Antidiabetic Properties of Plant Secondary Metabolites
Abstract
1. Introduction
2. Materials and Methods
3. Interaction of Intestinal Microbiota and Herbal Medicines in the Treatment of DM
4. Main Plants Used to Treat Diabetes
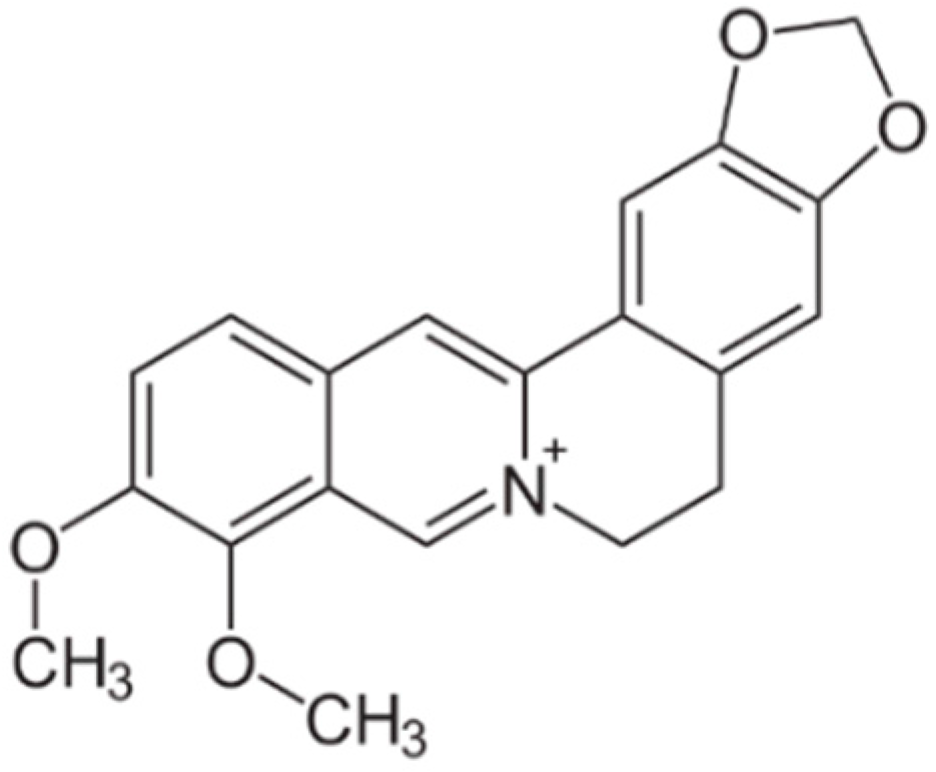
4.1. Barberry (Berberis)
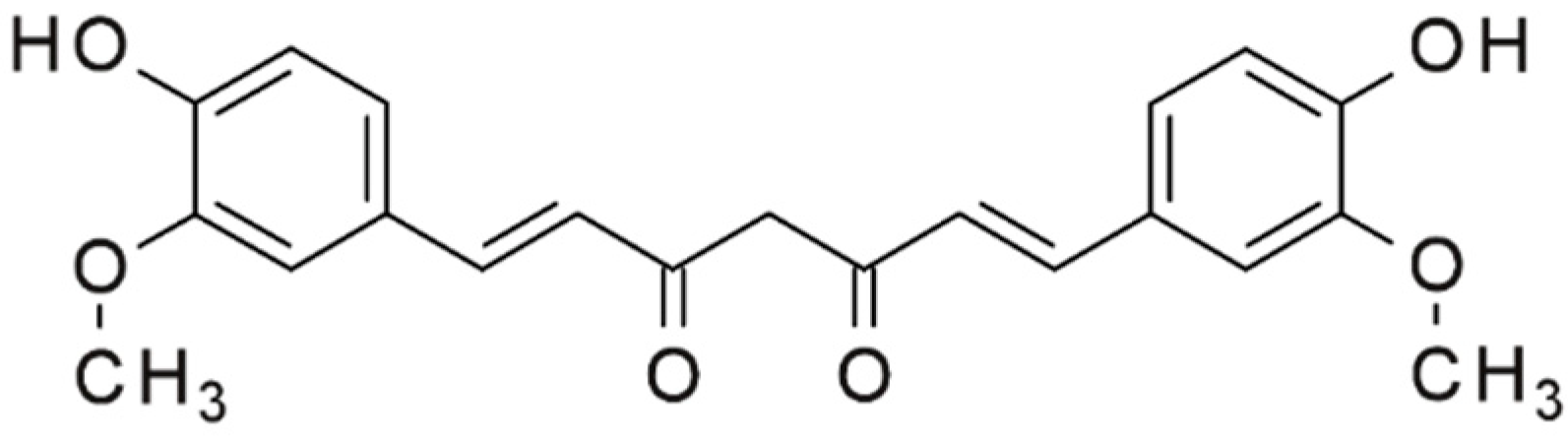
4.2. Turmeric (Curcuma longa)
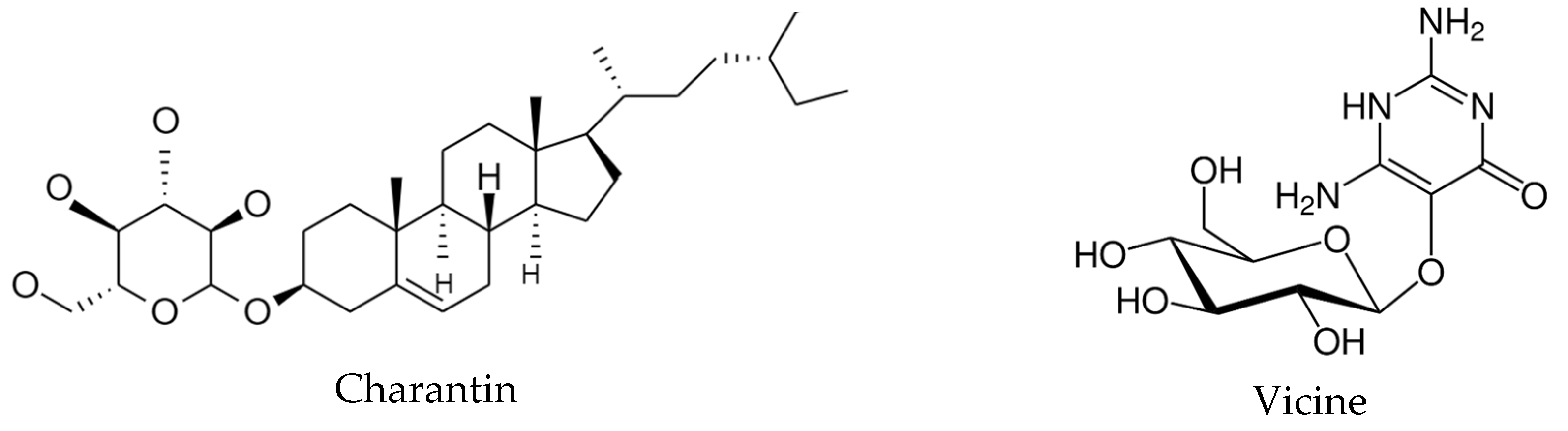
4.3. Bitter melon (Momordica charantia)
4.4. Ginseng (Panax)
4.5. Siberian Ginseng (Eleutherococcus)
4.6. Golden root (Rhodiola rosea L.)
4.7. Stevia (Stevia rebaudiana Bertoni)
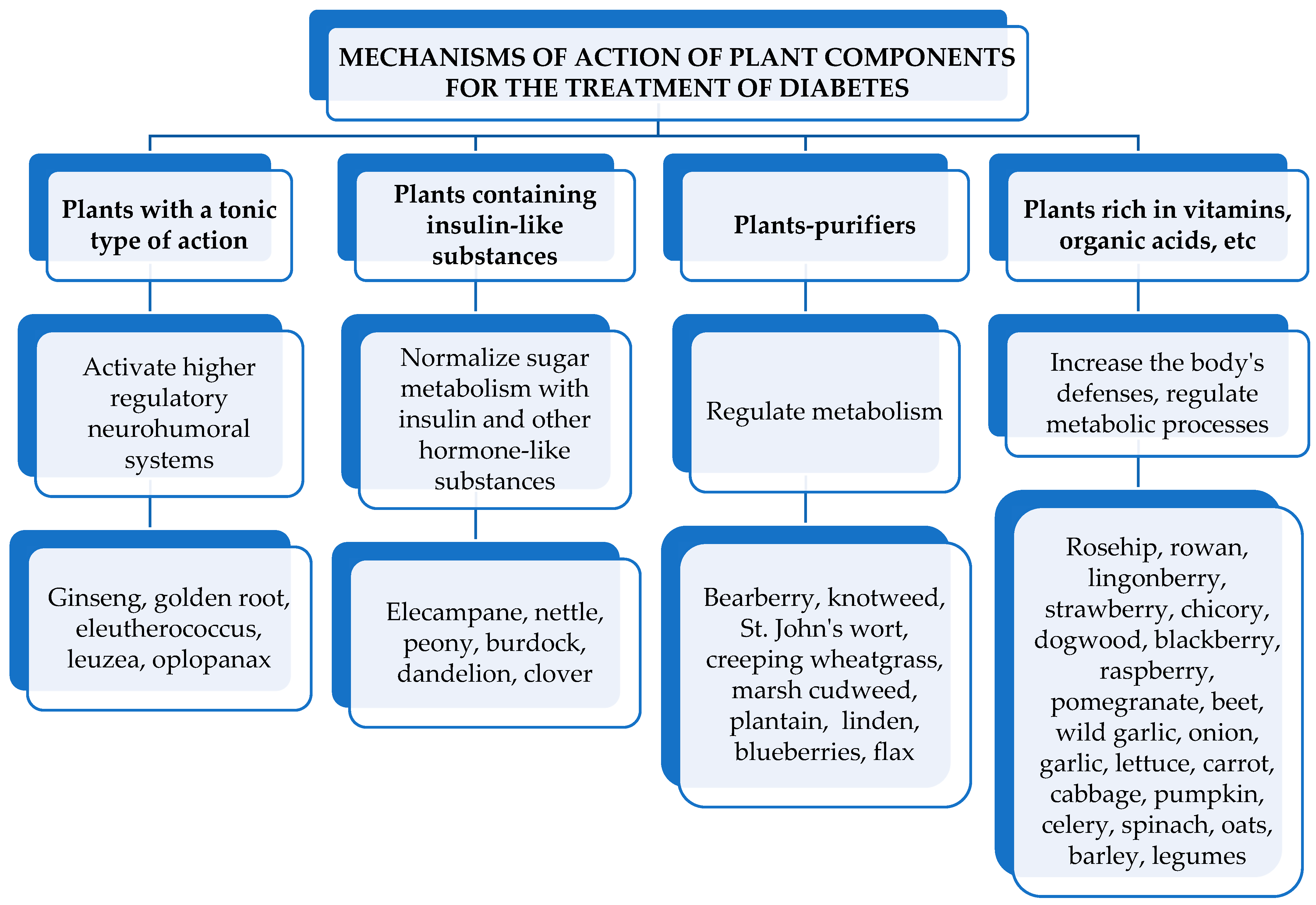
5. Mechanisms of Action of Medicinal Plant Components on the Course of DM
6. Groups of Biologically Active Substances of Medicinal Plants Used for the Treatment of DM
6.1. Insulin-like Substances of Plants, Their Mechanism of Lowering Blood Sugar
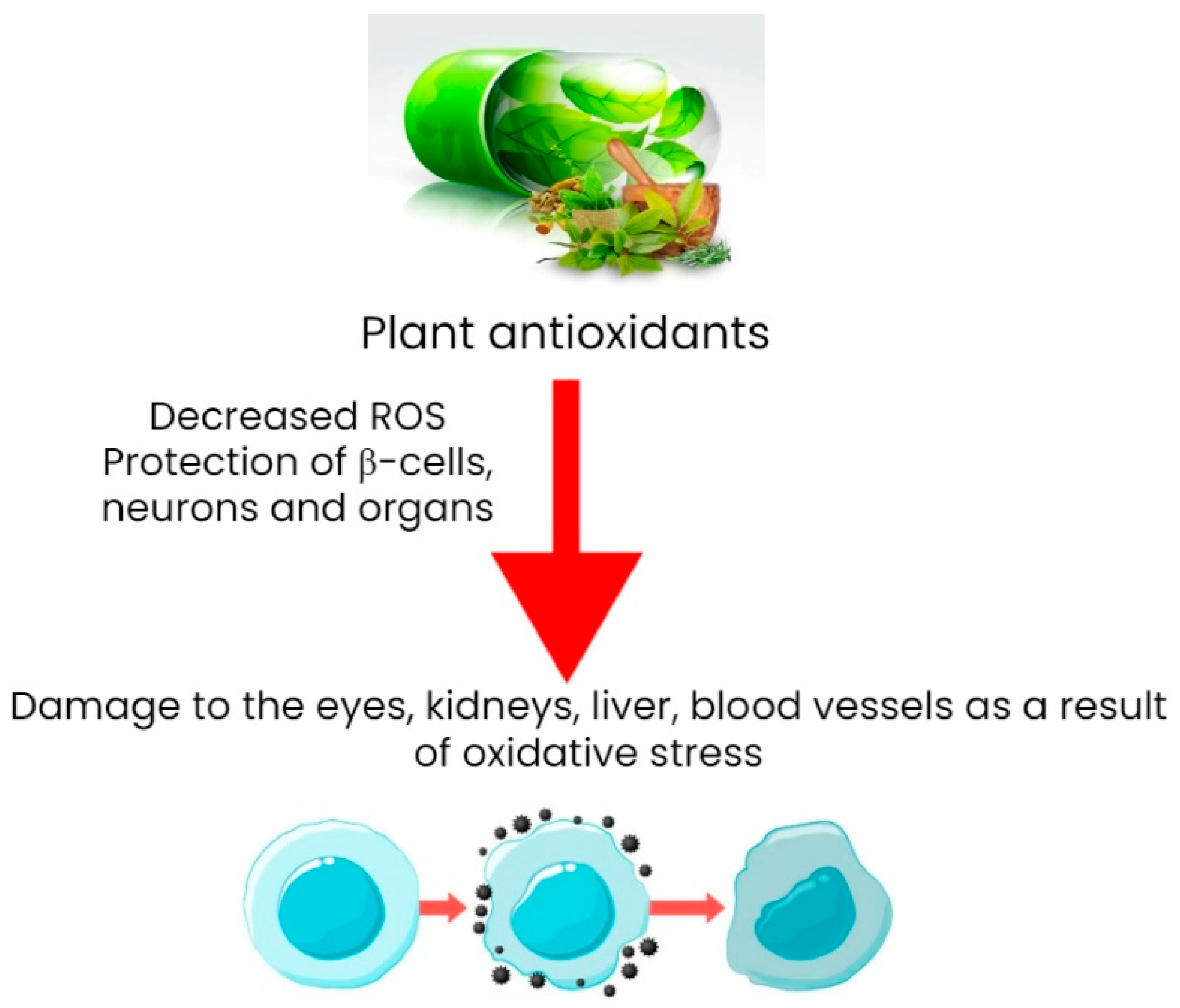
6.2. Plant Antioxidants and Their Antidiabetic Properties
6.3. Plant Polysaccharides with Antidiabetic Properties
6.3.1. Increased Insulin Levels and Decreased Pancreatic Glucagon Levels
6.3.2. Increased Insulin Sensitivity
6.3.3. Inhibition of α-Amylase and α-Glycosidase Enzymes
6.3.4. Increased Hepatic Glycogen Content
6.3.5. Normalized Intestinal Microflora
6.3.6. Decreased Blood Glucose Levels
6.3.7. Oxidative Stress Protection
6.4. Plant Alkaloids with Antidiabetic Properties
7. Complications of DM and the Effect of Medicinal Plants and Their Phytocomponents on Them
8. Current Progress and Prospects for the Use of Medicinal Plants and Phytocomponents in the Treatment of Diabetes
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, region-al, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Saini, D.C.; Kochar, A.; Poonia, R. Clinical correlation of diabetic retinopathy with nephropathy and neuropathy. Indian J. Ophthalmol. 2021, 69, 3364. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.M.M.; Chua, Z.J.Y.; Tan, J.C.; Yang, Y.; Liao, Z.; Zhao, Y. From pre-diabetes to diabetes: Diagnosis, treatments and translational research. Medicina 2019, 55, 546. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Wareham, N.J. Epidemiology of diabetes. Medicine 2019, 47, 22–27. [Google Scholar] [CrossRef]
- Lee, J.; Noh, S.; Lim, S.; Kim, B. Plant extracts for type 2 diabetes: From traditional medicine to modern drug discovery. Antioxidants 2021, 10, 81. [Google Scholar] [CrossRef]
- Turtinen, M.; Härkönen, T.; Ilonen, J.; Parkkola, A.; Knip, M.; Finnish Pediatric Diabetes Register. Seasonality in the manifestation of type 1 diabetes varies according to age at diagnosis in Finnish children. Acta Paediatr. 2022, 111, 1061–1069. [Google Scholar] [CrossRef]
- Moalem, S.; Storey, K.B.; Percy, M.E.; Peros, M.C.; Perl, D.P. The sweet thing about type 1 diabetes: A cryoprotective evolutionary adaptation. Med. Hypotheses 2005, 65, 8–16. [Google Scholar] [CrossRef]
- Chung, W.K.; Erion, K.; Florez, J.C.; Hattersley, A.T.; Hivert, M.F.; Lee, C.G.; McCarthy, M.I.; Nolan, J.J.; Norris, J.M.; Pearson, E.R.; et al. Precision medicine in diabetes: A consensus report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020, 43, 1617–1635. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- He, J.H.; Chen, L.X.; Li, H. Progress in the discovery of naturally occurring antidiabetic medications and in the identification of their molecular targets. Fitoterapia 2019, 134, 270–289. [Google Scholar] [CrossRef] [PubMed]
- Balbaa, M.; El-Zeftawy, M.; Abdulmalek, S.A. Therapeutic Screening of Herbal Remedies for the Management of Diabetes. Molecules 2021, 26, 6836. [Google Scholar] [CrossRef] [PubMed]
- Terechuk, L.; Starovoytova, K.; Babich, O.; Dyshlyuk, L.; Sergeeva, I.; Pavsky, V.; Ivanova, S.; Prosekov, A. Sea Buckthorn and Rosehip Oils with Chokeberry Extract to Prevent Hypercholesterolemia in Mice Caused by a High-Fat Diet In Vivo. Nutrients 2020, 12, 2941. [Google Scholar] [CrossRef] [PubMed]
- Xiao, E.; Luo, L. Alternative Therapies for Diabetes: A Comparison of Western and Traditional Chinese Medicine (TCM) Approaches. Curr. Diabetes Rev. 2018, 14, 487–496. [Google Scholar] [CrossRef]
- Shikov, A.N.; Narkevich, I.A.; Akamova, A.V.; Nemyatykh, O.D.; Flisyuk, E.V.; Luzhanin, V.G.; Povydysh, M.N.; Mikhailova, I.V.; Pozharitskaya, O.N. Medical Species Used in Russia for the Management of Diabetes and Related Disorders. Front. Pharmacol. 2021, 12, 697411. [Google Scholar] [CrossRef]
- Mabate, B.; Daub, C.D.; Malgas, S.; Edkins, A.L.; Pletschke, B.I. Fucoidan Structure and Its Impact on Glucose Metabolism: Implications for Diabetes and Cancer Therapy. Mar. Drugs 2021, 19, 30. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of Bioactivities of Fucoidan from the Brown Seaweed Fucus vesiculosus L. of the Barents Sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef]
- Oduro, P.K.; Fang, J.; Niu, L.; Li, Y.; Li, L.; Zhao, X.; Wang, Q. Pharmacological management of vascular endothelial dysfunction in diabetes: TCM and western medicine compared based on biomarkers and biochemical parameters. Pharmacol. Res. 2020, 158, 104893. [Google Scholar] [CrossRef]
- Samarakoon, D.N.A.W.; Uluwaduge, D.I.; Siriwardhene, M.A. Mechanisms of action of Sri Lankan herbal medicines used in the treatment of diabetes: A review. J. Integr. Med. 2020, 18, 14–20. [Google Scholar] [CrossRef]
- Khosravifar, M.; Sajadimajd, S.; Bahrami, G. Anti-diabetic Effects of Macronutrients via Modulation of Angiogenesis: A Comprehensive Review on Carbohydrates and Proteins. Cur. Mol. Med. 2023, 23, 250–265. [Google Scholar]
- Menezes, R.; Matafome, P.; Freitas, M.; García-Conesa, M.-T. Updated Information of the Effects of (Poly)phenols against Type-2 Diabetes Mellitus in Humans: Reinforcing the Recommendations for Future Research. Nutrients 2022, 14, 3563. [Google Scholar] [CrossRef] [PubMed]
