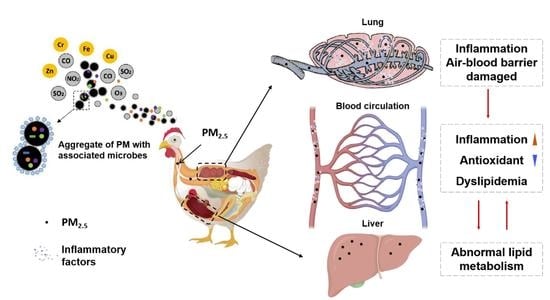
Exposure to Particulate Matter in the Broiler House Causes Dyslipidemia and Exacerbates It by Damaging Lung Tissue in Broilers
Abstract
1. Introduction
2. Materials and Methods
2.1. PM Collection
2.2. Animals
2.3. Experimental Design
2.4. Exposure Treatment
2.5. Sample Collection
2.6. Histomorphology Examination
2.7. Inflammatory Cytokines and Serum Biochemical Assay
2.8. RT-PCR Assay
2.9. Statistical Analysis
3. Results
3.1. Growth Performance
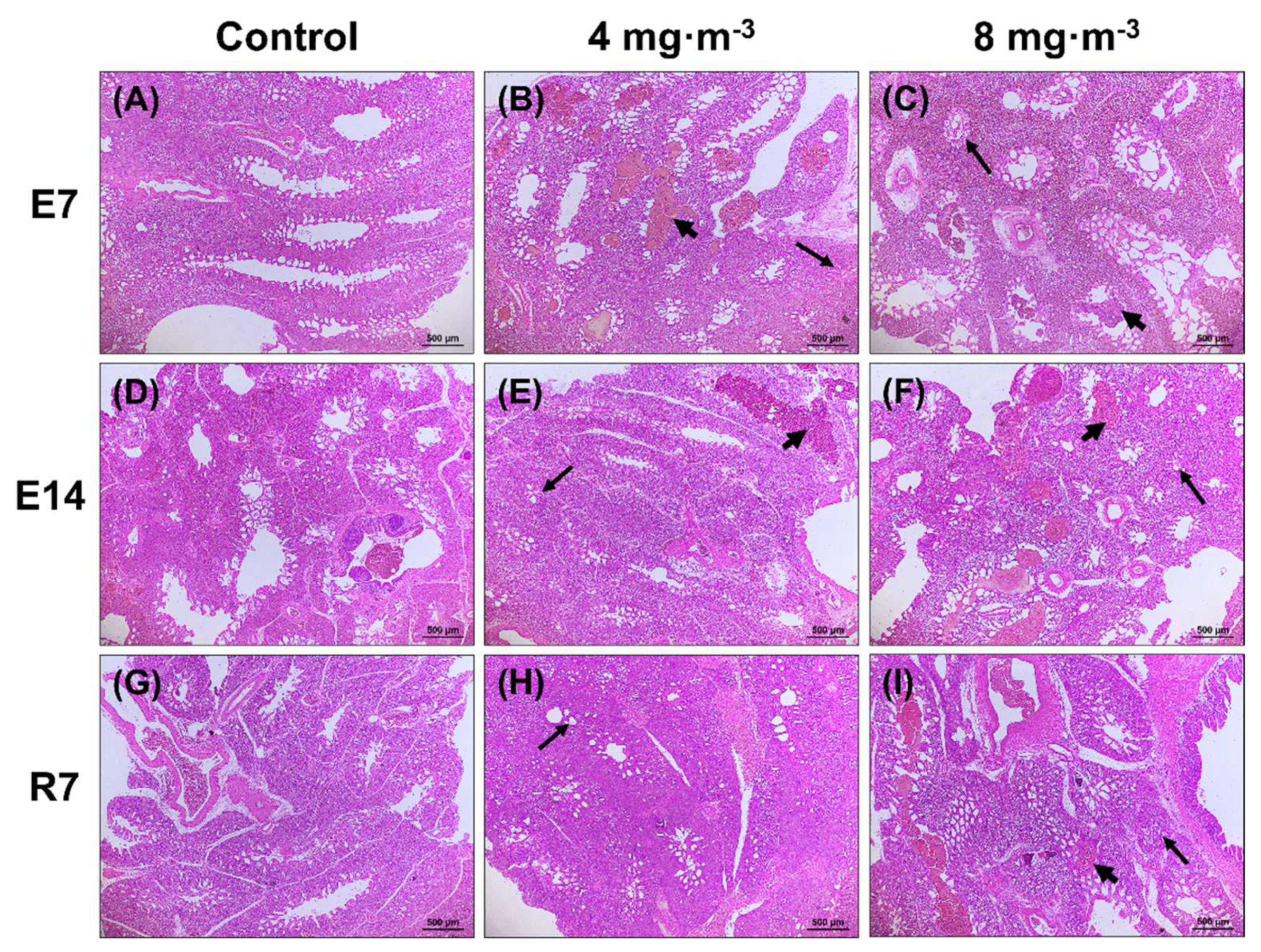
3.2. Lung and Liver Histomorphology
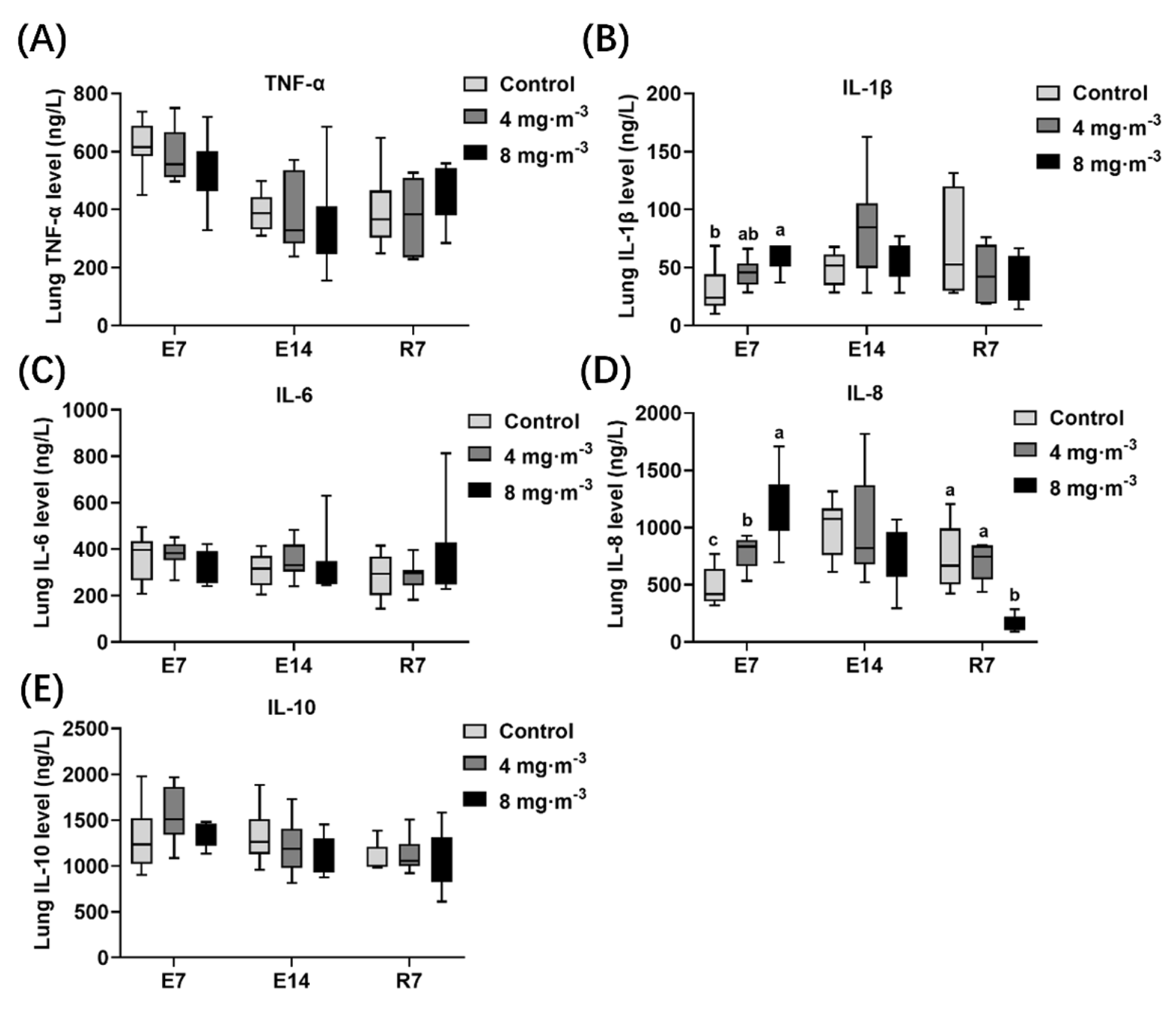
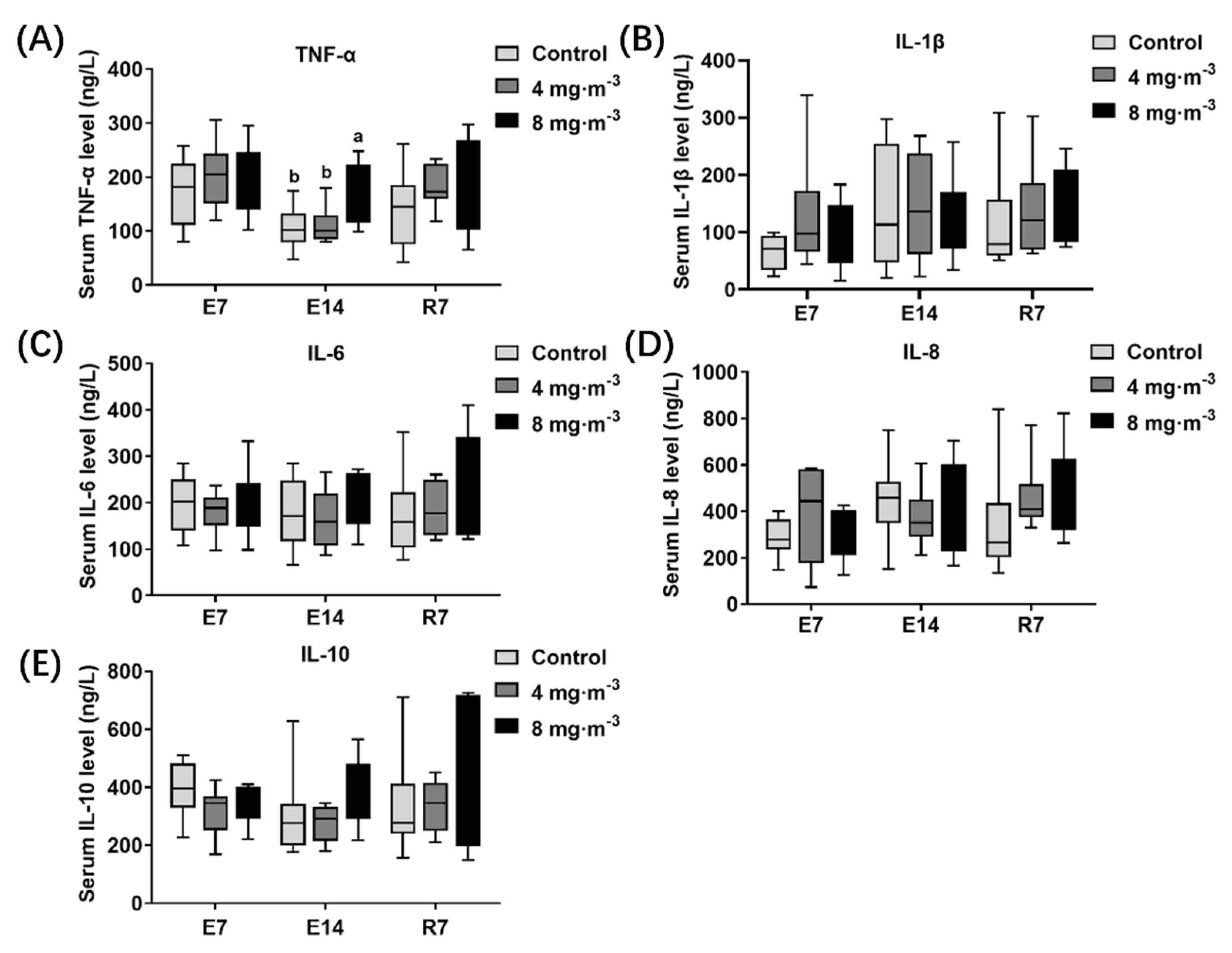
3.3. Inflammatory Factor Levels
3.4. Antioxidant Capacity
3.5. Liver Function
