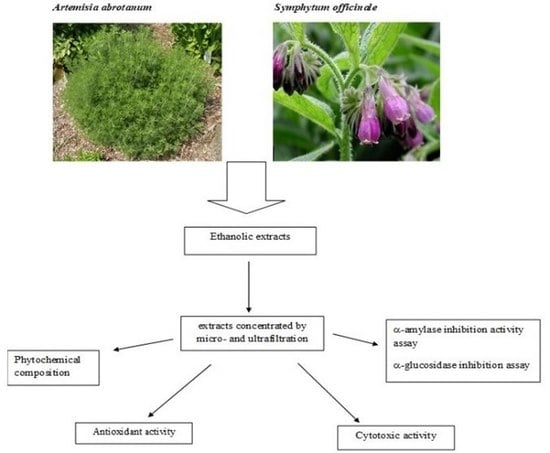
Artemisia abrotanum and Symphytum officinale Polyphenolic Compounds-Rich Extracts with Potential Application in Diabetes Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Material
2.3. Obtaining and Concentrating Extracts
2.4. Phytochemical Characterization
2.4.1. Polyphenolic Compound Content Determination
2.4.2. Flavonoid Content Determination
2.4.3. HPLC Analysis
2.5. Antioxidant Assays
2.5.1. DPPH Radical Scavenging Activity
2.5.2. Reducing Power Activity (Iron (III) to Iron (II) Reduction)
2.6. Enzymes Inhibition Activity
2.6.1. α-Amylase Inhibition
2.6.2. α-Glucosidase Inhibition
2.7. Testing Extracts’ Cytotoxic Activity In Vitro
- Cell Viability
- Neutral Red Test
- Light Microscopy
2.8. Statistical Analysis
3. Results and Discussions
3.1. Polyphenols and Flavonoid Content Determination
3.2. HPLC Analysis
3.3. Antioxidant Activity Determination
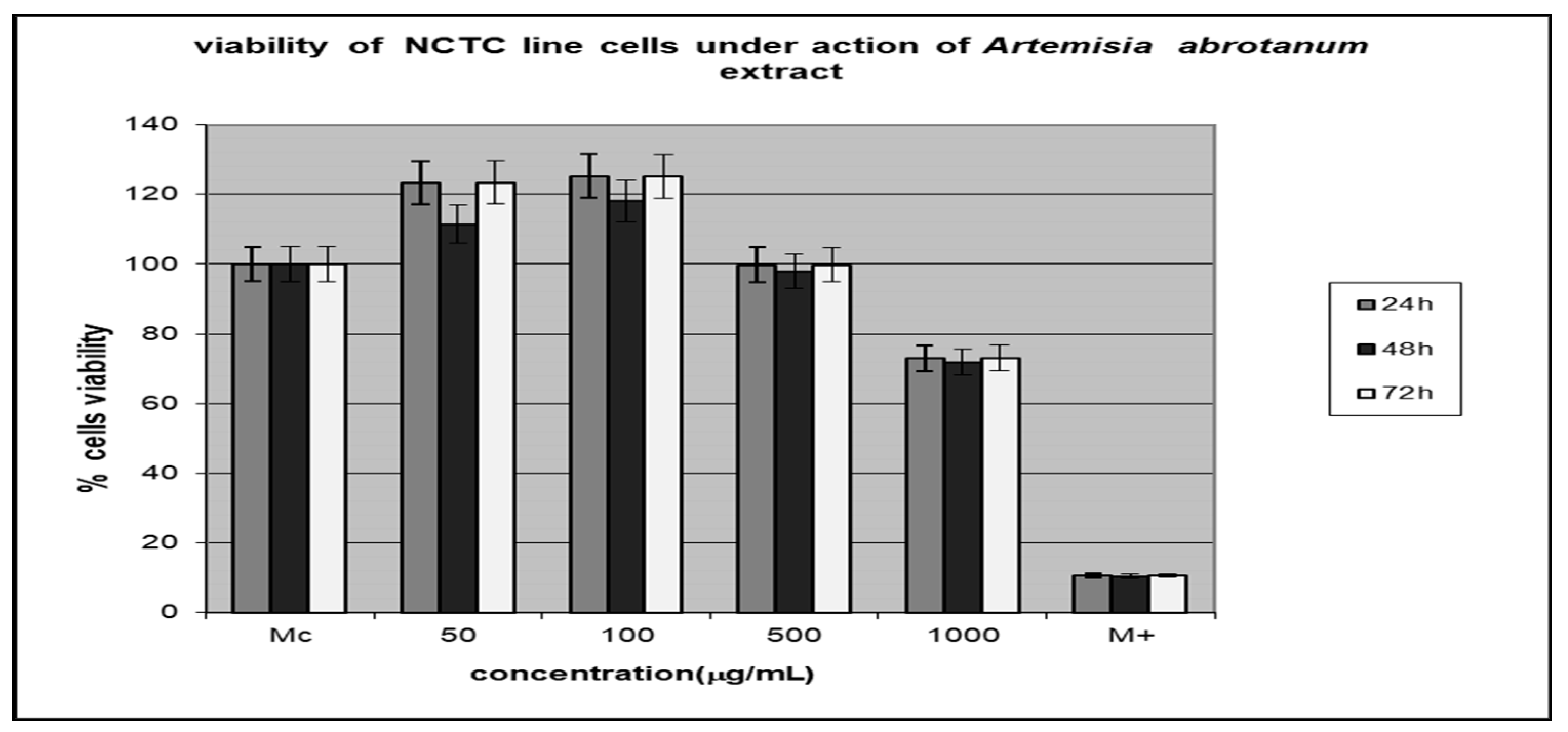
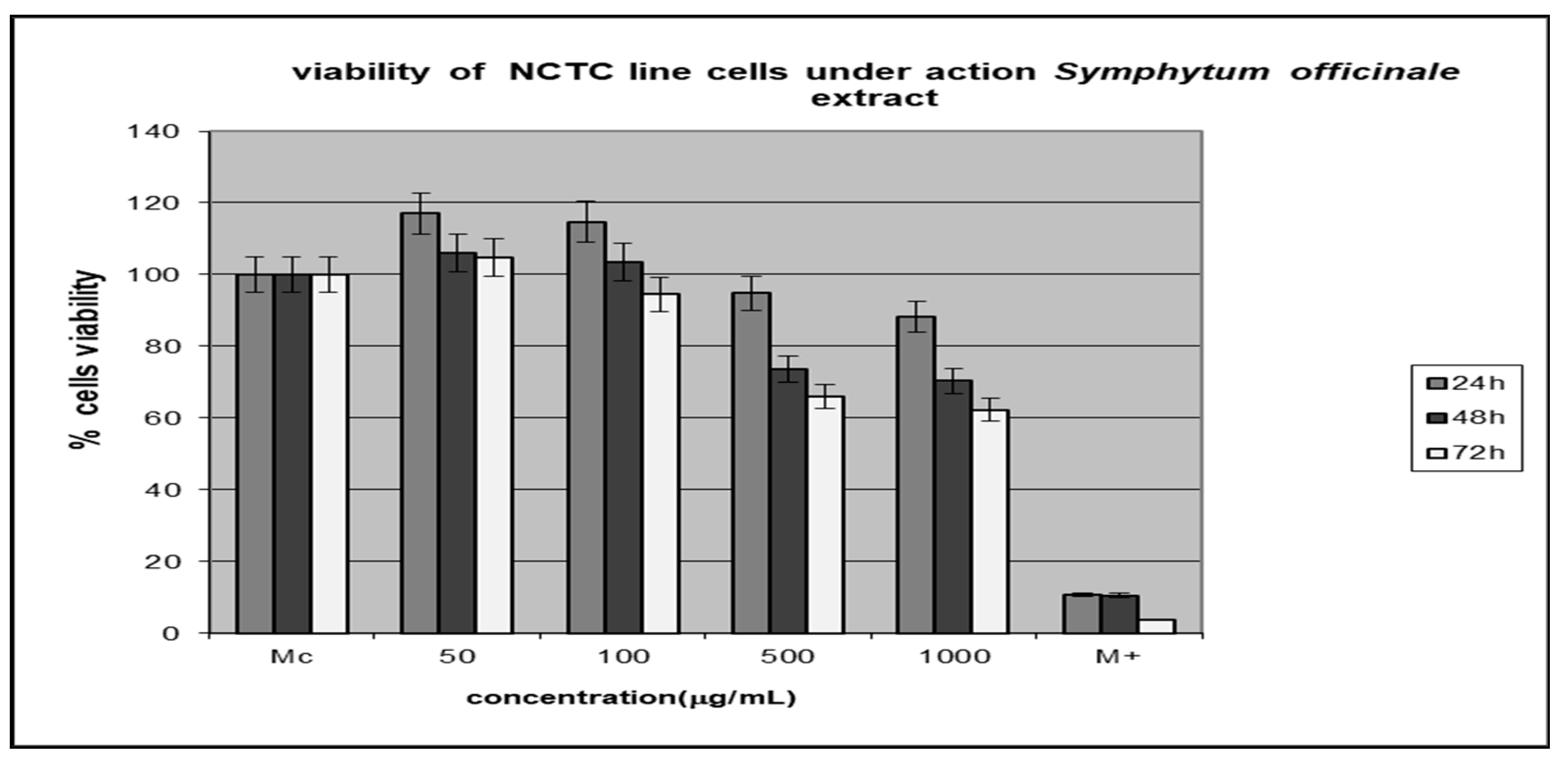
3.4. Testing the Cytotoxic Activity of the Extracts
Morphological Characterization of Cell Lines
3.5. α-Amylase and α-Glucosidase Inhibition Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, D.K.; Prasad, S.K.; Kumar, R.; Hemalatha, S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac. J. Trop. Biomed. 2012, 2, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Irudayaraj, S.S.; Stalin, A.; Sunil, C.; Duraipandiyan, V.; Al-Dhabi, N.A.; Ignacimuthu, S. Antioxidant, antilipidemic and antidiabetic effects of ficusin with their effects on GLUT4 translocation and PPARγ expression in type 2 diabetic rats. Chem. Biol. Interact. 2016, 256, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, M.R.; Jong-Anurakkun, N.; Hong, G.; Kawabata, J. α-Glucosidase and α-amylase inhibitory activities of Nepalese medicinal herb Pakhanbhed (Bergenia ciliata, Haw.). Food Chem. 2008, 106, 247–252. [Google Scholar] [CrossRef]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.; Soliternik, J.; Mazzola, N. Nutritional supplements for the prevention of diabetes mellitus and its complications. J. Nutr. Intermed. Metab. 2018, 14, 16–21. [Google Scholar] [CrossRef]
