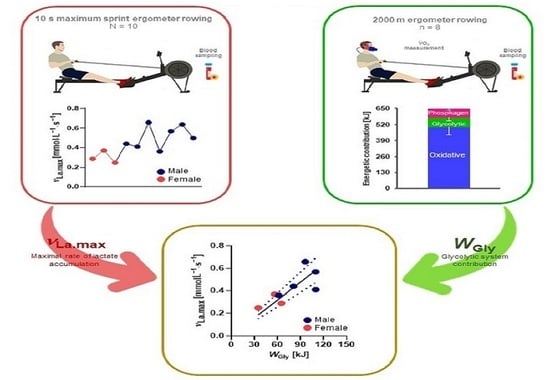
Diagnostics of νLa.max and Glycolytic Energy Contribution Indicate Individual Characteristics of Anaerobic Glycolytic Energy Metabolism Contributing to Rowing Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Lactate Diagnostics
2.3. Cardio Pulmonary Exercise Testing (CPET)
2.4. Rowing Ergometers
2.5. Heart Rate
2.6. Laboratory Conditions
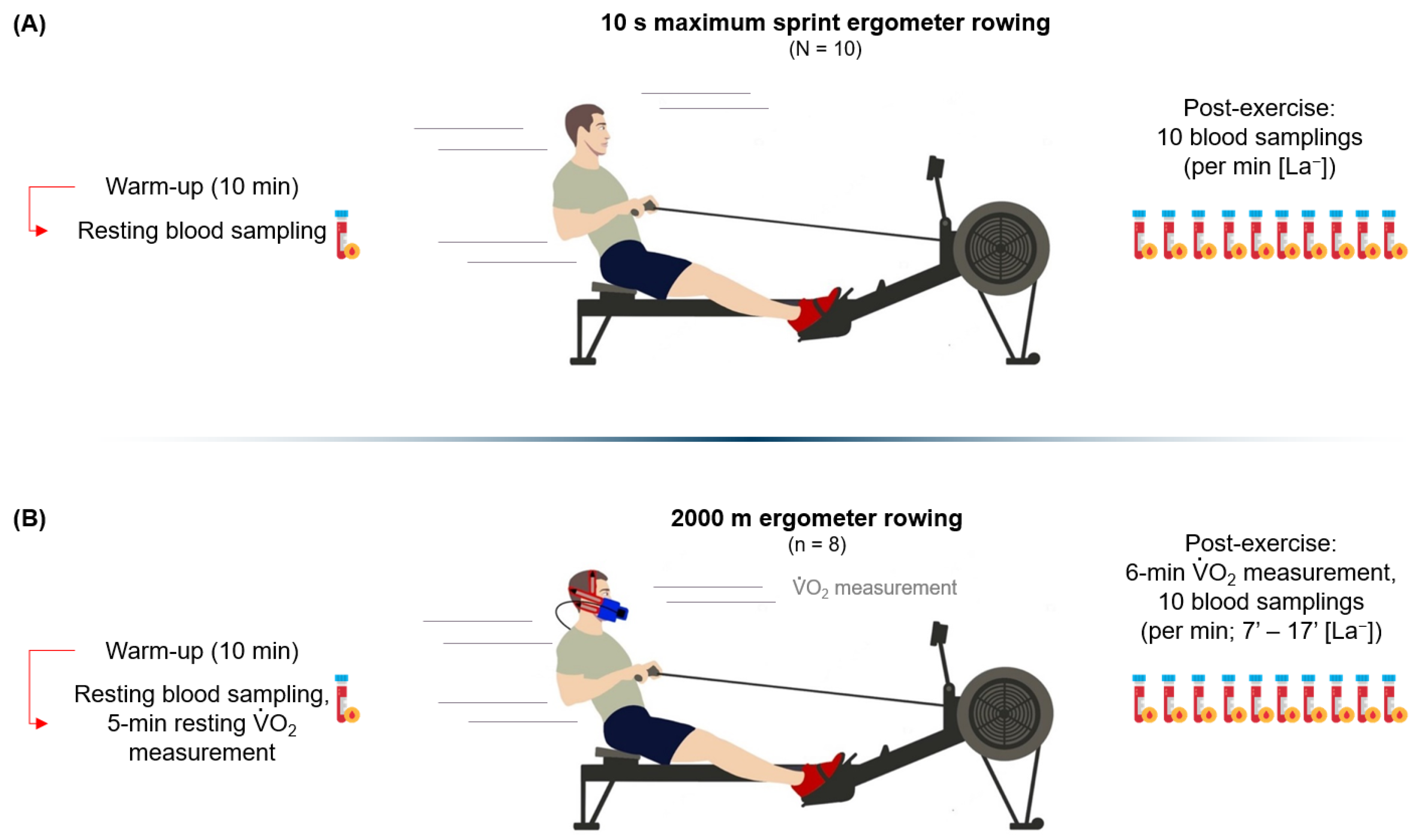
2.7. The 10 s RST
2.8. The 2000 m RTT
- (1)
- 2000 m RTT performance (s).
- (2)
- Average power over the first and last 300 m of 2000 m RTT (P300first and P300last in W).
- (3)
- Mechanical power output difference between 300 m first and last (∆300 first-last).
- (4)
- O2 (litres O2·min−1) before, during, and after 2000 m RTT.
- (5)
- Resting lactate and peak blood lactate concentration after 2000 m RTT.
