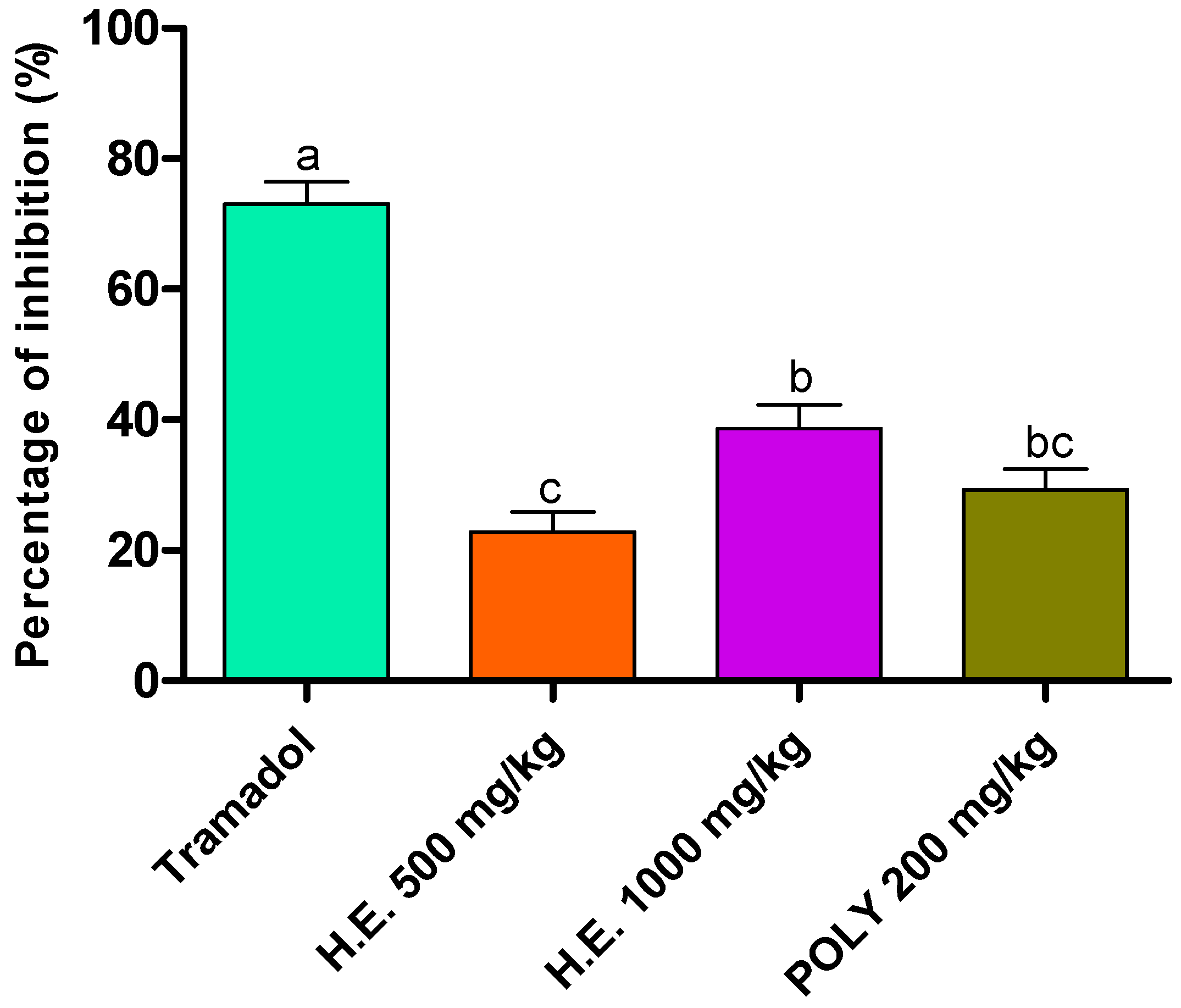Abstract
Parsley (Petroselinum sativum Hoffm.) is renowned for its ethnomedicinal uses including managing pain, wound, and dermal diseases. We previously highlighted the estrogenic and anti-inflammatory properties of parsley and profiled the phytochemistry of its polyphenolic fraction using HPLC-DAD. To extend our investigation, we here characterized the phytochemical composition of the hydro-ethanolic extract using LC-MS/MS and GC-MS upon silylation, and evaluated the antioxidant, analgesic, antimicrobial, and wound healing activities of its hydro-ethanolic and polyphenolic fraction. The antioxidant property was assessed using FRAP, DPPH, and TAC assays. The antimicrobial activity was tested against four wound infectious microbes (Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans). The analgesic effect was studied using acetic acid (counting the number of writhes) and formalin (recording the licking and biting times) injections while the wound healing activity was evaluated using burn model in vivo. The LC-MS/MS showed that the hydro-ethanolic contains four polyphenols (oleuropein, arbutin, myricetin, and naringin) while GC-MS revealed that it contains 20 compounds including malic acid, D-glucose, and galactofuranoside. The hydro-ethanolic (1000 mg/kg) decreased abdominal writhes (38.96%) and licking time (37.34%). It also elicited a strong antioxidant activity using DPPH method (IC50 = 19.38 ± 0.15 µg/mL). Polyphenols exhibited a good antimicrobial effect (MIC = 3.125–12.5 mg/mL). Moreover, both extracts showed high wound contraction by 97.17% and 94.98%, respectively. This study provides evidence that P. sativum could serve as a source of bio-compounds exhibiting analgesic effect and their promising application in mitigating ROS-related disorders, impeding wound infections, and enhancing burn healing.
1. Introduction