- Debnath, B.; Manna, K. Formulating anti-diabetic nutraceutical tablets based on edible plants from Tripura, India. Foods Raw Mater. 2022, 10, 227–234. [Google Scholar] [CrossRef]
- Rahman, M.M.; Dhar, P.S.; Anika, F.; Ahmed, L.; Islam, M.R.; Sultana, N.A.; Cavalu, S.; Pop, O.; Rauf, A. Exploring the plant-derived bioactive substances as antidiabetic agent: An extensive review. Biomed Pharm. 2022, 152, 113217. [Google Scholar] [CrossRef] [PubMed]
- Dhakshayani, G.M.; Priya, S.J.A. A comparative study of phytochemical, antioxidant, anticarcinogenic, and antidiabetic potential of coriander (Coriandrum sativum L.): Microgreen and mature plant. Foods Raw Mater. 2022, 10, 283–294. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Sorini, C.; Cosorich, I.; Lo Conte, M.; De Giorgi, L.; Facciotti, F.; Lucianò, R. Loss of gut barrier integrity triggers activation of islet-reactive T cells and autoimmune diabetes. Proc. Natl. Acad. Sci. USA 2019, 116, 15140–15149. [Google Scholar] [CrossRef]
- Wu, Y.P.; Tang, L.; Wang, B.K.; Sun, Q.M.; Zhao, P.W.; Li, W.F. The role of autophagy in maintaining intestinal mucosal barrier. J. Cell. Physiol. 2019, 234, 19406–19419. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 2017, 49, e338. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Zhang, B.; Yue, R.; Chen, Y.; Yang, M.; Huang, X.; Shui, J. Gut microbiota, a potential new target for chinese herbal medicines in treating diabetes mellitus. Evid.-Based Complement. Altern. Med. 2019, 2019, 2634898. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Creely, S.J.; McTernan, P.G.; Kusminski, C.M.; Fisher, F.M.; Da Silva, N.F.; Khanolkar, M. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E740–E747. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.; Clemente-Casares, X.; Zhou, A.C.; Lei, H.; Ahn, J.J.; Chan, Y.T. Insulin receptor-mediated stimulation boosts T cell immunity during inflammation and infection. Cell Metab. 2018, 28, 922–934.e4. [Google Scholar] [CrossRef]
- Xia, C.; Rao, X.Q.; Zhong, J.X. Role of T lymphocytes in type 2 diabetes and diabetes-associated inflammation. J. Diabetes Res. 2017, 2017, 6494795. [Google Scholar] [CrossRef]
- Raphael, I.; Nalawade, S.; Eagar, T.N.; Forsthuber, T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015, 74, 5–17. [Google Scholar] [CrossRef]
- Kesarwani, K.; Gupta, R.; Mukerjee, A. Bioavailability enhancers of herbal origin: An overview. Asian Pac. J. Trop. Biomed. 2013, 3, 253–266. [Google Scholar] [CrossRef]
- Khashayar, A.; Bahari, Z.; Elliyeh, M.; Ghasemi, M. Therapeutic effects of berberine in metabolic diseases and diabetes mellitus. Rev. Bras. Farmacogn. 2021, 31, 272–281. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Atkin, S.L.; Nikiforov, N.G.; Sahebkar, A. Therapeutic role of curcumin in diabetes: An analysis based on bioinformatic findings. Nutrients 2022, 14, 3244. [Google Scholar] [CrossRef]
- Saeed, F.; Sultan, M.T.; Riaz, A.; Ahmed, S.; Bigiu, N.; Amarowicz, R.; Manea, R. Bitter melon (Momordica charantia L.) fruit bioactives charantin and vicinee potential for diabetes prophylaxis and treatment. Plants 2021, 10, 730. [Google Scholar] [CrossRef]
- Jovanovski, E.; Komishon, A.; Au-Yeung, F.; Zurbau, A.; Jenkins, A.L.; Sung, M.K.; Josse, R.; Vuksan, V. Vascular effects of combined enriched Korean Red ginseng (Panax Ginseng) and American ginseng (Panax Quinquefolius) administration in individuals with hypertension and type 2 diabetes: A randomized controlled trial. Complement. Ther. Med. 2020, 49, 102338. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Park, Y.H.; Kwon, C.J.; Ham, H.J.; Jeong, H.N.; Kim, K.H.; Ahn, Y.S. Antidiabetic and Hypoglycemic Effect of Eleutherococcus spp. J. Korean Soc. Food Sci. Nutr. 2010, 39, 1761–1768. [Google Scholar] [CrossRef]
- Teng, H.; Deng, H.; Wu, Y.; Zhang, C.; Ai, C.; Cao, H.; Xiao, J.; Chen, L. Effects of Rhodiola rosea and its major compounds on insulin resistance in Caenorhabditis elegans. J. Fut. Foods 2022, 2, 365–371. [Google Scholar] [CrossRef]
- Song, D.; Hao, J.; Fan, D. Biological properties and clinical applications of berberine. Front. Med. 2020, 14, 564–582. [Google Scholar] [CrossRef]
- Habtemariam, S. The quest to enhance the efficacy of berberine for type-2 diabetes and associated diseases: Physicochemical modification approaches. Biomedicines 2020, 8, 90. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, Y.; Ren, H.; Wang, S.; Zhong, H.; Zhao, X.; Ma, J.; Gu, X.; Xue, Y.; Huang, S.; et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat. Commun. 2020, 11, 5015. [Google Scholar] [CrossRef] [PubMed]
- Shinjyo, N.; Parkinson, J.; Bell, J.; Katsuno, T.; Bligh, A. Berberine for prevention of dementia associated with diabetes and its comorbidities: A systematic review. J. Integr. Med. 2020, 18, 125–151. [Google Scholar] [CrossRef]
- Panda, D.S.; Eid, H.M.; Elkomy, M.H.; Khames, A.; Hassan, R.M.; Abo El-Ela, F.I.; Yassin, H.A. Berberine encapsulated lecithin–chitosan nanoparticles as innovative wound healing agent in type II diabetes. Pharmaceutics 2021, 13, 1197. [Google Scholar] [CrossRef]
- Zhou, R.; Xiang, C.; Cao, G.; Xu, H.; Zhang, Y.; Yang, H.; Zhang, J. Berberine accelerated wound healing by restoring TrxR1/JNK in diabetes. Clin. Sci. 2021, 135, 613–627. [Google Scholar] [CrossRef]
- Mi, J.; He, W.; Lv, J.; Zhuang, K.; Huang, H.; Quan, S. Effect of berberine on the HPA-axis pathway and skeletal muscle GLUT4 in type 2 diabetes mellitus rats. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 1717. [Google Scholar] [CrossRef] [PubMed]
- Pivari, F.; Mingione, A.; Brasacchio, C.; Soldati, L. Curcumin and type 2 diabetes mellitus: Prevention and treatment. Nutrients 2019, 11, 1837. [Google Scholar] [CrossRef] [PubMed]
