Metabolomic Changes in Patients Affected by Multiple Sclerosis and Treated with Fingolimod
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Sample Preparation
2.3. H-NMR Analysis and Data Processing
2.4. Statistical Analysis
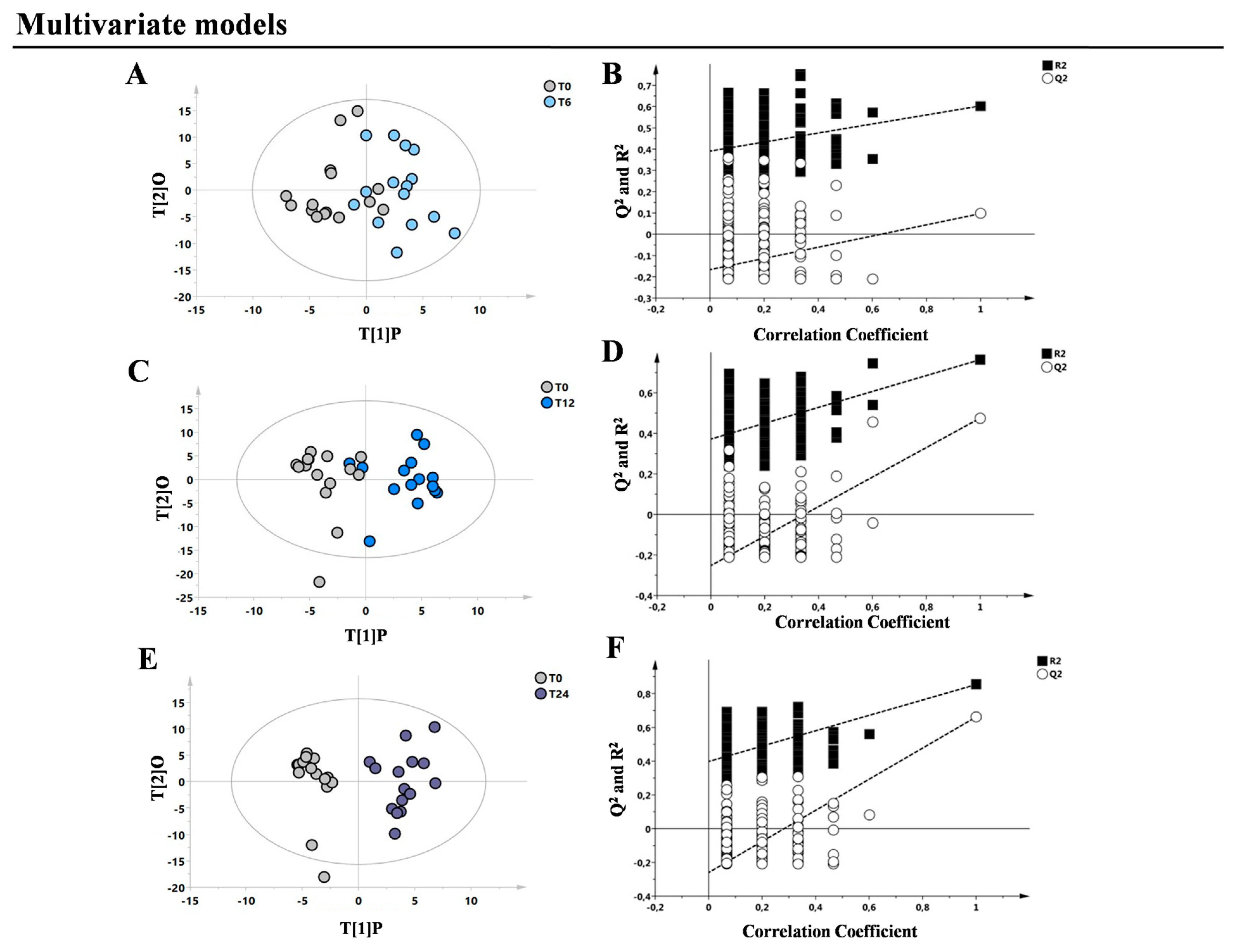
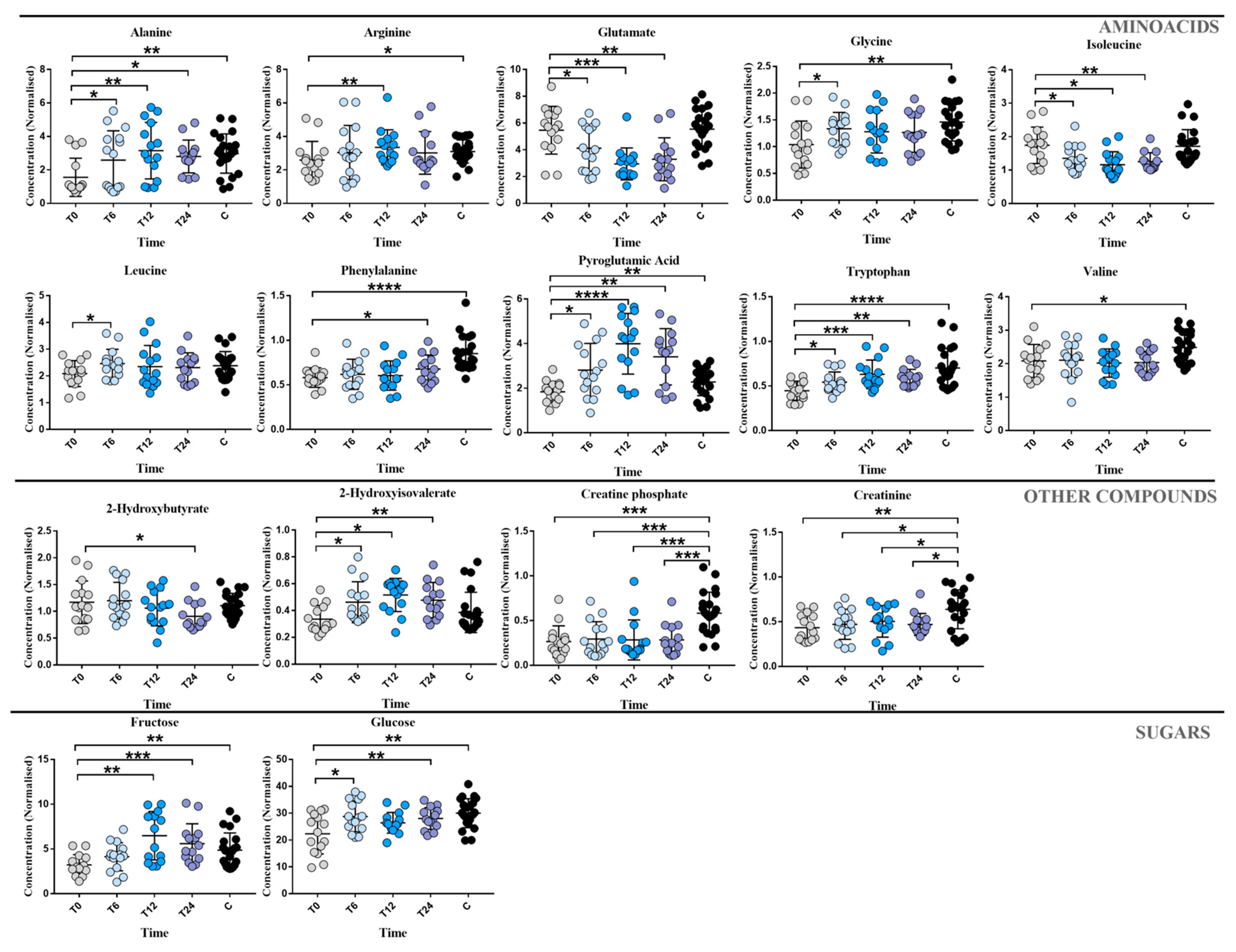
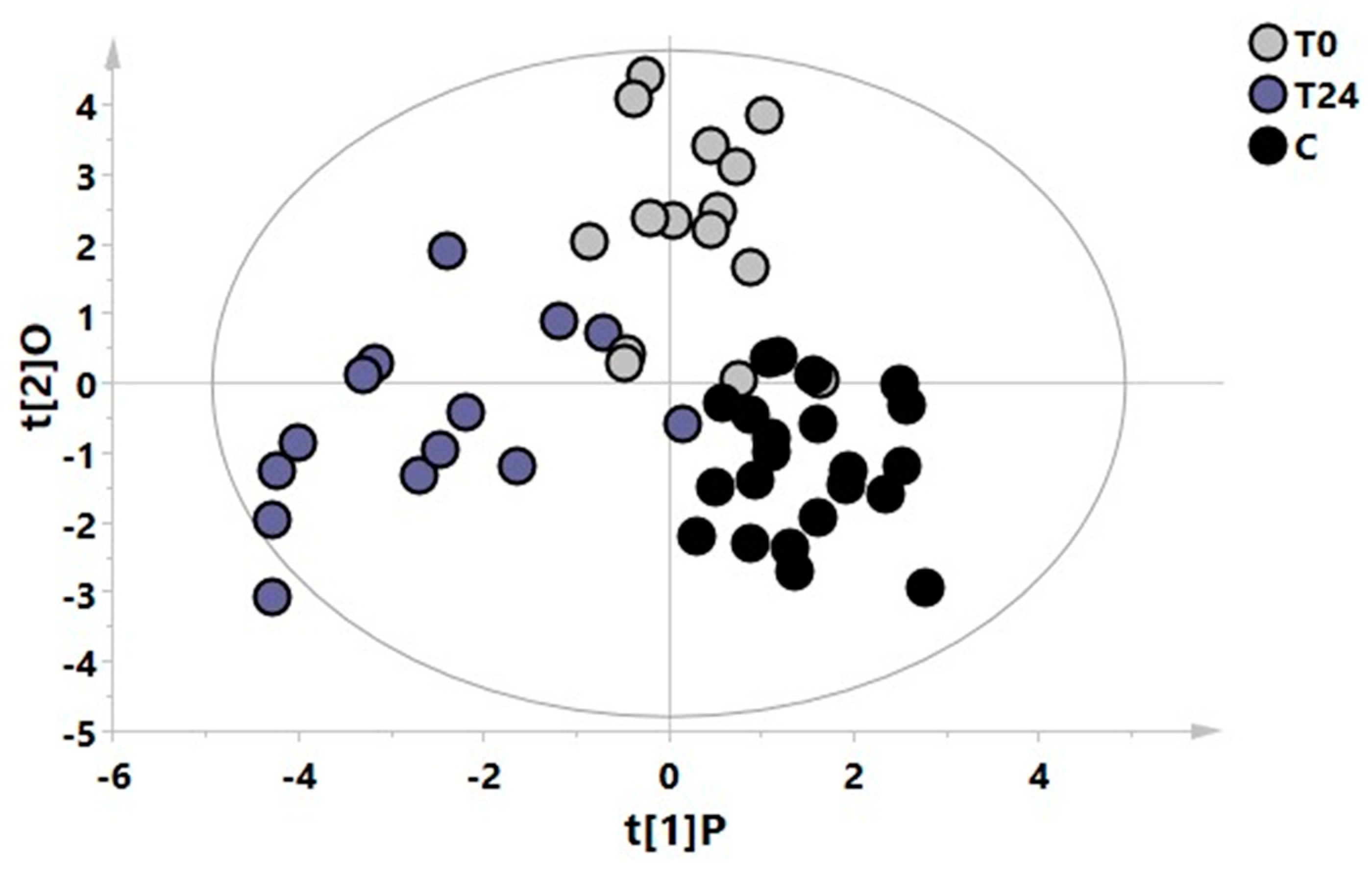
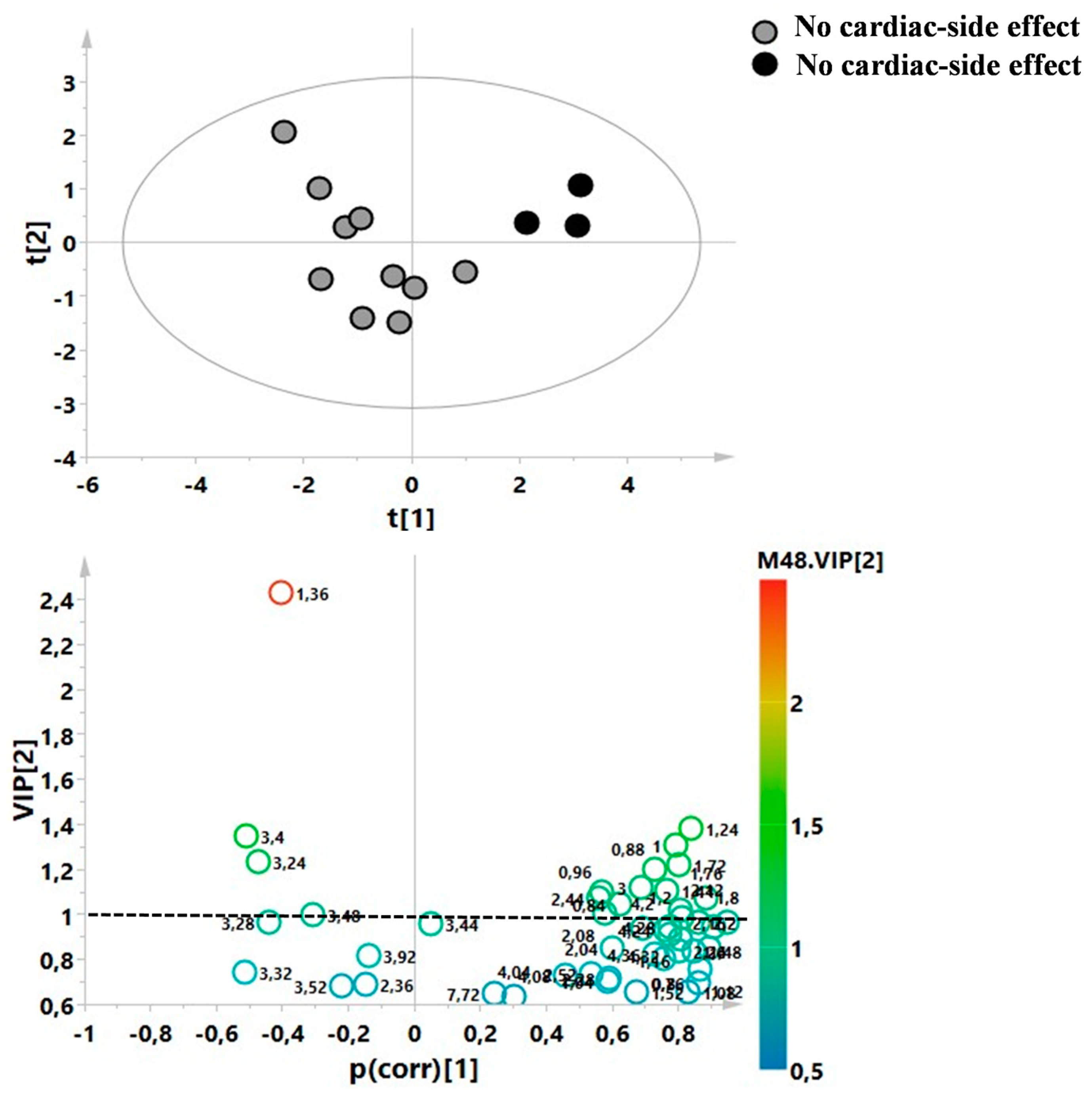
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milo, R.; Miller, A. Revised diagnostic criteria of multiple sclerosis. Autoimmun. Rev. 2014, 13, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef] [PubMed]
- Loma, I.; Heyman, R. Multiple sclerosis: Pathogenesis and treatment. Curr. Neuropharmacol. 2011, 9, 409–416. [Google Scholar] [CrossRef] [PubMed]
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and Treatment of Multiple Sclerosis: A Review. JAMA 2021, 325, 765–779. [Google Scholar] [CrossRef]
- Tedeschi, G.; Gallo, A. Multiple sclerosis patients and immunomodulation therapies: The potential role of new MRI techniques to assess responders versus non-responders. Neurol. Sci. 2005, 26, s209–s212. [Google Scholar] [CrossRef] [PubMed]
- Ziemssen, T.; Akgün, K.; Brück, W. Molecular biomarkers in multiple sclerosis. J. Neuroinflamm. 2019, 16, 272. [Google Scholar] [CrossRef]
- Harris, V.K.; Sadiq, S.A. Biomarkers of therapeutic response in multiple sclerosis: Current status. Mol. Diagn. Ther. 2014, 18, 605–617. [Google Scholar] [CrossRef]
- Kappos, L.; Radue, E.W.; O’Connor, P.; Polman, C.; Hohlfeld, R.; Calabresi, P.; Selmaj, K.; Agoropoulou, C.; Leyk, M.; Zhang-Auberson, L.; et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 387–401. [Google Scholar] [CrossRef]
- Cohen, J.A.; Barkhof, F.; Comi, G.; Hartung, H.P.; Khatri, B.O.; Montalban, X.; Pelletier, J.; Capra, R.; Gallo, P.; Izquierdo, G.; et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 402–415. [Google Scholar] [CrossRef]