2.9. The Incremental Step Test
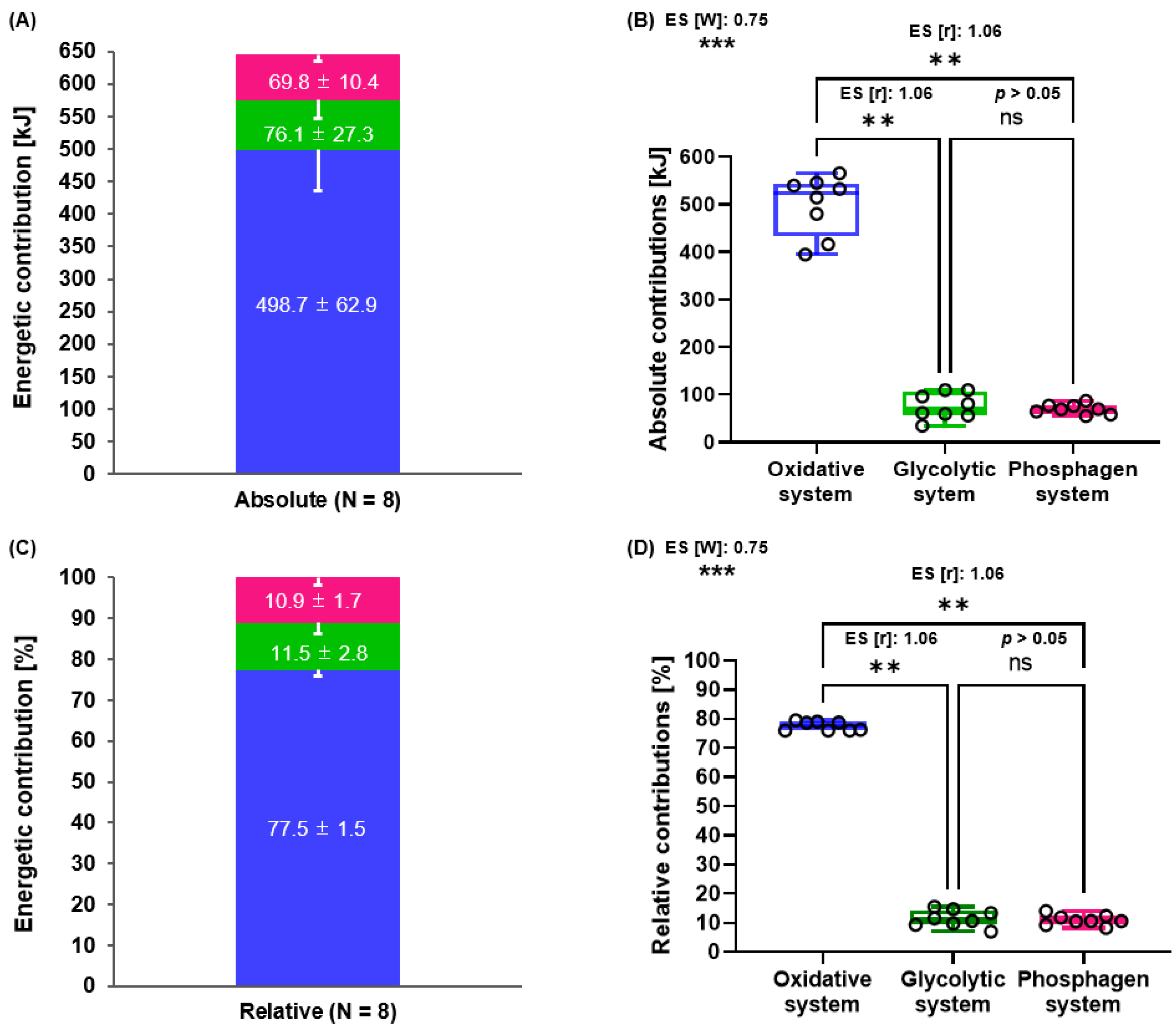
2.10. Calculations of Energetic Contributions during 2000 m RTT
2.11. Statistical Analyses
3. Results
3.1. Calculation of the Three Energy System Contributions (PCr-La−-O2 Method) during 2000 m RTT
3.2. νLa.max and tPCr of 10 s RST, P300first, and P300last of 2000 m RTT
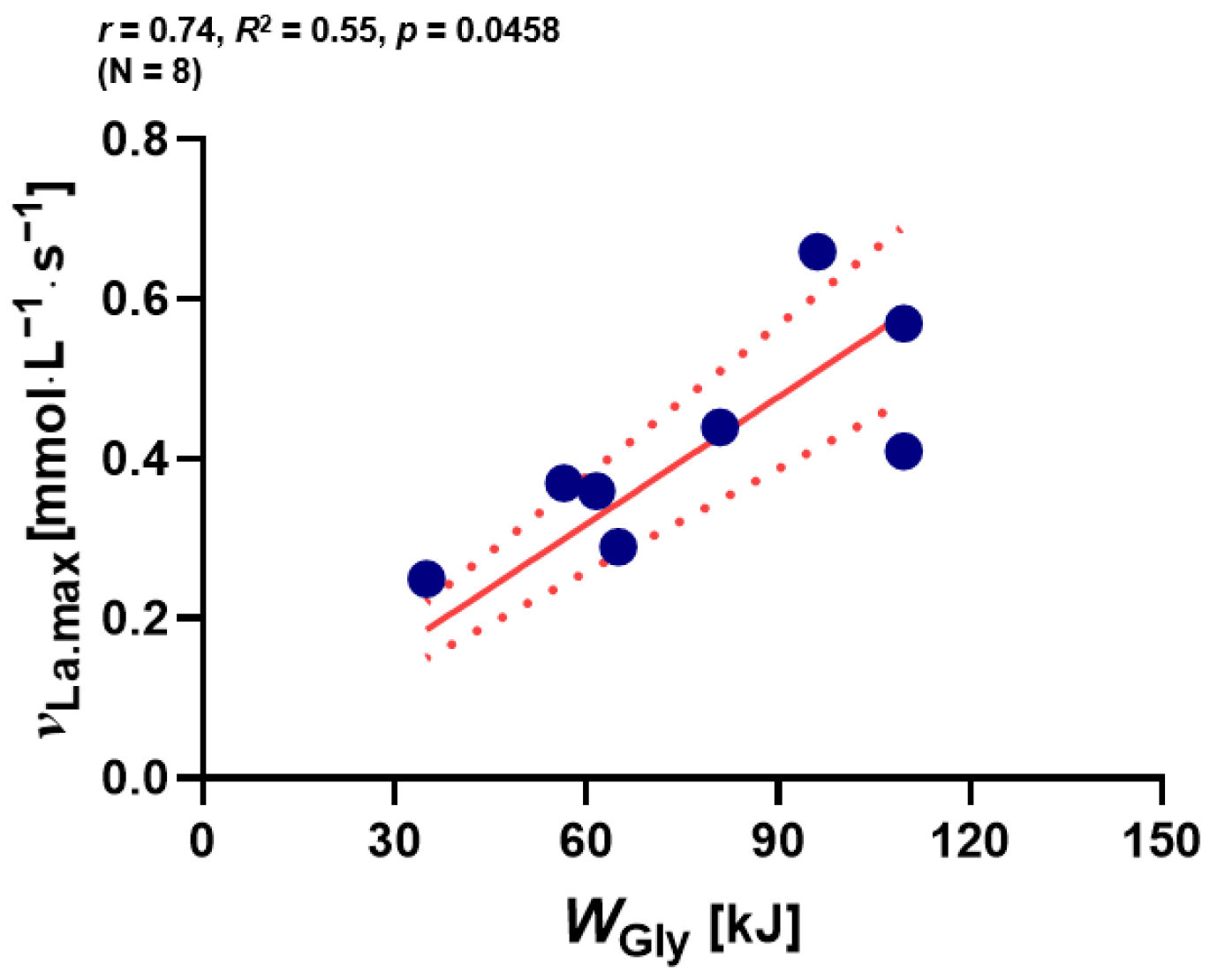
3.3. The Relationship between νLa.max and WGly over 2000 m RTT
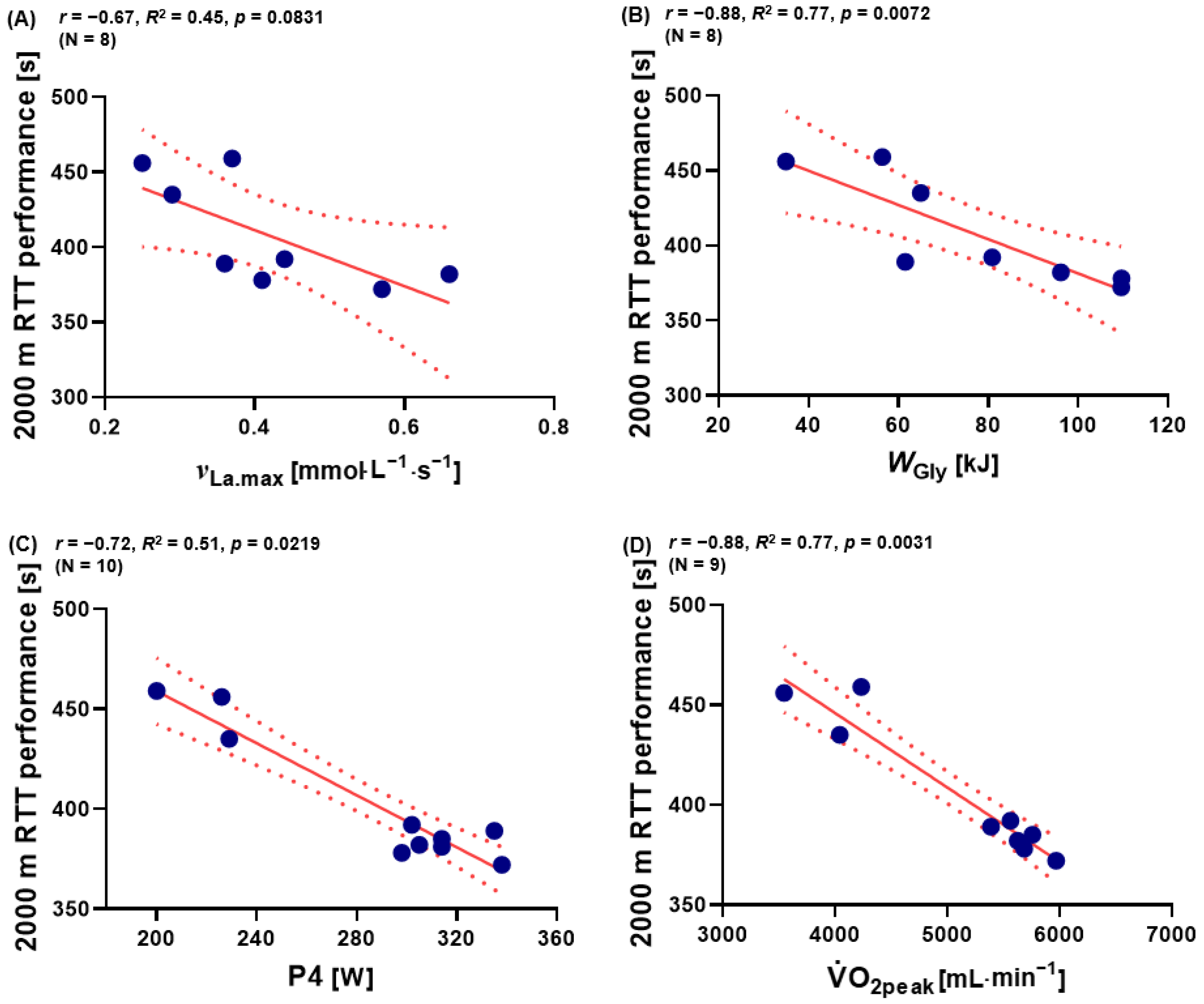
3.4. The Relationship between νLa.max, WGly, P4, Absolute O2peak, and 2000 m RTT Performance
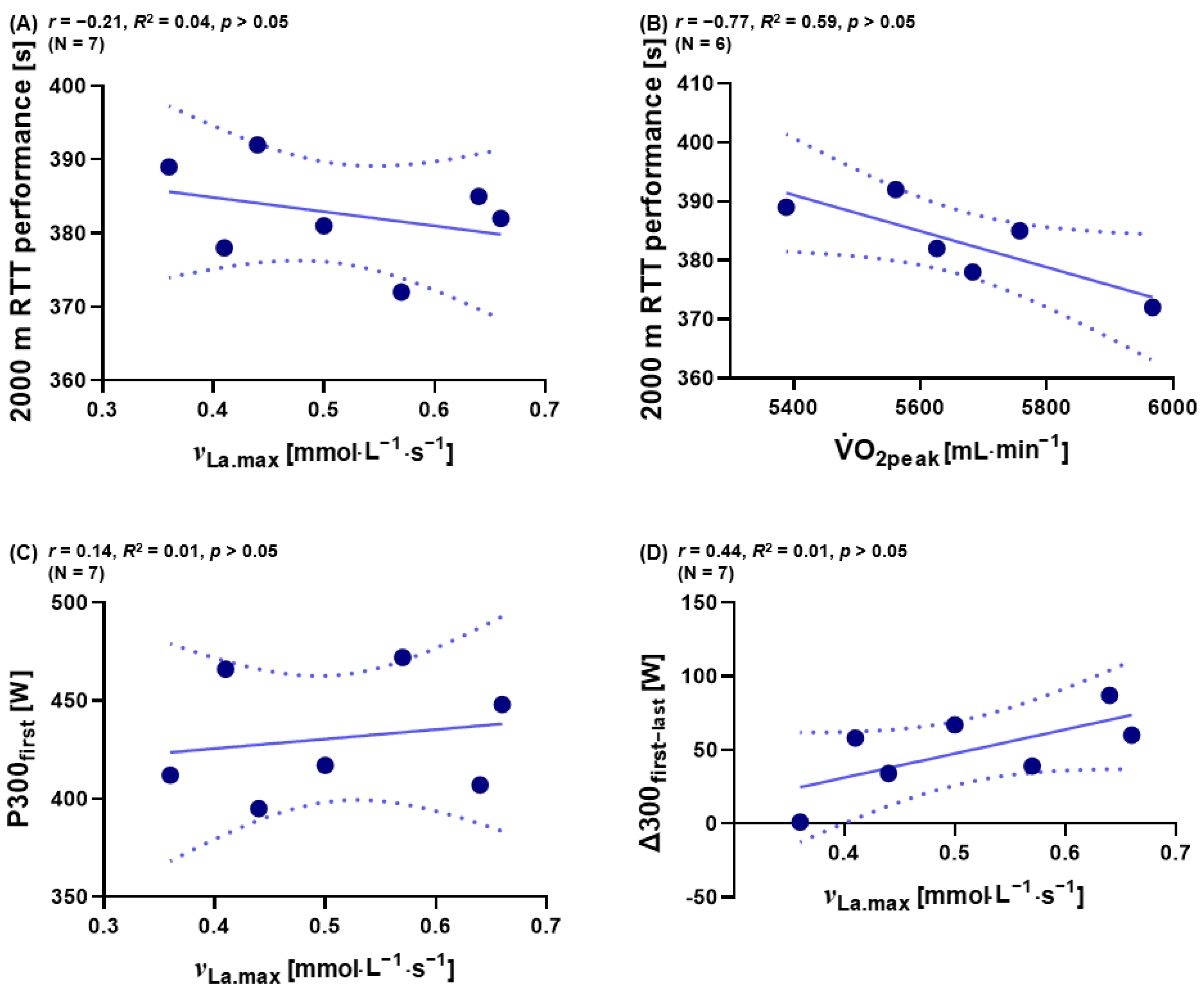
3.5. The Influence of νLa.max on Specific Sections of 2000 m RTT
3.6. Separate Classification of νLa.max, O2peak, and Performance over P300first and ∆300first−last for Male Athletes
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kleshnev, V. Biomechanics of Rowing: A Unique Insight into the Technical and Tactical Aspects of Elite Rowing; The Crowood Press: Marlborough, UK, 2020. [Google Scholar]
- Ingham, S.A.; Whyte, G.P.; Jones, K.; Nevill, A.M. Determinants of 2,000 m rowing ergometer performance in elite rowers. Eur. J. Appl. Physiol. 2002, 88, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Yoshiga, C.C.; Higuchi, M. Rowing performance of female and male rowers. Scand J. Med. Sci. Sports 2003, 13, 317–321. [Google Scholar] [CrossRef]
- Larsson, L.; Forsberg, A. Morphological muscle characteristics in rowers. Can. J. Appl. Sport Sci 1980, 5, 239–244. [Google Scholar]
- Hagerman, F.C.; Connors, M.C.; Gault, J.A.; Hagerman, G.R.; Polinski, W.J. Energy expenditure during simulated rowing. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1978, 45, 87–93. [Google Scholar] [CrossRef]
- Mikulic, P.; Bralic, N. Elite status maintained: A 12-year physiological and performance follow-up of two Olympic champion rowers. J. Sports Sci. 2018, 36, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Treff, G.; Schmidt, W.; Wachsmuth, N.; Völzke, C.; Steinacker, J.M. Total haemoglobin mass, maximal and submaximal power in elite rowers. Int. J. Sports Med. 2014, 35, 571–574. [Google Scholar] [CrossRef]
- Cosgrove, M.J.; Wilson, J.; Watt, D.; Grant, S.F. The relationship between selected physiological variables of rowers and rowing performance as determined by a 2000 m ergometer test. J. Sports Sci. 1999, 17, 845–852. [Google Scholar] [CrossRef]
- Secher, N.H. The physiology of rowing. J. Sport. Sci. 1983, 1, 23–53. [Google Scholar] [CrossRef]
- Gillies, E.; Bell, G. The relationship of physical and physiological parameters to 2000 m simulated rowing performance. Res. Sport. Med. Int. J. 2000, 9, 277–288. [Google Scholar] [CrossRef]