- Ghadge, A.A.; Kuvalekar, A.A. Controversy of oral hypoglycemic agents in type 2 diabetes mellitus: Novel move towards combination therapies. Diabetes Metab. Syndr. 2017, 11, S5–S13. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Matthews, D.R. Management of hyperglycemia in type 2 diabetes, 2015: A patient-centered approach: Update to a position statement of the american diabetes association and the european association for the study of diabetes. Diabetes Care 2015, 38, 140–149. [Google Scholar] [CrossRef]
- Nicholson, G.; Hall, G.M. Diabetes mellitus: New drugs for a new epidemic. Br. J. Anaesth. 2011, 107, 65–73. [Google Scholar] [CrossRef]
- Saadi, T.; Waterman, M.; Yassin, H.; Baruch, Y. Metformin-induced mixed hepatocellu-lar and cholestatic hepatic injury: Case report and literature review. Int. J. Gen. Med. 2013, 6, 703–706. [Google Scholar] [CrossRef]
- Sarkar, A.; Tiwari, A.; Bhasin, P.S.; Mitra, M. Pharmacological and pharmaceutical pro-file of gliclazide: A review. J. Appl. Pharm. Sci. 2011, 1, 11–19. [Google Scholar]
- Kim, K.-T.; Rioux, L.-E.; Turgeon, S.L. Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry 2014, 98, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.M.; Sales, P.M.; Simeoni, L.A.; Silva, E.C.; Silveira, D.; de Oliveira Magalhães, P. Inhibitory activity of α-amylase and α-glucosidase by plant extracts from the Brazilian cerrado. Planta Med. 2012, 78, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Rhabasa-Lhoret, J.L.; Chiasson, R. Alpha-glucosidase inhibitors. In International Textbook of Diabetes Mellitus, 3rd ed.; De-fronzo, R.A., Ferrannini, E., Keen, H., Zimmet, P., Eds.; John Wiley: New York, NY, USA, 2004; Volume 1. [Google Scholar]
- Gao, H.; Huang, Y.N.; Gao, B.; Xu, P.Y.; Inagaki, C.; Kawabata, J. α-Glucosidase inhibitory effect by the flower buds of Tussilago farfara L. Food Chem. 2008, 106, 1195–1201. [Google Scholar] [CrossRef]
- Taleghani, A.; Emami, S.A.; Tayarani-Najaran, Z. Artemisia: A promising plant for the treatment of cancer. Bioorganic Med. Chem. 2020, 28, 115180. [Google Scholar] [CrossRef]
- Suresh, J.; Vasav, R.A.; Dhanya, R.; Ihsanullah, M.; Nayeemmullah, M.K. Antimicrobial Activity of Artemisia abrotanum and Artemisia pallens. Int. J. Pharm. Phytochem. Res. 2010, 3, 18–21. [Google Scholar]
- Trifan, A.; Skalicka-Woźniak, K.; Granica, S.; Czerwińska, M.E.; Kruk, A.; Marcourt, L.; Wolfender, J.-L.; Wolfram, E.; Esslinger, N.; Grubelnik, A.; et al. Symphytum officinale L.: Liquid-liquid chromatography isolation of caffeic acid oligomers and evaluation of their influence on pro-inflammatory cytokine release in LPS-stimulated neutrophils. J. Ethnopharmacol. 2020, 262, 113169. [Google Scholar] [CrossRef]
