evolving Epidemiology of IBD. Curr. Gastroenterol. Rep. 2019, 21, 40. [Google Scholar] [CrossRef]
- Ballini, A.; Santacroce, L.; Cantore, S.; Bottalico, L.; Dipalma, G.; Topi, S.; Saini, R.; De Vito, D.; Inchingolo, F. Probiotics Efficacy on Oxidative Stress Values in Inflammatory Bowel Disease: A Randomized Double-Blinded Placebo-Controlled Pilot Study. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxid. Med. Cell. Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef]
- Jopkiewicz, S. Oxidative stress. Part I. Oxidative stress as a factor in the development of civilization diseases. Med. Srod. 2018, 21, 48–52. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Li, C. Perspectives of New Advances in the Pathogenesis of Vitiligo: From Oxidative Stress to Autoimmunity. Med. Sci. Monit. 2019, 6, 1017–1023. [Google Scholar] [CrossRef]
- Jopkiewicz, S. Oxidative stress Part II. Prevention of free radical damage. Med. Srod. 2018, 21, 53–59. [Google Scholar] [CrossRef]
- Kim, G.; Kim, J.; Rhie, S.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef]
- Kulkarni, A.; Kuppusamy, P.; Parinandi, N. Oxygen, the lead actor in the pathophysiologic drama: Enactment of the trinity of normoxia, hypoxia, and hyperoxia in disease and therapy. Antioxid. Redox Signal. 2007, 9, 1717–1730. [Google Scholar] [CrossRef]
- O’Neill, S.; Brault, J.; Stasia, M.; Knaus, U. Genetic disorders coupled to ROS deficiency. Redox Biol. 2015, 6, 135–156. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: Eustress and Distress in Redox Homeostasis. In Stress: Physiology, Biochemistry, and Pathology, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 153–163. [Google Scholar]
- Hajam, Y.; Rani, R.; Ganie, S.; Sheikh, T.; Javaid, D.; Qadri, S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell. 2021, 81, 3691–3707. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, C.; Feng, C.; Yan, C.; Yu, Y.; Chen, Z.; Guo, C.; Wang, X. Role of mitochondrial reactive oxygen species in homeostasis regulation. Redox Rep. 2022, 27, 45–52. [Google Scholar] [CrossRef]
- Patel, R.; Rinker, L.; Peng, J.; Chilian, W.M. Reactive Oxygen Species: The Good and the Bad. In Reactive Oxygen Species (ROS) in Living Cells; Books on Demand: Paris, France, 2018. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: Role and Response of Short Guanine Tracts at Genomic Locations. Int. J. Mol. Sci. 2019, 20, 4258. [Google Scholar] [CrossRef] [PubMed]
- Sadasivam, N.; Kim, Y.; Radhakrishnan, K.; Kim, D. Oxidative Stress Genomic Integrity, and Liver Diseases. Molecules 2022, 27, 3159. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef] [PubMed]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of Oxidative Stress in Metabolic Syndrome and Associated Diseases. Oxid. Med. Cell. Longev. 2019, 5, 8267234. [Google Scholar] [CrossRef]
- Kloska, M.; Mańkowska-Wierzbicka, D.; Człapka-Matyasik, M.; Dobrowolska, A.; Grzymisławski, M. Stres oksydacyjny w etiopatogenezie nieswoistych chorób zapalnych jelit [Oxidative stress in etiopathogenesis of inflammatory bowel diseases]. Postepy Biochem. 2020, 66, 143–150. [Google Scholar] [CrossRef]
- Arno, B.; Martin, F.; Klaas, F.; Andreas, P. Oxidative Stress and Redox-Modulating. Trends Mol. Med. 2020, 26, 1034–1046. [Google Scholar] [CrossRef]
- Costantino, S.; Paneni, F.; Cosentino, F. Ageing, metabolism and cardiovascular disease. J. Physiol. 2016, 594, 2061–2073. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Luo, L.; Namani, A.; Wang, X.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Korac, B.; Kalezic, A.; Pekovic-Vaughan, V.; Korac, A.; Jankovic, A. Redox changes in obesity, metabolic syndrome, and diabetes. Redox Biol. 2021, 42, 101887. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, Y. Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: Updated experimental and clinical evidence. Exp. Biol. Med. 2012, 237, 474–480. [Google Scholar] [CrossRef]
- Pereira, C.; Grácio, D.; Teixeira, J.; Magro, F. Oxidative Stress and DNA Damage: Implications in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 2403–2417. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, K.; Papadakis, K. Inflammatory Bowel Disease: Updates on Molecular Targets for Biologics. Gut Liver. 2017, 11, 455–463. [Google Scholar] [CrossRef]