- Kramer, J.F.; Leger, A.; Paterson, D.H.; Morrow, A. Rowing performance and selected descriptive, field, and laboratory variables. Can. J. Appl. Physiol. 1994, 19, 174–184. [Google Scholar] [CrossRef]
- de Campos Mello, F.; de Moraes Bertuzzi, R.C.; Grangeiro, P.M.; Franchini, E. Energy systems contributions in 2,000 m race simulation: A comparison among rowing ergometers and water. Eur. J. Appl. Physiol. 2009, 107, 615–619. [Google Scholar] [CrossRef]
- Nielsen, H.B. pH after competitive rowing: The lower physiological range? Acta Physiol. Scand 1999, 165, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Maciejewski, H.; Bourdin, M.; Lacour, J.R.; Denis, C.; Moyen, B.; Messonnier, L. Lactate accumulation in response to supramaximal exercise in rowers. Scand J. Med. Sci. Sports 2013, 23, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Maciejewski, H.; Bourdin, M.; Féasson, L.; Dubouchaud, H.; Messonnier, L.A. Non-oxidative Energy Supply Correlates with Lactate Transport and Removal in Trained Rowers. Int. J. Sports Med. 2020, 41, 936–943. [Google Scholar] [CrossRef]
- Pripstein, L.P.; Rhodes, E.C.; McKenzie, D.C.; Coutts, K.D. Aerobic and anaerobic energy during a 2-km race simulation in female rowers. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 79, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Diry, A.; Ratel, S.; Nevill, A.; Maciejewski, H. What is the physiological impact of reducing the 2000 m Olympic distance in rowing to 1500 m and 1000 m for French young competitive rowers? Insights from the energy system contribution. Front. Physiol. 2022, 13, 896975. [Google Scholar] [CrossRef] [PubMed]
- Gastin, P.B. Energy system interaction and relative contribution during maximal exercise. Sports Med. 2001, 31, 725–741. [Google Scholar] [CrossRef]
- Mader, A. Glycolysis and oxidative phosphorylation as a function of cytosolic phosphorylation state and power output of the muscle cell. Eur. J. Appl. Physiol. 2003, 88, 317–338. [Google Scholar] [CrossRef]
- Heck, H.; Schulz, H.; Bartmus, U. Diagnostics of anaerobic power and capacity. Eur. J. Sport Sci. 2003, 3, 1–23. [Google Scholar] [CrossRef]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Baker, J.S.; McCormick, M.C.; Robergs, R.A. Interaction among Skeletal Muscle Metabolic Energy Systems during Intense Exercise. J. Nutr. Metab. 2010, 2010, 905612. [Google Scholar] [CrossRef]
- Mader, A. Zur beurteilung der sportartspezifischen ausdauerleistungsfahigkeit im labor. Sportarzt. Sportmed. 1976, 27, 80–88. [Google Scholar]
- Poole, D.C.; Rossiter, H.B.; Brooks, G.A.; Gladden, L.B. The anaerobic threshold: 50+ years of controversy. J. Physiol. 2021, 599, 737–767. [Google Scholar] [CrossRef]