- Frost, R.; O’Meara, S.; MacPherson, H. The external use of comfrey: A practitioner survey. Complement. Ther. Clin. Pract. 2014, 20, 347–355. [Google Scholar] [CrossRef]
- Grube, B.; Grunwald, J.; Krug, L.; Staiger, C. Efficacy of a comfrey root (Symphytioffic. radix) extract ointment in the treatment of patients with painful osteoarthritis of the knee: Results of a double-blind, randomised, bicenter, placebo-controlled trial. Phytomedicine 2007, 14, 2–10. [Google Scholar] [CrossRef]
- EMA/HMPC/893108/2011; Public Statement on the Use of Herbal Medicinal Products Containing Toxic, Unsaturated Pyrrolizidine Alkaloids (PAs). HMPC: Valetta, Malta, 2014.
- Oberlies, N.H.; Kim, N.C.; Brine, D.R.; Collins, B.J.; Handy, R.W.; Sparacino, C.M.; Wani, M.C.; Wall, M.E. Analysis of herbal teas made from the leaves of comfrey (Symphytum officinale): Reduction of N-oxides results in order of magnitude increases in the measurable concentration of pyrrolizidine alkaloids. Public Health Nutr. 2004, 7, 919–924. [Google Scholar] [CrossRef]
- Gerke, I.B.B.; Hamerski, F.; Scheer, A.P.; Silvab, V.R. Clarification of crude extract of yerba mate (Ilex paraguariensis) by membrane processes: Analysis of fouling and loss of bioactive compounds. Food Bioprod. Process. 2017, 102, 204–212. [Google Scholar] [CrossRef]
- Habert, A.C.; Borges, C.P.; Nobrega, R. Processos de Separacão por Membranas, 1st ed.; E-papers: Rio de Janeiro, Brazil, 2006. [Google Scholar]
- Díaz-Reinoso, B. Chapter 14—Concentration and purification of seaweed extracts using membrane technologies. In Sustainable Seaweed Technologies, Cultivation, Biorefinery, and Applications, 1st ed.; Advances in Green and Sustainable Chemistry series; Maria Dolores Torres, M.D., Kraan, S., Herminia Dominguez, H., Eds.; Elsevier: Oxford, UK, 2020; pp. 371–390. [Google Scholar]
- Dushkova, M.; Mihalev, K.; Dinchev, A.; Vasilev, K.; Georgiev, D.; Terziyska, M. Concentration of polyphenolic antioxidants in apple juice and extract using ultrafiltra-tion. Membranes 2022, 12, 1032. [Google Scholar] [CrossRef] [PubMed]
- Paun, G.; Neagu, E.; Moroeanu, V.; Albu, C.; Savin, S.; Radu, G.L. Chemical and bioactivity evaluation of eryngium planum and cnicus benedictus polyphenolic-rich extracts. BioMed Res. Int. 2019, 2019, 3692605. [Google Scholar] [CrossRef] [PubMed]
