Alterations of NMR-Based Lipoprotein Profile Distinguish Unstable Angina Patients with Different Severity of Coronary Lesions
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Measurements
2.3. Measurement of 1H NMR Spectra of Plasma
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
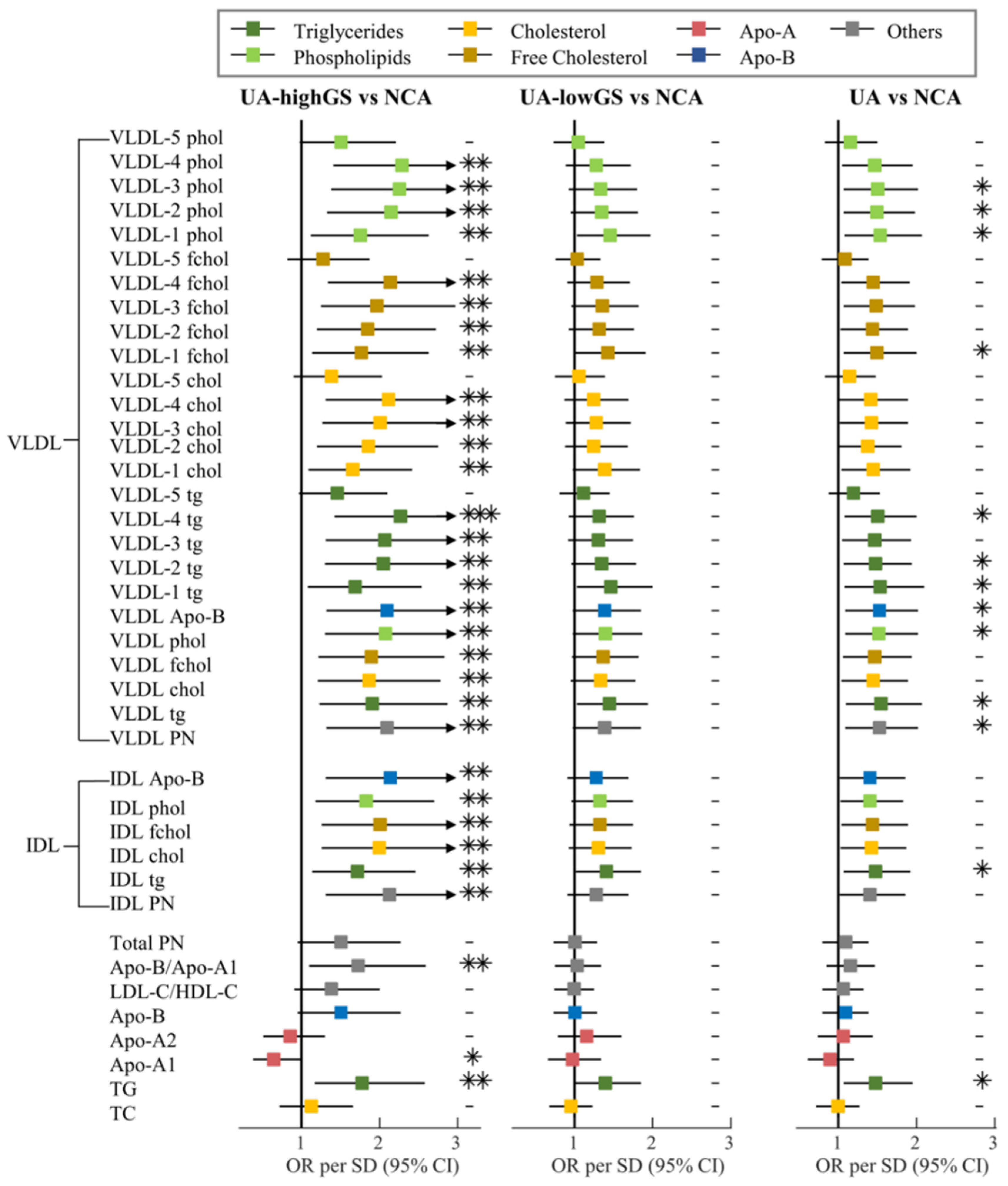
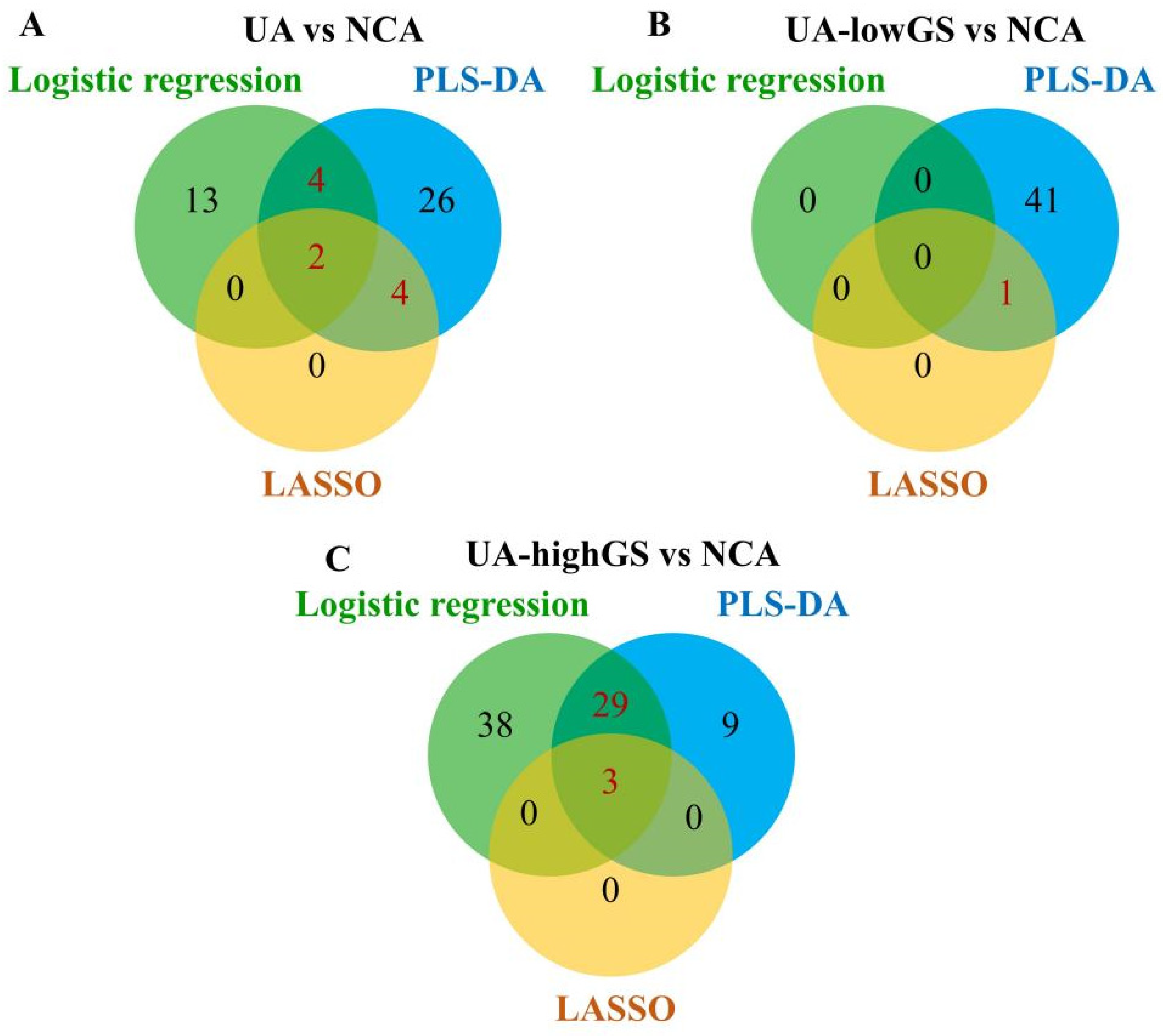
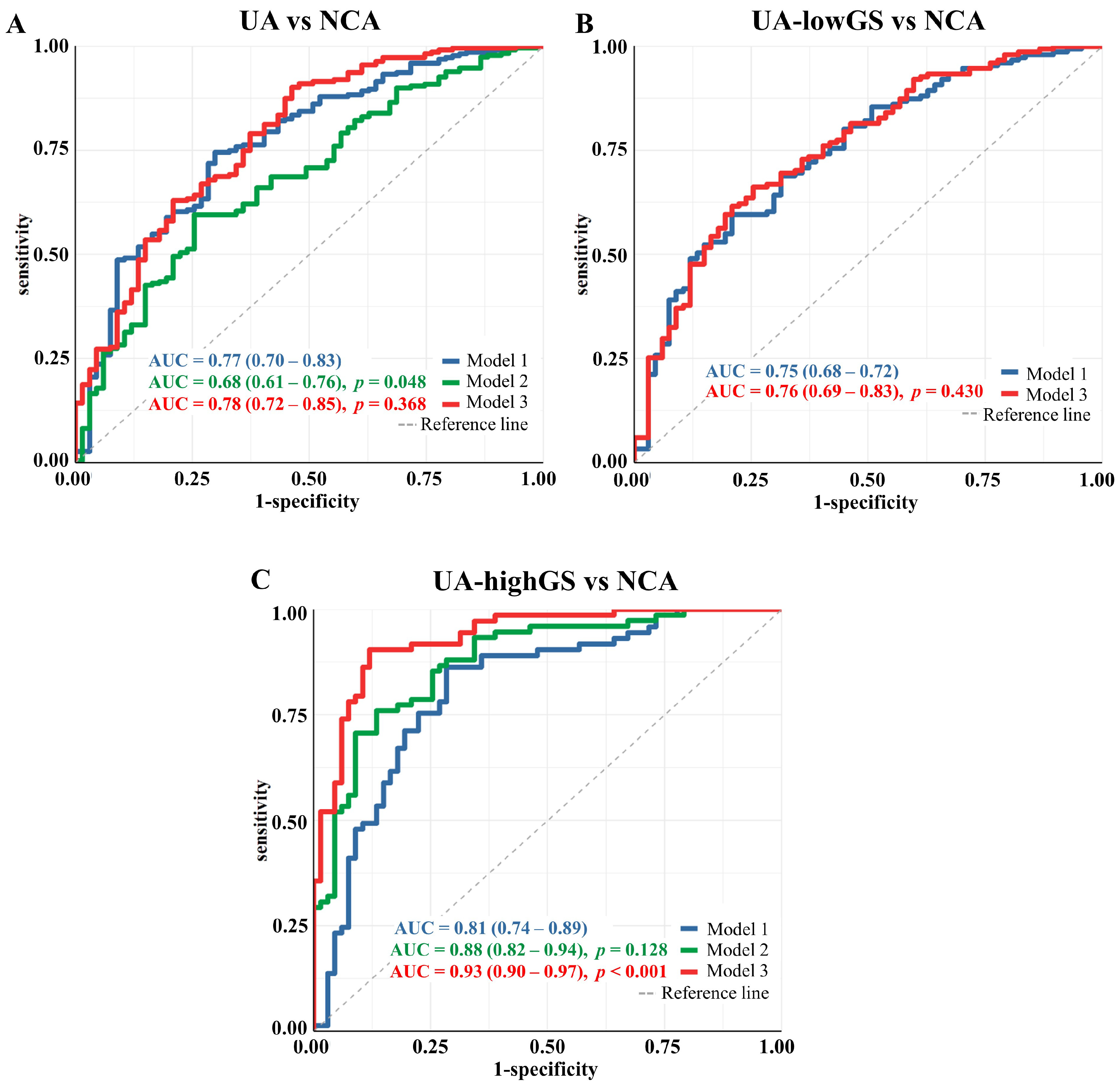
3.2. Lipoprotein Particles Discriminating Unstable Angina from Angiographically Normal Coronary Arteries Patients
3.3. Lipoprotein Particles Show Higher Performance in Discriminating UA Patients with High Gensini Scores from Angiographically Normal Coronary Arteries Patients
3.4. Sensitivity Analysis
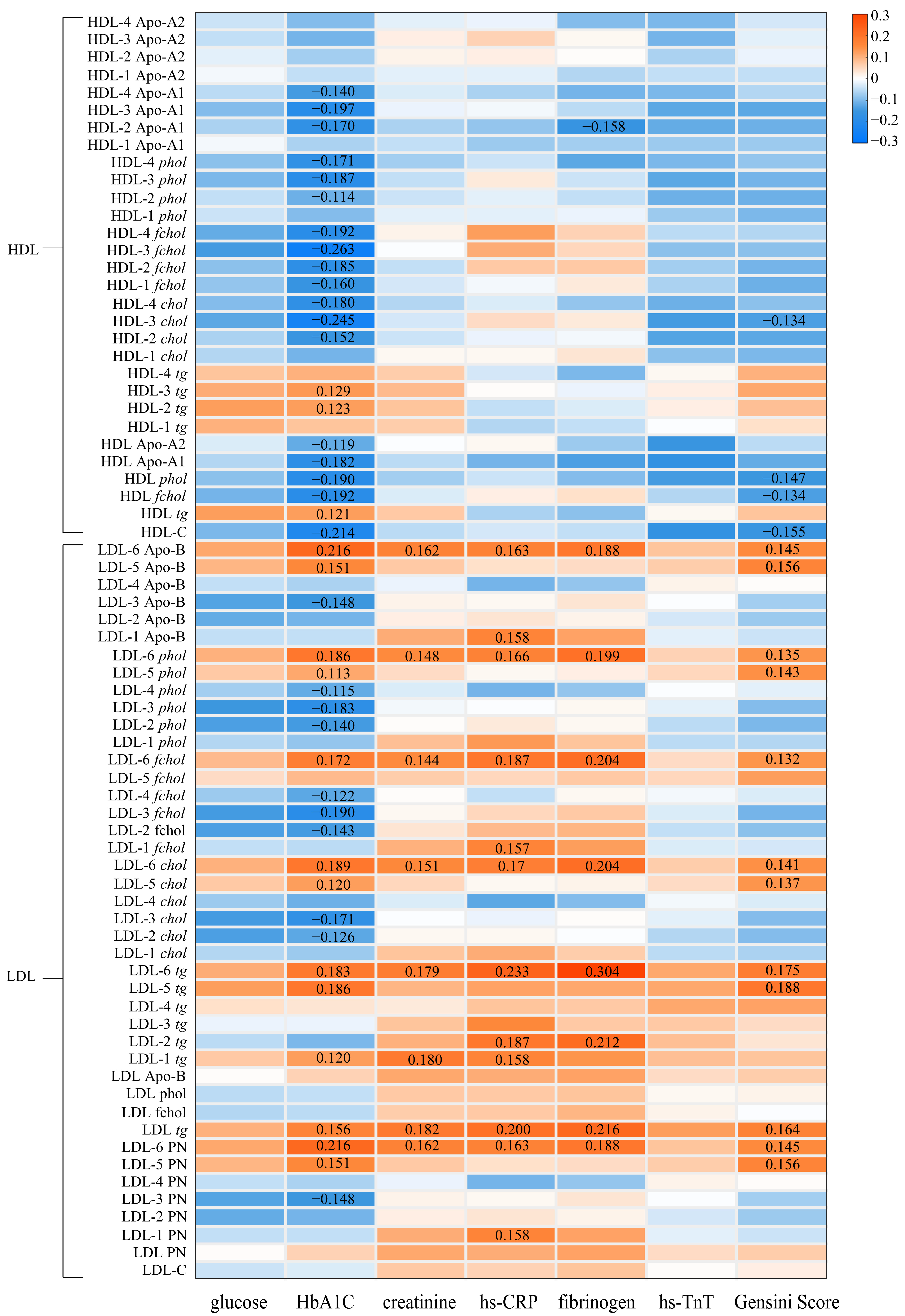
3.5. The Associations between Lipoproteins and Clinical Biomarkers of Cardiovascular Diseases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maddox, T.M.; Reid, K.J.; Rumsfeld, J.S.; Spertus, J.A. One-year health status outcomes of unstable angina versus myocardial infarction: A prospective, observational cohort study of ACS survivors. BMC Cardiovasc. Disord. 2007, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthelemy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2020, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.V.; Millwood, I.Y.; Kartsonaki, C.; Hill, M.R.; Bennett, D.A.; Boxall, R.; Guo, Y.; Xu, X.; Bian, Z.; Hu, R.; et al. Lipids, Lipoproteins, and Metabolites and Risk of Myocardial Infarction and Stroke. J. Am. Coll. Cardiol. 2018, 71, 620–632. [Google Scholar] [CrossRef]
- Martin, S.S.; Khokhar, A.A.; May, H.T.; Kulkarni, K.R.; Blaha, M.J.; Joshi, P.H.; Toth, P.P.; Muhlestein, J.B.; Anderson, J.L.; Knight, S.; et al. HDL cholesterol subclasses, myocardial infarction, and mortality in secondary prevention: The Lipoprotein Investigators Collaborative. Eur. Heart J. 2015, 36, 22–30. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Nicholls, S.J.; Riesmeyer, J.S.; Barter, P.J.; Brewer, H.B.; Fox, K.A.A.; Gibson, C.M.; Granger, C.; Menon, V.; Montalescot, G.; et al. Evacetrapib and Cardiovascular Outcomes in High-Risk Vascular Disease. N. Engl. J. Med. 2017, 376, 1933–1942. [Google Scholar] [CrossRef]