- Campbell, E.; Colgan, S. Control and dysregulation of redox signalling in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 106–120. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid. Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef] [PubMed]
- Andresen, L.; Jørgensen, V.; Perner, A.; Hansen, A.; Eugen-Olsen, J.; Rask-Madsen, J. Activation of nuclear factor kappaB in colonic mucosa from patients with collagenous and ulcerative colitis. Gut 2005, 54, 503–509. [Google Scholar] [CrossRef]
- Naito, Y.; Takagi, T.; Ishikawa, T.; Handa, O.; Matsumoto, N.; Yagi, N.; Matsuyama, K.; Yoshida, N.; Yoshikawa, T.; Kotake, Y. alpha-Phenyl-N-tert-butylnitrone provides protection from dextran sulfate sodium-induced colitis in mice. Antioxid. Redox Signal. 2002, 4, 195–206. [Google Scholar] [CrossRef]
- de Souza, H.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef]
- Guo, X.; Ko, J.; Mei, Q.; Cho, C. Aggravating effect of cigarette smoke exposure on experimental colitis is associated with leukotriene B(4) and reactive oxygen metabolites. Digestion 2001, 63, 180–187. [Google Scholar] [CrossRef]
- Bourgonje, A.; von Martels, J.; Bulthuis, M.; van Londen, M.; Faber, K.; Dijkstra, G.; van Goor, H. Crohn’s Disease in Clinical Remission Is Marked by Systemic Oxidative Stress. Front. Physiol. 2019, 10, 499. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Lan, S. Implications of Antioxidant Systems in Inflammatory Bowel Disease. Biomed. Res. Int. 2018, 2018, 1290179. [Google Scholar] [CrossRef] [PubMed]
- Krzystek-Korpacka, M.; Kempiński, R.; Bromke, M.; Neubauer, K. Oxidative Stress Markers in Inflammatory Bowel Diseases: Systematic Review. Diagnostics 2020, 10, 601. [Google Scholar] [CrossRef]
- Handa, O.; Naito, Y.; Yoshikawa, T. Helicobacter pylori: A ROS-inducing bacterial species in the stomach. Inflamm. Res. 2010, 59, 997–1003. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Parveen, A.; Akash, M.; Rehman, K.; Kyunn, W. Recent Investigations for Discovery of Natural Antioxidants: A Comprehensive Review. Crit. Rev. Eukaryot. Gene Expr. 2016, 26, 143–160. [Google Scholar] [CrossRef]
- Bouayed, J.; Bohn, T. Exogenous antioxidants--Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef]
- Aggarwal, B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the vitamin E of the 21st century: Its potential against cancer and other chronic diseases. Biochem. Pharmacol. 2010, 80, 1613–1631. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Kono, N. α-Tocopherol transfer protein (α-TTP). Free. Radic. Biol. Med. 2021, 176, 162–175. [Google Scholar] [CrossRef]
- Lewis, E.; Meydani, S.; Wu, D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life 2019, 71, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, K.; Hohjoh, H.; Inazumi, T.; Tsuchiya, S.; Sugimoto, Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim. Biophys. Acta 2015, 1851, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, K.; Inazumi, T.; Shimamoto, A.; Sugimoto, Y. Molecular mechanisms underlying prostaglandin E2-exacerbated inflammation and immune diseases. Int. Immunol. 2019, 31, 597–606. [Google Scholar] [CrossRef]
- Liu, K.; Nakatsu, C.; Jones-Hall, Y.; Kozik, A.; Jiang, Q. Vitamin E alpha- and gamma-tocopherol mitigate colitis, protect intestinal barrier function and modulate the gut microbiota in mice. Free. Radic. Biol. Med. 2021, 163, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Lee, H. Therapeutic Potential of the Combination of Pentoxifylline and Vitamin-E in Inflammatory Bowel Disease: Inhibition of Intestinal Fibrosis. J. Clin. Med. 2022, 11, 4713. [Google Scholar] [CrossRef]
- Chen, J.; Ruan, X.; Yuan, S.; Deng, M.; Zhang, H.; Sun, J.; Yu, L.; Satsangi, J.; Larsson, S.C.; Therdoratou, E.; et al. Antioxidants, minerals and vitamins in relation to Crohn’s disease and ulcerative colitis: A Mendelian randomization study. Aliment. Pharmacol. Ther. 2023, 57, 399–408. [Google Scholar] [CrossRef]