- Uyttebroek, M.; Vandezande, P.; Van Dael, M.; Vloemans, S.; Noten, B.; Bongers, B.; Lemmens, B. Concentration of phenolic compounds from apple pomace extracts by nanofiltration at lab and pilot scale with a technoeconomic assessment. J. Food. Process. Eng. 2018, 41, e12629. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Lin, J.-Y.; Tang, C.-Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007, 101, 140–147. [Google Scholar] [CrossRef]
- Alecu, A.; Albu, C.; Litescu, S.C.; Eremia, S.A.V.; Radu, G.L. Phenolic Anthocyanin Profile of Valea Calugareasca Red Wines by HPLC-PDA-MS, MALDI-TOF. Analysis. Food Analyt. Meth. 2016, 9, 300–310. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanism of antioxidant activity using the DPPH free radical method. Leb. Wiss Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Berker, K.; Guclu, K.; Tor, I.; Apak, R. Comparative evaluation of Fe (III) reducing power-based antioxidant capacity assays in the presence of phenanthroline, batho-phenanthroline, tripyridyltriazine (FRAP) and ferricyanide reagents. Talanta 2007, 72, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Ranilla, L.G.; Kwon, Y.I.; Apostolidis, E.; Shetty, K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour. Technol. 2010, 101, 4676–4689. [Google Scholar] [CrossRef]
- Queiroz, D.P.; Ferreira, A.G.; Lima, A.S.; Lima, E.S.; Lima, M.D. Isolation and identification of α-glucosidase, α-amylase and lipase inhibitors from hortia longifolia. Int. J. Pharm. Pharm. Sci. 2013, 5, 336–339. [Google Scholar]
- Borenfreund, E.; Puerner, J.A. A simple quantitative procedure using monolayer cultures for cytotoxicity assays. J. Tiss Culture Meth. 1984, 9, 7–9. [Google Scholar] [CrossRef]
- Liu, R. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. LC-MS/MS Characterization of Phenolic Metabolites and Their Antioxidant Activities from Australian Native Plants. Metabolites 2022, 12, 1016. [Google Scholar] [CrossRef] [PubMed]
- Cheplick, S.; Kwon, Y.; Bhowmik, P.; Shetty, K. Phenolic-linked variation in strawberry cultivars for potential dietary management of hyperglycemia and related complications of hypertension. Bioresource Technol. 2010, 101, 404–413. [Google Scholar] [CrossRef]
- Oboh, G.; Rocha, J.B.T. Antioxidant in Foods: A New Challenge for Food Processors: Leading Edge Antioxidants Research; Nova Science Publishers Inc.: New York, NY, USA, 2007. [Google Scholar]