- Navarese, E.P.; Robinson, J.G.; Kowalewski, M.; Kolodziejczak, M.; Andreotti, F.; Bliden, K.; Tantry, U.; Kubica, J.; Raggi, P.; Gurbel, P.A. Association Between Baseline LDL-C Level and Total and Cardiovascular Mortality After LDL-C Lowering: A Systematic Review and Meta-analysis. JAMA 2018, 319, 1566–1579. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Q.; Liu, X.C.; Lo, K.; Liu, L.; Yu, Y.L.; Chen, C.L.; Huang, J.Y.; Feng, Y.Q.; Zhang, B. The U Shaped Relationship Between High-Density Lipoprotein Cholesterol and All-Cause or Cause-Specific Mortality in Adult Population. Clin. Interv. Aging 2020, 15, 1883–1896. [Google Scholar] [CrossRef]
- Johannesen, C.D.L.; Langsted, A.; Mortensen, M.B.; Nordestgaard, B.G. Association between low density lipoprotein and all cause and cause specific mortality in Denmark: Prospective cohort study. BMJ 2020, 371, m4266. [Google Scholar] [CrossRef]
- Wurtz, P.; Havulinna, A.S.; Soininen, P.; Tynkkynen, T.; Prieto-Merino, D.; Tillin, T.; Ghorbani, A.; Artati, A.; Wang, Q.; Tiainen, M.; et al. Metabolite profiling and cardiovascular event risk: A prospective study of 3 population-based cohorts. Circulation 2015, 131, 774–785. [Google Scholar] [CrossRef]
- Boren, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef]
- Hua, J.; Malinski, T. Variable Effects Of LDL Subclasses Of Cholesterol On Endothelial Nitric Oxide/Peroxynitrite Balance—The Risks And Clinical Implications For Cardiovascular Disease. Int. J. Nanomed. 2019, 14, 8973–8987. [Google Scholar] [CrossRef] [PubMed]
- Khakimov, B.; Hoefsloot, H.C.J.; Mobaraki, N.; Aru, V.; Kristensen, M.; Lind, M.V.; Holm, L.; Castro-Mejía, J.L.; Nielsen, D.S.; Jacobs, D.M.; et al. Human Blood Lipoprotein Predictions from 1H NMR Spectra: Protocol, Model Performances, and Cage of Covariance. Anal. Chem. 2022, 94, 628–636. [Google Scholar] [CrossRef]
- Richardson, T.G.; Sanderson, E.; Palmer, T.M.; Ala-Korpela, M.; Ference, B.A.; Davey Smith, G.; Holmes, M.V. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: A multivariable Mendelian randomisation analysis. PLoS Med. 2020, 17, e1003062. [Google Scholar] [CrossRef] [PubMed]
- Otvos, J.D.; Guyton, J.R.; Connelly, M.A.; Akapame, S.; Bittner, V.; Kopecky, S.L.; Lacy, M.; Marcovina, S.M.; Muhlestein, J.B.; Boden, W.E. Relations of GlycA and lipoprotein particle subspecies with cardiovascular events and mortality: A post hoc analysis of the AIM-HIGH trial. J. Clin. Lipidol. 2018, 12, 348–355.e342. [Google Scholar] [CrossRef] [PubMed]