- Hodaei, H.; Adibian, M.; Nikpayam, O.; Hedayati, M.; Sohrab, G. The effect of curcumin supplementation on anthropometric indices, insulin resistance and oxidative stress in patients with type 2 diabetes: A randomized, double-blind clinical trial. Diabetol. Metab. Syndr. 2019, 11, 41. [Google Scholar] [CrossRef]
- Marton, L.T.; Pescinini-e-Salzedas, L.M.; Camargo, M.E.C.; Barbalho, S.M.; Haber, J.F.D.S.; Sinatora, R.V.; Detregiachi, C.R.P.; Girio, R.J.S.; Buchaim, D.V.; Cincotto dos Santos Bueno, P. The effects of curcumin on diabetes mellitus: A systematic review. Front. Endocrinol. 2021, 12, 669448. [Google Scholar] [CrossRef]
- Poolsup, N.; Suksomboon, N.; Kurnianta, P.D.M.; Deawjaroen, K. Effects of curcumin on glycemic control and lipid profile in prediabetes and type 2 diabetes mellitus: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0215840. [Google Scholar] [CrossRef]
- Asadi, S.; Gholami, M.S.; Siassi, F.; Qorbani, M.; Khamoshian, K.; Sotoudeh, G. Nano curcumin supplementation reduced the severity of diabetic sensorimotor polyneuropathy in patients with type 2 diabetes mellitus: A randomized double-blind placebo-controlled clinical trial. Complement. Ther. Med. 2019, 43, 253–260. [Google Scholar] [CrossRef]
- Adibian, M.; Hodaei, H.; Nikpayam, O.; Sohrab, G.; Hekmatdoost, A.; Hedayati, M. The effects of curcumin supplementation on high-sensitivity C-reactive protein, serum adiponectin, and lipid profile in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2019, 33, 1374–1383. [Google Scholar] [CrossRef]
- Bulboacă, A.E.; Boarescu, P.M.; Bolboacă, S.D.; Blidaru, M.; Feștilă, D.; Dogaru, G.; Nicula, C.A. Comparative effect of curcumin versus liposomal curcumin on systemic pro-inflammatory cytokines profile, MCP-1 and RANTES in experimental diabetes mellitus. Int. J. Nanomed. 2019, 14, 8961. [Google Scholar] [CrossRef]
- Peter, E.L.; Kasali, F.M.; Deyno, S.; Mtewa, A.; Nagendrappa, P.B.; Tolo, C.U.; Ogwang, P.E.; Sesaazi, D. Momordica charantia L. lowers elevated glycaemia in type 2 diabetes mellitus patients: Systematic review and meta-analysis. J. Ethnopharmacol. 2019, 231, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Peter, E.L.; Mtewa, A.G.; Nagendrappa, P.B.; Kaligirwa, A.; Sesaazi, C.D. Systematic review and meta-analysis protocol for efficacy and safety of Momordica charantia L. on animal models of type 2 diabetes mellitus. Syst. Rev. 2020, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gong, J.; Huang, W.; Lu, F.; Dong, H. The effect of Momordica charantia in the treatment of diabetes mellitus: A review. Evid. Based Complement. Altern. Med. 2021, 2021, 3796265. [Google Scholar] [CrossRef]
- Pahlavani, N.; Roudi, F.; Zakerian, M.; Ferns, G.A.; Navashenaq, J.G.; Mashkouri, A.; Ghayour-Mobarhan, M.; Rahimi, H. Possible molecular mechanisms of glucose-lowering activities of Momordica charantia (karela) in diabetes. J. Cell. Biochem. 2019, 120, 10921–10929. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Jung, J.; Jung, J.H.; Yoon, N.; Kang, S.S.; Roh, G.S.; Hahm, J.R. Hypoglycemic efficacy and safety of Momordica charantia (bitter melon) in patients with type 2 diabetes mellitus. Complement. Ther. Med. 2020, 52, 102524. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wen, J.J.; Hu, J.L.; Nie, Q.X.; Chen, H.H.; Xiong, T.; Nie, S.P.; Xie, M.Y. Fermented Momordica charantia L. juice modulates hyperglycemia, lipid profile, and gut microbiota in type 2 diabetic rats. Food Res. Int. 2019, 121, 367–378. [Google Scholar] [CrossRef]
- Malekshahi, H.; Bahrami, G.; Miraghaee, S.; Ahmadi, S.A.; Sajadimajd, S.; Hatami, R.; Mohammadi, B.; Keshavarzi, S. Momordica charantia reverses type II diabetes in rat. J. Food Biochem. 2019, 43, e13021. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, X.; Shi, F.; Liu, Y. Comparison of antidiabetic effects of saponins and polysaccharides from Momordica charantia L. in STZ-induced type 2 diabetic mice. Biomed. Pharmacother. 2019, 109, 744–750. [Google Scholar] [CrossRef]
- Liao, P.Y.; Lo, H.Y.; Liu, I.C.; Lo, L.C.; Hsiang, C.Y.; Ho, T.Y. A gastro-resistant peptide from Momordica charantia improves diabetic nephropathy in db/db mice via its novel reno-protective and anti-inflammatory activities. Food Funct. 2022, 13, 1822–1833. [Google Scholar] [CrossRef]
- Peter, E.L.; Nagendrappa, P.B.; Kaligirwa, A.; Ogwang, P.E.; Sesaazi, C.D. The safety and efficacy of Momordica charantia L. in animal models of type 2 diabetes mellitus: A systematic review and meta-analysis. Phytother. Res. 2021, 35, 637–656. [Google Scholar] [CrossRef]
- Jovanovski, E.; Smircic-Duvnjak, L.; Komishon, A.; Au-Yeung, F.R.; Sievenpiper, J.L.; Zurbau, A.; Jenkins, A.L.; Sung, M.K.; Josse, R.; Li, D.; et al. Effect of coadministration of enriched Korean Red Ginseng (Panax ginseng) and American ginseng (Panax quinquefolius L.) on cardiometabolic outcomes in type-2 diabetes: A randomized controlled trial. J. Ginseng Res. 2021, 45, 546–554. [Google Scholar] [CrossRef]
- Naseri, K.; Saadati, S.; Sadeghi, A.; Asbaghi, O.; Ghaemi, F.; Zafarani, F.; Li, H.B.; Gan, R.Y. The Efficacy of Ginseng (Panax) on Human Prediabetes and Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2401. [Google Scholar] [CrossRef] [PubMed]
- Vuksan, V.; Xu, Z.Z.; Jovanovski, E.; Jenkins, A.L.; Beljan-Zdravkovic, U.; Sievenpiper, J.L.; Stavro, P.M.; Zurbau, A.; Duvnjak, L.; Li, M.Z. Efficacy and safety of American ginseng (Panax quinquefolius L.) extract on glycemic control and cardiovascular risk factors in individuals with type 2 diabetes: A double-blind, randomized, cross-over clinical trial. Eur. J. Nutr. 2019, 58, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Povydysh, M.N.; Titova, M.V.; Ivanov, I.M.; Klushin, A.G.; Kochkin, D.V.; Galishev, B.A.; Popova, E.V.; Ivkin, D.Y.; Luzhanin, V.G.; Krasnova, M.V.; et al. Effect of phytopreparations based on bioreactor-grown cell biomass of dioscorea deltoidea, tribulus terrestris and panax japonicus on carbohydrate and lipid metabolism in type 2 diabetes mellitus. Nutrients 2021, 13, 3811. [Google Scholar] [CrossRef]
