Neogrisphenol A, a Potential Ovarian Cancer Inhibitor from a New Record Fungus Neohelicosporium griseum
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedure
2.2. Fungal Taxonomy and Identification
2.3. Fermentation, Extraction, and Isolation
2.4. Biological Assays
Antimicrobial and Cytotoxic Activities of Compounds 1–7
2.5. Effect of Neogrisphenol A on the Growth of A2780 Cells
2.5.1. Cell Culture and Compound Treatment
2.5.2. Cell Proliferation Assay
2.5.3. Cell Apoptosis Assay
2.5.4. Cell Cycle Assay
3. Results
3.1. Fungal Taxonomy
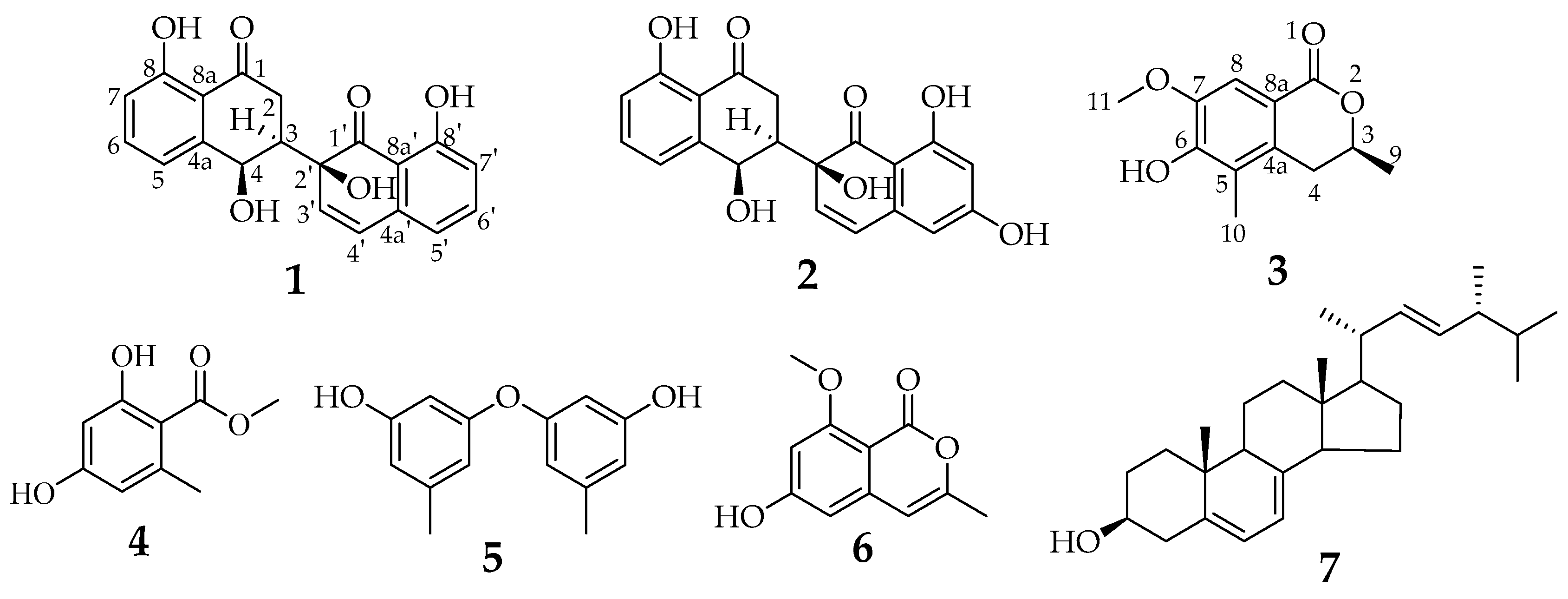
3.2. Spectroscopic Data
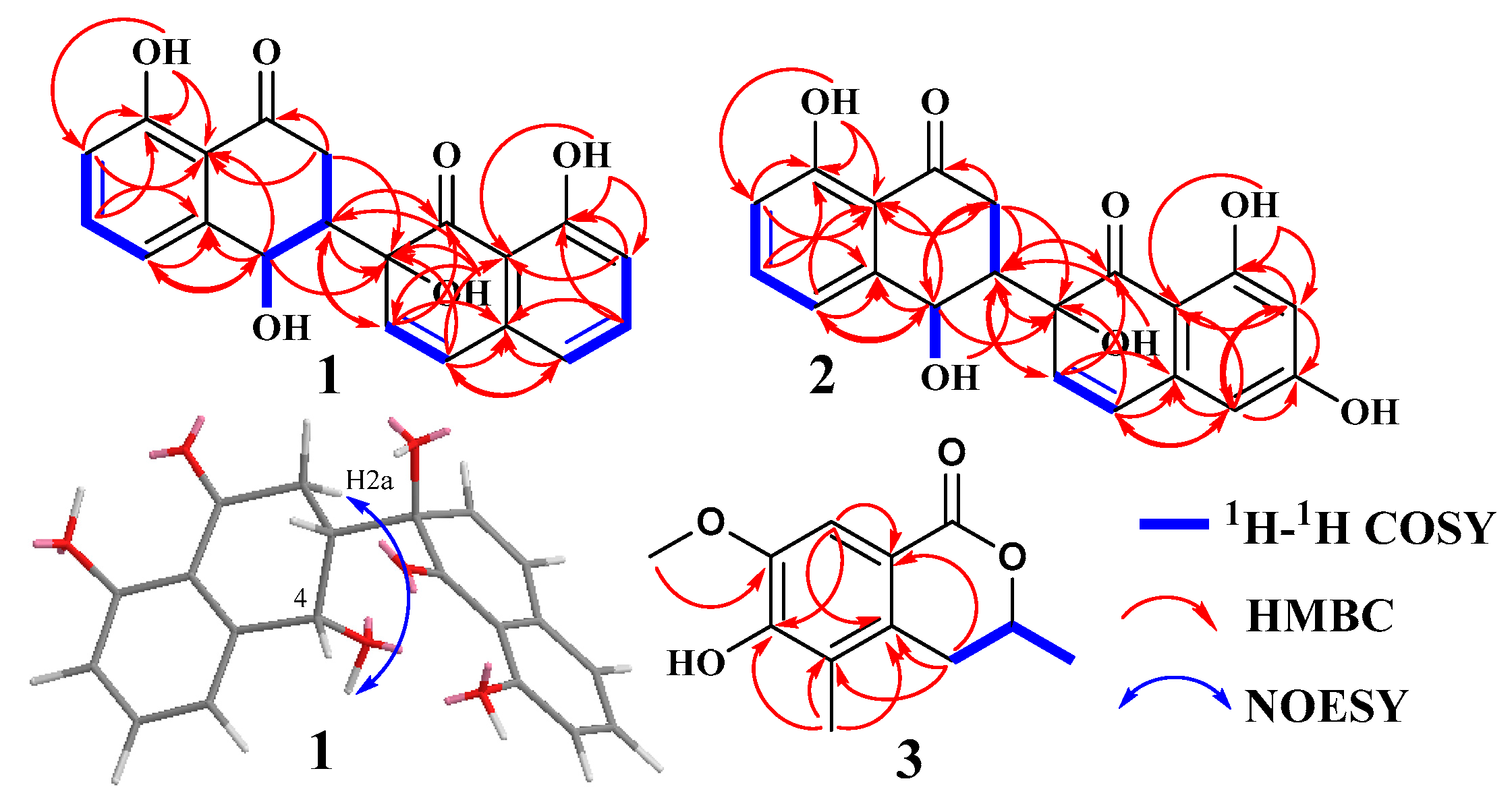
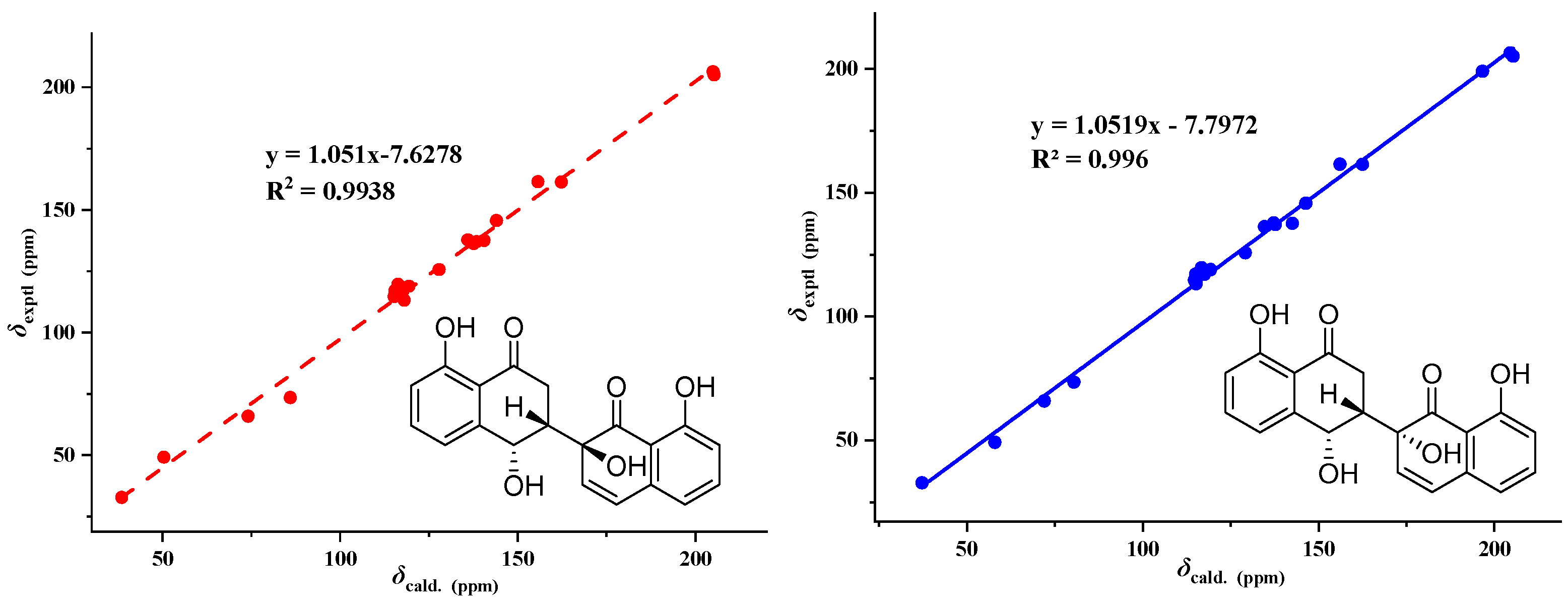
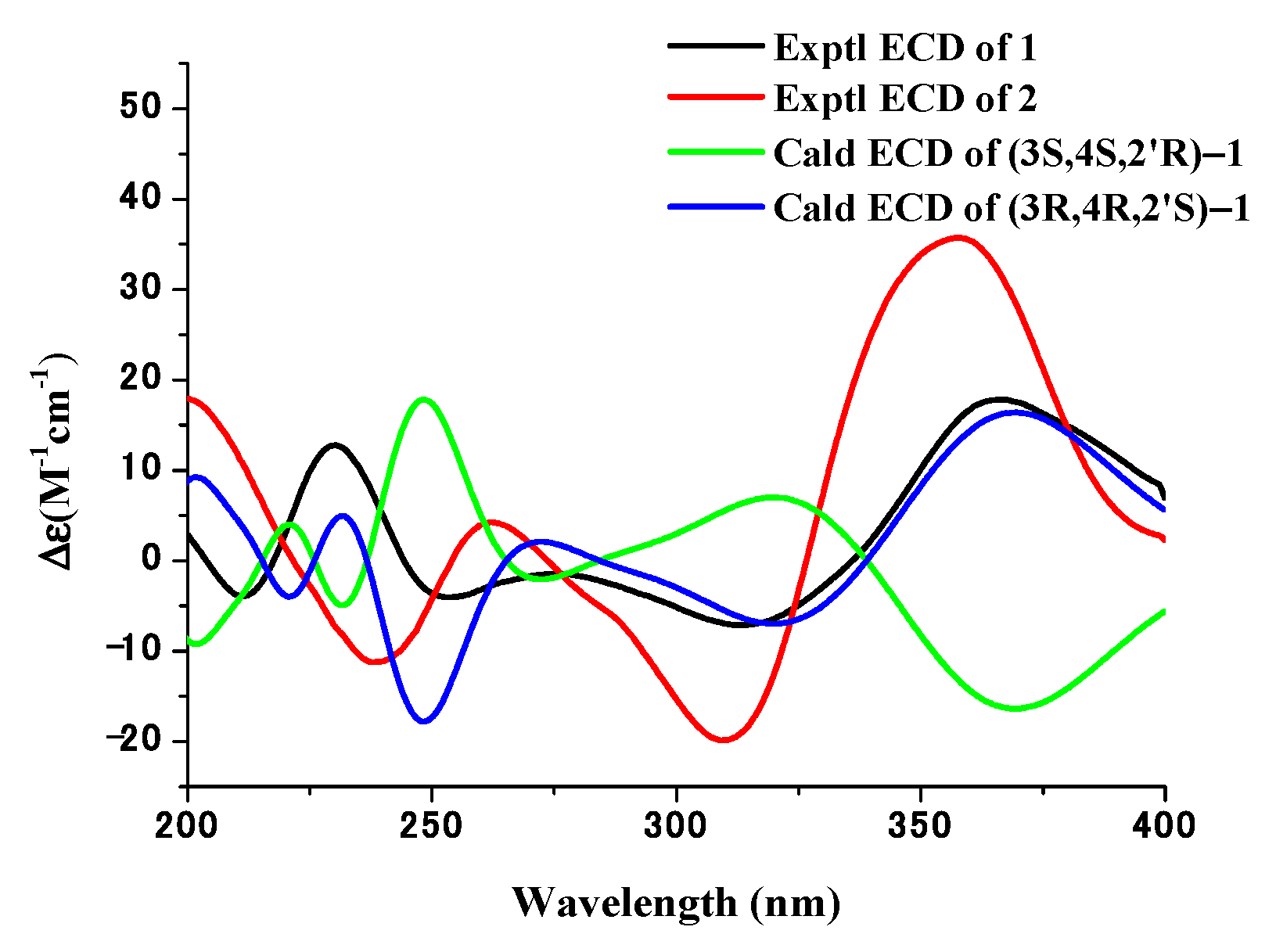
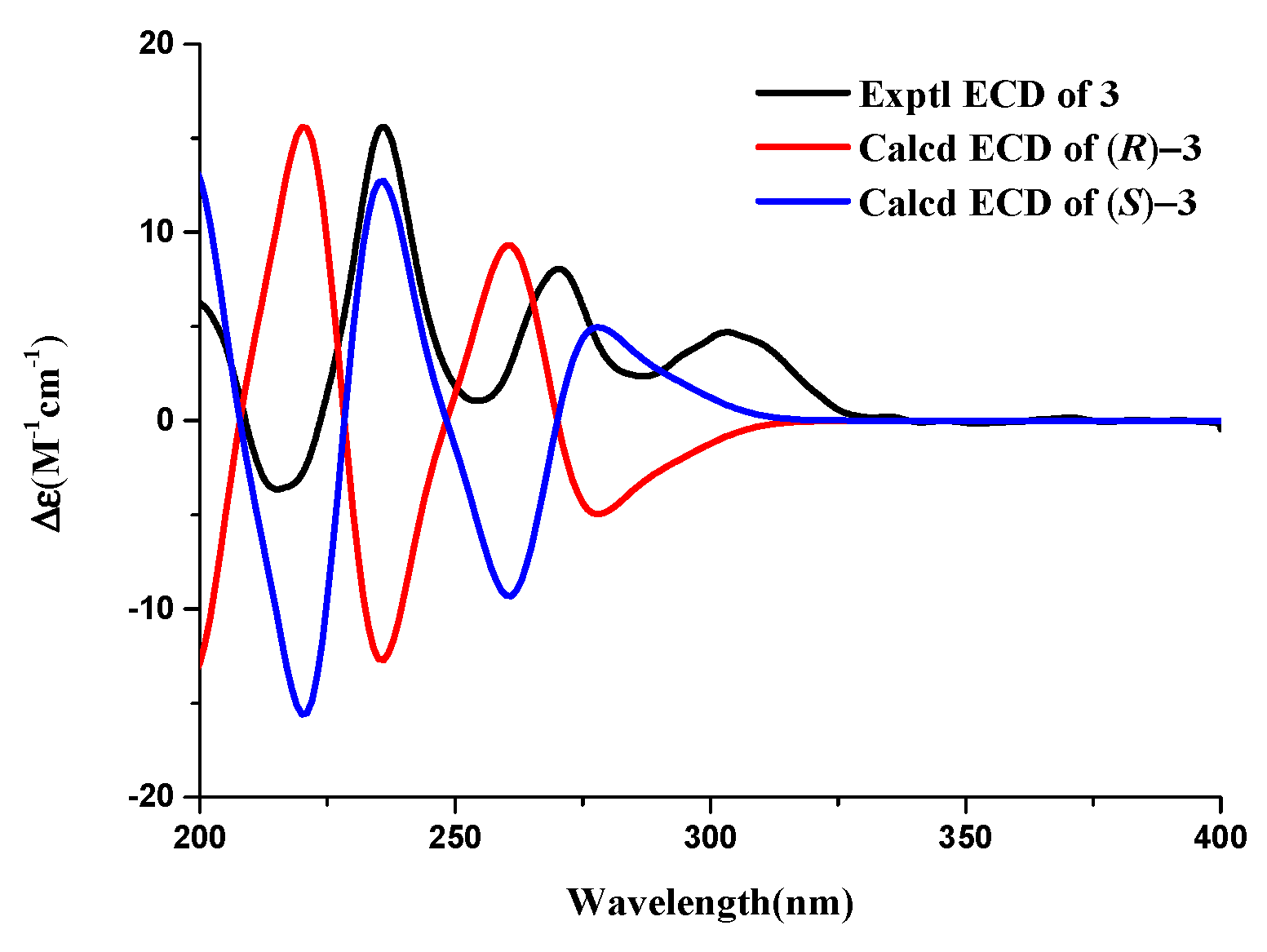
3.3. Structure Elucidation of Compounds 1–7
3.4. Antimicrobial and Cytotoxic Activities of Compounds 1–7
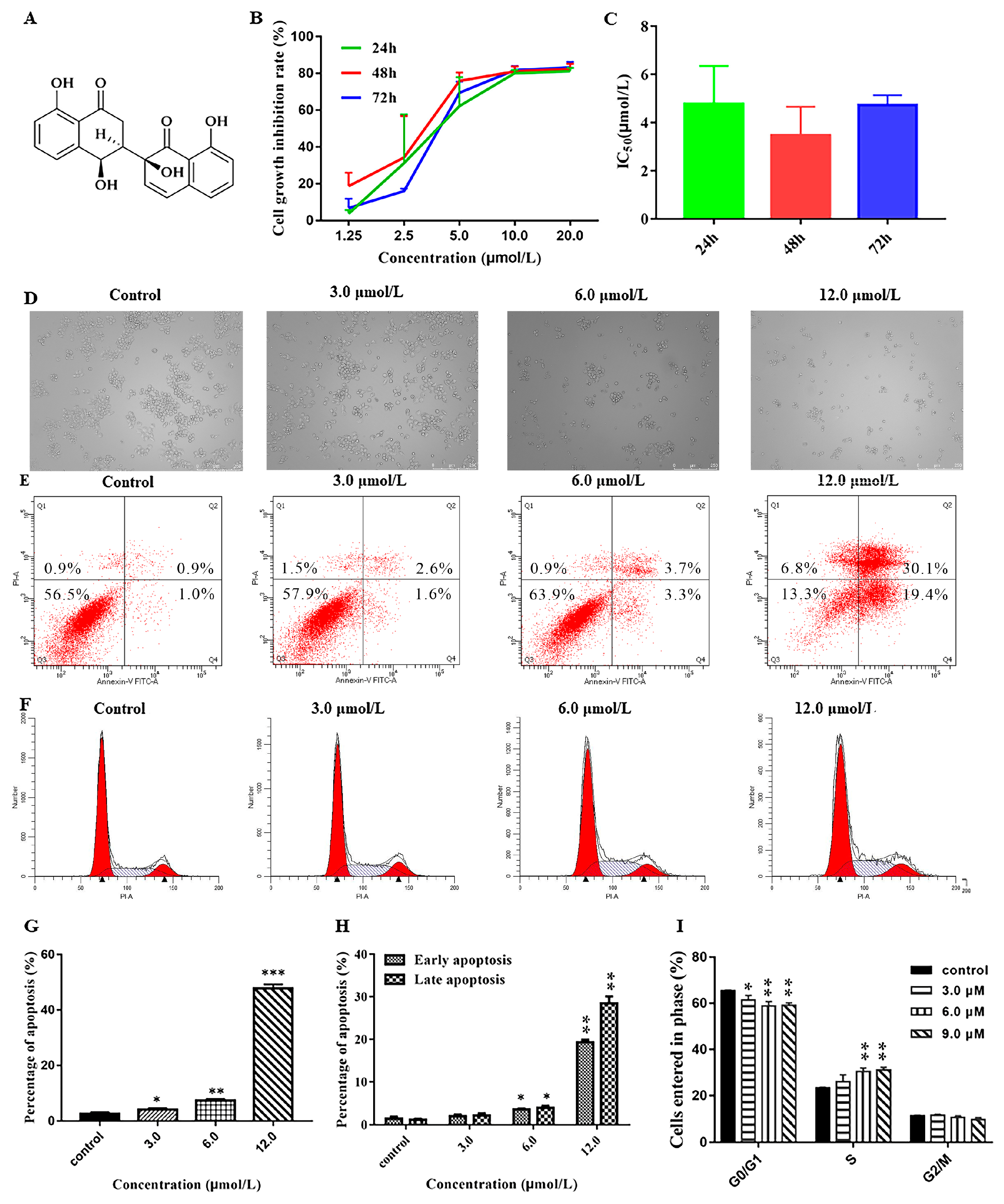
3.5. Effect of Neogrisphenol A on the Growth of A2780 Cells
3.5.1. Effect of Neogrisphenol A on the Cell Viability of A2780 Cells
3.5.2. Effect of Neogrisphenol A on Apoptosis of A2780 Cells
3.5.3. Effects of Different Concentrations of Neogrisphenol A on the Cycle of A2780 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Meena, H.; Hnamte, S.; Siddhardha, B. Secondary Metabolites from Endophytic Fungi: Chemical Diversity and Application. In Advances in Endophytic Fungal Research; Springer: Cham, Switzerland, 2019; pp. 145–169. [Google Scholar] [CrossRef]
- Hu, H.; Guo, H.; Li, E.; Liu, X.; Zhou, Y.; Che, Y. Decaspirones F−I, Bioactive Secondary Metabolites from the Saprophytic Fungus Helicoma viridis. J. Nat. Prod. 2006, 69, 1672–1675. [Google Scholar] [CrossRef] [PubMed]