- Karwatowska-Prokopczuk, E.; Tardif, J.C.; Gaudet, D.; Ballantyne, C.M.; Shapiro, M.D.; Moriarty, P.M.; Baum, S.J.; Amour, E.S.; Alexander, V.J.; Xia, S.; et al. Effect of olezarsen targeting APOC-III on lipoprotein size and particle number measured by NMR in patients with hypertriglyceridemia. J. Clin. Lipidol. 2022, 16, 617–625. [Google Scholar] [CrossRef]
- Yao, W.; Gao, Y.; Wan, Z. Serum Metabolomics Profiling to Identify Biomarkers for Unstable Angina. Biomed. Res. Int. 2017, 2017, 7657306. [Google Scholar] [CrossRef] [PubMed]
- Oyama, K.; Giugliano, R.P.; Blazing, M.A.; Park, J.G.; Tershakovec, A.M.; Sabatine, M.S.; Cannon, C.P.; Braunwald, E. Baseline Low-Density Lipoprotein Cholesterol and Clinical Outcomes of Combining Ezetimibe With Statin Therapy in IMPROVE-IT. J. Am. Coll. Cardiol. 2021, 78, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Zitnanova, I.; Oravec, S.; Janubova, M.; Konarikova, K.; Dvorakova, M.; Laubertova, L.; Kralova, M.; Simko, M.; Muchova, J. Gender differences in LDL and HDL subfractions in atherogenic and nonatherogenic phenotypes. Clin. Biochem. 2020, 79, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Grammer, T.B.; Kleber, M.E.; Marz, W.; Silbernagel, G.; Siekmeier, R.; Wieland, H.; Pilz, S.; Tomaschitz, A.; Koenig, W.; Scharnagl, H. Low-density lipoprotein particle diameter and mortality: The Ludwigshafen Risk and Cardiovascular Health Study. Eur. Heart J. 2015, 36, 31–38. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Wang, J.; Gao, J.; Guo, S.; Gao, K.; Man, H.; Wang, Y.; Chen, J.; Wang, W. Analysis of urinary metabolomic profiling for unstable angina pectoris disease based on nuclear magnetic resonance spectroscopy. Mol. Biosyst. 2015, 11, 3387–3396. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G. Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease: New Insights From Epidemiology, Genetics, and Biology. Circ. Res. 2016, 118, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Long, J.; Cheng, Y.; Chen, Y.; Zhao, S. Elevated plasma lipoprotein(a) levels were associated with increased risk of cardiovascular events in Chinese patients with stable coronary artery disease. Sci. Rep. 2018, 8, 7726. [Google Scholar] [CrossRef]