- Tang, X.; Huang, M.; Jiang, J.; Liang, X.; Li, X.; Meng, R.; Chen, L.; Li, Y. Panax notoginseng preparations as adjuvant therapy for diabetic kidney disease: A systematic review and meta-analysis. Pharm. Biol. 2020, 58, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Tan, R.Z.; Zhao, C.Y.; Li, J.C.; Zhong, X.; Diao, H.; Lin, X.; Duan, D.D.; Fan, J.M.; Xie, X.S.; et al. Astragalus mongholicus Bunge and Panax notoginseng (Burkill) FH chen formula for renal injury in diabetic Nephropathy—In Vivo and In Vitro evidence for autophagy regulation. Front. Pharmacol. 2020, 11, 732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, C.; Miao, L.; Tan, Y.; Zhou, Y.; Cheong, M.S.; Huang, Y.; Wang, Y.; Yu, H.; Cheang, W.S. Panax Notoginseng Protects against Diabetes-Associated Endothelial Dysfunction: Comparison between Ethanolic Extract and Total Saponin. Oxid. Med. Cell. Longev. 2021, 2021, 4722797. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Liu, J.; Sun, Z.; Meng, L.; Zhu, J.; Wang, M.; Su, J. Effects of Self-Made Prescription Compound Rhodiola on the Ultrastructure of Podocytes in Rats with Type 2 Diabetic Nephropathy. Int. J. Emerg. Med. 2022, 2022, 3417557. [Google Scholar] [CrossRef]
- Huang, Y.H.; Li, J.T.; Zan, K.; Wang, J.; Fu, Q. The traditional uses, secondary metabolites, and pharmacology of Eleutherococcus species. Phytochem. Rev. 2022, 21, 1081–1184. [Google Scholar] [CrossRef]
- Lee, K.Y.; Hong, S.Y.; Jeong, H.J.; Lee, J.H.; Lim, S.H.; Heo, N.K.; Kim, S.; Kim, H.Y. Isolation and identification of α-glucosidase inhibitory compounds, hyperoside, and isoquercetin from Eleutherococcus senticosus leaves. J. Korean Soc. Food Sci. Nutr. 2014, 43, 1858–1864. [Google Scholar] [CrossRef]
- Zheng, T.; Bian, F.; Chen, L.; Wang, Q.; Jin, S. Beneficial effects of Rhodiola and salidroside in diabetes: Potential role of AMP-activated protein kinase. Mol. Diagn. Ther. 2019, 23, 489–498. [Google Scholar] [CrossRef]
- Pu, W.L.; Zhang, M.Y.; Bai, R.Y.; Sun, L.K.; Li, W.H.; Yu, Y.L.; Zhang, Y.; Song, L.; Wang, Z.; Peng, Y.; et al. Anti-inflammatory effects of Rhodiola rosea L.: A review. Biomed. Pharmacother. 2020, 121, 109552. [Google Scholar] [CrossRef]
- Qi, S.S.; Shao, M.L.; Ze, S.; Zheng, H.X. Salidroside from Rhodiola rosea L. attenuates diabetic nephropathy in STZ induced diabetic rats via anti-oxidative stress, anti-inflammation, and inhibiting TGF-β1/Smad pathway. J. Funct. Foods 2021, 77, 104329. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, M.; Yuan, S.; Fu, S.; Li, Y.; Li, Y.; Wang, Q.; Cao, Y.; Liu, L.; Zhang, Q. Rhodiola rosea: A therapeutic candidate on cardiovascular diseases. Oxid. Med. Cell. Longev. 2022, 2022, 1348795. [Google Scholar] [CrossRef]
- Zheng, H.X. Protective effect of rhodiola rosea extract on alveolar bone loss in diabetic rats. Chin. Pharmacol. Bull. 2019, 35, 850–853. [Google Scholar] [CrossRef]
- Dandin, E.; Üstündağ, Ü.V.; Ünal, İ.; Ateş-Kalkan, P.S.; Cansız, D.; Beler, M.; Ak, E.; Alturfan, A.A.; Emekli-Alturfan, E. Stevioside ameliorates hyperglycemia and glucose intolerance, in a diet-induced obese zebrafish model, through epigenetic, oxidative stress and inflammatory regulation. Obes. Res. Clin. Pract. 2022, 16, 23–29. [Google Scholar] [CrossRef]
- Aswar, U.; Gogawale, V.; Miniyar, P.; Patil, Y. Beneficial effects of Stevioside on AGEs, blood glucose, lipid profile and renal status in streptozotocin-induced diabetic rats. J. Appl. Biomed. 2019, 17, 190–197. [Google Scholar] [CrossRef]
- Deenadayalan, A.; Subramanian, V.; Paramasivan, V.; Veeraraghavan, V.P.; Rengasamy, G.; Coiambatore Sadagopan, J.; Rajagopal, P.; Jayaraman, S. Stevioside attenuates insulin resistance in skeletal muscle by facilitating IR/IRS-1/Akt/GLUT 4 signaling pathways: An in vivo and in silico approach. Molecules 2021, 26, 7689. [Google Scholar] [CrossRef] [PubMed]
- Rashad, N.M.; Abdelsamad, M.A.; Amer, A.M.; Sitohy, M.Z.; Mousa, M.M. The impact of stevioside supplementation on glycemic control and lipid profile in patients with type 2 diabetes: A controlled clinical trial. Egypt. J. Intern. Med. 2019, 31, 22–30. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Choi, K.; Kim, Y.B. Molecular mechanism of insulin resistance in obesity and type 2 diabete. Korean J. Intern. Med. 2010, 25, 119. [Google Scholar] [CrossRef] [PubMed]
- Perseghin, G.; Petersen, K.; Shulman, G.I. Cellular mechanism of insulin resistance: Potential links with inflammation. Int. J. Obes. 2003, 27, S6–S11. [Google Scholar] [CrossRef] [PubMed]
- Costa, I.S.; Medeiros, A.F.; Piuvezam, G.; Medeiros, G.C.; Maciel, B.L.; Morais, A.H.A. Insulin-like proteins in plant sources: A systematic review. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3421. [Google Scholar] [CrossRef]
- Paula, P.C.; Oliveira, J.T.A.D.; Sousa, D.D.O.B.D.; Alves, B.G.T.; Carvalho, A.F.U.; Franco, O.L.; Vasconcelos, I.M. Insulin-like plant proteins as potential innovative medications to treat diabetes—The Moringa oleifera case study. New Biotechnol. 2017, 39, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zeng, Y.; Hou, W.; Zhang, S.; Li, L.; Luo, X.; Xi, W.; Chen, Z.; Xiang, M. The soybean peptide aglycin regulates glucose homeostasis in type 2 diabetic mice via IR/IRS1 pathway. J. Nutr. Biochem. 2012, 23, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Dun, X.P.; Wang, J.H.; Chen, L.; Lu, J.; Li, F.F.; Zhao, Y.Y.; Cederlund, E.; Bryzgalova, G.; Efendic, S.; Jörnvall, H.; et al. Activity of the plant peptide aglycin in mammalian systems. FEBS J. 2007, 274, 751–759. [Google Scholar] [CrossRef]