- Itazaki, H.; Nagashima, K.; Sugita, K.; Yoshida, H.; Kawamura, Y.; Yasuda, Y.; Matsumoto, K.; Ishii, K.; Uotani, N.; Nakai, H.; et al. Isolation and structural elucidation of new cyclotetrapeptides, trapoxins A and B, having detransformation activities as antitumor agents. J. Antibiot. 1990, 43, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Nakai, H.; Nagashima, K.; Itazaki, H. Structure of a new cyclotetrapeptide trapoxin A. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1991, 47, 1496–1499. [Google Scholar] [CrossRef]
- Ohtsu, Y.; Sasamura, H.; Shibata, T.; Nakajima, H.; Hino, M.; Fujii, T. The novel gluconeogenesis inhibitors FR225659 and related compounds that originate from Helicomyces sp. No. 19353. II. Biological profiles. J. Antibiot. 2003, 56, 689–693. [Google Scholar] [CrossRef]
- Qian, S.Y.; Zeng, X.Y.; Qian, Y.X.; Lu, Y.Z.; He, Z.J.; Kang, J.C. A saprophytic fungus Tubeufia rubra Produces novel rubracin D and E reversing multidrug resistance in cancer cells. J. Fungi 2023, 9, 309. [Google Scholar] [CrossRef]
- Zeng, X.; Qian, S.; Lu, Y.; Li, Y.; Chen, L.; Qian, Y.; He, Z.; Kang, J. A Novel Nitrogen-containing Glyceride from Fungal Saprobe Tubeufia rubra Reverses MDR of Tumor Cell Lines to Doxorubicin. Rec. Nat. Prod. 2022, 16, 622–632. [Google Scholar] [CrossRef]
- Zheng, W.; Han, L.; He, Z.J.; Kang, J.C. A new alkaloid derivative from the saprophytic fungus Neohelicomyces hyalosporus PF11-1. Nat. Prod. Res. 2023, 1–5. [Google Scholar] [CrossRef]
- Lu, Y.Z.; Liu, J.K.; Hyde, K.D.; Jeewon, R.; Kang, J.C.; Fan, C.; Boonmee, S.; Bhat, D.J.; Luo, Z.L.; Lin, C.G. A taxonomic reassessment of Tubeufiales based on multi-locus phylogeny and morphology. Fungal Divers. 2018, 92, 131–344. [Google Scholar] [CrossRef]