- Gensini, G.G. A more meaningful scoring system for determining the severity of coronary heart disease. Am. J. Cardiol. 1983, 51, 606. [Google Scholar] [CrossRef]
- Li, X.; Lin, Y.; Wang, S.; Zhou, S.; Ju, J.; Wang, X.; Chen, Y.; Xia, M. Extracellular Superoxide Dismutase Is Associated With Left Ventricular Geometry and Heart Failure in Patients With Cardiovascular Disease. J. Am. Heart Assoc. 2020, 9, e016862. [Google Scholar] [CrossRef]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G.; Tada, T.; Fusaro, M.; Cassese, S.; King, L.; Hadamitzky, M.; Haase, H.U.; Schomig, A.; Kastrati, A.; Pache, J. Association of coronary atherosclerotic burden with clinical presentation and prognosis in patients with stable and unstable coronary artery disease. Clin. Res. Cardiol. 2012, 101, 1003–1011. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Y.; Chen, Y.; Zhao, Y.J.; Liu, L.W.; Li, J.; Wang, S.L.; Alolga, R.N.; Yin, Y.; Wang, X.M.; et al. Comprehensive Metabolomic Characterization of Coronary Artery Diseases. J. Am. Coll. Cardiol. 2016, 68, 1281–1293. [Google Scholar] [CrossRef]
- Monsonis Centelles, S.; Hoefsloot, H.C.J.; Khakimov, B.; Ebrahimi, P.; Lind, M.V.; Kristensen, M.; de Roo, N.; Jacobs, D.M.; van Duynhoven, J.; Cannet, C.; et al. Toward Reliable Lipoprotein Particle Predictions from NMR Spectra of Human Blood: An Interlaboratory Ring Test. Anal. Chem. 2017, 89, 8004–8012. [Google Scholar] [CrossRef]
- Jimenez, B.; Holmes, E.; Heude, C.; Tolson, R.F.; Harvey, N.; Lodge, S.L.; Chetwynd, A.J.; Cannet, C.; Fang, F.; Pearce, J.T.M.; et al. Quantitative Lipoprotein Subclass and Low Molecular Weight Metabolite Analysis in Human Serum and Plasma by (1)H NMR Spectroscopy in a Multilaboratory Trial. Anal. Chem. 2018, 90, 11962–11971. [Google Scholar] [CrossRef]
- Smilde, A.K.; Jansen, J.J.; Hoefsloot, H.C.; Lamers, R.J.; van der Greef, J.; Timmerman, M.E. ANOVA-simultaneous component analysis (ASCA): A new tool for analyzing designed metabolomics data. Bioinformatics 2005, 21, 3043–3048. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate—A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B—Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression Shrinkage and Selection Via the Lasso. J. R. Stat. Soc. Ser. B 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Ståhle, L.; Wold, S. Partial least squares analysis with cross-validation for the two-class problem: A Monte Carlo study. J. Chemom. 1987, 1, 185–196. [Google Scholar] [CrossRef]