- Panda, S.K.; Peng, V.; Sudan, R.; Ulezko Antonova, A.; Di Luccia, B.; Ohara, T.E.; Fachi, J.L.; Grajales-Reyes, G.E.; Jaeger, N.; Trsan, T.; et al. Repression of the aryl-hydrocarbon receptor prevents oxidative stress and ferroptosis of intestinal intraepithelial lymphocytes. Immunity 2023, 56, 797–812.e4. [Google Scholar] [CrossRef]
- Suzuki, N.; Niikura, R.; Ihara, S.; Hikiba, Y.; Kinoshita, H.; Higashishima, N.; Hayakawa, Y.; Yamada, A.; Hirata, Y.; Nakata, R.; et al. Alpha-Blockers As Colorectal Cancer Chemopreventive: Findings from a Case-Control Study, Human Cell Cultures, and In Vivo Preclinical Testing. Cancer Prev. Res. 2019, 12, 185–194. [Google Scholar] [CrossRef]
- Fan, X.; Yin, J.; Yin, J.; Weng, X.; Ding, R. Comparison of the anti-inflammatory effects of vitamin E and vitamin D on a rat model of dextran sulfate sodium-induced ulcerative colitis. Exp. Ther. Med. 2023, 25, 98. [Google Scholar] [CrossRef]
- Hiratsuka, T.; Inomata, M.; Hagiwara, S.; Kono, Y.; Shiraishi, N.; Noguchi, T.; Kitano, S. Bolus injection of newly synthesized vitamin E derivative ETS-GS for the treatment of acute severe ulcerative colitis in a mouse model. New vitamin E derivative for acute severe UC. Int. J. Colorectal. Dis. 2013, 28, 305–311. [Google Scholar] [CrossRef]
- Traber, M.; Head, B. Vitamin E: How much is enough, too much and why! Free Radic. Biol. Med. 2021, 177, 212–225. [Google Scholar] [CrossRef]
- Carr, A.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- Spoelstra-de Man, A.; Elbers, P.; Oudemans-Van Straaten, H. Vitamin C: Should we supplement? Curr. Opin. Crit. Care 2018, 24, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Caritá, A.C.; Fonseca-Santos, B.; Shultz, J.D.; Michniak-Kohn, B.; Chorilli, M.; Leonardi, G.R. Vitamin C: One compound, several uses. Advances for delivery, efficiency and stability. Nanomedicine 2020, 24, 102117. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B. Two Faces of Vitamin C-Antioxidative and Pro-Oxidative Agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef] [PubMed]
- Moritz, B.; Schmitz, A.; Rodrigues, A.; Dafre, A.; Cunha, M. The role of vitamin C in stress-related disorders. J. Nutr. Biochem. 2020, 85, 108459. [Google Scholar] [CrossRef]
- Elste, V.; Troesch, B.; Eggersdorfer, M.; Weber, P. Emerging Evidence on Neutrophil Motility Supporting Its Usefulness to Define Vitamin C Intake Requirements. Nutrients 2017, 9, 503. [Google Scholar] [CrossRef]
- Gordon, B.; Galati, J.; Yang, S.; Longman, R.; Lukin, D.; Scherl, E.; Battat, R. Prevalence and factors associated with vitamin C deficiency in inflammatory bowel disease. World J. Gastroenterol. 2022, 28, 4834–4845. [Google Scholar] [CrossRef]
- Dunleavy, K.; Ungaro, R.; Manning, L.; Gold, S.; Novak, J.; Colombel, J. Vitamin C Deficiency in Inflammatory Bowel Disease: The Forgotten Micronutrient. Crohns Colitis 360 2021, 3, otab009. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Tanaka, K.; Nagata, C.; Furukawa, S.; Andoh, A.; Yokoyama, T.; Yoshimura, N.; Mori, K.; Ninomiya, T.; Yamamoto, Y.; et al. Japan Ulcerative Colitis Study Group. Dietary intake of vegetables, fruit, and antioxidants and risk of ulcerative colitis: A case-control study in Japan. Nutrition 2021, 91–92, 111378. [Google Scholar] [CrossRef] [PubMed]
- Vahid, F.; Rashvand, S.; Sadeghi, M.; Hekmatdoost, A. The association between index of nutritional quality and ulcerative colitis: A case-control study. J. Res. Med. Sci. 2018, 23, 67. [Google Scholar] [CrossRef] [PubMed]
- Filippi, J.; Al-Jaouni, R.; Wiroth, J.; Hébuterne, X.; Schneider, S. Nutritional deficiencies in patients with Crohn’s disease in remission. Inflamm. Bowel Dis. 2006, 12, 185–191. [Google Scholar] [CrossRef]
- Jo, H.; Lee, D.; Go, C.; Jang, Y.; Chu, N.; Bae, S.; Kang, D.; Im, J.P.; Kim, Y.; Kang, J.S. Preventive Effect of Vitamin C on Dextran Sulfate Sodium (DSS)-Induced Colitis via the Regulation of IL-22 and IL-6 Production in Gulo(-/-) Mice. Int. J. Mol. Sci. 2022, 23, 10612. [Google Scholar] [CrossRef]
- Chang, Y.L.; Rossetti, M.; Vlamakis, H.; Casero, D.; Sunga, G.; Harre, N.; Miller, S.; Humphries, R.; Stappenbeck, T.; Simpson, K.W.; et al. A screen of Crohn’s disease-associated microbial metabolites identifies ascorbate as a novel metabolic inhibitor of activated human T cells. Mucosal Immunol. 2019, 12, 457–467. [Google Scholar] [CrossRef]
