Abstract
Microalgae are attracting attention as a next-generation alternative source of protein and essential fatty acids that do not consume large amounts of water or land. Chaetoceros gracilis (C. gracilis)—a marine microalga—is rich in proteins, fucoxanthin, and eicosapentaenoic acid (EPA). Growing evidence indicates that dietary fucoxanthin and EPA have beneficial effects in humans. However, none of these studies have shown that dietary C. gracilis has beneficial effects in mammals. In this study, we investigated the effects of dietary C. gracilis on lipid abnormalities in Sprague-Dawley rats fed a high-sucrose cholesterol-containing diet. Dried C. gracilis was added to the control diet at a final dose of 2 or 5% (w/w). After four weeks, the soleus muscle weights were found to be dose-responsive to C. gracilis and showed a tendency to increase. The hepatic triglyceride and total cholesterol levels were significantly reduced by C. gracilis feeding compared to those in the control group. The activities of FAS and G6PDH, which are related to fatty acid de novo synthesis, were found to be dose-responsive to C. gracilis and tended to decrease. The hepatic glycerol content was also significantly decreased by C. gracilis feeding, and the serum HDL cholesterol levels were significantly increased, whereas the serum levels of cholesterol absorption markers (i.e., campesterol and β-sitosterol) and the hepatic mRNA levels of Scarb1 were significantly decreased. Water-soluble metabolite analysis showed that the muscular contents of several amino acids, including leucine, were significantly increased by C. gracilis feeding. The tendency toward an increase in the weight of the soleus muscle as a result of C. gracilis feeding may be due to the enhancement of muscle protein synthesis centered on leucine. Collectively, these results show that the oral administration of C. gracilis alleviates hepatic lipid accumulation in rats fed a high-sucrose and cholesterol-containing diet, indicating the potential use of C. gracilis as a food resource.
1. Introduction
Numerous algae species are found worldwide and are classified into two groups—macroalgae (also known as seaweeds) and microalgae—depending on the complexity of their biological organization [1]. Microalgae are microscopic, unicellular, and photosynthetic organisms distributed in both freshwater and marine ecosystems [1,2]. They have attracted attention as promising candidates for the industrial exploitation of food and biofuels due to their high productivity per unit area compared to agricultural crops, their high adaptability to various cultivation conditions, and their capacity to grow rapidly [1,2]. From the perspective of sustainable food supply and security, microalgae are also attracting attention as next-generation alternative sources of protein and essential fatty acids that do not require the consumption of large amounts of water or land [2]. Currently, the majority of microalgae dominating the global market are freshwater species that are inexpensive and easy to grow, such as Spirulina and Chlorella, which can be produced in open ponds with little risk of contamination [3]. On the other hand, marine microalgae species are known to be efficient producers of bioactive compounds, such as carotenoids and n-3 polyunsaturated fatty acids [4]. Compared to fish oils, marine microalgae oils have several advantages, such as no unpleasant smell, less contamination with heavy metals, and no variations in fatty acid composition under controlled cultivation [5]. In Commission Implementing Regulation (EU) 2018/1023, the European Union’s list of novel foods was established to include several microalgae, including dried biomass and extracted oils [6].
Among the marine microalgae, Chaetoceros gracilis (C. gracilis) is classified as a diatom and is characterized by high protein, fucoxanthin, and eicosapentaenoic acid (EPA) contents, in addition to photosynthetic pigments such as chlorophyll a, c1, and c2 [4,7,8]. Fucoxanthin is a non-provitamin-A carotenoid that belongs to the xanthophyll family and has an allene structure. Fucoxanthin has been reported to have anti-proliferative and apoptosis-inducing effects on cancer cells [9,10], an anti-obesity effect [11], and an anti-diabetic effect [12,13], suggesting that the allene structure is involved in the expression of physiological functions. EPA is an essential fatty acid that humans are unable to produce and has both a lipid-lowering effect and an anti-inflammatory effect, leading to a lower risk of cardiovascular diseases [14,15]. Although C. gracilis is not currently included in the European Union’s list of novel foods, the intake of C. gracilis—which is rich in these bioactive compounds—is expected to have these beneficial effects.
To the best of our knowledge, no studies have reported the effects of C. gracilis as a dietary supplement against lipid abnormalities. Therefore, to explore the potential use of C. gracilis as a food resource, the present study examines the effects of dietary C. gracilis on lipid abnormalities in rats fed a high-sucrose, cholesterol-containing diet.
2. Experimental Design
2.1. Materials
C. gracilis was cultured, as described previously [7], at Blue Scientific Shinkamigoto Co., Ltd. (Nagasaki, Japan). Cultured C. gracilis was centrifuged at 7000 rpm at 25 °C at a flow rate of 0.8 L/min using a continuous high-speed centrifuge (H-660 type; KOKUSAN Co., Ltd., Saitama, Japan). Separated C. gracilis was freeze-dried for 3 days using a freeze dryer (EYELA FDU-1110; Tokyo Rikakikai Co., Ltd., Tokyo, Japan) and then stored at −20 °C until use. The nutritional composition of C. gracilis was analyzed by the Institute of Food Hygiene, the Nagasaki Food Hygiene Association, and the Food and Environment Research Center (Nagasaki, Japan) (Table 1). The compositions of amino acids [16] and fatty acids [17] in C. gracilis were also analyzed by the aforementioned institute. Their compositions are shown in Table S1 and Table S2, respectively. The pigments in C. gracilis were analyzed using a liquid chromatography mass spectrometer (LCMS), as previously described [7], and determined to be as follows (per g dry weight): 15.2 mg chlorophyll a, 5.73 mg chlorophyll c1, 0.868 mg chlorophyll c2, 11.8 mg fucoxanthin, 0.650 mg diadinoxanthin, and 0.455 mg diatoxanthin.

Table 1.
Nutritional composition of C. gracilis.