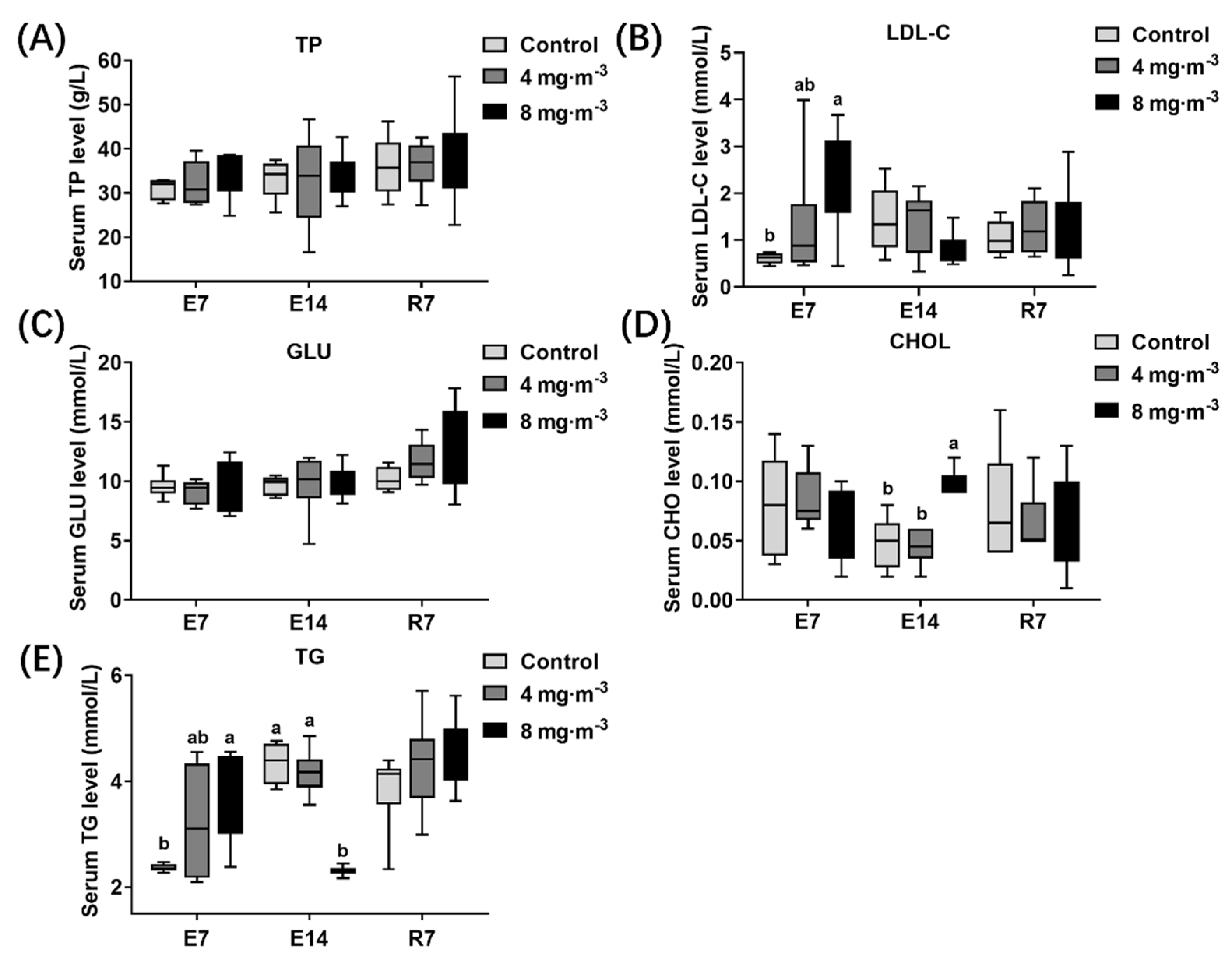
3.6. Lipid Concentration in Serum
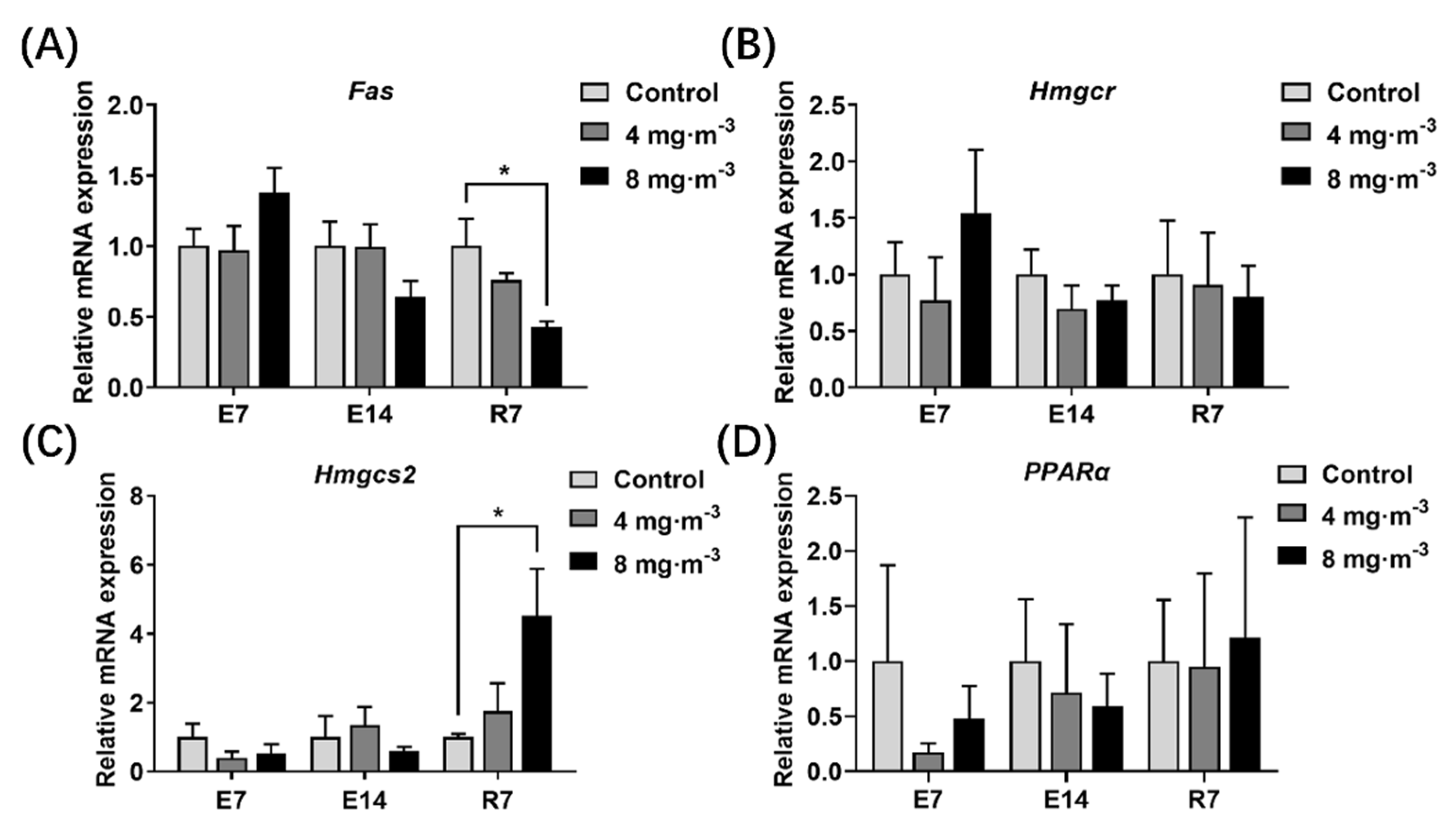
3.7. Expression Levels of Genes Related to Lipid Metabolism in the Liver
4. Discussion
4.1. Characteristics of PM during Broiler Rearing
4.2. Effects of PM Exposure on Productivity and Histomorphology in the Lung and Liver of Broilers
4.3. Effects of PM Exposure on Inflammation Level and Antioxidant Capacity in Broilers
4.4. Effects of PM Exposure on Liver Function and Lipid Metabolism in Broilers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Achilleos, S.; Kioumourtzoglou, M.A.; Wu, C.D.; Schwartz, J.D.; Koutrakis, P.; Papatheodorou, S.I. Acute Effects of Fine Particulate Matter Constituents On