- Szymańska, E.; Saccenti, E.; Smilde, A.K.; Westerhuis, J.A. Double-check: Validation of diagnostic statistics for PLS-DA models in metabolomics studies. Metabolomics 2012, 8, 3–16. [Google Scholar] [CrossRef]
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef]
- Pokharel, Y.; Tang, Y.; Bhardwaj, B.; Patel, K.K.; Qintar, M.; O’Keefe, J.H., Jr.; Kulkarni, K.R.; Jones, P.H.; Martin, S.S.; Virani, S.S.; et al. Association of low-density lipoprotein pattern with mortality after myocardial infarction: Insights from the TRIUMPH study. J. Clin. Lipidol. 2017, 11, 1458–1470.e1454. [Google Scholar] [CrossRef] [PubMed]
- Otvos, J.D.; Jeyarajah, E.J.; Cromwell, W.C. Measurement issues related to lipoprotein heterogeneity. Am. J. Cardiol. 2002, 90, 22i–29i. [Google Scholar] [CrossRef]
- Pichler, G.; Amigo, N.; Tellez-Plaza, M.; Pardo-Cea, M.A.; Dominguez-Lucas, A.; Marrachelli, V.G.; Monleon, D.; Martin-Escudero, J.C.; Ascaso, J.F.; Chaves, F.J.; et al. LDL particle size and composition and incident cardiovascular disease in a South-European population: The Hortega-Liposcale Follow-up Study. Int. J. Cardiol. 2018, 264, 172–178. [Google Scholar] [CrossRef]
- Saether, J.; Madssen, E.; Vesterbekkmo, E.; Giskeodegaard, G.; Gjaere, S.; Wiseth, R.; Baathen, T.F.; Bye, A.; CERG. P1547 Circulating lipoprotein subfractions as new non-invasive biomarkers of coronary atherosclerosis. Eur. Heart J. 2019, 40 (Suppl. S1). [Google Scholar] [CrossRef]
- Xu, R.X.; Li, S.; Li, X.L.; Zhang, Y.; Guo, Y.L.; Zhu, C.G.; Wu, N.Q.; Qing, P.; Sun, J.; Dong, Q.; et al. High-density lipoprotein subfractions in relation with the severity of coronary artery disease: A Gensini score assessment. J. Clin. Lipidol. 2015, 9, 26–34. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, D.; Xu, R.X.; Guo, Y.L.; Zhu, C.G.; Wu, N.Q.; Zhang, Y.; Li, S.; Gao, Y.; Liu, G.; et al. Low-density lipoprotein-associated variables and the severity of coronary artery disease: An untreated Chinese cohort study. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem. 2018, 23, 647–653. [Google Scholar] [CrossRef]