- Huang, M.H.; Lin, J.L.; Wang, M.; Ma, Y.; Wang, L.F. High-level Expression and Activity Determination of Hypoglycemic Peptide Aglycin. Mod. Food Sci. Technol. 2020, 36, 143–149. [Google Scholar] [CrossRef]
- Rashid, K.I. Detection of insulin-like protein and some active compounds in Bauhinia variegata Linn. leaf ethanolic extracts and the effect in reducing blood glucose levels in Mice. J. Biotechnol. Res. Cent. 2014, 8, 76–83. [Google Scholar] [CrossRef]
- van der Schaft, N.; Schoufour, J.D.; Nano, J.; Kiefte-de Jong, J.C.; Muka, T.; Sijbrands, E.J.; Ikram, M.A.; Franco, O.H.; Voortman, T. Dietary antioxidant capacity and risk of type 2 diabetes mellitus, prediabetes and insulin resistance: The Rotterdam Study. Eur. J. Epidemiol. 2019, 34, 853–861. [Google Scholar] [CrossRef]
- Rajendiran, D.; Packirisamy, S.; Gunasekaran, K. A review on role of antioxidants in diabetes. Asian J. Pharm. Clin. Res. 2018, 11, 48–53. [Google Scholar] [CrossRef]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative stress and diabetes: Antioxidative strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox. Biol. 2020, 37, 101799. [Google Scholar] [CrossRef] [PubMed]
- De Geest, B.; Mishra, M. Role of oxidative stress in diabetic cardiomyopathy. Antioxidants 2022, 11, 784. [Google Scholar] [CrossRef]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The role of oxidative stress and antioxidants in diabetic wound healing. Oxid. Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef]
- Kanwugu, O.N.; Glukhareva, T.V.; Danilova, I.G.; Kovaleva, E.G. Natural antioxidants in diabetes treatment and management: Prospects of astaxanthin. Crit. Rev. Food Sci. Nutr. 2022, 62, 5005–5028. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Wu, N.Y.; Kornelius, E.; Huang, C.N.; Yang, N.C. A randomized, double-blind, placebo-controlled trial to evaluate the hypoglycemic efficacy of the mcIRBP-19-containing Momordica charantia L. fruit extracts in the type 2 diabetic subjects. Food Nutr. Res. 2022, 66, 3685. [Google Scholar] [CrossRef]
- Dwijayanti, D.R.; Shimada, T.; Ishii, T.; Okuyama, T.; Ikeya, Y.; Mukai, E.; Nishizawa, M. Bitter melon fruit extract has a hypoglycemic effect and reduces hepatic lipid accumulation in ob/ob mice. Phytother. Res. 2020, 34, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Cabral, B.; Bortolin, R.H.; Gonçalves, T.A.F.; Maciel, P.M.P.; de Arruda, A.V.; de Carvalho, T.G.; Abboud, K.Y.; Alves, J.S.F.; Cordeiro, L.M.C.; Almeida de Medeiros, I.; et al. Hypoglycemic and vasorelaxant effect of Passiflora edulis fruit peel by-product. Plant Foods Hum. Nutr. 2021, 76, 466–471. [Google Scholar] [CrossRef]
- Dou, Z.M.; Chen, C.; Huang, Q.; Fu, X. The structure, conformation, and hypoglycemic activity of a novel heteropolysaccharide from the blackberry fruit. Food Funct. 2021, 12, 5451–5464. [Google Scholar] [CrossRef]
- Dai, K.; Wei, Y.; Jiang, S.; Xu, F.; Wang, H.; Zhang, X.; Shao, X. Lab Scale Extracted Conditions of Polyphenols from Thinned Peach Fruit Have Antioxidant, Hypoglycemic, and Hypolipidemic Properties. Foods 2022, 11, 99. [Google Scholar] [CrossRef]
- Giambanelli, E.; Gómez-Caravaca, A.M.; Ruiz-Torralba, A.; Guerra-Hernández, E.J.; Figueroa-Hurtado, J.G.; García-Villanova, B.; Verardo, V. New advances in the determination of free and bound phenolic compounds of Banana Passion Fruit Pulp (Passiflora tripartita, var. Mollissima (Kunth) LH Bailey) and their in vitro antioxidant and hypoglycemic capacities. Antioxidants 2020, 9, 628. [Google Scholar] [CrossRef]
- Tan, S.S.; Tan, S.T.; Tan, C.X. Antioxidant, hypoglycemic and anti-hypertensive properties of extracts derived from peel, fruit and kernel of Salak. Br. Food J. 2020, 122, 3029–3038. [Google Scholar] [CrossRef]
- Jiang, S.; Zhao, X.; Liu, C.; Dong, Q.; Mei, L.; Chen, C.; Shao, Y.; Tao, Y.; Yue, H. Identification of phenolic compounds in fruits of Ribes stenocarpum Maxim. By UHPLC-QTOF/MS and their hypoglycemic effects in vitro and in vivo. Food Chem. 2021, 344, 128568. [Google Scholar] [CrossRef] [PubMed]
- Haruna, M.; Abdulmumin, Y.; Abdulmumin, T.M.; Murtala, M.; Dalhatu, M.M. Hypoglycemic and Antihyperlipidemic Effects of Ethanolic Fruit Peel Extract of Carica papaya (Pawpaw) in an Alloxan-induced Diabetic Rats. J. Adv. Med. Pharm. Sci. 2022, 24, 31–41. [Google Scholar] [CrossRef]
- Khalil, N.; Alfaris, N.A.; Altamimi, J.Z. Potential health effects of tomato (lycopersicon esculentum) juice and hypoglycemic amelioration in the atherogenic indices between diabetic animal models. Food Sci. Technol. 2022, 42, e88222. [Google Scholar] [CrossRef]
- Kim, K.J.; Park, S.Y.; Kim, J.T.; Lee, H.J.; Jung, S.K.; Kim, Y.J.; Lee, C.H.; Byun, S.; Kim, J.Y. In vitro and in vivo postprandial hypoglycemic effects and comprehensive metabolite profiling of Dangjo chili pepper (Capsicum annuum L. cv. Dangjo). Food Biosci. 2023, 51, 102180. [Google Scholar] [CrossRef]
- Mans, K.; Aburjai, T. Accessing the hypoglycemic effects of seed extract from celery (Apium graveolens) in alloxan-induced diabetic rats. J. Pharm. Res. Int. 2019, 26, 1–10. [Google Scholar] [CrossRef]
- Ogbonna, J.I.; Umeh, O.N.; Mbah, C.C.; Ofoefule, S.I.; Ozumba, B.C. Hypoglycemic activities and biochemical parameters modulation of herbal formulations of Allium cepa L. in alloxanized diabetic rats. J. Sci. Res. Essay 2019, 14, 74–85. [Google Scholar] [CrossRef]
- Sun, C.; Liu, Y.; Zhan, L.; Rayat, G.R.; Xiao, J.; Jiang, H.; Li, H.; Chen, K. Antidiabetic effects of natural antioxidants from fruits. Trends Food Sci. Technol. 2021, 117, 3–14. [Google Scholar] [CrossRef]
- Bhadra, P. In-silico Analysis of Effects of Stevia Extracts on Diabetes. Indian J. Nat. Prod. Resour. 2020, 10, 20710–20719. [Google Scholar]