- Wackerhage, H.; Gehlert, S.; Schulz, H.; Weber, S.; Ring-Dimitriou, S.; Heine, O. Lactate Thresholds and the Simulation of Human Energy Metabolism: Contributions by the Cologne Sports Medicine Group in the 1970s and 1980s. Front. Physiol. 2022, 13, 899670. [Google Scholar] [CrossRef]
- Roth, W.; Schwanitz, P.; Pas, P.; Bauer, P. Force-time characteristics of the rowing stroke and corresponding physiological muscle adaptations. Int. J. Sports Med. 1993, 14, S32–S34. [Google Scholar] [CrossRef]
- Akça, F. Prediction of rowing ergometer performance from functional anaerobic power, strength and anthropometric components. J. Hum. Kinet. 2014, 41, 133–142. [Google Scholar] [CrossRef]
- Izquierdo-Gabarren, M.; Expósito, R.G.; de Villarreal, E.S.; Izquierdo, M. Physiological factors to predict on traditional rowing performance. Eur. J. Appl. Physiol. 2010, 108, 83–92. [Google Scholar] [CrossRef]
- Cataldo, A.; Cerasola, D.; Russo, G.; Zangla, D.; Traina, M. Mean power during 20 sec all-out test to predict 2000 m rowing ergometer performance in national level young rowers. J. Sports Med. Phys. Fit. 2015, 55, 872–877. [Google Scholar]
- Heck, H.; Mader, A.; Hess, G.; Mucke, S.; Muller, R.; Hollmann, W. Justification of the 4-mmol/l lactate threshold. Int. J. Sports Med. 1985, 6, 117–130. [Google Scholar] [CrossRef]
- Mader, A. Eine Theorie zur Berechnung der Dynamik und des steady state von Phosphorylierungszustand und Stoffwechselaktivität der Muskelzelle als Folge des Energiebedarfs; German Sport University Cologne: Cologne, Germany, 1984. [Google Scholar]
- Julio, U.F.; Panissa, V.L.G.; Esteves, J.V.; Cury, R.L.; Agostinho, M.F.; Franchini, E. Energy-System Contributions to Simulated Judo Matches. Int. J. Sports Physiol. Perform. 2017, 12, 676–683. [Google Scholar] [CrossRef]
- Yang, W.H.; Park, J.H.; Shin, Y.C.; Kim, J. Physiological Profiling and Energy System Contributions During Simulated Epée Matches in Elite Fencers. Int. J. Sports Physiol. Perform. 2022, 17, 943–950. [Google Scholar] [CrossRef]
- Yang, W.-H.; Heine, O.; Grau, M. Rapid weight reduction does not impair athletic performance of Taekwondo athletes–A pilot study. PLoS ONE 2018, 13, e0196568. [Google Scholar] [CrossRef]
- Treff, G.; Mentz, L.; Mayer, B.; Winkert, K.; Engleder, T.; Steinacker, J.M. Initial Evaluation of the Concept-2 Rowing Ergometer’s Accuracy Using a Motorized Test Rig. Front. Sports Act. Living 2021, 3, 801617. [Google Scholar] [CrossRef]
- Quittmann, O.J.; Appelhans, D.; Abel, T.; Strüder, H.K. Evaluation of a sport-specific field test to determine maximal lactate accumulation rate and sprint performance parameters in running. J. Sci. Med. Sport 2020, 23, 27–34. [Google Scholar] [CrossRef]
- Beneke, R.; Beyer, T.; Jachner, C.; Erasmus, J.; Hütler, M. Energetics of karate kumite. Eur. J. Appl. Physiol. 2004, 92, 518–523. [Google Scholar] [CrossRef]