- Aronson, D.; Rayfield, E.J. How hyperglycemia promotes atherosclerosis: Molecular mechanisms. Cardiovasc. Diabetol. 2002, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D. Disorders of lipid metabolism in nephrotic syndrome: Mechanisms and consequences. Kidney Int. 2016, 90, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wang, Y.; Li, B.; Yu, S.; Wang, A.; Wang, W.; Gao, Z.; Tang, X.; Yan, L.; Wan, Q.; et al. Triglycerides to high-density lipoprotein cholesterol ratio is superior to triglycerides and other lipid ratios as an indicator of increased urinary albumin-to-creatinine ratio in the general population of China: A cross-sectional study. Lipids Health Dis. 2021, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Casanova, F.; Wood, A.R.; Yaghootkar, H.; Beaumont, R.N.; Jones, S.E.; Gooding, K.M.; Aizawa, K.; Strain, W.D.; Hattersley, A.T.; Khan, F.; et al. A Mendelian Randomization Study Provides Evidence That Adiposity and Dyslipidemia Lead to Lower Urinary Albumin-to-Creatinine Ratio, a Marker of Microvascular Function. Diabetes 2020, 69, 1072–1082. [Google Scholar] [CrossRef]
- Singh, S.K.; Agrawal, A. Functionality of C-Reactive Protein for Atheroprotection. Front. Immunol. 2019, 10, 1655. [Google Scholar] [CrossRef]
- Luyendyk, J.P.; Schoenecker, J.G.; Flick, M.J. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood 2019, 133, 511–520. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, M.; Cheng, X.; Shen, J.; Khound, R.; Zhang, K.; Su, Q. CREBH mediates metabolic inflammation to hepatic VLDL overproduction and hyperlipoproteinemia. J. Mol. Med. 2017, 95, 839–849. [Google Scholar] [CrossRef]
- Ivanova, E.A.; Myasoedova, V.A.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. Small Dense Low-Density Lipoprotein as Biomarker for Atherosclerotic Diseases. Oxid. Med. Cell Longev. 2017, 2017, 1273042. [Google Scholar] [CrossRef]
- Baumstark, M.W.; Kreutz, W.; Berg, A.; Frey, I.; Keul, J. Structure of human low-density lipoprotein subfractions, determined by X-ray small-angle scattering. Biochim. Biophys. Acta 1990, 1037, 48–57. [Google Scholar] [CrossRef]
- Van der Graaf, A.; Rodenburg, J.; Vissers, M.N.; Hutten, B.A.; Wiegman, A.; Trip, M.D.; Stroes, E.S.; Wijburg, F.A.; Otvos, J.D.; Kastelein, J.J. Atherogenic lipoprotein particle size and concentrations and the effect of pravastatin in children with familial hypercholesterolemia. J. Pediatr. 2008, 152, 873–878. [Google Scholar] [CrossRef]