- Tian, X.G.; Karunarathna, S.C.; Xu, R.; Lu, Y.; Suwannarach, N.; Mapook, A.; Bao, D.; Xu, J.; Tibpromma, S. Three New Species, Two New Records and Four New Collections of Tubeufiaceae from Thailand and China. J. Fungi 2022, 8, 206. [Google Scholar] [CrossRef]
- Lu, Y.-Z.; Ma, J.; Xiao, X.-J.; Zhang, L.-J.; Xiao, Y.-P.; Kang, J.-C. Four new species and three new records of helicosporous hyphomycetes from China and their multi-gene phylogenies. Front. Microbiol. 2022, 13, 1053849. [Google Scholar] [CrossRef]
- Wang, K.B.; Jiang, S.S.; Pu, T.; Fan, L.M.; Su, F.W.; Ye, M. Antifungal activity of phenolic monoterpenes and structure-related compounds against plant pathogenic fungi. Nat. Prod. Res. 2018, 33, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Marin-Felix, Y.; Surup, F.; Stchigel, A.M.; Stadler, M. Seven New Cytotoxic and Antimicrobial Xanthoquinodins from Jugulospora vestita. J. Fungi 2020, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Goos, R.D. On the anamorph genera Helicosporium and Drepanospora. Mycologia 1989, 81, 356–374. [Google Scholar] [CrossRef]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An Improved Probability for the Stereochemical Assignment of Isomeric Compounds Using Quantum Chemical Calculations of NMR Shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.W.; Liang, W.Z.; Zhang, Z.B.; Wang, Y.; Zhang, S.S.; Liu, J.T.; Chang, J.; Ji, C.J.; Zhu, D. Polyketide Derivatives from the Endophytic Fungus Phaeosphaeria sp. LF5 Isolated from Huperzia serrata and Their Acetylcholinesterase Inhibitory Activities. J. Fungi 2022, 8, 232. [Google Scholar] [CrossRef]