- Hausen, M.; Soares, P.P.; Araújo, M.P.; Porto, F.; Franchini, E.; Bridge, C.A.; Gurgel, J. Physiological responses and external validity of a new setting for taekwondo combat simulation. PLoS ONE 2017, 12, e0171553. [Google Scholar]
- Campos, F.A.D.; Bertuzzi, R.; Dourado, A.C.; Santos, V.G.F.; Franchini, E. Energy demands in taekwondo athletes during combat simulation. Eur. J. Appl. Physiol. 2012, 112, 1221–1228. [Google Scholar] [CrossRef]
- Lopes-Silva, J.P.; Da Silva Santos, J.F.; Artioli, G.G.; Loturco, I.; Abbiss, C.; Franchini, E. Sodium bicarbonate ingestion increases glycolytic contribution and improves performance during simulated taekwondo combat. Eur. J. Sport Sci. 2018, 18, 431–440. [Google Scholar] [CrossRef]
- di Prampero, P.E.; Ferretti, G. The energetics of anaerobic muscle metabolism: A reappraisal of older and recent concepts. Respir. Physiol. 1999, 118, 103–115. [Google Scholar] [CrossRef]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef]
- Andrade, V.L.; Zagatto, A.M.; Kalva-Filho, C.A.; Mendes, O.C.; Gobatto, C.A.; Campos, E.Z.; Papoti, M. Running-based Anaerobic Sprint Test as a Procedure to Evaluate Anaerobic Power. Int. J. Sports Med. 2015, 36, 1156–1162. [Google Scholar] [CrossRef]
- Keir, D.A.; Thériault, F.; Serresse, O. Evaluation of the running-based anaerobic sprint test as a measure of repeated sprint ability in collegiate-level soccer players. J. Strength Cond. Res. 2013, 27, 1671–1678. [Google Scholar] [CrossRef]
- Meckel, Y.; Gottlieb, R.; Eliakim, A. Repeated sprint tests in young basketball players at different game stages. Eur. J. Appl. Physiol. 2009, 107, 273–279. [Google Scholar] [CrossRef]
- Quittmann, O.J.; Abel, T.; Vafa, R.; Mester, J.; Schwarz, Y.M.; Strüder, H.K. Maximal lactate accumulation rate and post-exercise lactate kinetics in handcycling and cycling. Eur. J. Sport Sci. 2021, 21, 539–551. [Google Scholar] [CrossRef]
- van Loon, L.J.; Greenhaff, P.L.; Constantin-Teodosiu, D.; Saris, W.H.; Wagenmakers, A.J. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J. Physiol. 2001, 536, 295–304. [Google Scholar] [CrossRef]
- Astridge, D.J.; Peeling, P.; Goods, P.S.R.; Girard, O.; Hewlett, J.; Rice, A.J.; Binnie, M.J. Rowing in Los Angeles: Performance Considerations for the Change to 1500 m at the 2028 Olympic Games. Int. J. Sports Physiol. Perform. 2022, 18, 104–107. [Google Scholar] [CrossRef]
- Medbø, J.I.; Mohn, A.C.; Tabata, I.; Bahr, R.; Vaage, O.; Sejersted, O.M. Anaerobic capacity determined by maximal accumulated O2 deficit. J. Appl. Physiol. 1988, 64, 50–60. [Google Scholar] [CrossRef]
- Lacour, J.R.; Bouvat, E.; Barthélémy, J.C. Post-competition blood lactate concentrations as indicators of anaerobic energy expenditure during 400-m and 800-m races. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 61, 172–176. [Google Scholar] [CrossRef]