2.2. Animals and Diets
All animal experiments were conducted in accordance with the Guidelines for Animal Experiments of University of Nagasaki, Siebold, and Law no. 105 and Notification no. 6 of the government of Japan. The animal protocol used in this study was approved by the Institutional Review Board of University of Nagasaki, Siebold (authorization no. R02-12). Five-week-old male Sprague-Dawley rats (Jcl:SD) were purchased from CLEA Japan Inc. (Osaka, Japan). The rats were housed individually in metal cages in an air-conditioned room at 22 ± 1 °C and 55 ± 5% humidity under a 12 h light-dark cycle. After a one-week adaptation period on a powder chow diet (CE-2; CLEA Japan, Inc.), 19 rats were assigned to one of three groups (n = 6–7/group). The experimental diets were prepared according to the AIN-76 formula [18] with several modifications. Dried C. gracilis was added to the control diet at a final dose of 2 or 5% (w/w). The protein, fat, and other components (i.e., carbohydrate, ash, moisture, and sodium) in the diets containing C. gracilis were adjusted with casein, corn oil, and sucrose, respectively, to match the control diet. Diets containing high sucrose and cholesterol levels were used to induce lipid abnormalities. Further details regarding the composition of the experimental diets can be found in Table 2. The rats were provided free access to food and water for four weeks. Then, their feces were collected over a period of 48 h at the end of the experiment. At the end of the four-week feeding period and after a six-hour starvation period, the rats were euthanized by decapitation. To obtain serum, blood was incubated at room temperature for 30 min and then centrifuged at 1200× g for 15 min at 4 °C. The liver, soleus muscle, and abdominal (epididymal, perirenal, and mesenteric) white adipose tissue (WAT) and brown adipose tissue were excised immediately and weighed within 4 h. The collected samples were stored at −80 °C until further analysis.

Table 2.
Composition of experimental diets in the present study.
2.3. Measurement of Serum Biochemical Parameters
The serum levels of triglycerides (TG), total cholesterol, phospholipids (PL), non-esterified fatty acids (NEFAs), glucose, and alanine aminotransferase (ALT) were measured using commercial enzyme assay kits (TG E-test Wako, Cholesterol E-test Wako, Phospholipid C-test Wako, NEFA C-test Wako, Glucose CII-test Wako, and Transaminase CII-test Wako; FUJIFILM Wako Pure Chemical Co., Osaka, Japan). Serum high-density lipoprotein (HDL) fractions were separated as described previously [19]. The serum cholesterol levels in the HDL fraction were measured using a commercial kit (Cholesterol E-test; Wako). The serum non-HDL cholesterol levels were calculated as the difference between the total cholesterol and HDL cholesterol levels. The serum levels of C-peptide, insulin, and adiponectin were measured using commercial rat ELISA kits (LBIS rat C-peptide ELISA kit and LBIS rat insulin ELISA kit; Shibayagi, Gunma, Japan; mouse/rat adiponectin ELISA kit; Otsuka Pharmaceutical, Tokyo, Japan).
2.4. Measurement of Triglycerides, Cholesterol, Phospholipids, and Glycogen Contents in the Liver
Total lipids from the liver were extracted using the Bligh and Dyer method, with some modifications, as described previously [20]. The extracted lipids were then dissolved in 2-propanol. The hepatic TG and total cholesterol contents were measured using commercial kits (TG E-test Wako and Cholesterol E-test Wako) [21]. The hepatic phospholipid content was measured according to the method described by Rouser et al. [22]. The hepatic glycogen content was measured according to the method described by Lo et al. [23].
2.5. Assays for Hepatic Enzyme Activities
The activities of fatty acid synthase (FAS), malic enzyme (ME), and glucose-6-phosphate dehydrogenase (G6PDH) in the cytosolic fraction, along with that of carnitine palmitoyltransferase (CPT) in the mitochondrial fraction, were determined as previously described [24]. The protein concentration of each fraction was determined according to the method described by Lowry et al. [25], using bovine serum albumin as the standard.
2.6. Measurement of Serum Steroid Levels
Biomarkers related to cholesterol metabolism—such as lathosterol, campesterol, and β-sitosterol [26,27]—in serum were measured by a gas chromatography-mass spectrometry (GC-MS) system using the Shimadzu GCMS-QP2010 Ultra (Shimadzu Corporation, Kyoto, Japan) equipped with an InertCap 5MS/NP capillary column (30 m × 0.25 mm i.d., 0.25 µm thickness, GL Sciences Inc., Tokyo, Japan) and using 5α-cholestane (Cayman Chemical Company, MI, USA) as an internal standard. Briefly, 250 µL of serum was added to 25 µg of 5α-cholestane. Then, the samples were saponified with ethanolic KOH and the steroids were extracted. The extracted steroids were converted to trimethylsilyl (TMS) ethers using the TMS derivatization reagent (N,O-Bis(trimethylsilyl)trifluoroacetamide (BSTFA)-trimethylchlorosilane (TMCS), (99:1); Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) before injection into the GC-MS system. The following program was applied using helium as a carrier gas at a flow rate of 1.08 mL/min: 180 °C for 1 min, from 180 °C to 250 °C (20 °C/min), from 250 °C to 290 °C (5 °C/min), and 290 °C for 17.5 min. The total run time was 30 min. The injector was operated at a split ratio of 1:30 and was maintained at 300 °C. The interface temperature was 250 °C. The ion source temperature was 290 °C. The mass spectrometer was operated in the electron impact (EI) mode (70 eV). The mass scan range was 35–700 m/z.