- Fonseca, J. Fingolimod Real World Experience: Efficacy and Safety in Clinical Practice. Neurosci. J. 2015, 2015, 389360. [Google Scholar] [CrossRef]
- Cocco, E.; Murgia, F.; Lorefice, L.; Barberini, L.; Poddighe, S.; Frau, J.; Fenu, G.; Coghe, G.; Murru, M.R.; Murru, R.; et al. 1H-NMR analysis provides a metabolomic profile of patients with multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2015, 3, e185. [Google Scholar] [CrossRef]
- Lorefice, L.; Murgia, F.; Fenu, G.; Frau, J.; Coghe, G.; Murru, M.R.; Tranquilli, S.; Visconti, A.; Marrosu, M.G.; Atzori, L.; et al. Assessing the metabolomic profile of multiple sclerosis patients treated with interferon beta 1a by 1H-NMR spectroscopy. Neurother. J. 2019, 16, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Byrne, T.; Johansson, E.; Trygg, J.; Vikstrom, C. Multi- and Megavariate Data Analysis Basic Principles and Applications, 3rd ed.; MKS Umetrics AB: Umea, Sweden, 2013; Volume 1. [Google Scholar]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Trygg, J.; Holmes, E.; Lundstedt, T. Chemometrics in metabonomics. J. Proteome Res. 2007, 6, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, F.; Hansen, B.; Karcher, W.; Sjöström, M.; Eriksson, L. Model validation by permutation tests: Applications to variable selection. J. Chemom. 1996, 10, 521–532. [Google Scholar] [CrossRef]
- Weljie, A.M.; Newton, J.; Mercier, P.; Carlson, E.; Slupsky, C.M. Targeted profiling: Quantitative analysis of 1H NMR metabolomics data. Anal. Chem. 2006, 78, 4430–4442. [Google Scholar] [CrossRef] [PubMed]
- Ingwersen, J.; Aktas, O.; Kuery, P.; Kieseier, B.; Boyko, A.; Hartung, H.P. Fingolimod in multiple sclerosis: Mechanisms of action and clinical efficacy. Clin. Immunol. 2012, 142, 15–24. [Google Scholar] [CrossRef]