- Minda, D.; Ghiulai, R.; Banciu, C.D.; Pavel, I.Z.; Danciu, C.; Racoviceanu, R.; Soica, C.; Budu, O.D.; Muntean, D.; Diaconeasa, Z.; et al. Phytochemical Pro-file, Antioxidant and Wound Healing Potential of Three Artemisia Species: In Vitro and In Ovo Evaluation. Appl. Sci. 2022, 12, 1359. [Google Scholar] [CrossRef]
- Baiceanu, E.; Vlase, L.; Baiceanu, A.; Nanes, M.; Rusu, D.; Crisan, G. New polyphenols identified in Artemisiae abrotani herba extract. Molecules 2015, 20, 11063–11075. [Google Scholar] [CrossRef] [PubMed]
- Sowa, I.; Paduch, R.; Strzemski, M.; Zielińska, S.; Rydzik-Strzemska, E.; Sawicki, J.; Kocjan, R.; Polkowski, J.; Matkowski, A.; Latalski, M.; et al. Proliferative and antioxidant activity of Symphytum officinale root extract. Nat. Prod. Res. 2018, 32, 605–609. [Google Scholar] [CrossRef]
- Trifan, A.; Zengin, G.; Sinan, K.I.; Wolfram, E.; Skalicka-Woźniak, K.; Luca, S.V. LC-HRMS/MS phytochemical profiling of symphytum officinale L. and Anchusa ochroleuca M. bieb. (boraginaceae): Unveiling their multi-biological potential via an integrated approach. J. Pharm. Biomed. Anal. 2021, 204, 114283. [Google Scholar] [CrossRef]
- Nastić, N.; Borrás-Linares, I.; Lozano-Sánchez, J.; Švarc-Gajić, J.; Segura-Carretero, A. Comparative Assessment of Phytochemical Profiles of Comfrey (Symphytum officinale L.) Root Extracts Obtained by Different Extraction Techniques. Molecules 2020, 25, 837. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Olszowy, M. What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol. Biochem. 2019, 144, 135–143. [Google Scholar] [CrossRef]
- Siddharthan, S.; Yi-Zhong, C.; Harold, C.; Mei, S. Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food Chem. 2007, 102, 938–953. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure–antioxidant activity relationships of flavonoids and phenolic acids. Free. Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef] [PubMed]
- Zengina, G.; Sarikurkcub, A.; Aktumseka, R.; Ceylana, O. Ceylan, A comprehensive study on phytochemical characterization of Haplophyl lummyrti folium Boiss. endemic to Turkey C, and its inhibitory potential against key enzymes involved in Alzheimer, skin diseases and type II diabetes. Ind. Crop Prod. 2014, 53, 244–251. [Google Scholar] [CrossRef]