- Tram, N.T.T.; Anh, D.H.; Thuc, H.H.; Tuan, N.T. Investigation of chemical constituents and cytotoxic activity of the lichen Usnea undulata. Vietnam. J. Chem. 2020, 58, 63–66. [Google Scholar] [CrossRef]
- Tian, Y.Q.; Lin, X.P.; Liu, J.; Kaliyaperumal, K.; Ai, W.; Ju, Z.R.; Yang, B.; Wang, J.; Yang, X.W.; Liu, Y. Ascomycotin A, a new citromycetin analogue produced by Ascomycota sp. Ind19F07 isolated from deep sea sediment. Nat. Prod. Res. 2014, 29, 820–826. [Google Scholar] [CrossRef]
- Yurchenko, A.N.; Smetanina, O.F.; Kalinovsky, A.I.; Pivkin, M.V.; Dmitrenok, P.S.; Kuznetsova, T.A. A new meroterpenoid from the marine fungus Aspergillus versicolor (Vuill.) Tirab. Russ. Chem. Bull. 2010, 59, 852–856. [Google Scholar] [CrossRef]
- Wu, J.S.; Shi, X.H.; Zhang, Y.H.; Yu, J.Y.; Fu, X.M.; Li, X.; Chen, K.X.; Guo, Y.W.; Shao, C.L.; Wang, C.Y. Co-cultivation with 5-Azacytidine Induced New Metabolites from the Zoanthid-Derived Fungus Cochliobolus lunatus. Front. Chem. 2019, 7, 763. [Google Scholar] [CrossRef]
- Wang, F.; Tan, J.; Liu, J. Vibratilicin: A Novel Compound from the Basidiomycete Cortinarius vibratilis. Helvetica Chim. Acta 2010, 87, 1912–1915. [Google Scholar] [CrossRef]
- Sakagami, Y.; Sano, A.; Hara, O.; Mikawa, T.; Marumo, S. Cladosporol, β-1, 3-glucan biosynthesis inhibitor, isolated from fungus, Cladosporium cladosporioides. Tetrahedron Lett. 1995, 36, 1469–1472. [Google Scholar] [CrossRef]
- Weigenand, O.; Hussein, A.A.; Lall, N.; Meyer, J. Antibacterial activity of naphthoquinones and triterpenoids from Euclea natalensis root bark. J. Nat. Prod. 2004, 67, 1936–1938. [Google Scholar] [CrossRef] [PubMed]