- Patel, M.R.; Peterson, E.D.; Dai, D.; Brennan, J.M.; Redberg, R.F.; Anderson, H.V.; Brindis, R.G.; Douglas, P.S. Low diagnostic yield of elective coronary angiography. N. Engl. J. Med. 2010, 362, 886–895. [Google Scholar] [CrossRef] [PubMed]



| NCA (n = 67) | UA (n = 230) | p1 | LowGS (n = 155) | HighGS (n = 75) | p2 | |
|---|---|---|---|---|---|---|
| Age, years | 61.6 ± 7.25 | 65.35 ± 7.25 | <0.01 | 65.14 ± 7.76 | 65.77 ± 6.11 | 0.81 |
| Female | 43 (64.2) | 85 (37.0) | <0.01 | 66 (42.6) | 19 (25.3) | 0.01 |
| BMI, kg/m2 | 23.3 ± 3.92 | 24.34 ± 2.87 | 0.04 | 24.21 ± 2.82 | 24.62 ± 2.97 | 0.63 |
| Blood Pressure, mmHg | ||||||
| Systolic | 128.91 ± 21.29 | 132.97 ± 20.25 | 0.11 | 131.5 ± 19.78 | 136 ± 21.01 | 0.26 |
| Diastolic | 76.82 ± 9.92 | 79.43 ± 11.38 | 0.20 | 79.11 ± 11.41 | 80.08 ± 11.36 | 0.81 |
| Current smoker | 9 (13.4) | 64 (27.8) | 0.04 | 42 (27.1) | 22 (29.3) | 0.57 |
| Comorbidity | ||||||
| Hypertension | 28 (41.8) | 139 (60.4) | 0.02 | 93 (60.0) | 46 (61.3) | 0.85 |
| Type 2 diabetes | 7 (10.5) | 56 (24.4) | <0.01 | 32 (20.7) | 24 (32.0) | 0.06 |
| Lipid-lowering drugs | 6 (9.0) | 68 (29.6) | <0.01 | 49 (31.6) | 19 (25.3) | 0.33 |
| Gensini score | 0 | 25.4 ± 25.16 | <0.01 | 12.36 ± 6.61 | 52.35 ± 27.87 | <0.01 |
| Number of affected arteries | <0.01 | <0.01 | ||||
| 0 | 67 (100) | 7 (3.0) | 7 (4.5) | 0 (0) | ||
| 1 | 0 | 42 (18.3) | 39 (25.2) | 3 (4.0) | ||
| 2 | 0 | 72 (31.3) | 54 (34.8) | 18 (24.0) | ||
| 3 | 0 | 109 (47.4) | 55 (35.5) | 54 (72.0) | ||
| Stenosis location | ||||||
| Left main artery | 0 | 23 (10.0) | <0.01 | 4 (2.6) | 19 (25.3) | <0.01 |
| Left anterior descending artery | 0 | 205 (89.1) | <0.01 | 133 (85.8) | 72 (96.0) | 0.02 |
| Circumflex coronary artery | 0 | 140 (60.9) | <0.01 | 74 (47.7) | 66 (88.0) | <0.01 |
| Right coronary artery | 0 | 168 (73.0) | <0.01 | 105 (67.7) | 63 (84.0) | <0.01 |
| Laboratory data | ||||||
| ALT, units/L | 20.15 ± 8.77 | 22.71 ± 12.49 | 0.25 | 23.06 ± 13.58 | 21.95 ± 9.83 | 0.80 |
| AST, units/L | 19.94 ± 7.01 | 22.03 ± 11.59 | 0.27 | 22.45 ± 11.65 | 21.15 ± 11.5 | 0.69 |
| Total cholesterol, mmol/L | 4.75 ± 1.03 | 4.38 ± 1.08 | 0.05 | 4.36 ± 1.07 | 4.43 ± 1.10 | 0.88 |
| Triglycerides, mmol/L | 1.40 ± 0.71 | 1.65 ± 1.07 | 0.19 | 1.63 ± 1.20 | 1.68 ± 0.74 | 0.94 |