2.7. Analysis of Hepatic mRNA Levels
Total RNA was extracted from frozen liver tissue using RNAzol® RT reagent (Molecular Research Center, Inc., Cincinnati, OH, USA) with 4-bromoanisole (Molecular Research Center, Inc.) and converted to cDNA using PrimeScript™ RT Master Mix (Perfect Real Time) (Takara Bio Inc., Shiga, Japan), according to the manufacturer’s instructions. Polymerase chain reaction (PCR) amplification was performed in a final volume of 20 µL, which contained SYBR Green (THUNDERBIRD® SYBR® qPCR Mix; Toyobo Co., Ltd., Osaka, Japan), 0.3 µM of each primer (Fasmac Co., Ltd., Kanagawa, Japan), and 20 ng of cDNA, using a real-time PCR system (LightCycler®96; Nippon Genetics Co., Ltd., Tokyo, Japan). The reaction conditions were as follows: hot start at 95 °C for 60 s, followed by 45 cycles of denaturation at 95 °C for 15 s, and annealing/extension at 60 °C for 45 s. The relative mRNA levels were determined using the ΔΔCT method [28] using ribosomal protein L4 (Rpl4) as the housekeeping gene. The sequences of the primers used in this study are listed in Table S3.
2.8. Analysis of Water-Soluble Metabolites in the Liver and Soleus Muscle (Non-Target Metabolome Analysis)
Water-soluble metabolites in the liver and soleus muscle of rats in the two groups (control group and 5%Chaeto group) were analyzed according to the protocol described by Tsugawa et al. [29], with some modifications. Prior to metabolomic analysis, frozen liver (20 mg) or soleus muscle (50 mg) was homogenized using a ShakeMan 6 (Bio Medical Science, Tokyo, Japan) with zirconia beads. Homogenized samples were mixed with 250 µL of mixed solvent (methanol–H2O–chloroform = 5:2:2) and 10 µL of an internal standard solution (0.5 mg/mL) (2-isopropylmalic acid; Sigma-Aldrich Co., MO, USA). After vortexing, the samples were shaken using a shaking apparatus at 37 °C for 30 min. After centrifugation at 16,000× g and 4 °C for 3 min, the supernatants (180 µL) were collected, placed in new microtubes with 200 µL of H2O, and mixed using a vortex. The tubes were centrifuged at 16,000× g at 4 °C for 3 min. The resulting supernatants (250 µL) were collected into new microtubes for analysis.
To prepare quality control (QC) samples, 50 µL of each sample was mixed and dispensed into new tubes (200 µL/microtube). The analysis and QC samples were condensed using a centrifugal evaporator (EYELA CVE-2200; Tokyo Rikakikai Co., Ltd.) with a cold trap (EYELA UT-1010; Tokyo Rikakikai Co., Ltd.) at room temperature until the solvent evaporated. Methoxyamine hydrochloride (Sigma-Aldrich Co.) in anhydrous pyridine (20 mg/mL) was added to each tube, mixed using a vortex, and sonicated in an ice-cold water bath for 20 min with an ultrasonic cleaner. The mixture was shaken using a shaking apparatus for 90 min at 30 °C for oxidation. Next, 30 µL of N-methyl-N-trimethylsilyltrifluoroacetamine (MSTFA; GL Sciences Inc., Tokyo, Japan) was added to each tube and shaken with a shaking apparatus for 30 min at 37 °C to prepare the trimethylsilyl (TMS) derivatives. After centrifugation (16,000× g, room temperature, 5 min), the supernatant (80 µL) was collected into a 100 µL spitz attached to a 1.5 mL vial, which was sealed with a septum and a screw cap. The derivatized samples were analyzed with a GC-MS system (GCMS-QP2010 Ultra; Shimadzu) equipped with an InertCap 5MS/NP capillary column. The following program was applied using helium as a carrier gas at a flow rate of 1.12 mL/min: 80 °C for 2 min, from 80 °C to 330 °C (5 °C/min), and 330 °C for 6 min. The total run time was 58 min. The injector was operated at a split ratio of 1:20 and was maintained at 230 °C. The interface temperature was maintained at 250 °C. The ion-source temperature was maintained at 200 °C. The mass spectrometer was operated in EI mode (70 eV). The mass scan range was 85–500 m/z.
The obtained MS data (qgd-format) were converted to netCDF format. The data (netCDF format) were converted to the analysis base file (ABF) format by using an ABF converter (https://www.reifycs.com/AbfConverter/, accessed on 11 March 2023). Data processing was performed using MS-DIAL (version 4.92) [30,31]. Peak identification and prediction were also performed using MS-DIAL. The relative quantity of each metabolite was calculated using the peak area of each metabolite relative to that of an internal standard (2-isopropylmalic acid).
2.9. Statistical Analysis
All values are expressed as the mean ± standard error of the mean (SEM). All data were analyzed using Bartlett’s test (among three groups) or the F-test (between two groups) for equality of variance. When the data were recognized as unequal variances, they were normalized by logarithmic transformation and then re-analyzed using Bartlett’s test or the F-test. Parametric tests were used for further statistical analysis to confirm the homogeneity of all data. Comparisons among three groups (control, 2%Chaeto, and 5%Chaeto groups) were performed using one-way analysis of variance (ANOVA), followed by Dunnett’s multiple comparison post hoc test. The dose dependency of the Chaeto groups (2% and 5%) was examined using the Jonckheere–Terpstra trend test. Comparisons between two groups (control and 5%Chaeto groups) were performed using Student’s t-test for data with equal variances or Welch’s t-test for data with unequal variances. Results with p < 0.05 were considered statistically significant, and 0.05 ≤ p < 0.1 was considered a tendency.
Statistical analysis was performed using EZR, which is a graphical user interface for R (version 4.0.4) (The R Foundation for Statistical Computing, Vienna, Austria) [32], as well as MetaboAnalyst 5.0—a free web-based software platform [33]. Metabolites significantly affected by the 5%Chaeto diet compared to the control diet were subjected to pathway and enrichment analyses using MetaboAnalyst 5.0.