- Mader, A.; Hartmann, U.; Hollmann, W. Der Einfluß der Ausdauer auf die 6minütige maximale anaerobe und aerobe Arbeitskapazität eines Eliteruderers. In Rudern; Springer: Berlin/Heidelberg, Germany, 1988; pp. 62–78. [Google Scholar]
- Lee, K.H.; Ju, H.M.; Yang, W.H. Metabolic Energy Contributions During High-Intensity Hatha Yoga and Physiological Comparisons Between Active and Passive (Savasana) Recovery. Front. Physiol. 2021, 12, 743859. [Google Scholar] [CrossRef]
- Yang, W.-H.; Park, J.-H.; Park, S.-Y.; Park, Y. Energetic Contributions Including Gender Differences and Metabolic Flexibility in the General Population and Athletes. Metabolites 2022, 12, 965. [Google Scholar] [CrossRef]
- Adelnia, F.; Ubaida-Mohien, C.; Moaddel, R.; Shardell, M.; Lyashkov, A.; Fishbein, K.W.; Aon, M.A.; Spencer, R.G.; Ferrucci, L. Proteomic signatures of in vivo muscle oxidative capacity in healthy adults. Aging Cell 2020, 19, e13124. [Google Scholar] [CrossRef] [PubMed]
- Zagatto, A.M.; Beck, W.R.; Gobatto, C.A. Validity of the running anaerobic sprint test for assessing anaerobic power and predicting short-distance performances. J. Strength Cond. Res. 2009, 23, 1820–1827. [Google Scholar] [CrossRef] [PubMed]
- Nitzsche, N.; Baumgärtel, L.; Schulz, H. Comparison of Maximum Lactate Formation Rates in Ergometer Sprint and Maximum Strength Loads. Ger. J. Sports Med. 2018, 69, 13–18. [Google Scholar] [CrossRef]
- Dobson, G.P.; Yamamoto, E.; Hochachka, P.W. Phosphofructokinase control in muscle: Nature and reversal of pH-dependent ATP inhibition. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1986, 250, R71–R76. [Google Scholar] [CrossRef] [PubMed]
- Beneke, R.; Leithäuser, R.M.; Ochentel, O. Blood lactate diagnostics in exercise testing and training. Int. J. Sports Physiol. Perform. 2011, 6, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Hauser, T.; Adam, J.; Schulz, H. Comparison of calculated and experimental power in maximal lactate-steady state during cycling. Theor. Biol. Med. Model 2014, 11, 25. [Google Scholar] [CrossRef]
- Weber, S. Berechnung Leistungsbestimmender Parameter der Metabolischen Aktivität auf Zellulärer Ebene Mittels Fahrradergometrischer Untersuchungen. Diploma Thesis, German Sport University Cologne, Cologne, Germany, 2003. [Google Scholar]
- Esbjörnsson-Liljedahl, M.; Sundberg, C.J.; Norman, B.; Jansson, E. Metabolic response in type I and type II muscle fibers during a 30-s cycle sprint in men and women. J. Appl. Physiol. 1999, 87, 1326–1332. [Google Scholar] [CrossRef]
- Henriksson, J.; Chi, M.M.; Hintz, C.S.; Young, D.A.; Kaiser, K.K.; Salmons, S.; Lowry, O.H. Chronic stimulation of mammalian muscle: Changes in enzymes of six metabolic pathways. Am. J. Physiol. 1986, 251, C614–C632. [Google Scholar] [CrossRef]