- La Mantia, L.; Tramacere, I.; Firwana, B.; Pacchetti, I.; Palumbo, R.; Filippini, G. Fingolimod for relapsing-remitting multiple sclerosis. Cochrane Database Syst. Rev. 2016, 4, CD009371. [Google Scholar] [CrossRef]
- Correale, J. Immunosuppressive Amino-Acid Catabolizing Enzymes in Multiple Sclerosis. Front. Immunol. 2021, 11, 600428. [Google Scholar] [CrossRef]
- Negrotto, L.; Correale, J. Amino Acid Catabolism in Multiple Sclerosis Affects Immune Homeostasis. J. Immunol. 2017, 198, 1900–1909. [Google Scholar] [CrossRef]
- Fitzgerald, K.C.; Smith, M.D.; Kim, S.; Sotirchis, E.S.; Kornberg, M.D.; Douglas, M.; Nourbakhsh, B.; Graves, J.; Rattan, R.; Poisson, L.; et al. Multi-omic evaluation of metabolic alterations in multiple sclerosis identifies shifts in aromatic amino acid metabolism. Cell Rep. Med. 2021, 2, 100424. [Google Scholar] [CrossRef]
- Carmans, S.; Hendriks, J.J.; Thewissen, K.; Van den Eynden, J.; Stinissen, P.; Rigo, J.M.; Hellings, N. The inhibitory neurotransmitter glycine modulates macrophage activity by activation of neutral amino acid transporters. J. Neurosci. Res. 2010, 88, 2420–2430. [Google Scholar] [CrossRef]
- Ďurfinová, M.; Bartová, R.; Orešanská, K.; Turecký, L.; Procházková, L.; Petrleničová, D.; Líška, B. Increased glycine levels in cerebrospinal fluid of patients with multiple sclerosis. Clin. Chim. Acta 2018, 486, 86–87. [Google Scholar] [CrossRef] [PubMed]
- Groom, A.J.; Smith, T.; Turski, L. Multiple sclerosis and glutamate. Ann. N. Y. Acad. Sci. 2003, 993, 229–275. [Google Scholar] [CrossRef] [PubMed]
- Pitt, D.; Nagelmeier, I.E.; Wilson, H.C.; Raine, C.S. Glutamate uptake by oligodendrocytes: Implications for excitotoxicity in multiple sclerosis. Neurology 2003, 61, 1113–1120. [Google Scholar] [CrossRef]
- Sarchielli, P.; Greco, L.; Floridi, A.; Floridi, A.; Gallai, V. Excitatory amino acids and multiple sclerosis: Evidence from cerebrospinal fluid. Arch. Neurol. 2003, 60, 1082–1088. [Google Scholar] [CrossRef]