3. Results
3.1. Effects of C. gracilis Feeding on Morphometric Variables in SD Rats
At the time of autopsy, the color of white adipose tissues of rats fed C. gracilis was found to turn “orange-like” (Figure 1). Table 3 summarizes the morphometric variables of SD rats after a four-week feeding period. Furthermore, no significant differences were observed in the final body weight, food intake, food efficiency, and organ or tissue weights—including the liver, several white adipose tissues, and brown adipose tissue—among the three groups. In contrast, dose dependency was observed in the weight of soleus muscle in the 2% and 5%Chaeto groups (p < 0.05, Jonckheere–Terpstra trend test). The soleus muscle weight tended to be higher in rats fed the 5%Chaeto diet than in those fed the control diet (p = 0.0916). Similarly, dose dependency was observed in the fecal weights in the 2% and 5%Chaeto groups (p < 0.05, Jonckheere–Terpstra trend test). Furthermore, a significant difference in fecal weight was observed between the control group and the 5%Chaeto group (p < 0.05, Dunnett’s multiple comparison test).
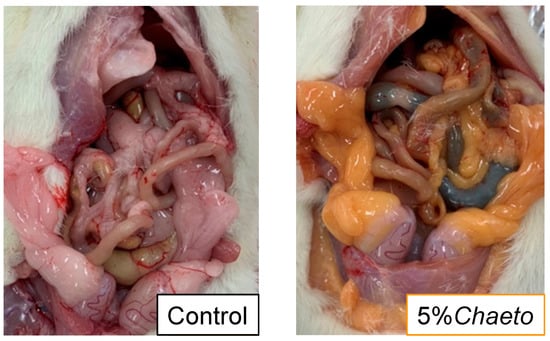
Figure 1.
Macroscopic changes in the abdominal cavity of SD rats fed C. gracilis.

Table 3.
Effects of C. gracilis feeding on morphometric variables of SD rats.
3.2. Effects of C. gracilis Feeding on Hepatic Lipid and Glycogen Contents and Serum Biochemical Parameters in SD Rats
Table 4 summarizes the hepatic lipid and glycogen contents and serum biochemical parameters in SD rats after a four-week feeding period. A dose dependency was observed in the hepatic TG contents in the 2% and 5%Chaeto groups (p < 0.05, Jonckheere–Terpstra trend test). The hepatic TG contents were significantly lower in the 2% and 5%Chaeto groups than in the control group. Dose dependency was also observed in the hepatic total cholesterol contents in the 2% and 5%Chaeto groups (p < 0.05, Jonckheere–Terpstra trend test). The hepatic total cholesterol content was significantly lower in the 5%Chaeto group than in the control group. However, no significant differences in hepatic PL and glycogen contents were observed among the three groups.

Table 4.
Effects of C. gracilis feeding on hepatic lipid and glycogen contents and serum biochemical parameters in SD rats.
Dose dependencies were observed in the serum total cholesterol and HDL cholesterol levels in the 2% and 5%Chaeto groups (p < 0.05, Jonckheere–Terpstra trend test). The serum levels of total and HDL cholesterol were significantly higher in the 2% and 5%Chaeto groups than in the control group. In contrast, no significant differences were observed in the serum levels of TG, PL, NEFAs, glucose, C-peptide, insulin, adiponectin, or ALT among the three groups.
3.3. Effects of C. gracilis Feeding on Activities of Hepatic Enzymes and mRNA Levels Related to Fatty Acid Metabolism in SD Rats
Enzymatic activities related to hepatic fatty acid de novo synthesis and fatty acid β-oxidation in rats are summarized in Table 5. Dose dependencies were observed for the FAS and G6PDH activities in the 2% and 5%Chaeto groups (p < 0.05, Jonckheere–Terpstra trend test). FAS activity tended to be lower in rats fed the 5%Chaeto diet than in those fed the control diet (p = 0.0865). G6PDH activity was significantly lower in the 5%Chaeto group than in the control group. However, no significant differences were observed in the ME and CPT activities among the three groups. The hepatic mRNA levels of Fasn and G6pd did not differ significantly among the three groups (Table 5).

Table 5.
Effects of C. gracilis feeding on activities of hepatic enzymes related to fatty acid metabolism, serum levels of steroids, and relative levels of hepatic mRNA related to cholesterol metabolism in SD rats.
3.4. Effects of C. gracilis Feeding on Serum Levels of Steroids and Relative Levels of Hepatic mRNA Related to Cholesterol Metabolism in SD Rats
The serum steroid levels and relative levels of hepatic mRNA related to cholesterol metabolism in rats are summarized in Table 5. A dose dependency was observed in the serum levels of cholesterol absorption markers, such as campesterol and β-sitosterol in the 2% and 5%Chaeto groups (p < 0.05, Jonckheere–Terpstra trend test). The serum levels of campesterol and β-sitosterol were significantly lower in the 2% and 5%Chaeto groups than in the control group. In contrast, the serum levels of lathosterol—a cholesterol synthesis marker—did not significantly differ among the three groups. In addition, dose dependency was observed in the hepatic mRNA levels of Scarb1, known as the HDL receptor, in the 2% and 5%Chaeto groups (p < 0.05, Jonckheere–Terpstra trend test). The Scarb1 mRNA levels were significantly lower in the 5%Chaeto group than in the control group. In contrast, the hepatic mRNA levels of Hmgcr, Soat1, and Abca1 did not significantly differ among the three groups.
3.5. Effects of C. gracilis Feeding on Water-Soluble Metabolites in the Liver of SD Rats
Among the 66 metabolites identified and semi-quantified in the liver (Table S4), the levels of three metabolites (glycerol, hypotaurine, and inositol) were significantly reduced in the 5%Chaeto group compared to those in the control group (Table 6).

Table 6.
Effects of C. gracilis feeding on levels of identified water-soluble metabolites in the liver and soleus muscle of SD rats.
3.6. Effects of C. gracilis Feeding on Water-Soluble Metabolites in the Soleus Muscle of SD Rats
Among the 50 metabolites identified and semi-quantified in the soleus muscle (Table S5), the levels of 19 metabolites (2-aminoethanol, 3-hydroxypyruvate, β-alanine, cadaverine, creatine, glycerol, glycine, hypoxanthine, iminodiacetate, isoleucine, leucine, lysine, nicotinamide, O-phosphoethanolamine, phenylalanine, serine, threonine, uracil, and valine) were significantly increased, whereas the levels of one metabolite (oxalate) were significantly reduced in the 5%Chaeto group compared with those in the control group (Table 6).