- Jarmakiewicz-Czaja, S.; Piątek, D.; Filip, R. The Influence of Nutrients on Inflammatory Bowel Diseases. J. Nutr. Metab. 2020, 2020, 2894169. [Google Scholar] [CrossRef]
- Sanna, A.; Firinu, D.; Zavattari, P.; Valera, P. Zinc Status and Autoimmunity: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 68. [Google Scholar] [CrossRef]
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef]
- Maywald, M.; Wessels, I.; Rink, L. Zinc Signals and Immunity. Int. J. Mol. Sci. 2017, 18, 2222. [Google Scholar] [CrossRef]
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef]
- Gammoh, N.; Rink, L. Zinc in Infection and Inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef]
- Choi, S.; Liu, X.; Pan, Z. Zinc deficiency and cellular oxidative stress: Prognostic implications in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, B. The Impact of Zinc and Zinc Homeostasis on the Intestinal Mucosal Barrier and Intestinal Diseases. Biomolecules 2022, 12, 900. [Google Scholar] [CrossRef]
- Camilleri, M. Human Intestinal Barrier: Effects of Stressors, Diet, Prebiotics, and Probiotics. Clin. Transl. Gastroenterol. 2021, 12, e00308. [Google Scholar] [CrossRef]
- Islam, T.; Albracht-Schulte, K.; Ramalingam, L.; Schlabritz-Lutsevich, N.; Park, O.; Zabet-Moghaddam, M.; Kalupahana, N.; Moustaid-Moussa, N. Anti-inflammatory mechanisms of polyphenols in adipose tissue: Role of gut microbiota, intestinal barrier integrity and zinc homeostasis. J. Nutr. Biochem. 2022, 115, 109242. [Google Scholar] [CrossRef]
- Soltani, Z.; Rafiei, F.; Ebrahim, A.; Rafiei, R. The Prevalence of Zinc Deficiency in Crohn’s Disease Patients. Maedica 2021, 16, 29–33. [Google Scholar] [CrossRef]
- Dragasevic, S.; Stankovic, B.; Kotur, N.; Milutinovic, A.S.; Milovanovic, T.; Stojkovic Lalosevic, M.; Stojanovic, M.; Pavlovic, S.; Popovic, D. Genetic Aspects of Micronutrients Important for Inflammatory Bowel Disease. Life 2022, 18, 1623. [Google Scholar] [CrossRef]
- Moon, N.; Figgins, B.; Altshuler, E.; Pham, A.; Kamel, A. Concurrent zinc and vitamin B6 deficiencies in acutely exacerbated inflammatory bowel disease: Case reports. Nutr. Clin. Pract. 2022, 37, 203–208. [Google Scholar] [CrossRef]
- Ye, R.; Huang, J.; Wang, Z.; Chen, Y.; Dong, Y. Trace Element Selenium Effectively Alleviates Intestinal Diseases. Int. J. Mol. Sci. 2021, 28, 11708. [Google Scholar] [CrossRef]
- Mehdi, Y.; Hornick, J.; Istasse, L.; Dufrasne, I. Selenium in the environment, metabolism and involvement in body functions. Molecules 2013, 13, 3292–3311. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Rose, A.; Hoffmann, P. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef] [PubMed]
- Avery, J.; Hoffmann, P. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef]
- Maggini, S.; Pierre, A.; Calder, P. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M. Selenium Fascinating Microelement, Properties and Sources in Food. Molecules. 2019, 24, 1298. [Google Scholar] [CrossRef]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Saeed, F.; Nadeem, M.; Ahmed, R.; Nadeem, M.; Arshad, M.; Ullah, A. Studying the impact of nutritional immunology underlying the modulation of immune responses by nutritional compounds—A review. Food Agric. Immunol. 2016, 27, 205–229. [Google Scholar] [CrossRef]
- Rayman, M. Selenium intake, status, and health: A complex relationship. Hormones 2020, 19, 9–14. [Google Scholar] [CrossRef]
- Huang, L.J.; Mao, X.T.; Li, Y.Y.; Liu, D.D.; Fan, K.Q.; Liu, R.B.; Wu, T.T.; Wang, H.L.; Zhang, Y.; Yang, B.; et al. Multiomics analyses reveal a critical role of selenium in controlling T cell differentiation in Crohn’s disease. Immunity 2021, 10, 1728–1744. [Google Scholar] [CrossRef]
- Weisshof, R.; Chermesh, I. Micronutrient deficiencies in inflammatory bowel disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 576–581. [Google Scholar] [CrossRef]
- Sahebari, M.; Rezaieyazdi, Z.; Khodashahi, M. Selenium and Autoimmune Diseases: A Review Article. Curr. Rheumatol. Rev. 2019, 15, 123–134. [Google Scholar] [CrossRef]