- Lall, N.; Weiganand, O.; Hussein, A.A.; Meyer, J.J.M. Antifungal activity of naphthoquinones and triterpenes isolated from the root bark of Euclea natalensis. S. Afr. J. Bot. 2006, 72, 579–583. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, L.; Kong, F.; Ma, Q.; Xie, Q.; Li, J.; Dai, H.; Guo, L.; Zhao, Y. Altertoxins with Quorum Sensing Inhibitory Activities from The Marine-Derived Fungus Cladosporium sp. KFD33. Mar. Drugs 2020, 18, 67. [Google Scholar] [CrossRef]
- Li, H.L.; Li, X.M.; Mandi, A.; Antus, S.; Li, X.; Zhang, P.; Liu, Y.; Kurtan, T.; Wang, B.G. Characterization of Cladosporols from the Marine Algal-Derived Endophytic Fungus Cladosporium cladosporioides EN-399 and Configurational Revision of the Previously Reported Cladosporol Derivatives. J. Org. Chem. 2017, 82, 9946–9954. [Google Scholar] [CrossRef]
- Rapuano, R.; Ziccardi, P.; Cioffi, V.; Dallavalle, S.; Moricca, S.; Lupo, A. Cladosporols A and B, two natural peroxisome proliferator-activated receptor gamma (PPARγ) agonists, inhibit adipogenesis in 3T3-L1 preadipocytes and cause a conditioned-culture-medium-dependent arrest of HT-29 cell proliferation. Biochim. Biophys. Acta (BBA) Gen. Subj. 2021, 1865, 129973. [Google Scholar] [CrossRef]
- Blacutt, A.; Ginnan, N.; Dang, T.; Bodaghi, S.; Vidalakis, G.; Ruegger, P.; Peacock, B.; Viravathana, P.; Vieira, F.C.; Drozd, C.; et al. An In Vitro Pipeline for Screening and Selection of Citrus-Associated Microbiota with Potential Anti-“Candidatus Liberibacter asiaticus” Properties. Appl. Environ. Microbiol. 2020, 86, e02883-19. [Google Scholar] [CrossRef]