- Erukainure, O.L.; Chukwuma, C.I.; Sanni, O.; Matsabisa, M.G.; Islam, M.S. Histochemistry, phenolic content, antioxidant, and anti-diabetic activities of Vernonia amygdalina leaf extract. J. Food Biochem. 2019, 43, e12737. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative stress, plant natural antioxidants, and obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- Tang, T.; Duan, X.; Ke, Y.; Zhang, L.; Shen, Y.; Hu, B.; Liu, A.; Chen, H.; Li, C.; Wu, W.; et al. Antidiabetic activities of polysaccharides from Anoectochilus roxburghii and Anoectochilus formosanus in STZ-induced diabetic mice. Int. J. Biol. Macromol. 2018, 112, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, S.; Epifano, F.; Preziuso, F.; Taddeo, V.A.; Genovese, S. Selenylated plant polysaccharides: A survey of their chemical and pharmacological properties. Phytochemistry 2018, 153, 1–10. [Google Scholar] [CrossRef]
- Wang, X.F.; Chen, X.; Tang, Y.; Wu, J.M.; Qin, D.L.; Yu, L.; Zhou, X.G.; Wu, A.G. The therapeutic potential of plant polysaccharides in metabolic diseases. Pharmaceuticals 2022, 15, 1329. [Google Scholar] [CrossRef]
- Hu, J.L.; Nie, S.P.; Xie, M.Y. Antidiabetic mechanism of dietary polysaccharides based on their gastrointestinal functions. J. Agric. Food Chem. 2018, 66, 4781–4786. [Google Scholar] [CrossRef] [PubMed]
- Sajadimajd, S.; Bahrami, G.; Daglia, M.; Nabavi, S.M.; Naseri, R.; Farzaei, M.H. Plant-derived supplementary carbohydrates, polysaccharides and oligosaccharides in management of diabetes mellitus: A comprehensive review. Food Rev. Int. 2019, 35, 563–586. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Zhang, Y.; Liu, X.; Wu, Z.; Gilbert, R.G.; Deng, B.; Wang, K. Dendrobium officinale polysaccharide ameliorates diabetic hepatic glucose metabolism via glucagon-mediated signaling pathways and modifying liver-glycogen structure. J. Ethnopharmacol. 2020, 248, 112308. [Google Scholar] [CrossRef]
- Kuang, M.T.; Li, J.Y.; Yang, X.B.; Yang, L.; Xu, J.Y.; Yan, S.; Lv, Y.F.; Ren, F.C.; Hu, J.M.; Zhou, J. Structural characterization and hypoglycemic effect via stimulating glucagon-like peptide-1 secretion of two polysaccharides from Dendrobium officinale. Carbohydr. Polym. 2020, 241, 116326. [Google Scholar] [CrossRef]
- Xie, F.; Zou, T.; Chen, J.; Liang, P.; Wang, Z.; You, J. Polysaccharides from Enteromorpha prolifera improves insulin sensitivity and promotes adipose thermogenesis in diet-induced obese mice associated with activation of PGC-1α-FNDC5/irisin pathway. J. Funct. Foods 2022, 90, 104994. [Google Scholar] [CrossRef]
- Yang, S.; Yan, J.; Yang, L.; Meng, Y.; Wang, N.; He, C.; Fan, Y.; Zhou, Y. Alkali-soluble polysaccharides from mushroom fruiting bodies improve insulin resistance. Int. J. Biol. Macromol. 2019, 126, 466–474. [Google Scholar] [CrossRef]
- Liu, D.; Gao, H.; Tang, W.; Nie, S. Plant non-starch polysaccharides that inhibit key enzymes linked to type 2 diabetes mellitus. Ann. N. Y. Acad. Sci. 2017, 1401, 28–36. [Google Scholar] [CrossRef]
- Song, J.; Wu, Y.; Ma, X.; Feng, L.; Wang, Z.; Jiang, G.; Tong, H. Structural characterization and α-glycosidase inhibitory activity of a novel polysaccharide fraction from Aconitum coreanum. Carbohydr. Polym. 2020, 230, 115586. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.K.; Yu, Y.B.; Wang, C.; Cai, W.D.; Wu, L.X.; Yang, Y.; Zhang, H.N. Production, physicochemical characteristics, and in vitro biological activities of polysaccharides obtained from fresh bitter gourd (Momordica charantia L.) via room temperature extraction techniques. Food Chem. 2021, 337, 127798. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.K.; Wang, C.; Yu, Y.B.; Wu, L.X.; Chen, T.T.; Wang, Z.W. Physicochemical characteristics and in vitro biological activities of polysaccharides derived from raw garlic (Allium sativum L.) bulbs via three-phase partitioning combined with gradient ethanol precipitation method. Food Chem. 2021, 339, 128081. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Peng, B.; Wei, W.; Tian, X.; Wu, Z. Antioxidant and antidiabetic activities of polysaccharides from guava leaves. Molecules 2019, 24, 1343. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Zhang, W.; Sun, M.; Zhang, Z. Hypoglycemic effect of inulin combined with ganoderma lucidum polysaccharides in T2DM rats. J. Funct. Foods 2019, 55, 381–390. [Google Scholar] [CrossRef]
- Zhong, Q.W.; Zhou, T.S.; Qiu, W.H.; Wang, Y.K.; Xu, Q.L.; Ke, S.Z.; Wang, S.J.; Jin, W.H.; Chen, J.W.; Zhang, H.W.; et al. Characterization and hypoglycemic effects of sulfated polysaccharides derived from brown seaweed Undaria pinnatifida. Food Chem. 2021, 341, 128148. [Google Scholar] [CrossRef]
- Yu, W.; Zeng, D.; Xiong, Y.; Shan, S.; Yang, X.; Zhao, H.; Lu, W. Health benefits of functional plant polysaccharides in metabolic syndrome: An overview. J. Funct. Foods 2022, 95, 105154. [Google Scholar] [CrossRef]
- Dave, D.T.; Shah, G.B. Pharmacological potential of naturally occurring non-starch polysaccharides (NSP). J. Phytopharmacol. 2015, 4, 307–310. [Google Scholar] [CrossRef]
- Raish, M.; Ahmad, A.; Jan, B.L.; Alkharfy, K.M.; Ansari, M.A.; Mohsin, K.; Jenoobi, F.; Al-Mohizea, A. Momordica charantia polysaccharides mitigate the progression of STZ induced diabetic nephropathy in rats. Int. J. Biol. Sci. Macromol. 2016, 91, 394–399. [Google Scholar] [CrossRef]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Awasthi, A. Therapeutic potential of mushrooms in diabetes mellitus: Role of polysaccharides. Int. J. Biol. Macromol. 2020, 164, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Meenu, M.; Liu, H.; Xu, B. A concise review on the molecular structure and function relationship of β-glucan. Int. J. Mol. Sci. 2019, 20, 4032. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Khalil, A.A.; Shaukat, F.; Song, Y. Sources, extraction and biomedical properties of polysaccharides. Foods 2019, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. Antidiabetic effects and mechanisms of dietary polysaccharides. Molecules 2019, 24, 2556. [Google Scholar] [CrossRef]