Mortality: A Systematic Review and Meta-Regression Analysis. Environ. Int. 2017, 109, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Hedelin, A.S.; Sundblad, B.M.; Sahlander, K.; Wilkinson, K.; Seisenbaeva, G.; Kessler, V.; Larsson, K.; Palmberg, L. Comparing Human Respiratory Adverse Effects After Acute Exposure to Particulate Matter in Conventional and Particle-Reduced Swine Building Environments. Occup. Environ. Med. 2016, 73, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Viegas, S.; Mateus, V.; Almeida-Silva, M.; Carolino, E.; Viegas, C. Occupational Exposure to Particulate Matter and Respiratory Symptoms in Portuguese Swine Barn Workers. J. Toxicol. Env. Health Part A 2013, 76, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Audi, C.; Baiz, N.; Maesano, C.N.; Ramousse, O.; Reboulleau, D.; Magnan, A.; Caillaud, D.; Annesi-Maesano, I. Serum Cytokine Levels Related to Exposure to Volatile Organic Compounds and PM(2.5) in Dwellings and Workplaces in French Farmers—A Mechanism to Explain Nonsmoking Copd. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1363–1374. [Google Scholar] [CrossRef]
- Smit, L.; Boender, G.J.; de Steenhuijsen, P.W.; Hagenaars, T.J.; Huijskens, E.; Rossen, J.; Koopmans, M.; Nodelijk, G.; Sanders, E.; Yzermans, J.; et al. Increased Risk of Pneumonia in Residents Living Near Poultry Farms: Does the Upper Respiratory Tract Microbiota Play a Role? Pneumonia 2017, 9, 3. [Google Scholar] [CrossRef]
- Cambra-Lopez, M.; Aarnink, A.J.; Zhao, Y.; Calvet, S.; Torres, A.G. Airborne Particulate Matter From Livestock Production Systems: A Review of an Air Pollution Problem. Environ. Pollut. 2010, 158, 1–17. [Google Scholar] [CrossRef]
- Ali, M.Z. Common Respiratory Diseases of Poultry in Bangladesh: A Review. SAARC J. Agric. 2020, 18, 1–11. [Google Scholar] [CrossRef]
- Ellen, H.H.; Bottcher, R.W.; von Wachenfelt, E.; Takai, H. Dust Levels and Control Methods in Poultry Houses. J. Agric. Saf. Health. 2000, 6, 275–282. [Google Scholar] [CrossRef]
- Environmental Quality Standard for the Livestock and Poultry Farm. In NY/T 388-1999; Ministry of Agriculture of the People’s Republic of China: Beijing, China, 1999.