- Yan, W.; Meihao, W.; Zihan, S.; Lingjie, H.; Haotian, C.; Qian, C.; Lianli, S. Correlation Between Crohn’s Disease Activity and Serum Selenium Concentration. Clin. Ther. 2022, 44, 736–743. [Google Scholar] [CrossRef]
- Wu, Z.; Pan, D.; Jiang, M.; Sang, L.; Chang, B. Selenium-Enriched Lactobacillus acidophilus Ameliorates Dextran Sulfate Sodium-Induced Chronic Colitis in Mice by Regulating Inflammatory Cytokines and Intestinal Microbiota. Front. Med. 2021, 31, 716816. [Google Scholar] [CrossRef]
- Hu, Y.; Jin, X.; Gao, F.; Lin, T.; Zhu, H.; Hou, X.; Yin, Y.; Kan, S.; Chen, D. Selenium-enriched Bifidobacterium longum DD98 effectively ameliorates dextran sulfate sodium-induced ulcerative colitis in mice. Front. Microbiol. 2022, 5, 955112. [Google Scholar] [CrossRef]
- Khattab, A.; Darwish, A.; Othman, S.; Allam, A.; Alqhtani, H. Anti-inflammatory and Immunomodulatory Potency of Selenium-Enriched Probiotic Mutants in Mice with Induced Ulcerative Colitis. Biol. Trace Elem. Res. 2023, 201, 353–367. [Google Scholar] [CrossRef]
- Khazdouz, M.; Daryani, N.E.; Alborzi, F.; Jazayeri, M.H.; Farsi, F.; Hasani, M.; Heshmati, J.; Shidfar, F. Effect of Selenium Supplementation on Expression of SIRT1 and PGC-1α Genes in Ulcerative Colitis Patients: A Double Blind Randomized Clinical Trial. Clin. Nutr. Res. 2020, 26, 284–295. [Google Scholar] [CrossRef]
- Keshteli, A.H.; Valcheva, R.; Nickurak, C.; Park, H.; Mandal, R.; van Diepen, K.; Kroeker, K.I.; van Zanten, S.V.; Halloran, B.; Wishart, D.S.; et al. Anti-Inflammatory Diet Prevents Subclinical Colonic Inflammation and Alters Metabolomic Profile of Ulcerative Colitis Patients in Clinical Remission. Nutrients 2022, 14, 3294. [Google Scholar] [CrossRef]
- Castro Aguilar-Tablada, T.; Navarro-Alarcón, M.; Quesada Granados, J.; Samaniego Sánchez, C.; Rufián-Henares, J.Á.; Nogueras-Lopez, F. Ulcerative Colitis and Crohn’s Disease Are Associated with Decreased Serum Selenium Concentrations and Increased Cardiovascular Risk. Nutrients 2016, 8, 780. [Google Scholar] [CrossRef]
- Short, S.; Whitten-Barrett, C.; Williams, C. Selenoprotein P in colitis-associated carcinoma. Mol. Cell. Oncol. 2015, 3, e1075094. [Google Scholar] [CrossRef]
- Short, S.P.; Pilat, J.M.; Barrett, C.W.; Reddy, V.K.; Haberman, Y.; Hendren, J.R.; Marsh, B.J.; Keating, C.E.; Motley, A.K.; Hill, K.E.; et al. Colonic Epithelial-Derived Selenoprotein P Is the Source for Antioxidant-Mediated Protection in Colitis-Associated Cancer. Gastroenterology 2021, 160, 1694–1708.e3. [Google Scholar] [CrossRef]
- Bohn, T.; Desmarchelier, C.; El, S.N.; Keijer, J.; van Schothorst, E.; Rühl, R.; Borel, P. β-Carotene in the human body: Metabolic bioactivation pathways—From digestion to tissue distribution and excretion. Proc. Nutr. Soc. 2019, 78, 68–87. [Google Scholar] [CrossRef]
- Miazek, K.; Beton, K.; Śliwińska, A.; Brożek-Płuska, B. The Effect of β-Carotene, Tocopherols and Ascorbic Acid as Anti-Oxidant Molecules on Human and Animal In Vitro/In Vivo Studies: A Review of Research Design and Analytical Techniques Used. Biomolecules 2022, 12, 1087. [Google Scholar] [CrossRef] [PubMed]
- Sandhiya, L.; Zipse, H. Conformation-dependent antioxidant properties of β-carotene. Org. Biomol. Chem. 2021, 20, 152–162. [Google Scholar] [CrossRef]
- Kake, T.; Imai, M.; Takahashi, N. Effects of β-carotene on oxazolone-induced atopic dermatitis in hairless mice. Exp. Dermatol. 2019, 28, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Marcelino, G.; Machate, D.J.; Freitas, K.C.; Hiane, P.A.; Maldonade, I.R.; Pott, A.; Asato, M.A.; Candido, C.J.; Guimarães, R.C.A. β-Carotene: Preventive Role for Type 2 Diabetes Mellitus and Obesity: A Review. Molecules 2020, 9, 5803. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.; Dold, S.; Rehman, A.; Bird, J.K.; Steinert, R.E. Vitamins, the gut microbiome and gastrointestinal health in humans. Nutr. Res. 2021, 95, 35–53. [Google Scholar] [CrossRef]
- Honarbakhsh, M.; Malta, K.; Ericsson, A.; Holloway, C.; Kim, Y.K.; Hammerling, U.; Quadro, L. β-carotene improves fecal dysbiosis and intestinal dysfunctions in a mouse model of vitamin A deficiency. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids. 2022, 1867, 159122. [Google Scholar] [CrossRef]