- Matsui, T.; Tanaka, T.; Tamura, S.; Toshima, A.; Miyata, Y.; Tamaya, K.; Miyata, Y.; Tanaka, K.; Matsumoto, K. Alpha-glucosidase inhibitory profile of catechins and theafla-vins. J. Agric. Food Chem. 2007, 55, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Xiang, J.F.; Tang, Y.L. Screening α-glucosidase inhibitors from mulberry extracts via DOSY and relaxation-edited NMR. Talanta 2012, 97, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Rammohan, S.; Asmaw, M.Z.B.; Amirin, S. In vitro alpha-glucosidase and alpha-amylase enzyme inhibitory effects of Andrographis paniculata extract and andrographolide. Acta Biochim. Pol. 2008, 55, 391–398. [Google Scholar]
- Zuñiga, L.Y.; Aceves-de Aceves-de la Mora, M.C.; González-Ortiz, M.; Ramos-Núñez, J.L.; Martínez-Abundis, E. Effect of chlorogenic acid administration on glycemic control, insulin secretion, and insulin sensitivity in patients with impaired glucose tolerance. J. Med. Food 2018, 21, 469–473. [Google Scholar] [CrossRef]
- Jin, S.; Chang, C.; Zhang, L.; Liu, Y.; Huang, X.; Chen, Z. Chlorogenic acid improves late diabetes through adiponectin receptor signaling pathways in db/db mice. PLoS ONE 2015, 10, e120842. [Google Scholar] [CrossRef]
- Hunyadi, A.; Martins, A.; Hsieh, T.J.; Seres, A.; Zupkó, I. Chlorogenic acid and rutin play a major role in the in vivo anti-diabetic activity of Morus alba leaf extract on type II diabetic rats. PLoS ONE 2012, 7, e50619. [Google Scholar] [CrossRef]
- Ngo, Y.L.; Lau, C.H.; Chua, L.S. Review on rosmarinic acid extraction, fractiona-tion and its anti-diabetic potential. Food Chem. Toxicol. 2018, 121, 687–700. [Google Scholar] [CrossRef]
- Oboh, G.; Akinyemi, A.J.; Ademiluyi, A.O.; Bello, F.O. Inhibition of α-amylase and α-glucosidase activities by ethanolic extract of Amaranthus cruentus leaf as affected by blanching. Afr. J. Pharm. Pharmacol. 2013, 7, 1026–1032. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Oboh, G.; Aragbaiye, F.P.; Oyeleye, S.I.; Ogunsuyi, O.B. Antioxidant properties and in vitro a-amylase and a-glucosidase inhibitory properties of phenolics constituents from different varieties of Cor-chorus spp. J. Taibah Univ. Med. Sci. 2015, 10, 278–287. [Google Scholar]