- Shen, D.; Wu, S.; Dai, P.Y.; Li, Y.S.; Li, C.M. Distribution of Particulate Matter and Ammonia and Physicochemical Properties of Fine Particulate Matter in a Layer House. Poult. Sci. 2018, 97, 4137–4149. [Google Scholar] [CrossRef]
- Qiu, X.; Duan, L.; Gao, J.; Wang, S.; Chai, F.; Hu, J.; Zhang, J.; Yun, Y. Chemical Composition and Source Apportionment of Pm10 and Pm2.5 in Different Functional Areas of Lanzhou, China. J. Environ. Sci. 2016, 40, 75–83. [Google Scholar] [CrossRef]
- Mostafa, E.; Nannen, C.; Henseler, J.; Diekmann, B.; Gates, R.; Buescher, W. Physical Properties of Particulate Matter From Animal Houses-Empirical Studies to Improve Emission Modelling. Environ. Sci. Pollut. Res. 2016, 23, 12253–12263. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Shen, D.; Tang, Q.; Huang, K.; Li, C. PM(2.5) From a Broiler Breeding Production System: The Characteristics and Microbial Community Analysis. Environ. Pollut. 2020, 256, 113368. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.F.; Wang-Li, L.; Liu, Z.; Jayanty, R.K.; Shah, S.B.; Bloomfield, P. Major Ionic Compositions of Fine Particulate Matter in an Animal Feeding Operation Facility and its Vicinity. J. Air Waste Manag. Assoc. 2014, 64, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Gao, D.; Liao, F.; Zhou, F.; Wang, X. The Health Effects of Ambient PM2.5 and Potential Mechanisms. Ecotoxicol. Environ. Saf. 2016, 128, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K.; Loridas, S. Pulmonary Oxidative Stress, Inflammation and Cancer: Respirable Particulate Matter, Fibrous Dusts and Ozone as Major Causes of Lung Carcinogenesis through Reactive Oxygen Species Mechanisms. Int. J. Environ. Res. Public Health. 2013, 10, 3886–3907. [Google Scholar] [CrossRef]
- Wei, H.; Yuan, W.; Yu, H.; Geng, H. Cytotoxicity Induced by Fine Particulate Matter (PM(2.5)) Via Mitochondria-Mediated Apoptosis Pathway in Rat Alveolar Macrophages. Environ. Sci. Pollut. Res. 2021, 28, 25819–25829. [Google Scholar] [CrossRef]
- Li, J.; An, Z.; Song, J.; Du, J.; Zhang, L.; Jiang, J.; Ma, Y.; Wang, C.; Zhang, J.; Wu, W. Fine Particulate Matter-Induced Lung in Fl Ammation is Mediated by Pyroptosis in Mice. Ecotoxicol. Environ. Saf. 2021, 219, 112351. [Google Scholar] [CrossRef]
- Wu, Y.F.; Li, Z.Y.; Dong, L.L.; Li, W.J.; Wu, Y.P.; Wang, J.; Chen, H.P.; Liu, H.W.; Li, M.; Jin, C.L.; et al. Inactivation of Mtor Promotes Autophagy-Mediated Epithelial Injury in Particulate Matter-Induced Airway Inflammation. Autophagy 2020, 16, 435–450. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.; Zeng, H. Interactions of Particulate Matter and Pulmonary Surfactant: Implications for Human Health. Adv. Colloid Interface Sci. 2020, 284, 102244. [Google Scholar] [CrossRef]
- Homidan, A.A.; Robertson, J.F.; Petchey, A.M. Review of the Effect of Ammonia and Dust Concentrations on Broiler Performance. World’s Poult. Sci. J. 2003, 59, 340–349. [Google Scholar] [CrossRef]
- Almuhanna, E.A.; Ahmed, A.S.; Al-Yousif, Y.M. Effect of Air Contaminants on Poultry Immunological and Production Performance. Int. J. Poult. Sci. 2011, 10, 461–470. [Google Scholar] [CrossRef]
- Lai, H.T.; Nieuwland, M.G.; Aarnink, A.J.; Kemp, B.; Parmentier, H.K. Effects of 2 Size Classes of Intratracheally Administered Airborne Dust Particles On Primary and Secondary Specific Antibody Responses and Body Weight Gain of Broilers: A Pilot Study On the Effects of Naturally Occurring Dust. Poult. Sci. 2012, 91, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.N.; Wang, G.H.; Zhou, F.; Hao, J.J.; Tian, L.; Guan, L.F.; Geng, X.K.; Ding, Y.C.; Wu, H.W.; Zhang, K.Z. Pm2.5 Induces Liver Fibrosis Via Triggering Ros-Mediated Mitophagy. Ecotoxicol. Environ. Saf. 2019, 167, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.X.; Qin, Y.T.; Lou, D.S.; Li, Q.; Li, Y.Y.; Wang, Z.M.; Yang, W.W.; Wang, M.; Liu, N.; Wang, Z.; et al. Irhom2 Deficiency Relieves Tnf-Alpha Associated Hepatic Dyslipidemia in Long-Term Pm2.5-Exposed Mice. Biochem. Biophys. Res. Commun. 2017, 493, 1402–1409. [Google Scholar] [CrossRef]