- Bai, M.; Zheng, C.-J.; Tang, D.-Q.; Zhang, F.; Wang, H.-Y.; Chen, G.-Y. Two new secondary metabolites from a mangrove-derived fungus Cladosporium sp. JS1-2. J. Antibiot. 2019, 72, 779–782. [Google Scholar] [CrossRef]
- Yamazaki, H.; Yagi, A.; Akaishi, M.; Kirikoshi, R.; Takahashi, O.; Abe, T.; Chiba, S.; Takahashi, K.; Iwakura, N.; Namikoshi, M.; et al. Halogenated cladosporols produced by the sodium halide-supplemented fermentation of the plant-associated fungus Cladosporium sp. TMPU1621. Tetrahedron Lett. 2018, 59, 1913–1915. [Google Scholar] [CrossRef]
- Nasini, G.; Arnone, A.; Assante, G.; Bava, A.; Moricca, S.; Ragazzi, A. Secondary mould metabolites of Cladosporium tenuissimum, a hyperparasite of rust fungi. Phytochemistry 2004, 65, 2107–2111. [Google Scholar] [CrossRef] [PubMed]

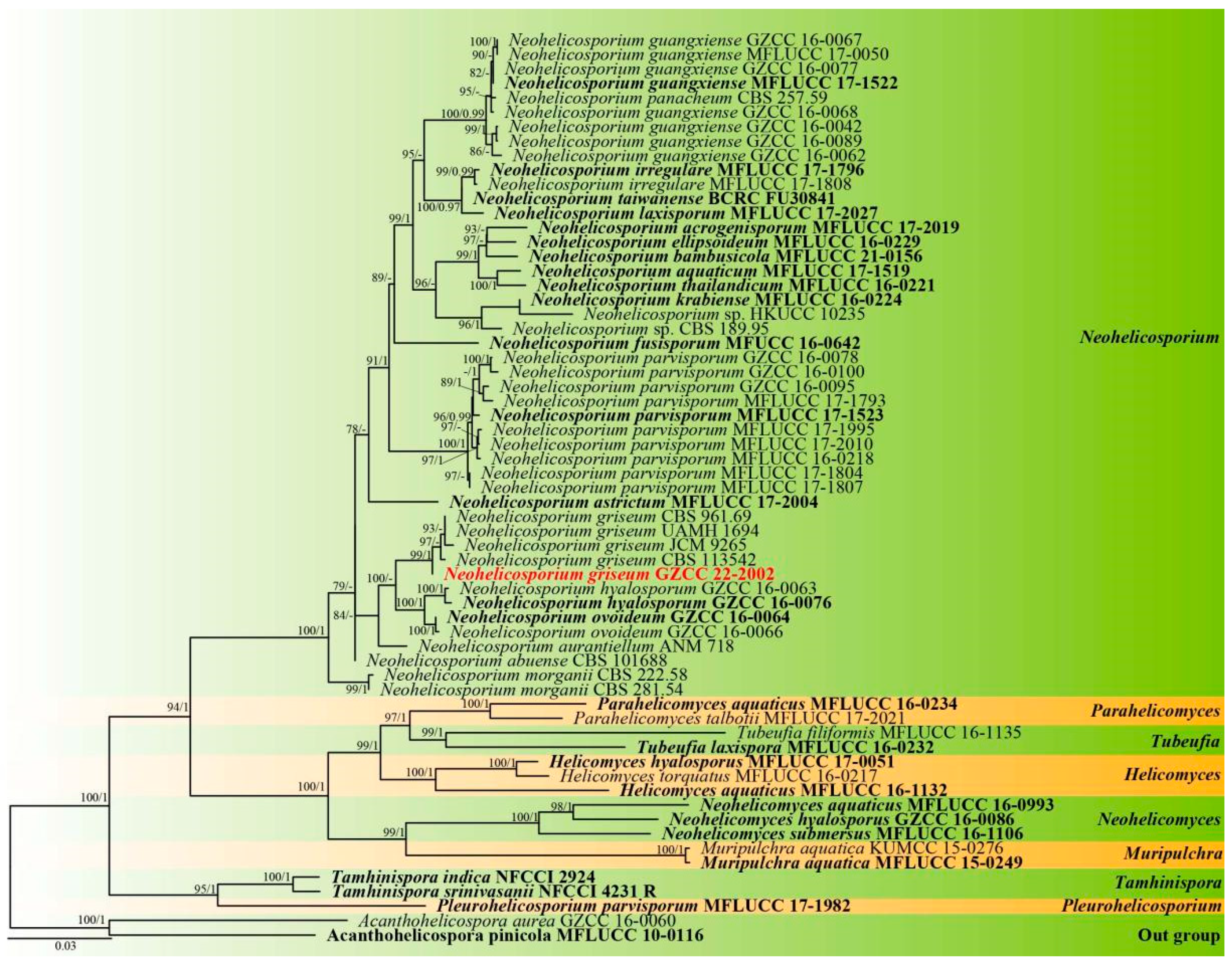

| Strains No. | ITS Sequence | LSU Sequence | ||||
|---|---|---|---|---|---|---|
| Position 410 | Position 458 | Position 460 | Position 7 | Position 161 | Position 496 | |
| GZCC 22-2002 | T | G | G | C | C | C |
| CBS 113542 | T | T | A | C | C | T |
| CBS 961.69 | T | G | A | T | C | T |
| JCM 9265 | C | G | A | - | T | T |
| UAMH 1694 | C | G | A | T | C | T |
| 1 | 2 | |||
|---|---|---|---|---|
| No | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) |
| 1 | 205.1, C | 205.0, C | ||
| 2 | 32.8, CH2 | 3.14, dd (17.7, 13.3), Ha 2.49, dd (17.7, 3.9), Hb | 33.2, CH2 | 3.03, dd (17.6, 13.4), Ha 2.25, dd (17.6, 3.8), Hb |
| 3 | 49.2, CH | 2.61, ddd (13.3, 3.9, 2.0) | 48.8, CH | 2.57, ddd (13.4, 3.8, 2.0) |
| 4 | 65.9, CH | 4.87, d (4.2) | 65.7, CH | 4.98, d (4.9) |
| 4a | 145.7, C | 146.0, C | ||
| 5 | 119.7, CH | 6.88, overlap | 119.5, CH | 6.89, overlap |