- Cheng, J.; Balbuena, E.; Miller, B.; Eroglu, A. The Role of β-Carotene in Colonic Inflammation and Intestinal Barrier Integrity. Front. Nutr. 2021, 27, 723480. [Google Scholar] [CrossRef]
- Grar, H.; Dib, W.; Gourine, H.; Negaoui, H.; Taleb, B.H.F.; Louaar, A.; Ouldhocine, S.; Kaddouri, H.; Kheroua, O.; Saidi, D. β-Carotene improves intestinal barrier function by modulating proinflammatory cytokines and improving antioxidant capacity in β-lactoglobulin-sensitize. J. Biol. Regul. Homeost. Agents 2020, 34, 1689–1697. [Google Scholar] [CrossRef]
- Yang, Y.; Li, R.; Hui, J.; Li, L.; Zheng, X. β-Carotene attenuates LPS-induced rat intestinal inflammation via modulating autophagy and regulating the JAK2/STAT3 and JNK/p38 MAPK signaling pathways. J. Food Biochem. 2021, 45, e13544. [Google Scholar] [CrossRef]
- Xu, G.; Ma, T.; Zhou, C.; Zhao, F.; Peng, K.; Li, B. β-Carotene Attenuates Apoptosis and Autophagy via PI3K/AKT/mTOR Signaling Pathway in Necrotizing Enterocolitis Model Cells IEC-6. Evid. Based Complement. Alternat Med. 2022, 17, 2502263. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Song, Y.; Liu, H.; Wu, M.; Gong, H.; Lan, H.; Zheng, X. Gut microbiota regulation and anti-inflammatory effect of β-carotene in dextran sulfate sodium-stimulated ulcerative colitis in rats. J. Food Sci. 2021, 86, 2118–2130. [Google Scholar] [CrossRef]
- Kuang, H.; Ma, Y.; Liu, Y. Protective effect of β-carotene on OVA-induced food allergy in mice by strengthening intestinal epithelial barrier function and regulating intestinal microflora. Food Funct. 2022, 13, 12330–12341. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Dias, M.; Pinto, D.; Silva, A. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Kopustinskiene, D. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 26, 965. [Google Scholar] [CrossRef]
- Li, G.; Ding, K.; Qiao, Y.; Zhang, L.; Zheng, L.; Pan, T.; Zhang, L. Flavonoids Regulate Inflammation and Oxidative Stress in Cancer. Molecules 2020, 25, 5628. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Liu, Q.; Zhou, X.; Wang, X.; Li, H.; Zhang, W.; Yuan, H.; Sun, C. Flavonoids regulate tumor-associated macrophages—From structure-activity relationship to clinical potential (Review). Pharmacol. Res. 2022, 184, 106419. [Google Scholar] [CrossRef]
- Yi, Y. Regulatory Roles of Flavonoids on Inflammasome Activation during Inflammatory Responses. Mol. Nutr. Food Res. 2018, 62, e1800147. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, H.; Wen, X.; Ho, C.; Li, S. Citrus flavonoids and the intestinal barrier: Interactions and effects. Compr. Rev. Food Sci. Food Saf. 2021, 20, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, M.; Kang, G.; Huang, H. The Potential Role of Phytonutrients Flavonoids Influencing Gut Microbiota in the Prophylaxis and Treatment of Inflammatory Bowel Disease. Front. Nutr. 2021, 14, 798038. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Lv, Q.; Miao, Y.; Qiao, S.; Dai, Y.; Wei, Z. Cardamonin, a natural flavone, alleviates inflammatory bowel disease by the inhibition of NLRP3 inflammasome activation via an AhR/Nrf2/NQO1 pathway. Biochem. Pharmacol. 2018, 155, 494–509. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhao, M.; Li, W.; Wei, P.; Liu, Q.; Chen, S.; Zeng, J.; Ma, X.; Tang, J. Preclinical evidence for quercetin against inflammatory bowel disease: A meta-analysis and systematic review. Inflammopharmacology 2022, 30, 2035–2050. [Google Scholar] [CrossRef]
- Farzaei, M.H.; El-Senduny, F.F.; Momtaz, S.; Parvizi, F.; Iranpanah, A.; Tewari, D.; Naseri, R.; Abdolghaffari, A.H.; Rezaei, N. An update on dietary consideration in inflammatory bowel disease: Anthocyanins and more. Expert. Rev. Gastroenterol. Hepatol. 2018, 12, 1007–1024. [Google Scholar] [CrossRef]
- Ciumărnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, Ș.C.; Răchișan, A.L.; Negrean, V.; Perné, M.G.; Donca, V.I.; Alexescu, T.G.; Para, I.; et al. Dogaru. The Effects of Flavonoids in Cardiovascular Diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Alzoghaibi, M. Concepts of oxidative stress and antioxidant defense in Crohn’s disease. World J. Gastroenterol. 2013, 19, 6540–6547. [Google Scholar] [CrossRef]