These 20 significant metabolites were subjected to pathway and enrichment analyses using MetaboAnalyst 5.0. The associated metabolic pathways are shown in Figure 2a, wherein nine pathways were found to be significantly related to C. gracilis feeding: “aminoacyl-tRNA biosynthesis,” “valine, leucine and isoleucine biosynthesis”, “pantothenate and CoA biosynthesis”, “glycine, serine and threonine metabolism,” “valine, leucine and isoleucine degradation”, “β-alanine metabolism”, “glutathione metabolism”, “phenylalanine, tyrosine and tryptophan biosynthesis,” and “glyoxylate and dicarboxylate metabolism”. Similarly, the enrichment ratio and p-values suggested that these nine pathways were significantly enriched (Figure 2b).
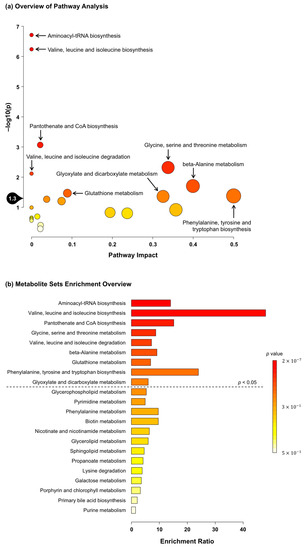
Figure 2.
(a) Summary of MetaboAnalyst 5.0 pathway analysis for metabolites significantly different between the control and 5%Chaeto groups. The pathway impact on the x axis from 0 (low impact) to 1 (strong impact) represents the values from the pathway topology analysis. The circle diameter covaries with pathway impact, and the fill color intensity (white to red) reflects statistical significance (decreasing p-value). The black drop with 1.3 on the y axis indicates the threshold of significance at p < 0.05. (b) Summary of MetaboAnalyst 5.0 enrichment analysis for metabolites significantly different between the control and 5%Chaeto groups. The enrichment ratio is calculated as the number of hits within a particular metabolic pathway divided by the expected number of hits. Each bar shows a pathway, and the fill color intensity (white to red) reflects statistical significance (decreasing p-value). The pathways above the dotted line indicate significance at p < 0.05.
4. Discussion
To explore the potential use of C. gracilis as a food resource, the effects of dietary C. gracilis on lipid abnormalities were investigated in rats fed the high-sucrose and cholesterol-containing diet.
In a previous study that evaluated the safety of long-term administration of high-dose fucoxanthin (500 mg and 1000 mg/kg of body weight) in mice, increases in the levels of cholesterol and phospholipids in the blood, as well as in the weight of the liver were observed [34]. In the present study, the fucoxanthin intake calculated from its content in C. gracilis was approximately 40 mg/kg of body weight, and no findings other than increased serum total and HDL cholesterol levels were observed (Table 4). Although EPA has a TG-lowering effect [14], the intake of 2–5%Chaeto (including EPA) did not affect the serum TG levels (Table 4). In addition, the color of the white adipose tissues of rats fed C. gracilis was observed to turn “orange-like” (Figure 1). This was consistent with previous studies in which fucoxanthin was administered alone [34]. Hashimoto et al. showed that, in mice, dietary fucoxanthin undergoes metabolic conversion to amarouciaxanthin A in the liver via fucoxanthinol and preferentially accumulates as amarouciaxanthin A in the adipose tissue [35], suggesting that amarouciaxanthin A is involved in the orange coloration of the adipose tissue. Taken together, we believe that the fucoxanthin contained in C. gracilis contributed greatly to the outcomes of this study.
In the present study, the soleus muscle weights were found to be dose-responsive to C. gracilis and showed a tendency to increase (Table 3). Dietary intake of protein is known to be a prerequisite for the day-to-day maintenance of skeletal muscle mass, which stimulates an increase in muscle protein synthesis and attenuates muscle protein breakdown [36]. In a previous study, dietary fucoxanthin was found to protect against dexamethasone-induced muscle atrophy in mice [37]. Therefore, the intake of C. gracilis, which is rich in protein and fucoxanthin, may be effective in maintaining muscle mass.
As shown in Table 4, the hepatic TG content was significantly reduced in the 2% and 5%Chaeto groups compared with that in the control group. To understand the mechanisms underlying the hepatic TG-lowering action of C. gracilis, the activities of hepatic enzymes related to fatty acid metabolism were analyzed. Although the activities of CPT responsible for fatty acid β-oxidation in the mitochondria did not differ among the three groups, the activities of FAS and G6PDH—which are related to fatty acid de novo synthesis in the cytosol—were found to be dose-responsive to C. gracilis and showed a tendency to decrease (Table 5). The decreased activities of FAS and G6PDH were consistent with the results of a previous study in which mice were fed high-fat diets with fucoxanthin (0.05% and 0.2%) [38]. The hepatic mRNA levels of Fasn and G6pd did not differ among the three groups (Table 5), suggesting that the changes in FAS and G6PDH activities resulting from C. gracilis feeding represent post-translational regulation, but not transcriptional regulation. According to the results of metabolomic analysis in the liver, the glycerol content was significantly reduced in the 5%Chaeto group compared to that in the control group (Table 6). These results suggest that the reduction in hepatic TG content by C. gracilis feeding was attributable to the reduction in both fatty acids and glycerol, which are the substrates of TG.