- Wende, A.R.; Schaeffer, P.J.; Parker, G.J.; Zechner, C.; Han, D.H.; Chen, M.M.; Hancock, C.R.; Lehman, J.J.; Huss, J.M.; McClain, D.A.; et al. A role for the transcriptional coactivator PGC-1alpha in muscle refueling. J. Biol. Chem. 2007, 282, 36642–36651. [Google Scholar] [CrossRef]
- Linossier, M.T.; Dormois, D.; Perier, C.; Frey, J.; Geyssant, A.; Denis, C. Enzyme adaptations of human skeletal muscle during bicycle short-sprint training and detraining. Acta Physiol. Scand 1997, 161, 439–445. [Google Scholar] [CrossRef]
- Tesch, P.A.; Komi, P.V.; Häkkinen, K. Enzymatic adaptations consequent to long-term strength training. Int. J. Sports Med. 1987, 8, 66–69. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, J.D.; Hicks, A.L.; MacDonald, J.R.; McKelvie, R.S.; Green, H.J.; Smith, K.M. Muscle performance and enzymatic adaptations to sprint interval training. J. Appl. Physiol. 1998, 84, 2138–2142. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, M.; Takeda, M. Lactate as a Signaling Molecule That Regulates Exercise-Induced Adaptations. Biology 2016, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Jensen-Urstad, M.; Svedenhag, J.; Sahlin, K. Effect of muscle mass on lactate formation during exercise in humans. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 69, 189–195. [Google Scholar] [CrossRef]
- Plotkin, D.L.; Roberts, M.D.; Haun, C.T.; Schoenfeld, B.J. Muscle Fiber Type Transitions with Exercise Training: Shifting Perspectives. Sports 2021, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Gehlert, S.; Weber, S.; Weidmann, B.; Gutsche, K.; Platen, P.; Graf, C.; Kappes-Horn, K.; Bloch, W. Cycling exercise-induced myofiber transitions in skeletal muscle depend on basal fiber type distribution. Eur. J. Appl. Physiol. 2012, 112, 2393–2402. [Google Scholar] [CrossRef] [PubMed]
- Nitzsche, N.; Lenz, J.C.; Voronoi, P.; Schulz, H. Adaption of Maximal Glycolysis Rate after Resistance Exercise with Different Volume Load. Sports Med. Int. Open 2020, 4, E39–E44. [Google Scholar] [CrossRef]
- Parra, J.; Cadefau, J.A.; Rodas, G.; Amigó, N.; Cussó, R. The distribution of rest periods affects performance and adaptations of energy metabolism induced by high-intensity training in human muscle. Acta Physiol. Scand 2000, 169, 157–165. [Google Scholar] [CrossRef]
- Millet, G.P.; Roels, B.; Schmitt, L.; Woorons, X.; Richalet, J.P. Combining hypoxic methods for peak performance. Sports Med. 2010, 40, 1–25. [Google Scholar] [CrossRef]
- Webster, K.A. Evolution of the coordinate regulation of glycolytic enzyme genes by hypoxia. J. Exp. Biol. 2003, 206, 2911–2922. [Google Scholar] [CrossRef]