- Ji, X.; Peng, B.; Ding, H.; Cui, B.; Nie, H.; Yan, Y. Purification, structure and biological activity of pumpkin polysaccharides: A review. Food Rev. Int. 2021, in press. [Google Scholar] [CrossRef]
- Rasouli, H.; Yarani, R.; Pociot, F.; Popović-Djordjević, J. Antidiabetic potential of plant alkaloids: Revisiting current findings and future perspectives. Pharmacol. Res. 2020, 155, 104723. [Google Scholar] [CrossRef] [PubMed]
- Ajebli, M.; Khan, H.; Eddouks, M. Natural alkaloids and diabetes mellitus: A review. Endocr. Metab. Immune. Disord. Drug Targets 2021, 21, 111–130. [Google Scholar] [CrossRef]
- Adhikari, B. Roles of alkaloids from medicinal plants in the management of diabetes mellitus. J. Chem. 2021, 2021, 2691525. [Google Scholar] [CrossRef]
- Zhang, H.; Hui, J.; Yang, J.; Deng, J.; Fan, D. Eurocristatine, a plant alkaloid from Eurotium cristatum, alleviates insulin resistance in db/db diabetic mice via activation of PI3K/AKT signaling pathway. Eur. J. Pharmacol. 2020, 887, 173557. [Google Scholar] [CrossRef]
- Belwal, T.; Bisht, A.; Devkota, H.P.; Ullah, H.; Khan, H.; Pandey, A.; Bhatt, I.D.; Echeverría, J. Phytopharmacology and clinical updates of Berberis species against diabetes and other metabolic diseases. Front. Pharmacol. 2020, 11, 41. [Google Scholar] [CrossRef]
- Bharti, S.K.; Krishnan, S.; Kumar, A.; Kumar, A. Antidiabetic phytoconstituents and their mode of action on metabolic pathways. Ther. Adv. Endocrinol. Metab. 2018, 9, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, M.; Grdović, N.; Mihailović, M.; Jovanović, J.A.; Uskoković, A.; Rajić, J.; Sinadinović, M.; Tolić, A.; Mišić, D.; Šiler, B. Centaurium erythraea extract improves survival and functionality of pancreatic beta-cells in diabetes through multiple routes of action. J. Ethnopharmacol. 2019, 242, 112043. [Google Scholar] [CrossRef] [PubMed]
- Unuofin, J.O.; Lebelo, S.L. Antioxidant Effects and Mechanisms of Medicinal Plants and Their Bioactive Compounds for the Prevention and Treatment of Type 2 Diabetes: An Updated Review. Oxid. Med. Cell. Longev. 2020, 2020, 1356893. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, M.; Grdović, N.; Mihailović, M.; Jovanović, J.A.; Uskoković, A.; Rajić, J.; Đorđević, M.; Tolić, A.; Mišić, D.; Šiler, B. Centaurium erythraea extract reduces redox imbalance and improves insulin expression and secretion in pancreatic β-cells exposed to oxidative and nitrosative stress. Arch. Biol. Sci. 2020, 72, 117–128. [Google Scholar]
- Sharma, B.; Mittal, A.; Dabur, R. Mechanistic approach of anti-diabetic compounds identified from natural sources. Chem. Biol. Lett. 2018, 5, 63–99. [Google Scholar]
- Ubasheev, I.O. Morphofunctional Bases of Regenerative Therapy with Natural Medicines for Experimental Liver Damage; Publishing House of the Belarusian Scientific Center of the Siberian Branch of the Russian Academy of Sciences: Ulan-Ude, Russia, 2005. [Google Scholar]
- Telyatiev, V.V. Healing Treasures of Central Siberia; Scientific and Practical Laboratory of Phytotherapy and Biochemistry Plants SB RAS (SIFIBR): Irkutsk, Russia, 2004; 999p. [Google Scholar]
- Pokrovsky, M.V. Endothelioprotective effects of L-arginine in modeling nitric oxide deficiency. Exp. Clin. Pharmacol. 2008, 71, 29–31. [Google Scholar]
- Maznev, N.I. Medicinal Plants; XXI Century: Moscow, Russia, 2006. [Google Scholar]
- Lapinskaya, E.S.; Kopytko, Y.F. Study of the composition of the lipophilic fraction of tinctures of homeopathic matrix stinging nettle (Urtica dioica L.) and stinging nettle (Urtica urens L.). Khim.-Farm. Mag. 2008, 42, 26–29. [Google Scholar]
- Miaffo, D.; Kamgue, O.; Guessom, T.; Temhoul, C.; Kamanyi, A. Antidiabetic and antioxidant potentials of Vitellaria para-doxa barks in alloxan-induced diabetic rats. Clin. Phytosci. 2019, 5, 44. [Google Scholar] [CrossRef]
- Mihailović, M.; Uskoković, A.; Jovanović, J.A.; Grdović, N.; Dinić, S.; Poznanović, G.; Franić, A.; Đorđević, M.; Vidaković, M. Treatment of streptozotocin-induced diabetic rats with Castanea sativa and Lactarius deterrimus extracts decreases liver damage by initiating activation of the Akt prosurvival kinase. Arch. Biol. Sci. 2020, 72, 233–242. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef] [PubMed]
- Rotter, S.; Beronius, A.; Boobis, A.R.; Hanberg, A.; van Klaveren, J.; Luijten, M.; Machera, K.; Nikolopoulou, D.; van der Voet, H.; Zilliacus, J. Overview on legislation and scientific approaches for risk assessment of combined expo-sure to multiple chemicals: The potential EuroMix contribution. Crit. Rev. Toxicol. 2018, 48, 796–814. [Google Scholar] [CrossRef] [PubMed]
- Mihailović, M.; Dinić, S.; Arambašić Jovanović, J.; Uskoković, A.; Grdović, N.; Vidaković, M. The Influence of Plant Extracts and Phytoconstituents on Antioxidant Enzymes Activity and Gene Expression in the Prevention and Treatment of Impaired Glucose Homeostasis and Diabetes Complications. Antioxidants 2021, 18, 480. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Prasad, S.K.; Kumar, R.; Hemalatha, S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac. J. Trop. Biomed. 2012, 2, 320–330. [Google Scholar] [CrossRef]
- Rivellese, A.A.; Maffettone, A. Dietary Fiber in the Treatment of Metabolic Diseases. Eur. J. Clin. Nutr. 1995, 49, 110–112. [Google Scholar]
- Moreno Franco, B.; Leon Latre, M.; Andres Esteban, E.M.; Ordovas, J.M.; Casasnovas, J.A.; Penalvo, J.L. Soluble and insoluble dietary fibre intake and risk factors for metabolic syndrome and cardiovascular disease in middle-aged adults: The AWHS cohort. Nutr. Hosp. 2014, 30, 1279–1288. [Google Scholar] [CrossRef]