- Li, K.L.; Lin, Y.C. Pm2.5 Induced Cardiac Hypertrophy Via Creb/Gsk3B/Sos1 Pathway and Metabolomics Alterations. Oncotarget 2018, 9, 30748–30760. [Google Scholar] [CrossRef][Green Version]
- Jeong, S.; Park, S.A.; Park, I.; Kim, P.; Cho, N.H.; Hyun, J.W.; Hyun, Y.M. Pm2.5 Exposure in the Respiratory System Induces Distinct Inflammatory Signaling in the Lung and the Liver of Mice. J. Immunol. Res. 2019, 2019, 3486841. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, L.; Yang, G.; Zhang, C.; Liu, X.; Sun, X.; Chen, X.; Wang, N. The Influence of PM(2.5) Exposure On Non-Alcoholic Fatty Liver Disease. Life Sci. 2021, 270, 119135. [Google Scholar] [CrossRef]
- Xu, M.X.; Ge, C.X.; Qin, Y.T.; Gu, T.T.; Lou, D.S.; Li, Q.; Hu, L.F.; Feng, J.; Huang, P.; Tan, J. Prolonged Pm2.5 Exposure Elevates Risk of Oxidative Stress-Driven Nonalcoholic Fatty Liver Disease by Triggering Increase of Dyslipidemia. Free Radic. Biol. Med. 2019, 130, 542–556. [Google Scholar] [CrossRef]
- Roussan, D.A.; Haddad, R.; Khawaldeh, G. Molecular Survey of Avian Respiratory Pathogens in Commercial Broiler Chicken Flocks with Respiratory Diseases in Jordan. Poult. Sci. 2008, 87, 444–448. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.F.; Xu, Y.H.; Shi, M.H.; Lian, Y.X. The Impact of PM2.5 On the Human Respiratory System. J. Thorac. Dis. 2016, 8, E69–E74. [Google Scholar] [CrossRef] [PubMed]
- Air Quality-Particle Size Fraction Definitions for Health-Related Sampling. In 7708; International Organization for Standardization: Virginia, USA, 1995; p. 9.
- Hayter, R.B.; Besch, E.L. Airborne-Particle Deposition in the Respiratory Tract of Chickens. Poult. Sci. 1974, 53, 1507–1511. [Google Scholar] [CrossRef] [PubMed]
- Corbanie, E.A.; Matthijs, M.G.; van Eck, J.H.; Remon, J.P.; Landman, W.J.; Vervaet, C. Deposition of Differently Sized Airborne Microspheres in the Respiratory Tract of Chickens. Avian Pathol. 2006, 35, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Glencross, D.A.; Ho, T.R.; Camina, N.; Hawrylowicz, C.M.; Pfeffer, P.E. Air Pollution and its Effects On the Immune System. Free Radic. Biol. Med. 2020, 151, 56–68. [Google Scholar] [CrossRef]
- Hermanns, M.I.; Kasper, J.; Dubruel, P.; Pohl, C.; Uboldi, C.; Vermeersch, V.; Fuchs, S.; Unger, R.E.; Kirkpatrick, C.J. An Impaired Alveolar-Capillary Barrier in Vitro: Effect of Proinflammatory Cytokines and Consequences on Nanocarrier Interaction. J. R. Soc. Interface 2010, 7 (Suppl. S1), S41–S54. [Google Scholar] [CrossRef]
- Dai, P.; Shen, D.; Shen, J.; Tang, Q.; Xi, M.; Li, Y.; Li, C. The Roles of Nrf2 and Autophagy in Modulating Inflammation Mediated by TLR4—NFκB in A549 Cell Exposed to Layer House Particulate Matter 2.5 (PM(2.5)). Chemosphere 2019, 235, 1134–1145. [Google Scholar] [CrossRef]
- Tang, Q.; Huang, K.; Liu, J.; Wu, S.; Shen, D.; Dai, P.; Li, C. Fine Particulate Matter From Pig House Induced Immune Response by Activating TLR4/MAPK/NFκB Pathway and NLRP3 Inflammasome in Alveolar Macrophages. Chemosphere 2019, 236, 124373. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, B.; Wang, Y.; Zhu, J.; Liu, J.; Yu, G.; Qin, J.; Song, W.; Ding, C. Time-and Dose-Resolved Proteome of PM2.5-Exposure-Induced Lung Injury and Repair in Rats. J. Proteome Res. 2020, 19, 3162–3175. [Google Scholar] [CrossRef]
- Arango, D.G.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Cui, J.; Zhou, Q.; Yu, M.; Liu, Y.; Teng, X.; Gu, X. 4-tert-butylphenol triggers common carp hepatocytes ferroptosis via oxidative stress, iron overload, SLC7A11/GSH/GPX4 axis, and ATF4/HSPA5/GPX4 axis. Ecotoxicol. Environ. Saf. 2022, 242, 113944. [Google Scholar] [CrossRef]