As shown in Table 4, compared with the control group, the hepatic total cholesterol content was significantly reduced in the 5%Chaeto group. To gain insights into the effects of C. gracilis feeding on cholesterol metabolism, we analyzed the serum levels of cholesterol, biomarkers, and hepatic mRNA levels related to cholesterol metabolism. Lathosterol is a precursor of de novo cholesterol synthesis, and its serum levels can be used as an index of cholesterol synthesis in the body [26,27]. In addition, plant sterols—such as campesterol and β-sitosterol—are sterol isomers that cannot be synthesized in animal bodies, and their serum levels are positively correlated with cholesterol absorption rates [26,27].
Although the serum levels of lathosterol and the hepatic mRNA levels of Hmgcr did not differ among the three groups, the serum levels of campesterol and β-sitosterol were found to be dose-responsive to C. gracilis and showed a significant decrease (Table 4 and Table 5). Additionally, as shown in Table 4, the serum total cholesterol levels were significantly higher in the 2% and 5%Chaeto groups than in the control group. Higher total cholesterol levels were associated with significantly increased HDL cholesterol levels. A previous study showed that high-fat diets with fucoxanthin (0.05% and 0.2%) reduced the hepatic cholesterol content and HMG-CoA reductase activities and increased the plasma HDL cholesterol levels and fecal cholesterol contents in mice [38]. This behavior, except for cholesterol synthesis in the previous study, was consistent with the results of our study. This difference in cholesterol synthesis was thought to be due to the differences in animal species (rats in the present study versus mice in the previous study), dietary fat (10% fat based on corn oil in the present study versus 10% lard + 10% corn oil in the previous study), and cholesterol content. Unfortunately, the quality of the lard used in the diet of the previous study was unknown, and the cholesterol contents in the diets of the present and previous studies could not be accurately compared. In the present study, no significant difference in the serum non-HDL cholesterol levels was observed (Table 4), indicating that C. gracilis supplementation did not affect the secretion of cholesterol from the liver into the bloodstream. Beppu et al. reported that fucoxanthin intake increases the serum HDL cholesterol levels by decreasing the protein expression of SR-B1—a receptor of HDL—in the livers of mice [39]. As shown in Table 5, the hepatic Scarb1 mRNA levels were significantly decreased in the 5%Chaeto group, supporting the results of a previous study. Taken together, these data suggest that the decreases in intestinal cholesterol absorption and HDL uptake from the bloodstream into the liver caused by C. gracilis feeding contribute to the reduction in hepatic total cholesterol content in rats. From the perspective of the utilization and safety of C. gracilis rich in fucoxanthin as a food resource, further studies using LDL animals—such as hamsters, which have a lipoprotein metabolism similar to that of humans—are needed to evaluate whether the increase in the total and HDL blood cholesterol levels is specific to HDL animals, such as rats and mice. The health benefits of n-3 PUFAs such as EPA and DHA on lipid metabolism are well known [14,15]. A systematic review of the differential effects of dietary EPA and DHA on cardiometabolic risk factors indicates that dietary DHA—but not EPA—increases blood HDL-cholesterol levels [40]. Thus, we consider that fucoxanthin (but not EPA) contained in C. gracilis contributes greatly to the changes in lipid metabolism observed in this study.
Since C. gracilis feeding tended to increase the soleus muscle weight of rats, water-soluble metabolite analysis in the soleus muscle was performed. As a result, compared to the water-soluble metabolite analysis in the liver, many metabolites in the muscle were significantly changed between the two groups (Table 6). The muscular contents of several amino acids, including branched-chain amino acids (BCAAs), were significantly increased in the 5%Chaeto group (Table 6). The magnitude of the muscle protein synthesis response to an ingested protein source is regulated at multiple levels, including dietary protein digestion and amino acid absorption, splanchnic amino acid retention, postprandial insulin release, transport, and uptake of amino acids into skeletal muscles [41]. The experimental diets used in this study were adjusted to ensure equal protein contents (Table 2). In terms of the amino acid contents of the 5%Chaeto diet, the contents of aspartic acid, glycine, alanine, arginine, and cystine were slightly higher and the contents of other amino acids were slightly lower compared to the control diet (Table S6). As the increase in the amino acid contents of muscles after C. gracilis feeding did not match the contents in the diet, this increase may be attributed to increases in protein digestion in C. gracilis and amino acid absorption, as well as amino acid uptake by the muscle. Leucine has been shown to upregulate the muscle protein synthesis machinery by activating the mechanistic target of the rapamycin complex 1 (mTORC1) signaling pathway [42]. Pathway and enrichment analyses associated with the significantly altered metabolites revealed that nine metabolic pathways were potentially affected by C. gracilis feeding. “Aminoacyl-tRNA biosynthesis” was one of the pathways found in the analysis (Figure 2a,b). Aminoacyl-tRNA synthetases are a family of essential enzymes used for protein synthesis that play pivotal roles in the ligation of tRNA with their cognate amino acids [43]. Therefore, these results suggest that the tendency toward an increase in the weight of the soleus muscle after C. gracilis feeding may be due to the enhancement of muscle protein synthesis centered on leucine. As several pathways that may be affected by C. gracilis feeding were identified in the present study, future studies should seek to focus on these pathways.
In conclusion, this study is the first to report that the oral administration of the marine microalga C. gracilis alleviates hepatic lipid accumulation in rats fed a high-sucrose and cholesterol-containing diet, indicating its potential use as a food resource. Through further comparative research with other marine microalgae containing bioactive compounds similar to those of C. gracilis, determining whether or not C. gracilis intake has a unique beneficial effect would be interesting. Although C. gracilis is rich in protein, its amino acid score is inferior to casein and egg white protein, which have scores of 100 [24], with tryptophan as the first limiting amino acid. Therefore, when considering C. gracilis as a dietary protein source, C. gracilis intake would ideally need to be combined with other protein sources.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/metabo13030436/s1, Table S1: Amino acid contents of C. gracilis; Table S2: Fatty acid contents of C. gracilis; Table S3: Sequences of primers used for real-time PCR; Table S4: Levels of identified water-soluble metabolites in the liver of SD rats; Table S5: Levels of identified water-soluble metabolites in the soleus muscle of SD rats; Table S6: Contents of amino acids in experimental diets.