Pain and inflammation are strongly involved in the healing process of the wound. Therefore, conventional medicines are often used to mitigate the intensity of pain and inflammation [1]. After an injury, the skin regenerates through the process of wound healing. The interaction between several cellular elements (fibroblasts, keratinocytes, endothelial cells, and macrophages/monocytes), and the constituents of the extracellular environment (collagen and fibronectin) during the wound healing phases, promote the wound contraction, and restore tissue integrity [2]. The wound healing provides the ideal micro-environment at the injured surface to achieve the maximum skin repair [3]. However, several disorders may affect the ability of healing such as mechanical stresses, toxic agents, or infections. In most situations, the molecular events that accelerate the nociception response are similar whether the pain is of extrinsic or intrinsic origin [2].
By reacting with biological components such as proteins and nucleic acids, excessive levels of reactive oxygen species (ROS) disrupt intrinsic tissue disability and lead to loss of function [4,5]. For instance, high concentrations of H2O2 can cause oxidative damage and thus delay healing, while low concentrations can act as a signaling molecule and promotes healing. However, all the advancements achieved against oxidative stress, the production of ROS during injuries, and their impact on the healing process still constitute a major health challenge [6].
Parsley, whose scientific name is Petroselinum sativum Hoffm., is a plant belonging to the Apiaceae family. Currently, it is cultivated all over the world and has been used as food, cosmetic ingredient, and for pharmaceutical purposes [7]. Parsley has several biological and pharmacological activities, mainly spasmolytic, antioxidant, immunomodulating, gastrointestinal, and antidiabetic attributes [8]. These various virtues are due to its bioactive phytoconstituents such as carotenoids, flavonoids, coumarin, and vitamins [9].