- Song, Y.; Manson, J.E.; Buring, J.E.; Sesso, H.D.; Liu, S. Association of dietary flavo-noids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflam-mation in women: A prospective study and cross sectional analysis. J. Am. Coll. Nutr. 2005, 24, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Molnar, V.; Garai, J. Plant-derived anti-inflammatory compounds affect MIF tautomer-ase activity. Intern Immunopharmacol. 2005, 5, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Shodehinde, S.A.; Oboh, G. Antioxidant properties of aqueous extracts of unripe (Musa paradisiaca) on sodium nitroprusside induced lipid peroxidation in rat pancreas in vitro. Asian Pac. J. Trop. Biomed. 2013, 3, 449–457. [Google Scholar] [CrossRef]
- Ramu, R.; Shirahatti, P.; Zameer, F.; Ranganatha, L.V.; Nagendra, M.; Prasad, N. Inhibitory effect of banana (Musa sp. var. Nanjangud rasa bale) flower extract and its constitu-ents Umbelliferone and Lupeol on α-glucosidase, aldose reductase and glycation at multi-ple stages. S. Afr. J. Bot. 2014, 95, 54–63. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Tiller, C.; Shen, J.K.; Wang, C.; Girouard, G.S.; Dennis, D.; Barrow, C.J.; Miao, M.S.; Ewart, H.S. Antidiabetic properties of polysaccharide and polyphenolic en-riched fractions from the brown seaweed Ascophyllum nodosum. Can. J. Physiol. Pharmacol. 2007, 85, 1116–1123. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant activity of food constituents: An overview. Arch. Toxikol. 2012, 86, 345–391. [Google Scholar] [CrossRef]
- Sales, P.M.; Souza, P.M.; Simeoni, L.A.; Silveira, D. α-Amylase inhibitors: A review of raw material and isolated compounds from plant source. J. Pharm. Pharm. Sci. 2012, 5, 141–183. [Google Scholar] [CrossRef]



| Sample | Polyphenols (GAEμg/mL) | Flavonoids (RE μg/mL) | |
|---|---|---|---|
| A. abrotanum | MF | 723.32 ± 25.32 | 403.51 ± 12.59 |
| extracts | concentrate | 977.75 ± 31.67 | 552.85 ± 15.36 |
| S. officinale | MF | 667.58 ± 17.64 | 84.53 ± 3.2 |
| extracts | concentrate | 896.95 ± 27.21 | 103.21 ± 5.16 |
| Compound | A. abrotanum Polyphenolic Compounds-Rich Extract, µg/mL | S. officinale Polyphenolic Compounds-Rich Extract, μg/mL | ||
|---|---|---|---|---|
| MF | Conc. | MF | Conc. | |
| Rutin | 9.39 ± 0.23 | 10.57 ± 0.6 | - | 1.06 ± 0.06 |
| Luteolin | 1.90 ± 0.09 | 4.35 ± 0.08 | - | 1.69 ± 0.05 |
| Quercetin | 3.17 ± 0.02 | 5.36 ± 0.27 | 1.87 ± 0.04 | 3.42 ± 0.07 |
| Quercetin 3-β-D-glucoside | 0.29 ± 0.01 | 1.07 ± 0.06 | - | - |
| Kaempferol | - | 1.33 ± 0.08 | 1.96 ± 0.07 | 6.79 ± 0.11 |
| Gallic acid | - | 2.83 ± 0.06 | - | - |
| Caffeic acid | 0.58 ± 0.02 | 1.43 ± 0.05 | 7.73 ± 0.23 | 9.42 ± 0.24 |
| Apigenin | 1.95 ± 0.09 | 3.84 ±0.16 | - | 0.71 ± 0.02 |