| Parameters | Total | Male | Female |
|---|---|---|---|
| N = 10 | n = 7 | n = 3 | |
| Age [years] | 19.80 ± 0.9 | 19.8 ± 0.9 | 19.6 ± 0.5 |
| Height [cm] | 183.20 ± 7.0 | 188.4 ± 3.2 | 175.6 ± 4.7 |
| Body mass [kg] | 79.9 ± 13.3 | 85.4 ± 11.2 | 67.2 ± 3.8 |
| Body fat [%] | 16.4 ± 5.1 | 14.8 ± 5.0 | 20.08 ± 3.1 |
| Participants (Sex) | 2000 m RTT Performance | Lapeak | O2peak | P4 |
|---|---|---|---|---|
| s | mmol·L−1 | ml·min−1 | W | |
| P1 (f) | 435 | 15.77 | 4042 | 229 |
| P2 (f) | 459 | 15.77 | 4233 | 200 |
| P3 (f) | 456 | 8.95 | 3546 | 226 |
| P4 (m) | 392 | 18.82 | 5561 | 302 |
| P5 (m) | 378 | 21.30 | 5683 | 298 |
| P6 (m) | 382 | 19.66 | 5626 | 305 |
| P7 (m) | 389 | 15.01 | 5388 | 335 |
| P8 (m) | 372 | 18.02 | 5967 | 338 |
| P9 (m) | 385 | 13.51 | 5757 | 314 |
| P10 (m) | 381 | 15.91 | - | 314 |
| Participants (Sex) | νLa.max | tPCr | P300first | P300last | ∆300 first-last |
|---|---|---|---|---|---|
| mmol·L−1·s−1 | s | W | W | W | |
| P1 (f) | 0.29 | 2.95 | 303 | 290 | 13 |
| P2 (f) | 0.37 | 2.95 | 253 | 233 | 20 |
| P3 (f) | 0.25 | 2.95 | 218 | 218 | 0 |
| P4 (m) | 0.44 | 2.95 | 395 | 361 | 34 |
| P5 (m) | 0.41 | 2.95 | 466 | 408 | 58 |
| P6 (m) | 0.66 | 2.95 | 448 | 388 | 60 |
| P7 (m) | 0.36 | 2.95 | 412 | 411 | 1 |
| P8 (m) | 0.57 | 2.95 | 472 | 433 | 39 |
| P9 (m) | 0.64 | 2.95 | 407 | 320 | 87 |
| P10 (m) | 0.50 | 2.95 | 417 | 350 | 67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schünemann, F.; Park, S.-Y.; Wawer, C.; Theis, C.; Yang, W.-H.; Gehlert, S. Diagnostics of νLa.max and Glycolytic Energy Contribution Indicate Individual Characteristics of Anaerobic Glycolytic Energy Metabolism Contributing to Rowing Performance. Metabolites 2023, 13, 317. https://doi.org/10.3390/metabo13030317
Schünemann F, Park S-Y, Wawer C, Theis C, Yang W-H, Gehlert S. Diagnostics of νLa.max and Glycolytic Energy Contribution Indicate Individual Characteristics of Anaerobic Glycolytic Energy Metabolism Contributing to Rowing Performance. Metabolites. 2023; 13(3):317. https://doi.org/10.3390/metabo13030317
Chicago/Turabian StyleSchünemann, Frederik, So-Young Park, Corinna Wawer, Christian Theis, Woo-Hwi Yang, and Sebastian Gehlert. 2023. "Diagnostics of νLa.max and Glycolytic Energy Contribution Indicate Individual Characteristics of Anaerobic Glycolytic Energy Metabolism Contributing to Rowing Performance" Metabolites 13, no. 3: 317. https://doi.org/10.3390/metabo13030317
APA StyleSchünemann, F., Park, S.-Y., Wawer, C., Theis, C., Yang, W.-H., & Gehlert, S. (2023). Diagnostics of νLa.max and Glycolytic Energy Contribution Indicate Individual Characteristics of Anaerobic Glycolytic Energy Metabolism Contributing to Rowing Performance. Metabolites, 13(3), 317. https://doi.org/10.3390/metabo13030317