- Noda, H.; Takeuchi, H.; Mizuno, T.; Suzumura, A. Fingolimod phosphate promotes the neuroprotective effects of microglia. J. Neuroimmunol. 2013, 256, 13–18. [Google Scholar] [CrossRef]
- Serpero, L.D.; Filaci, G.; Parodi, A.; Battaglia, F.; Kalli, F.; Brogi, D.; Mancardi, G.L.; Uccelli, A.; Fenoglio, D. Fingolimod modulates peripheral effector and regulatory T cells in MS patients. J. Neuroimmune Pharmacol. 2013, 8, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Centonze, D.; Muzio, L.; Rossi, S.; Furlan, R.; Bernardi, G.; Martino, G. The link between inflammation, synaptic transmission and neurodegeneration in multiple sclerosis. Cell Death Differ. 2010, 17, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Groves, A.; Kihara, Y.; Chun, J. Fingolimod: Direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. J. Neurol. Sci. 2013, 328, 9–18. [Google Scholar] [CrossRef]
- Pieragostino, D.; D’Alessandro, M.; di Ioia, M.; Rossi, C.; Zucchelli, M.; Urbani, A.; Di Ilio, C.; Lugaresi, A.; Sacchetta, P.; Del Boccio, P. An integrated metabolomics approach for the research of new cerebrospinal fluid biomarkers of multiple sclerosis. Mol. Biosyst. 2015, 11, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Villoslada, P.; Alonso, C.; Agirrezabal, I.; Kotelnikova, E.; Zubizarreta, I.; Pulido-Valdeolivaset, I.; Saiz, A.; Comabella, M.; Montalban, X.; Villar, L.; et al. Metabolomic signatures associated with disease severity in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e321. [Google Scholar] [CrossRef] [PubMed]