| Umbelliferone | 9.59 ± 0.03 | 17.03 ± 0.92 | 3.68 ± 0.12 | 4.28 ± 0.13 |
| Chlorogenic acid | 85.49 ± 2.12 | 103.47 ± 7.21 | 1.45 ± 0.08 | 2.30 ± 0.05 |
| Ellagic acid | - | - | 37.41 ± 1.23 | 43.79 ± 1.89 |
| Rosmarinic acid | - | - | 120.83 ± 1.25 | 136.14 ± 5.16 |
| Samples | Reducing Power Activity EC50 (mg/mL) | DPPH Radical Scavenging Activity IC50 (µg/mL) | |
|---|---|---|---|
| A. abrotanum | MF | 521.21 ± 4.8 * | 15.33 ± 1.23 * |
| extracts | concentrate | 92.14 ± 3.2 * | 9.31 ± 0.81 * |
| 1570.32 ± 6.5 * | 17.02 ± 0.89 * | ||
| S. officinale extracts | MF | 1091.13 ± 5.7 * | 13.06 ± 0.61 * |
| Ascorbic acid | concentrate | 1110.15 ± 4.8 | 11.76 ± 0.69 |
| Samples | α-Amylase Inhibition IC50 (mg/mL) | α-Glucosidase Inhibition IC50 (mg/mL) | |
|---|---|---|---|
| A. abrotanum extracts | MF | 2110.12 ± 9.2 * | 1450.32 ± 2.7 * |
| concentrate | 1881.21 ± 1.8 * | 1171.16 ± 6.5 * | |
| S. officinale extracts | MF | 28,270.35 ± 16.7 * | 413.02 ± 4.2 * |
| concentrate | 24,812.02 ± 12.9 * | 291.56 ± 2.1 * | |
| acarbose | 1110.25 ± 8.82 | 372.35 ± 3.2 | |
| quercetin | 351.25 ± 3.1 | 72.52 ± 1.5 | |
| chlorogenic acid | 192.31 ± 2.3 | 51.21 ± 1.8 | |
| rosmarinic acid | 95.63 ± 2.5 | 20.31 ± 0.9 | |
| kaempferol | 401.32 ± 3.9 | 62.36 ± 2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neagu, E.; Paun, G.; Albu, C.; Eremia, S.A.-M.V.; Radu, G.L. Artemisia abrotanum and Symphytum officinale Polyphenolic Compounds-Rich Extracts with Potential Application in Diabetes Management. Metabolites 2023, 13, 354. https://doi.org/10.3390/metabo13030354
Neagu E, Paun G, Albu C, Eremia SA-MV, Radu GL. Artemisia abrotanum and Symphytum officinale Polyphenolic Compounds-Rich Extracts with Potential Application in Diabetes Management. Metabolites. 2023; 13(3):354. https://doi.org/10.3390/metabo13030354
Chicago/Turabian StyleNeagu, Elena, Gabriela Paun, Camelia Albu, Sandra Ana-Maria Victoria Eremia, and Gabriel Lucian Radu. 2023. "Artemisia abrotanum and Symphytum officinale Polyphenolic Compounds-Rich Extracts with Potential Application in Diabetes Management" Metabolites 13, no. 3: 354. https://doi.org/10.3390/metabo13030354
APA StyleNeagu, E., Paun, G., Albu, C., Eremia, S. A.-M. V., & Radu, G. L. (2023). Artemisia abrotanum and Symphytum officinale Polyphenolic Compounds-Rich Extracts with Potential Application in Diabetes Management. Metabolites, 13(3), 354. https://doi.org/10.3390/metabo13030354