| 6 | 137.1, CH | 7.51, dd (8.4, 7.4) | 137.2, CH | 7.53, dd (7.9, 7.9) |
| 7 | 117.1, CH | 6.88, overlap | 117.0, CH | 6.89, overlap |
| 8 | 161.4, C | 161.4, C | ||
| 8a | 114.7, C | 114.7, C | ||
| 1′ | 206.4, C | 203.4, C | ||
| 2′ | 73.5, C | 73.6, C | ||
| 3′ | 136.3, CH | 6.35, d (10.0) | 136.8, CH | 6.43, d (10.0) |
| 4′ | 125.7, CH | 6.74, d (10.0) | 125.3, CH | 6.61, d (10.0) |
| 4a’ | 137.6, C | 139.6, C | ||
| 5′ | 118.9, CH | 6.88, overlap | 107.9, CH | 6.31, d (2.2) |
| 6′ | 137.8, CH | 7.56, dd (8.4, 7.4) | 166.2, C | |
| 7′ | 117.1, CH | 6.88, overlap | 101.5, CH | 6.17, d (2.2) |
| 8′ | 161.5, C | 164.8, C | ||
| 8a’ | 113.2, C | 106.7, C | ||
| 4-OH | 5.52, br d (4.2) | 5.50, br d (4.9) | ||
| 8-OH | 12.17, s | 12.12, s | ||
| 2′-OH | 6.10, br s | 6.03, br s | ||
| 6′-OH | 10.94, s | |||
| 8′-OH | 12.19, s | 12.68, s | ||
| 3 | ||
|---|---|---|
| No | δC, Type | δH (J in Hz) |
| 1 | 161.8, C | |
| 2 | ||
| 3 | 72.3, CH | 4.36, dqd (12.0, 6.2, 2.8) |
| 4 | 32.8, CH2 | 2.54, dd (16.5, 12.0), Ha 2.93, dd (16.5, 2.8), Hb |
| 4a | 112.6, C | |
| 5 | 142.0, C | |
| 6 | 160.7, C | |
| 7 | 160.5, C | |
| 8 | 97.8, CH | 6.47, s |
| 8a | 104.8, C | |
| 9 | 20.5, CH3 | 1.35, d (6.2) |
| 10 | 10.6, CH3 | 1.98, s |
| 11 | 55.4, OCH3 | 3.71, s |
| 6-OH | 10.41, br s | |
| Compound | B. subtilis | C. perfringens | R. solanacarum | MRSA Stain ATCC43300 | S. aureus |
|---|---|---|---|---|---|
| 1 | 15.63 | 31.25 | 15.63 | 31.25 | 31.25 |
| 2 | - | - | - | 31.25 | - |
| 5 | - | 250.00 | - | - | - |
| ciprofloxacin | 0.63 | 0.08 | 0.32 | 0.32 | 0.08 |
| Compound | P. nicotianae var. nicotianae | S. asclerotiorum |
|---|---|---|
| IC50 (µg/mL) | ||