- Bachhawat, A.K.; Yadav, S. The glutathione cycle: Glutathione metabolism beyond the γ-glutamyl cycle. IUBMB Life 2018, 70, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Murgia, F.; Lorefice, L.; Poddighe, S.; Fenu, G.; Secci, M.A.; Marrosu, M.G.; Cocco, E.; Atzori, L. Multi-Platform Characterization of Cerebrospinal Fluid and Serum Metabolome of Patients Affected by Relapsing-Remitting and Primary Progressive Multiple Sclerosis. J. Clin. Med. 2020, 9, 863. [Google Scholar] [CrossRef]
- Stojanovic, I.; Vojinovic, S.; Ljubisavljevic, S.; Pavlovic, R.; Basic, J.; Pavlovic, D.; Ilic, A.; Cvetkovic, T.; Stukalov, M. INF-β1b therapy modulates L-arginine and nitric oxide metabolism in patients with relapse remittent multiple sclerosis. J. Neurol. Sci. 2012, 323, 187–192. [Google Scholar] [CrossRef]
- Mangalam, A.; Poisson, L.; Nemutlu, E.; Datta, I.; Denic, A.; Dzeja, P.; Rodriguez, M.; Rattan, R.; Giri, S. Profile of Circulatory Metabolites in a Relapsing-remitting Animal Model of Multiple Sclerosis using Global Metabolomics. J. Clin. Cell. Immunol. 2013, 4, 3. [Google Scholar] [CrossRef]
- Pritzker, L.B.; Joshi, S.; Gowan, J.J.; Harauz, G.; Moscarello, M.A. Deimination of myelin basic protein. 1. Effect of deimination of arginyl residues of myelin basic protein on its structure and susceptibility to digestion by cathepsin D. Biochemistry 2000, 39, 5374–5381. [Google Scholar] [CrossRef]
- Rajda, C.; Majláth, Z.; Pukoli, D.; Vécsei, L. Kynurenines and Multiple Sclerosis: The Dialogue between the Immune System and the Central Nervous System. Int. J. Mol. Sci. 2015, 16, 18270–18282. [Google Scholar] [CrossRef]
- Moroni, F.; Cozzi, A.; Sili, M.; Mannaioni, G. Kynurenic acid: A metabolite with multiple actions and multiple targets in brain and periphery. J. Neural. Transm. 2012, 119, 133–139. [Google Scholar] [CrossRef]
- Guillemin, G.J. Quinolinic acid, the inescapable neurotoxin. FEBS J. 2012, 279, 1356–1365. [Google Scholar] [CrossRef]
- Vécsei, L.; Szalárdy, L.; Fülöp, F.; Toldi, J. Kynurenines in the CNS: Recent advances and new questions. Nat. Rev. Drug Discov. 2013, 12, 64–82. [Google Scholar] [CrossRef]