| HDL-C, mmol/L | 1.21 ± 0.30 | 1.06 ± 0.26 | <0.01 | 1.11 ± 0.27 | 0.96 ± 0.19 | <0.01 |
| LDL-C, mmol/L | 2.92 ± 0.76 | 2.71 ± 0.83 | 0.10 | 2.67 ± 0.82 | 2.81 ± 0.85 | 0.48 |
| Apo-A1, g/L | 1.26 ± 0.22 | 1.20 ± 0.21 | <0.01 | 1.23 ± 0.21 | 1.14 ± 0.20 | <0.01 |
| Apo-B, g/L | 0.84 ± 0.20 | 0.83 ± 0.23 | 0.10 | 0.80 ± 0.23 | 0.87 ± 0.22 | 0.08 |
| Apo-E, mg/L | 42.2 ± 9.97 | 39.67 ± 11.04 | 0.15 | 40.18 ± 11.48 | 38.55 ± 10.01 | 0.56 |
| Blood glucose, mmol/L | 4.97 ± 0.96 | 5.61 ± 1.81 | <0.01 | 5.45 ± 1.64 | 5.96 ± 2.10 | 0.08 |
| HbA1c, % | 5.75 ± 0.84 | 6.21 ± 1.26 | <0.01 | 6.03 ± 0.93 | 6.59 ± 1.71 | <0.01 |
| creatinine, μmol/L | 77.51 ± 14.88 | 85.63 ± 16.4 | <0.01 | 83.53 ± 15.92 | 90.09 ± 16.61 | 0.01 |
| hs-CRP, mg/L | 2.85 ± 5.47 | 4.6 ± 9.70 | 0.35 | 4.43 ± 10.10 | 4.95 ± 8.85 | 0.92 |
| Fibrinogen, g/L | 3.07 ± 0.82 | 3.17 ± 0.89 | 0.05 | 3.07 ± 0.78 | 3.36 ± 1.06 | 0.06 |
| hs-TnT, ng/L | 9.47 ± 9.88 | 15.53 ± 27.3 | <0.01 | 9.86 ± 8.09 | 27.25 ± 44.35 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Y.; Fan, J.; Chen, Z.; Li, X.; Wu, M.; Liu, W.; Zhou, S.; Rasmussen, M.A.; Engelsen, S.B.; Chen, Y.; et al. Alterations of NMR-Based Lipoprotein Profile Distinguish Unstable Angina Patients with Different Severity of Coronary Lesions. Metabolites 2023, 13, 273. https://doi.org/10.3390/metabo13020273
Ye Y, Fan J, Chen Z, Li X, Wu M, Liu W, Zhou S, Rasmussen MA, Engelsen SB, Chen Y, et al. Alterations of NMR-Based Lipoprotein Profile Distinguish Unstable Angina Patients with Different Severity of Coronary Lesions. Metabolites. 2023; 13(2):273. https://doi.org/10.3390/metabo13020273
Chicago/Turabian StyleYe, Yongxin, Jiahua Fan, Zhiteng Chen, Xiuwen Li, Maoxiong Wu, Wenhao Liu, Shiyi Zhou, Morten Arendt Rasmussen, Søren Balling Engelsen, Yangxin Chen, and et al. 2023. "Alterations of NMR-Based Lipoprotein Profile Distinguish Unstable Angina Patients with Different Severity of Coronary Lesions" Metabolites 13, no. 2: 273. https://doi.org/10.3390/metabo13020273
APA StyleYe, Y., Fan, J., Chen, Z., Li, X., Wu, M., Liu, W., Zhou, S., Rasmussen, M. A., Engelsen, S. B., Chen, Y., Khakimov, B., & Xia, M. (2023). Alterations of NMR-Based Lipoprotein Profile Distinguish Unstable Angina Patients with Different Severity of Coronary Lesions. Metabolites, 13(2), 273. https://doi.org/10.3390/metabo13020273