Several previous investigations have stated that herbal and plant-based ingredients activate the wound and cutaneous healing process. These include many medicinal and aromatic plants (MAP) such as turmeric (Curcuma longa), centella (Centella asiatica), tree peony (Paeonia suffruticosa), and aloe vera (Aloe barbadensis) [10,11]. Additionally, in traditional medicine, Petroselinum species including P. sativum were reported to be used in Anatolia, Turkey for wound healing purposes [12] as well as in treating some dermal diseases [13,14]. However, very limited studies were devoted to providing scientifically sound data to test this claim. Very recently, Thangavelu et al. (2022) showed that the leaf methanolic extracts of P. crispum, whose synonym is P. sativum, exhibited potent wound healing and anti-inflammatory activities on the human lung cancer cell lines by enhancing cell migration [15]. Interestingly, in another study, parsley was used as a maintenance diet for cutaneous closure of wounds in rabbits as a postoperative care after the surgery [16]. Elsewhere, P. crispum was also investigated for immunomodulatory and wound healing activities [17]. Moreover, the analgesic use of parsley in folklore medicine was also reported and demonstrated in vivo using seeds hydroalcoholic extract [14,18]. Other studies corroborated the same findings [19,20].
The growing antimicrobial resistance of microbes responsible for skin infections blew up the research on the potential of MAP preparations in antimicrobial therapeutics. In fact, many phytochemicals are shown to be effective against microbial infections [21,22]. In wounds, the skin barrier is breached and becomes susceptible to microbial infections by bacteria, fungi, and/or viruses, that delay the healing process [23]. These pathogens include Gram-positive bacteria such as Staphylococcus aureus and Streptococcus pyogenes, and Gram-negative bacteria including Escherichia coli, Pseudomonas aeruginosa, and Klebsiella species, and fungi—mainly Candida and Aspergillus [24]. Hence, minimizing the predisposition of wounds to infections is required in wound surveillance to reduce the infection rate [25].
We previously explored the estrogenic as well as the anti-inflammatory activities of P. sativum Hoffm. in vivo and characterized the chemical composition of its polyphenolic fraction [26]. Recognizing the promising therapeutic potential of this plant species and to follow up on our previous findings, we conducted this investigation that aimed to (1) annotate the phytoconstituents of the hydro-ethanolic extract of P. sativum Hoffm. using both LC-MS/MS and GC-MS to identify the maximum number of phytocompounds and (2) monitor the analgesic, antioxidant, antimicrobial, and burn healing effects of both the hydro-ethanolic extract and polyphenolic fraction of the plant. This study is the first to demonstrate the pharmacological relevance of parsley as a source of wound healing and analgesic biochemicals.
2. Materials and Methods
2.1. Plant Material
Aerial parts of P. sativum Hoffm. were collected before sunrise in the Taounate region (north of Morocco). The botanical name was checked by Pr. Bari Amina, at the laboratory of Biotechnology, Environment, Agro-Food, and Health, at Sidi Mohamed Ben Abdellah University. A sample was deposited under a voucher number (18TA5001) at the herbarium of the Faculty of Sciences-Fez.
2.2. Animal Material
In this study, we used Swiss albino mice rearing in an animal house that has a relative humidity of 50 to 55%, with an average temperature of 22 ± 2 °C, and a day/night cycle of about 12/12 h. Animals were given free access to water and food. The average weight of mice used was 30 ± 4 g. The handling and manipulation of animals were up to the standards of the directive EEC/86/EEC of the European community [26,27]. The experiment was approved by the institutional ethical committee of the Faculty of Sciences Dhar El Mehrez, Sidi Mohamed Ben Abdallah Fez University, Morocco (#04/2019/LBEAS).
2.3. Preparation of the Hydro-Ethanolic Extract
Aerial parts were left to the laboratory to dry for one week, then grinded using a blender. The grind (30 g) was macerated in 210 mL of ethanol 70% for three days [26]. The macerate was filtered, evaporated, and dried (rotary evaporator, 37 °C) [26,28].
2.4. Preparation of the Polyphenolic Fraction
One hundred grams of well ground P. sativum powder was subjected to three extractions in methanol (300 mL × 3) at 50 °C for 3 h. Subsequently, solvent was evaporated, and the obtained extract was dissolved in distilled water (500 mL) and extracted in hexane (200 mL × 3) and then in chloroform (200 mL × 3) to remove caffeine and chlorophyll residues. Next, the aqueous phase was extracted in ethyl acetate (200 mL × 3) which was evaporated later. Using 300 mL of distilled water, the residue was dissolved and then lyophilized [29,30].