- Mándi, Y.; Vécsei, L. The kynurenine system and immunoregulation. J. Neural. Transm. 2012, 119, 97–209. [Google Scholar] [CrossRef] [PubMed]
- Buttgereit, F.; Burmester, G.R.; Brand, M.D. Bioenergetics of immune functions: Fundamental and therapeutic aspects. Immunol. Today 2000, 21, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Straub, R.H.; Cutolo, M.; Buttgereit, F.; Pongratz, G. Energy regulation and neuroendocrine-immune control in chronic inflammatory diseases. J. Intern. Med. 2010, 267, 543–560. [Google Scholar] [CrossRef]
- Kim, H.H.; Jeong, I.H.; Hyun, J.S.; Kong, B.S.; Kim, H.J.; Park, S.J. Metabolomic profiling of CSF in multiple sclerosis and neuromyelitis optica spectrum disorder by nuclear magnetic resonance. PLoS ONE 2017, 12, e0181758. [Google Scholar] [CrossRef] [PubMed]
- Poddighe, S.; Murgia, F.; Lorefice, L.; Liggi, S.; Cocco, E.; Marrosu, M.G.; Atzori, L. Metabolomic analysis identifies altered metabolic pathways in Multiple Sclerosis. Int. J. Biochem. Cell. Biol. 2017, 93, 148–155. [Google Scholar] [CrossRef]


| Characteristics of Patients and Controls | ||||||
|---|---|---|---|---|---|---|
| MS patients | ||||||
| Patients | Age ± SD Range | F/M | MS duration (mean years) | EDSS score (mean) | Inclusion criteria | Exclusion criteria |
| 42 SM-RR | 39 ± 8.7 (22–56) | 23/19 | 10 ± 6 | 3 ± 1.7 | Adults ≥ 18 years of age | Corticosteroids exposure in the previous 30 days |
| MS diagnosis according to McDonald 2010 criteria | Presence of other chronic comorbidities | |||||
| Relapsing remitting course | Use of other chronic medications | |||||
| Scheduled Fingolimod treatment | ||||||
| Healthy controls | ||||||
| 22 C | 40.8 ± 13.8 (20–67) | 17/5 | Adults ≥ 18 years of age | No family history of MS | Presence of chronic disease | |
| Use of chronic medications | ||||||