- Mika, A.; Sledzinski, T. Alterations of Specific Lipid Groups in Serum of Obese Humans: A Review. Obes. Rev. 2017, 18, 247–272. [Google Scholar] [CrossRef] [PubMed]
- Schuck, R.N.; Zha, W.; Edin, M.L.; Gruzdev, A.; Vendrov, K.C.; Miller, T.M.; Xu, Z.; Lih, F.B.; DeGraff, L.M.; Tomer, K.B.; et al. The Cytochrome P450 Epoxygenase Pathway Regulates the Hepatic Inflammatory Response in Fatty Liver Disease. PLoS ONE 2014, 9, e110162. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.H.; Fiel, M.I.; Sun, Q.; Guo, J.; Gordon, R.E.; Chen, L.C.; Friedman, S.L.; Odin, J.A.; Allina, J. Kupffer Cell Activation by Ambient Air Particulate Matter Exposure May Exacerbate Non-Alcoholic Fatty Liver Disease. J. Immunotoxicol. 2009, 6, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Perveen, A.; Li, C. Effects of Maternal T-2 Toxin Exposure on the Hepatic Glycolipid Metabolism in Young Mice. Ecotoxicol. Environ. Saf 2020, 196, 110530. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, Y.J.; Jeong, Y.; Kim, Y.; Park, S.; Doh, K.; Kim, J. The Effect of Metformin on Liver Lipid Accumulation in Mice Fed a High-Fat Diet. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 198–205. [Google Scholar] [CrossRef]
- Mooli, R.; Ramakrishnan, S.K. Emerging Role of Hepatic Ketogenesis in Fatty Liver Disease. Front. Physiol. 2022, 13, 946474. [Google Scholar] [CrossRef]
- Cotter, D.G.; Ercal, B.; Huang, X.; Leid, J.M.; D’Avignon, D.A.; Graham, M.J.; Dietzen, D.J.; Brunt, E.M.; Patti, G.J.; Crawford, P.A. Ketogenesis Prevents Diet-Induced Fatty Liver Injury and Hyperglycemia. J. Clin. Investig. 2014, 124, 5175–5190. [Google Scholar] [CrossRef]
- Shen, D.; Guo, Z.; Huang, K.; Dai, P.; Jin, X.; Li, Y.; Li, C. Inflammation-Associated Pulmonary Microbiome and Metabolome Changes in Broilers Exposed to Particulate Matter in Broiler Houses. J. Hazard. Mater. 2022, 421, 126710. [Google Scholar] [CrossRef]



| Items | Control | 4 mg·m−3 | 8 mg·m−3 |
|---|---|---|---|
| E7 | |||
| Average daily gain (g/day) | 56.71 ± 2.73 A | 50.12 ± 2.21 AB | 44.35 ± 2.16 B |
| Average daily feed intake (g/day) | 95.53 | 97.76 | 90.75 |
| Feed/Gain | 1.68 | 2 | 1.85 |
| E14 | |||
| Average daily gain (g/day) | 67.52 ± 3.98 | 73.81 ± 3.04 | 65.05 ± 5.66 |
| Average daily feed intake (g/day) | 124.29 | 122.95 | 109.91 |
| Feed/Gain | 1.84 | 1.73 | 1.85 |
| R7 | |||
| Average daily gain (g/day) | 81.43 ± 5.34 | 92.14 ± 3.83 | 83.33 ± 4.56 |
| Average daily feed intake (g/day) | 264.29 | 279.52 | 275.37 |
| Feed/Gain | 2.78 | 2.60 | 2.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, D.; Guo, Q.; Huang, K.; Mao, W.; Wang, K.; Zeng, W.; Li, Y.; Guo, Z.; Nagaoka, K.; Li, C. Exposure to Particulate Matter in the Broiler House Causes Dyslipidemia and Exacerbates It by Damaging Lung Tissue in Broilers. Metabolites 2023, 13, 363. https://doi.org/10.3390/metabo13030363
Shen D, Guo Q, Huang K, Mao W, Wang K, Zeng W, Li Y, Guo Z, Nagaoka K, Li C. Exposure to Particulate Matter in the Broiler House Causes Dyslipidemia and Exacerbates It by Damaging Lung Tissue in Broilers. Metabolites. 2023; 13(3):363. https://doi.org/10.3390/metabo13030363
Chicago/Turabian StyleShen, Dan, Qi Guo, Kai Huang, Weijia Mao, Kai Wang, Wenjie Zeng, Yansen Li, Zhendong Guo, Kentaro Nagaoka, and Chunmei Li. 2023. "Exposure to Particulate Matter in the Broiler House Causes Dyslipidemia and Exacerbates It by Damaging Lung Tissue in Broilers" Metabolites 13, no. 3: 363. https://doi.org/10.3390/metabo13030363
APA StyleShen, D., Guo, Q., Huang, K., Mao, W., Wang, K., Zeng, W., Li, Y., Guo, Z., Nagaoka, K., & Li, C. (2023). Exposure to Particulate Matter in the Broiler House Causes Dyslipidemia and Exacerbates It by Damaging Lung Tissue in Broilers. Metabolites, 13(3), 363. https://doi.org/10.3390/metabo13030363