Author Contributions
Conceptualization, B.S.; data curation, B.S.; formal analysis, B.S., Y.K. (Yuri Kawahara), Y.K. (Yuka Kutsuna), M.H., M.O., S.M. and K.T.; funding acquisition, B.S.; investigation, B.S. and Y.K. (Yuri Kawahara); methodology, B.S.; project administration, B.S.; resources, N.N.; supervision, B.S.; visualization, B.S.; writing—original draft, B.S.; writing—review and editing, B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Project Research of University of Nagasaki (to B.S.).
Institutional Review Board Statement
All animal experiments were conducted in accordance with the Guidelines for Animal Experiments of University of Nagasaki, Siebold, and Law no. 105 and Notification no. 6 of the government of Japan. The animal protocol used in this study was approved by the Institutional Review Board of University of Nagasaki, Siebold (authorization no. R02-12).
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available because a public archive platform has not yet been set up for data sharing.
Acknowledgments
The authors thank Tomoyo Katayama (Graduate School of Agricultural and Life Science, The University of Tokyo) for analyzing the pigments in C. gracilis and Ayano Fukuda and Yukiko Shiota (University of Nagasaki, Siebold) for providing technical assistance with the rat dissection.
Conflicts of Interest
Norio Nagao is employee of Blue Scientific Shinkamigoto Co., Ltd. The paper reflects the views of the scientists, and not the company. The authors declare no conflict of interest.
References
- Garcia-Perez, P.; Cassani, L.; Garcia-Oliveira, P.; Xiao, J.; Simal-Gandara, J.; Prieto, M.A.; Lucini, L. Algal nutraceuticals: A perspective on metabolic diversity, current food applications, and prospects in the field of metabolomics. Food Chem. 2023, 409, 135295. [Google Scholar] [CrossRef]
- Barbosa, M.J.; Janssen, M.; Südfeld, C.; D’Adamo, S.; Wijffels, R.H. Hypes, hopes, and the way forward for microalgal biotechnology. Trends Biotechnol. 2023, 41, 452–471. [Google Scholar] [CrossRef]
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current status of the algae production industry in Europe: An emerging sector of the blue bioeconomy. Front. Mar. Sci. 2020, 7, 626389. [Google Scholar] [CrossRef]
- Katayama, T.; Rahman, N.A.; Khatoon, H.; Kasan, N.A.; Nagao, N.; Yamada, Y.; Takahashi, K.; Furuya, K.; Wahid, M.E.A.; Yusoff, F.M.; et al. Bioprospecting of tropical microalgae for high-value products: n-3 polyunsaturated fatty acids and carotenoids. Aquacult. Rep. 2022, 27, 101406. [Google Scholar] [CrossRef]
- Mayer, C.; Côme, M.; Ulmann, L.; Martin, I.; Zittelli, G.C.; Faraloni, C.; Ouguerram, K.; Chénais, B.; Mimouni, V. The potential of the marine microalga Diacronema lutheri in the prevention of obesity and metabolic syndrome in high-fat-fed Wistar rats. Molecules 2022, 27, 4246. [Google Scholar] [CrossRef]
- Commission Implementing Regulation (EU) 2018/1023 of 23 July 2018 Correcting Implementing Regulation (EU) 2017/2470 Establishing the Union List of Novel Foods. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2018:187:FULL&from=IT (accessed on 26 February 2023).
- Tachihana, S.; Nagao, N.; Katayama, T.; Hirahara, M.; Yusoff, F.M.; Banerjee, S.; Shariff, M.; Kurosawa, N.; Toda, T.; Furuya, K. High productivity of eicosapentaenoic acid and fucoxanthin by a marine diatom Chaetoceros gracilis in a semi-continuous culture. Front. Bioeng. Biotechnol. 2020, 8, 602721. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Du, L.; Hosokawa, M.; Miyashita, K. Total lipids content, lipid class and fatty acid composition of ten species of microalgae. J. Oleo Sci. 2020, 69, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J. Fucoxanthin and its metabolite fucoxanthinol in cancer prevention and treatment. Mar. Drugs 2015, 13, 4784–4798. [Google Scholar] [CrossRef] [PubMed]
- Lau, T.Y.; Kwan, H.Y. Fucoxanthin is a potential therapeutic agent for the treatment of breast cancer. Mar. Drugs 2022, 20, 370. [Google Scholar] [CrossRef]
- Gammone, M.A.; D’Orazio, N. Anti-obesity activity of the marine carotenoid fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef]
- Hosokawa, M.; Miyashita, T.; Nishikawa, S.; Emi, S.; Tsukui, T.; Beppu, F.; Okada, T.; Miyashita, K. Fucoxanthin regulates adipocytokine mRNA expression in white adipose tissue of diabetic/obese KK-Ay mice. Arch. Biochem. Biophys. 2010, 504, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, S.; Hosokawa, M.; Miyashita, K. Fucoxanthin promotes translocation and induction of glucose transporter 4 in skeletal muscles of diabetic/obese KK-A(y) mice. Phytomedicine 2012, 19, 389–394. [Google Scholar] [CrossRef]
- Skulas-Ray, A.C.; Wilson, P.W.F.; Harris, W.S.; Brinton, E.A.; Kris-Etherton, P.M.; Richter, C.K.; Jacobson, T.A.; Engler, M.B.; Miller, M.; Robinson, J.G.; et al. Omega-3 fatty acids for the management of hypertriglyceridemia: A science advisory from the American Heart Association. Circulation 2019, 140, e673–e691. [Google Scholar] [CrossRef] [PubMed]
- Djuricic, I.; Calder, P.C. Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef] [PubMed]
- The Pharmaceutical Society of Japan. 2.1.2.2 Amino Acids. In Methods of Analysis in Human Science; Kanehara & Co., Ltd.: Tokyo, Japan, 2020; pp. 211–214. [Google Scholar]
- Analytical Methods for Nutrients (March 30, 2015 Tsuuchi No.139 Attachment Consumer Affairs Agency Food Labelling Division). Available online: https://www.caa.go.jp/policies/policy/food_labeling/food_labeling_act/assets/food_labeling_cms201_220830_03.pdf (accessed on 11 March 2023).