- Liu, P.; Li, Y.; Wang, R.; Ren, F.; Wang, X. Oxidative Stress and Antioxidant Nanotherapeutic Approaches for Inflammatory Bowel Disease. Biomedicines 2021, 10, 85. [Google Scholar] [CrossRef]
- Rampon, C.; Volovitch, M.; Joliot, A.; Vriz, S. Hydrogen Peroxide and Redox Regulation of Developments. Antioxidants 2018, 7, 159. [Google Scholar] [CrossRef]
- Ferro, D.; Bakiu, R.; Pucciarelli, S.; Miceli, C.; Vallesi, A.; Irato, P.; Santovito, G. Molecular Characterization, Protein-Protein Interaction Network, and Evolution of Four Glutathione Peroxidases from Tetrahymena thermophila. Antioxidants 2020, 9, 949. [Google Scholar] [CrossRef]
- Chovanová, K.; Böhmer, M.; Poljovka, A.; Budiš, J.; Harichová, J.; Szemeš, T.; Zámocký, M. Parallel Molecular Evolution of Catalases and Superoxide Dismutases-Focus on Thermophilic Fungal Genomes. Antioxidants 2020, 9, 1047. [Google Scholar] [CrossRef]
- Marwicka, J.; Zięba, A. Antioxidants as a defence against reactive oxygen species. Aesth Cosmetol. Med. 2021, 10, 271–276. [Google Scholar] [CrossRef]
- Sakthivel, K.; Guruvayoorappan, C. Protective effect of Acacia ferruginea against ulcerative colitis via modulating inflammatory mediators, cytokine profile and NF-κB signal transduction pathways. J. Environ. Pathol. Toxicol. Oncol. 2014, 2, 83–98. [Google Scholar] [CrossRef]
- Szczeklik, K.; Krzysciak, W.; Domagala-Rodacka, R.; Mach, P.; Darczuk, D.; Cibor, D.; Pytko-Polonczyk, J.; Rodacki, T.; Owczarek, D. Alterations in glutathione peroxidase and superoxide dismutase activities in plasma and saliva in relation to disease activity in patients with Crohn’s disease. J. Physiol. Pharmacol. 2016, 67, 709–715. [Google Scholar]
- Alzoghaibi, M.; Al-Mofleh, I.; Al-Jebreen, A. Antioxidant activities for superoxide dismutase in patients with Crohn’s disease. J. Basic. Clin. Physiol. Pharmacol. 2014, 25, 59–62. [Google Scholar] [CrossRef]
- Mohammadi, E.; Qujeq, D.; Taheri, H.; Hajian-Tilaki, K. Evaluation of Serum Trace Element Levels and Superoxide Dismutase Activity in Patients with Inflammatory Bowel Disease: Translating Basic Research into Clinical Application. Biol. Trace Elem. Res. 2017, 177, 235–240. [Google Scholar] [CrossRef]
- Zielińska, A.K.; Sałaga, M.; Siwiński, P.; Włodarczyk, M.; Dziki, A.; Fichna, J. Oxidative Stress Does Not Influence Subjective Pain Sensation in Inflammatory Bowel Disease Patients. Antioxidants 2021, 10, 1237. [Google Scholar] [CrossRef]
- Tomusiak-Plebanek, A.; Heczko, P.; Skowron, B.; Baranowska, A.; Okoń, K.; Thor, P.J.; Strus, M. Lactobacilli with superoxide dismutase-like or catalase activity are more effective in alleviating inflammation in an inflammatory bowel disease mouse model. Drug. Des. Devel Ther. 2018, 28, 3221–3233. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Lin, Z.; Xu, Y.; Park, M.; Ji, G.E.; Johnston, T.V.; Ku, S.; Park, M.S. A recombinant Bifidobacterium bifidum BGN4 strain expressing the streptococcal superoxide dismutase gene ameliorates inflammatory bowel disease. Microb. Cell. Fact. 2022, 21, 113. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wen, K.; Chen, Y.; Fang, G.; Yang, S.; Li, Q. Oral Administration of Therapeutic Enzyme Capsule for the Management of Inflammatory Bowel Disease. Int. J. Nanomed. 2022, 17, 4843–4860. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.H.; Shi, M.; Chen, H.; Cui, S.; Wu, Y.; Gao, X.H.; Chen, H.D. Glutathione Peroxidase Level in Patients with Vitiligo: A Meta-Analysis. Biomed. Res. Int. 2016, 2016, 3029810. [Google Scholar] [CrossRef] [PubMed]
- Dayer, R.; Fischer, B.B.; Eggen, R.I.; Lemaire, S.D. The peroxiredoxin and glutathione peroxidase families in Chlamydomonas reinhardtii. Genetics 2008, 179, 41–57. [Google Scholar] [CrossRef]
- Socca, E.A.; Luiz-Ferreira, A.; de Faria, F.M.; de Almeida, A.C.; Dunder, R.J.; Manzo, L.P.; Brito, A.R. Inhibition of tumor necrosis factor-alpha and cyclooxigenase-2 by Isatin: A molecular mechanism of protection against TNBS-induced colitis in rats. Chem. Biol. Interact. 2014, 25, 48–55. [Google Scholar] [CrossRef]