| 5 | 52.36 ± 1.38 | 88.14 ± 2.21 |
| Compound | A2780 | PC-3 | MBA-MD-231 |
|---|---|---|---|
| 1 | 3.20 | 10.68 | 16.30 |
| 2 | 10.13 | >20 | >20 |
| 3 | >20 | >20 | >20 |
| 4 | >20 | >20 | >20 |
| 5 | >20 | >20 | >20 |
| 6 | >20 | >20 | >20 |
| 7 | >20 | >20 | >20 |
| cis-platinum | 9.34 | 7.53 | - |
| adriamycin | - | - | 12.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.-J.; Yang, M.-F.; Ma, J.; Xiao, X.-J.; Ma, X.-Y.; Zheng, D.-G.; Han, M.-Y.; Xia, M.-L.; Jayawardena, R.S.; Mapook, A.; et al. Neogrisphenol A, a Potential Ovarian Cancer Inhibitor from a New Record Fungus Neohelicosporium griseum. Metabolites 2023, 13, 435. https://doi.org/10.3390/metabo13030435
Zhang L-J, Yang M-F, Ma J, Xiao X-J, Ma X-Y, Zheng D-G, Han M-Y, Xia M-L, Jayawardena RS, Mapook A, et al. Neogrisphenol A, a Potential Ovarian Cancer Inhibitor from a New Record Fungus Neohelicosporium griseum. Metabolites. 2023; 13(3):435. https://doi.org/10.3390/metabo13030435
Chicago/Turabian StyleZhang, Li-Juan, Ming-Fei Yang, Jian Ma, Xing-Juan Xiao, Xiao-Yan Ma, De-Ge Zheng, Mei-Yan Han, Ming-Lei Xia, Ruvishika S. Jayawardena, Ausana Mapook, and et al. 2023. "Neogrisphenol A, a Potential Ovarian Cancer Inhibitor from a New Record Fungus Neohelicosporium griseum" Metabolites 13, no. 3: 435. https://doi.org/10.3390/metabo13030435
APA StyleZhang, L.-J., Yang, M.-F., Ma, J., Xiao, X.-J., Ma, X.-Y., Zheng, D.-G., Han, M.-Y., Xia, M.-L., Jayawardena, R. S., Mapook, A., Xiao, Y.-P., Kang, J.-C., & Lu, Y.-Z. (2023). Neogrisphenol A, a Potential Ovarian Cancer Inhibitor from a New Record Fungus Neohelicosporium griseum. Metabolites, 13(3), 435. https://doi.org/10.3390/metabo13030435