| SUPERVISED MODELS | ||||||
|---|---|---|---|---|---|---|
| N | R2X | R2Y | Q2 | p-Value | Permutation Test: Intercept R2/Q2 | |
| T0 vs. T6 vs. T12 vs. T24 | 60 | 0.52 | 0.39 | 0.27 | 0.02 | 0.2/−0.33 |
| T0 vs. T6 | 30 | 0.40 | 0.60 | 0.08 | ns | 0.38/−0.4 |
| T0 vs. T12 | 30 | 0.42 | 0.76 | 0.52 | <0.001 | 0.39/−0.55 |
| T0 vs. T24 | 30 | 0.51 | 0.90 | 0.72 | <0.0001 | 0.59/−0.7 |
| T0 vs. T24 vs. C | 52 | 0.44 | 0.67 | 0.49 | <0.0001 | 0.24/−0.35 |
| R vs. NR | 42 | 0.60 | 0.70 | 0.49 | 0.002 | 0.38/−0.6 |
| Baseline Characteristics | R Patients (n = 30) | NR Patients (n = 12) |
|---|---|---|
| Age (mean) ± SD | 37 ± 8.6 | 43 ± 7.8 |
| Gender F/M | 18/12 | 5/7 |
| MS duration (mean years) | 9 | 13 |
| EDSS score (mean) | 3 ± 1.8 | 3 ± 1.6 |
| MRI activity (Gd + lesions) | 0 | 8 (67%) |
| Author | Years | Sample Size | Biofluid | Technique | Results | Pathways |
|---|---|---|---|---|---|---|
| Basal metabolic profile | ||||||
| Cocco et al. [11] | 2015 | 161 subjects: 73 MS 77 controls | Plasma | 1H-NMR | Increase: 3-OH-butyrate, acetoacetate, acetone, alanine, choline Decrease: Glucose, 5-OH-tryptophan, tryptophan | Tryptophan metabolism Energy metabolism |
| Poddighe et al. [47] | 2017 | 65 subjects: 32 MS 33 controls | Plasma | GC-MS | Increase: asparagine, L-ornithine, glutamine, glutamate Decrease: Fructose, myo-inositol, pyroglutamate, threonate, leucine | Asparagine and Citrulline biosynthesis Energy metabolism |
| MS subtypes | ||||||
| Murgia et al. [35] | 2020 | 34 subjects 22 RRMS 12 PPMS | CSF Serum | 1H-NMR GC-MS L MS | Serum Increase: PC aa C34:3, PC ae C38:1, PC ae C38:2, methionine-Sulfoxide Decrease: PC aa C38:4, PC aa C40:5, SM C26:0, C5, alpha-aminoadipic acid, glutamate, valine, taurine, spermidine CSF Decrease: PCae C42:2, Ornithine Increase: Histidine, Phenylalanine, Threonine | Serum Glutathione metabolism, nitrogen