- Te Velde, A.A.; Pronk, I.; de Kort, F.; Stokkers, P.C. Glutathione peroxidase 2 and aquaporin 8 as new markers for colonic inflammation in experimental colitis and inflammatory bowel diseases: An important role for H2O2? Eur. J. Gastroenterol. Hepatol. 2008, 20, 555–560. [Google Scholar] [CrossRef]
- Esworthy, R.S.; Aranda, R.; Martín, M.G.; Doroshow, J.H.; Binder, S.W.; Chu, F.F. Mice with combined disruption of Gpx1 and Gpx2 genes have colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, 848–855. [Google Scholar] [CrossRef]
- Rana, S.; Sharma, S.; Prasad, K.; Sinha, S.; Singh, K. Role of oxidative stress & antioxidant defence in ulcerative colitis patients from north India. Indian J. Med. Res. 2014, 139, 568–571. [Google Scholar]
- Krzystek-Korpacka, M.; Neubauer, K.; Berdowska, I.; Zielinski, B.; Paradowski, L.; Gamian, A. Impaired erythrocyte antioxidant defense in active inflammatory bowel disease: Impact of anemia and treatment. Inflamm. Bowel Dis. 2010, 16, 1467–1475. [Google Scholar] [CrossRef]
- Weydert, C.; Cullen, J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef]
- Ekoue, D.; He, C.; Diamond, A.; Bonini, M. Manganese superoxide dismutase and glutathione peroxidase-1 contribute to the rise and fall of mitochondrial reactive oxygen species which drive oncogenesis. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 628–632. [Google Scholar] [CrossRef]
- Glorieux, C.; Calderon, P.B. Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef]
- Kahl, R.; Kampkötter, A.; Wätjen, W.; Chovolou, Y. Antioxidant Enzymes and Apoptosis. Drug. Metab. Rev. 2004, 36, 747–762. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Iborra, M.; Moret, I.; Busó, E.; García-Giménez, J.L.; Ricart, E.; Gisbert, J.P.; Cabré, E.; Esteve, M.; Márquez-Mosquera, L.; García-Planella, E.; et al. The Genetic Diversity and Dysfunctionality of Catalase Associated with a Worse Outcome in Crohn’s Disease. Int. J. Mol. Sci. 2022, 23, 15881. [Google Scholar] [CrossRef]
- Agrahari, G.; Sah, S.K.; Bang, C.H.; Kim, Y.H.; Kim, T.Y. Superoxide Dismutase 3 Controls the Activation and Differentiation of CD4+T Cells. Front. Immunol. 2021, 25, 628117. [Google Scholar] [CrossRef]
- Morris, G.; Gevezova, M.; Sarafian, V.; Maes, M. Redox regulation of the immune response. Cell. Mol. Immunol. 2022, 19, 1079–1101. [Google Scholar] [CrossRef]
- Rempel, J.; Grover, K.; El-Matary, W. Micronutrient Deficiencies and Anemia in Children with Inflammatory Bowel Disease. Nutrients 2021, 13, 236. [Google Scholar] [CrossRef]
- Fabisiak, N.; Fabisiak, A.; Watala, C.; Fichna, J. Fat-soluble Vitamin Deficiencies and Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2017, 51, 878–889. [Google Scholar] [CrossRef]
- İnanç, N.; Fırat, Y.Y.; Başmısırlı, E.; Çapar, A.G. Nutrient Intake of Crohn’s Patients: Is There Consistency between Crohn’s Disease Activity Index, Subjective Global Assessment and Body Mass Index? Iran. J. Public. Health 2021, 50, 2584–2592. [Google Scholar] [CrossRef] [PubMed]
| Substance | Mechanism of Action | |
|---|---|---|
| Endogenous antioxidants | Superoxide dismutase |
|
| Glutathione peroxidase (GPX) | ||
| Catalase |
| |
| Exogenous antioxidants | Vitamin E | |
| Vitamin C | ||
| Zinc | ||
| Selenium |
| |
| Betacarotene | ||
| Flavonoids |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarmakiewicz-Czaja, S.; Ferenc, K.; Filip, R. Antioxidants as Protection against Reactive Oxidative Stress in Inflammatory Bowel Disease. Metabolites 2023, 13, 573. https://doi.org/10.3390/metabo13040573
Jarmakiewicz-Czaja S, Ferenc K, Filip R. Antioxidants as Protection against Reactive Oxidative Stress in Inflammatory Bowel Disease. Metabolites. 2023; 13(4):573. https://doi.org/10.3390/metabo13040573
Chicago/Turabian StyleJarmakiewicz-Czaja, Sara, Katarzyna Ferenc, and Rafał Filip. 2023. "Antioxidants as Protection against Reactive Oxidative Stress in Inflammatory Bowel Disease" Metabolites 13, no. 4: 573. https://doi.org/10.3390/metabo13040573
APA StyleJarmakiewicz-Czaja, S., Ferenc, K., & Filip, R. (2023). Antioxidants as Protection against Reactive Oxidative Stress in Inflammatory Bowel Disease. Metabolites, 13(4), 573. https://doi.org/10.3390/metabo13040573