- Mann, J.I.; De Leeuw, I.; Hermansen, K.D.; Karamanos, B.; Karlström, B.; Katsilambros, N.; Riccardi, G.; Rivellese, A.A.; Rizkalla, S.; Slama, G. Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2004, 14, 373–394. [Google Scholar] [CrossRef]
- Gaikwad, S.B.; Krishna Mohan, G.; Sandhya Rani, M. Phytochemicals for Diabetes Management. Pharm. Crops 2014, 25, 11–28. [Google Scholar] [CrossRef]
- Chen, H.; Qu, Z.; Fu, L.; Dong, P.; Zhang, X. Physicochemical properties and antioxidant capacity of 3 polysaccharides from green tea, oolong tea, and black tea. J. Food Sci. 2009, 74, 469–474. [Google Scholar] [CrossRef]
- Chaudhary, S.; Semwal, A.; Kumar, H.; Verma, H.C.; Kumar, A. In-vivo study for anti-hyperglycemic potential of aqueous extract of Basil seeds (Ocimum basilicum Linn) and its influence on biochemical parameters, serum electrolytes and haematological indices. Biomed. Pharm. 2016, 84, 2008–2013. [Google Scholar] [CrossRef]
- Mirfeizi, M.; Mehdizadeh, T.Z.; Mirfeizi, S.Z.; Asghari, J.M.; Rezvani, H.R.; Afzali, M. Controlling type 2 diabetes mellitus with herbal medicines: A triple-blind randomized clinical trial of efficacy and safety. J. Diabetes 2016, 8, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Tapsell, L.C.; Hemphill, I.; Cobiac, L.; Patch, C.S.; Sullivan, D.R.; Fenech, M.; Roodenrys, S.; Keogh, J.B.; Clifton, P.M.; Williams, P.G.; et al. Health benefits of herbs and spices: The past, the present, the future. Med. J. Aust. 2006, 185, S1–S24. [Google Scholar] [CrossRef] [PubMed]
- Combs, G.F. Vitamin C. In The Vitamins, Fundamental Aspects in Nutrition and Health, 4th ed.; Elsevier: London, UK, 2012; pp. 233–261. [Google Scholar]
- Chen, H.; Karne, R.J.; Hall, G. High-dose oral vitamin C partially replenishes vitamin C levels in patients with type 2 diabetes and low vitamin C levels but does not improve endothelial dysfunction or insulin resistance. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei-Malazy, O.; Larijani, B.; Abdollahi, M. A systematic review of in vitro studies conducted on effect of herbal products on secretion of insulin from Langerhans islets. J. Pharm. Pharmacol. Sci. 2012, 15, 447–466. [Google Scholar]
- Jacques-Camarena, O.; González-Ortiz, M.; Martínez-Abundis, E.; López-Madrueño, J.P.; Medina-Santillán, R. Effect of vanadium on insulin sensitivity in patients with impaired glucose tolerance. Ann. Nutr. Metab. 2008, 53, 195–198. [Google Scholar] [CrossRef]
- Pandey, S.K.; Anand-Srivastava, M.B.; Srivastava, A.K. Vanadyl sulfate-stimulated glycogen synthesis is associated with activation of phosphatidylinositol 3-kinase and is independent of insulin receptor tyrosine phosphorylation. Biochemistry 1998, 37, 7006–7014. [Google Scholar] [CrossRef]
- Luo, J.; Rizkalla, S.W.; Boillot, J. Dietary (n−3) polyunsaturated fatty acids improve adipocyte insulin action and glucose metabolism in insulin resistant rats: Relation to membrane fatty acids. J. Nutr. 1996, 126, 1951–1958. [Google Scholar]
- Ghadge, A.A.; Kuvalekar, A.A. Controversy of oral hypoglycemic agents in type 2 diabetes mellitus: Novel move towards combination therapies. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, S5–S13. [Google Scholar] [CrossRef]
- Alhujaily, M.; Dhifi, W.; Mnif, W. An Overview of the Potential of Medicinal Plants Used in the Development of Nutraceuticals for the Management of Diabetes Mellitus: Proposed Biological Mechanisms. Processes 2022, 10, 2044. [Google Scholar] [CrossRef]
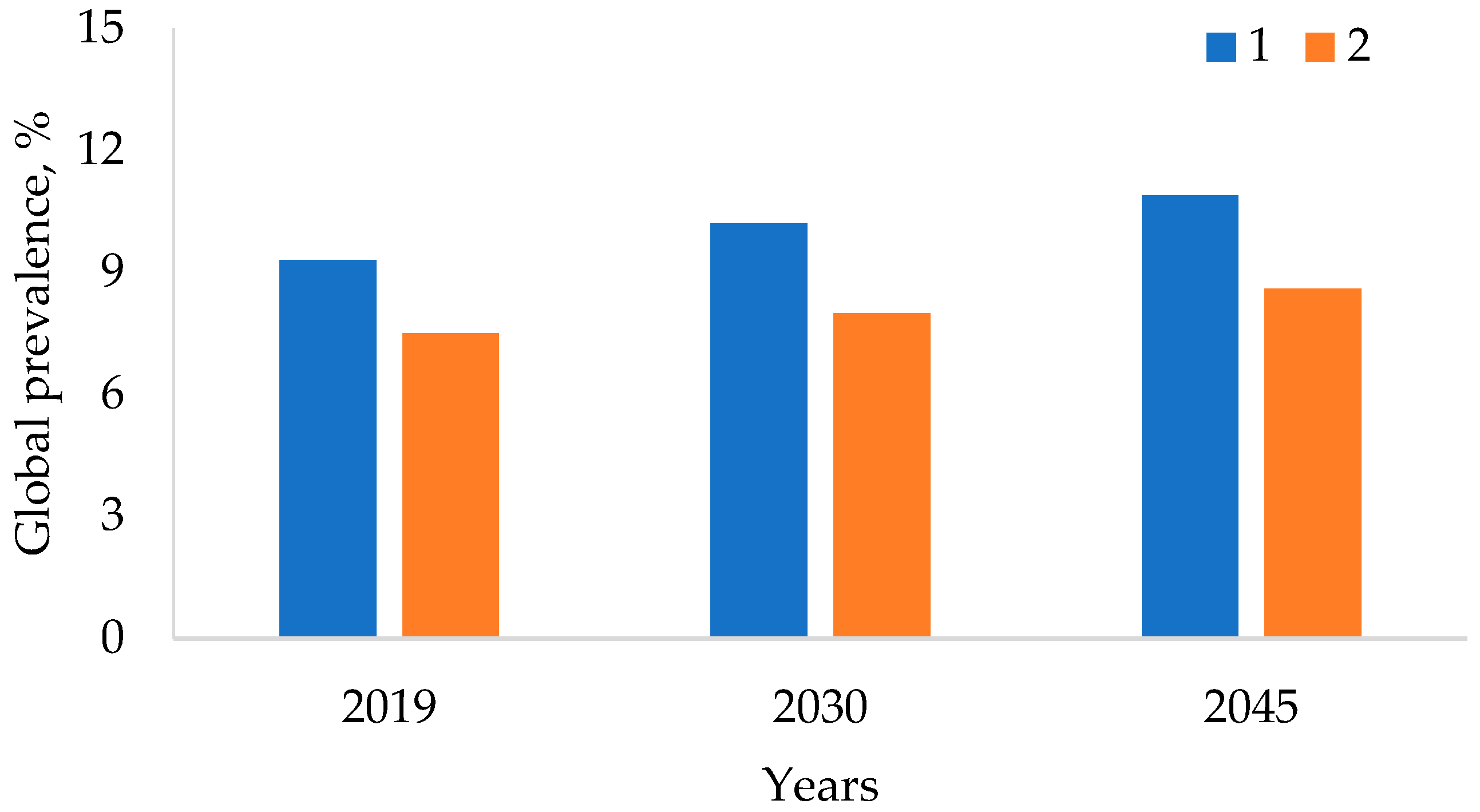
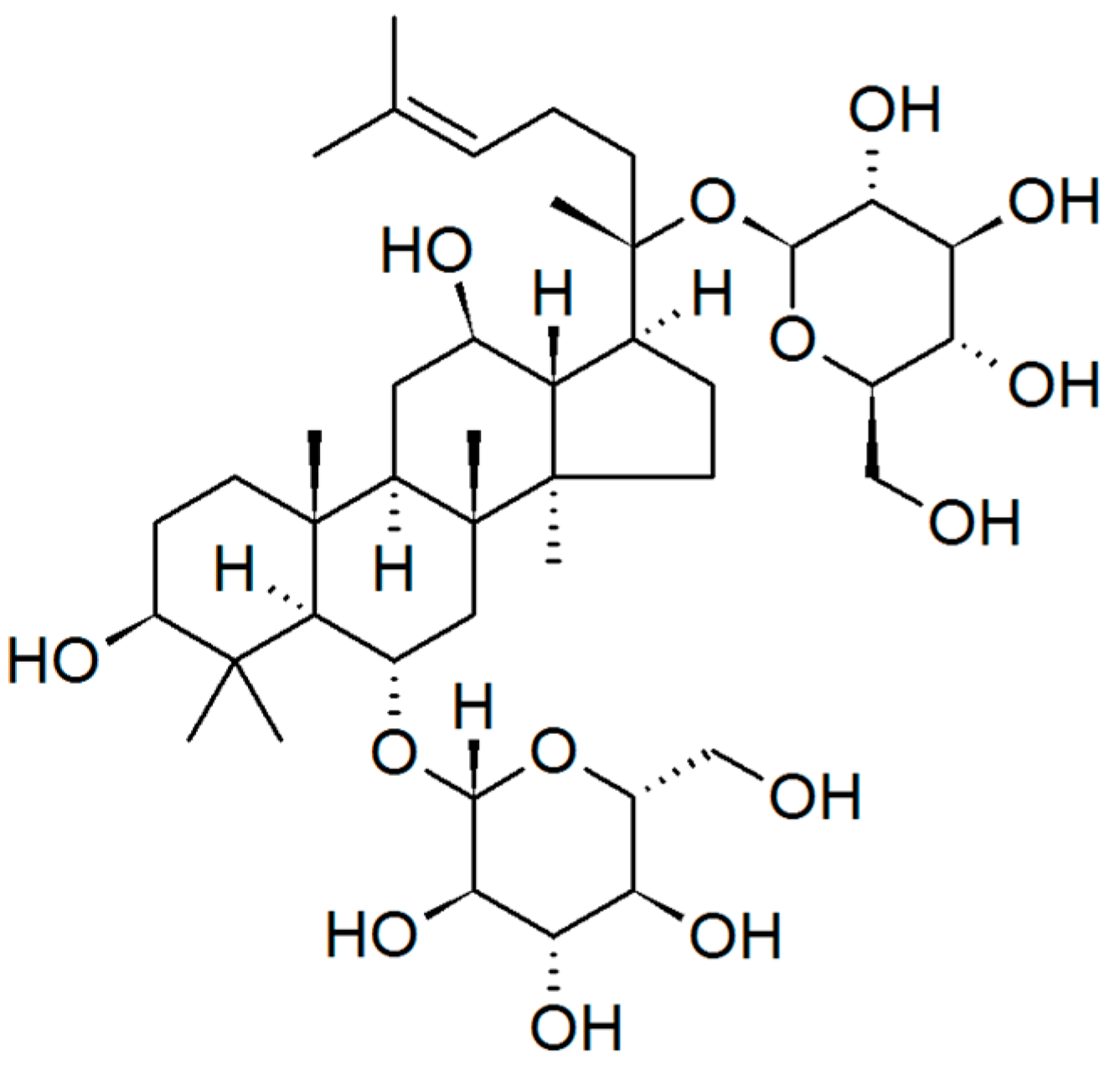
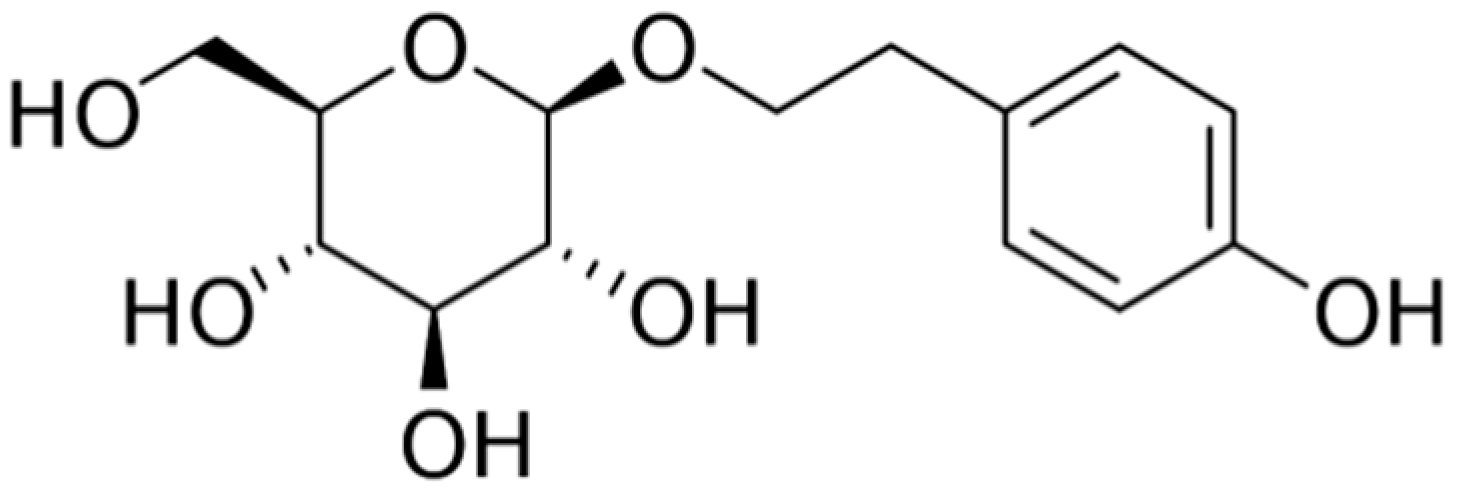
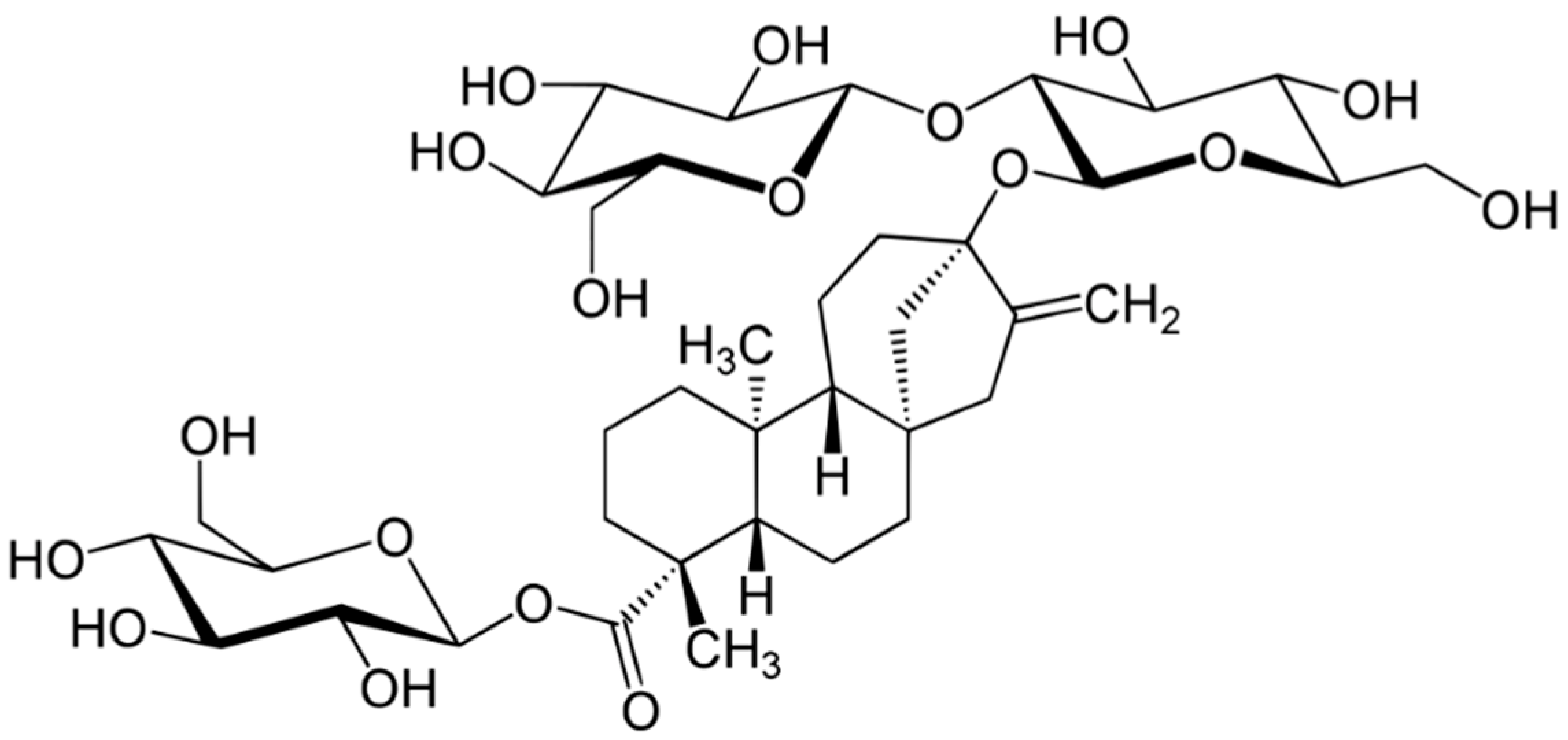

| Plant | Phytocomponents | Advantages | Disadvantages | Source |
|---|---|---|---|---|
| Dendrobium officinale | Polysaccharides | ↑ Glycogen synthesis ↓ Gluconeogenesis | ↓ Fatty acid synthesis | [172] |
| Anoectochilus roxburghii | Polysaccharides | ↓ Liver lipids ↑ Liver sensitivity to insulin | ↑ Fatty acid oxidation ↓ Glucose disposal by liver | [173] |
| Capsicum annuum | Alkaloids | ↑ Energy consumption ↓ Blood glucose ↑ Insulin sensitivity ↓ Serum lipids | ↑ Fatty acid oxidation | [170] |
| Costus igneus | Proteins/peptides | ↓ Fat intake, fatty acid synthesis ↑ Energy consumption | ↑ Fatty acid oxidation | [168] |
| Glycine max | Peptides | ↑ Adipocyte differentiation | ↑ Fat accumulation | [156] |
| Myrica gale L. | Polyphenols | ↓ Blood glucose ↑ Sensitivity to insulin | ↑ Glucose absorption | [174] |
| Vernonia amygdalina | Antioxidants | ↑ Glucose clearance | ↓ Glucose disposal by liver | [33] |
| Rosa majalis | Antioxidants | ↑ β-cell regeneration ↓ Glucose absorption | ↓ α- amylase and α- glucosidase | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukhikh, S.; Babich, O.; Prosekov, A.; Kalashnikova, O.; Noskova, S.; Bakhtiyarova, A.; Krol, O.; Tsvetkova, E.; Ivanova, S. Antidiabetic Properties of Plant Secondary Metabolites. Metabolites 2023, 13, 513. https://doi.org/10.3390/metabo13040513
Sukhikh S, Babich O, Prosekov A, Kalashnikova O, Noskova S, Bakhtiyarova A, Krol O, Tsvetkova E, Ivanova S. Antidiabetic Properties of Plant Secondary Metabolites. Metabolites. 2023; 13(4):513. https://doi.org/10.3390/metabo13040513
Chicago/Turabian StyleSukhikh, Stanislav, Olga Babich, Alexander Prosekov, Olga Kalashnikova, Svetlana Noskova, Alina Bakhtiyarova, Olesia Krol, Elena Tsvetkova, and Svetlana Ivanova. 2023. "Antidiabetic Properties of Plant Secondary Metabolites" Metabolites 13, no. 4: 513. https://doi.org/10.3390/metabo13040513
APA StyleSukhikh, S., Babich, O., Prosekov, A., Kalashnikova, O., Noskova, S., Bakhtiyarova, A., Krol, O., Tsvetkova, E., & Ivanova, S. (2023). Antidiabetic Properties of Plant Secondary Metabolites. Metabolites, 13(4), 513. https://doi.org/10.3390/metabo13040513