metabolism, arginine and proline metabolism, glutamine and glutamate metabolism, linoleic acid metabolism, taurine and hypotaurine metabolism alanine, aspartate, and glutamate metabolism. CSF Nitrogen metabolism, arginine and ornithine metabolism, branched chain amino acid (BCAAs) biosynthesis, phenylalanine, tyrosine and tryptophan biosynthesis and histidine metabolism. |
| Response to the IFN-β therapy | ||||||
| Lorefice et al. [12] | 2019 | 37 subjects: 21 MS 16 controls | Plasma | 1H-NMR | Decrease: Acetoacetate, acetone, 3-hydroxybutyrate, glutamate, methylmalonate Increase: Tryptophan | Energetic pathways Tryptophan metabolism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murgia, F.; Lorefice, L.; Noto, A.; Spada, M.; Frau, J.; Fenu, G.; Coghe, G.; Gagliano, A.; Atzori, L.; Cocco, E. Metabolomic Changes in Patients Affected by Multiple Sclerosis and Treated with Fingolimod. Metabolites 2023, 13, 428. https://doi.org/10.3390/metabo13030428
Murgia F, Lorefice L, Noto A, Spada M, Frau J, Fenu G, Coghe G, Gagliano A, Atzori L, Cocco E. Metabolomic Changes in Patients Affected by Multiple Sclerosis and Treated with Fingolimod. Metabolites. 2023; 13(3):428. https://doi.org/10.3390/metabo13030428
Chicago/Turabian StyleMurgia, Federica, Lorena Lorefice, Antonio Noto, Martina Spada, Jessica Frau, Giuseppe Fenu, Giancarlo Coghe, Antonella Gagliano, Luigi Atzori, and Eleonora Cocco. 2023. "Metabolomic Changes in Patients Affected by Multiple Sclerosis and Treated with Fingolimod" Metabolites 13, no. 3: 428. https://doi.org/10.3390/metabo13030428
APA StyleMurgia, F., Lorefice, L., Noto, A., Spada, M., Frau, J., Fenu, G., Coghe, G., Gagliano, A., Atzori, L., & Cocco, E. (2023). Metabolomic Changes in Patients Affected by Multiple Sclerosis and Treated with Fingolimod. Metabolites, 13(3), 428. https://doi.org/10.3390/metabo13030428