- American Institute of Nutrition. Report of the American Institute of Nutrition ad hoc Committee on Standards for Nutritional Studies. J. Nutr. 1977, 107, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Niibo, M.; Shirouchi, B.; Umegatani, M.; Morita, Y.; Ogawa, A.; Sakai, F.; Kadooka, Y.; Sato, M. Probiotic Lactobacillus gasseri SBT2055 improves insulin secretion in a diabetic rat model. J. Dairy Sci. 2019, 102, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Shirouchi, B.; Fukuda, A.; Akasaka, T. Unlike glycerophosphocholine or choline chloride, dietary phosphatidylcholine does not increase plasma trimethylamine-N-oxide levels in Sprague-Dawley rats. Metabolites 2022, 12, 64. [Google Scholar] [CrossRef]
- Carr, T.P.; Andresen, C.J.; Rudel, L.L. Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin. Biochem. 1993, 26, 39–42. [Google Scholar] [CrossRef]
- Rouser, G.; Siakotos, A.N.; Fleischer, S. Quantitative analysis of phospholipids by thin-layer chromatography and phosphorus analysis of spots. Lipids 1966, 1, 85–86. [Google Scholar] [CrossRef]
- Lo, S.; Russell, J.C.; Taylor, A.W. Determination of glycogen in small tissue samples. J. Appl. Physiol. 1970, 28, 234–236. [Google Scholar] [CrossRef]
- Matsuoka, R.; Shirouchi, B.; Umegatani, M.; Fukuda, M.; Muto, A.; Masuda, Y.; Kunou, M.; Sato, M. Dietary egg-white protein increases body protein mass and reduces body fat mass through an acceleration of hepatic β-oxidation in rats. Br. J. Nutr. 2017, 118, 423–430. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Miettinen, T.A.; Gylling, H.; Nissinen, M.J. The role of serum non-cholesterol sterols as surrogate markers of absolute cholesterol synthesis and absorption. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Gylling, H.; Hallikainen, M.; Simonen, P.; Miettinen, H.E.; Nissinen, M.J.; Miettinen, T.A. Serum and lipoprotein sitostanol and non-cholesterol sterols after an acute dose of plant stanol ester on its long-term consumption. Eur. J. Nutr. 2012, 51, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Bamba, T.; Shinohara, M.; Nishiumi, S.; Yoshida, M.; Fukusaki, E. Practical non-targeted gas chromatography/mass spectrometry-based metabolomics platform for metabolic phenotype analysis. J. Biosci. Bioeng. 2011, 112, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Tsugawa, H.; Wohlgemuth, G.; Mehta, S.; Mueller, M.; Zheng, Y.; Ogiwara, A.; Meissen, J.; Showalter, M.; Takeuchi, K.; et al. Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics. Nat. Methods 2018, 15, 53–56. [Google Scholar] [CrossRef]
- Tsugawa, H.; Ikeda, K.; Takahashi, M.; Satoh, A.; Mori, Y.; Uchino, H.; Okahashi, N.; Yamada, Y.; Tada, I.; Bonini, P.; et al. A lipidome atlas in MS-DIAL 4. Nat. Biotechnol. 2020, 38, 1159–1163. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- MetaboAnalyst. MetaboAnalyst 5.0—User-Friendly, Streamlined Metabolomics Data Analysis. Available online: https://www.metaboanalyst.ca/home.xhtml (accessed on 11 March 2023).
- Beppu, F.; Niwano, Y.; Tsukui, T.; Hosokawa, M.; Miyashita, K. Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice. J. Toxicol. Sci. 2009, 34, 501–510. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ozaki, Y.; Taminato, M.; Das, S.K.; Mizuno, M.; Yoshimura, K.; Maoka, T.; Kanazawa, K. The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. Br. J. Nutr. 2009, 102, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Deane, C.S.; Ely, I.A.; Wilkinson, D.J.; Smith, K.; Phillips, B.E.; Atherton, P.J. Dietary protein, exercise, ageing and physical inactivity: Interactive influences on skeletal muscle proteostasis. Proc. Nutr. Soc. 2021, 80, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Hosokawa, M.; Miyashita, K.; Nishino, H.; Hashimoto, T. Effects of fucoxanthin on the inhibition of dexamethasone-induced skeletal muscle loss in mice. Nutrients 2021, 13, 1079. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.N.; Jeon, S.M.; Kim, H.J.; Lee, M.K.; Shin, S.K.; Shin, Y.C.; Park, Y.B.; Choi, M.S. Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6N mice. Chem. Biol. Interact. 2010, 186, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Beppu, F.; Hosokawa, M.; Niwano, Y.; Miyashita, K. Effects of dietary fucoxanthin on cholesterol metabolism in diabetic/obese KK-A(y) mice. Lipids Health Dis. 2012, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.K.; Calder, P.C. The differential effects of eicosapentaenoic acid and docosahexaenoic acid on cardiometabolic risk factors: A systematic review. Int. J. Mol. Sci. 2018, 19, 532. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, S.H.; Rémond, D.; van Loon, L.J. The muscle protein synthetic response to food ingestion. Meat Sci. 2015, 109, 96–100. [Google Scholar] [CrossRef]
- Zaromskyte, G.; Prokopidis, K.; Ioannidis, T.; Tipton, K.D.; Witard, O.C. Evaluating the leucine trigger hypothesis to explain the post-prandial regulation of muscle protein synthesis in young and older adults: A systematic review. Front. Nutr. 2021, 8, 685165. [Google Scholar] [CrossRef]
- Yu, Y.C.; Han, J.M.; Kim, S. Aminoacyl-tRNA synthetases and amino acid signaling. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118889. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).