2.5. Phytochemical Analysis by LC-MS/MS
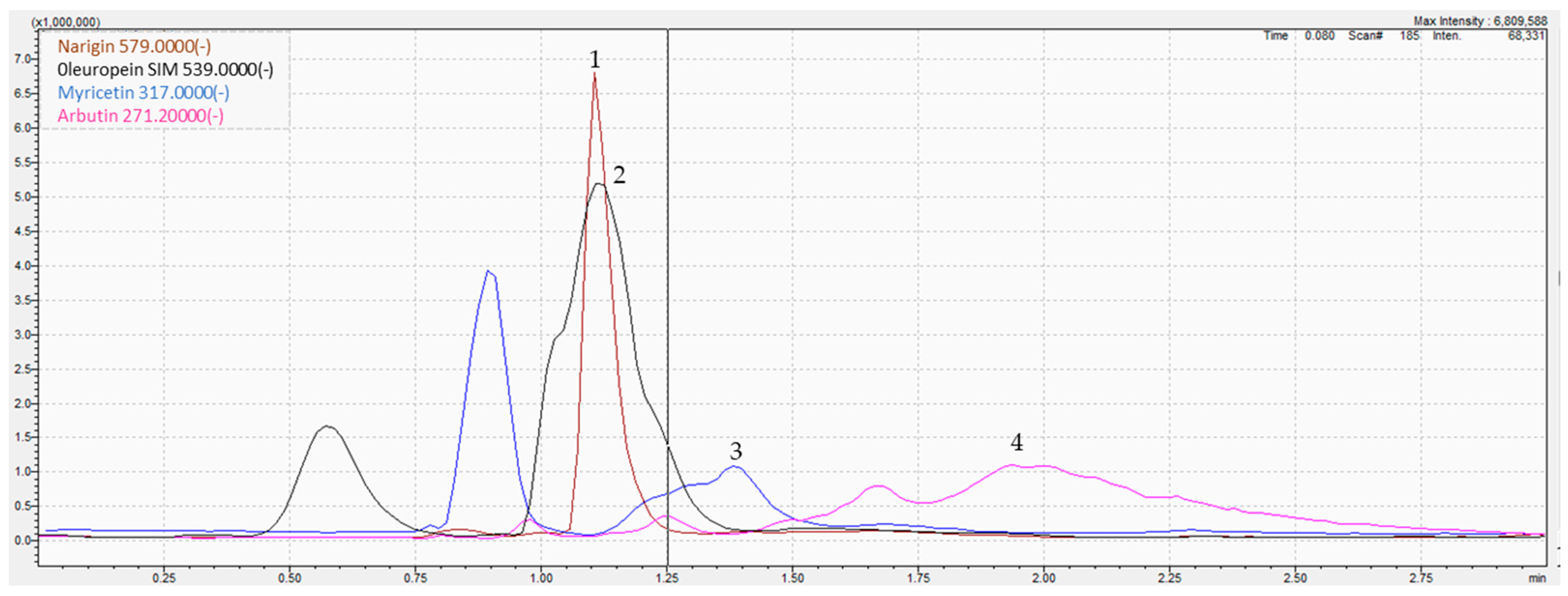
The chemical profiling of the hydro-ethanolic extract of P. sativum was performed using ultra-high performance liquid chromatography (Shimadzu, Nexera XR LC 40) coupled with mass spectrometry (LCMS 8060, Shimadzu Italy, Milan, Italy). The heating and nebulization gas flow was set to 10 and 2.9 L/min, respectively. The drying gas flow was at 10 L/min, the DL temperature was at 250 °C, the heating block temperature was 400 °C, and the interface temperature was 300 °C. Separation of compounds was carried out using C18 column, 3 × 100 mm, 2.6 μm (Phenomenex, Torrance, CA, USA). The mobile phase consisted of acetonitrile (A) and water containing 0.01% formic acid (B). The extract was added to acetonitrile and water (1:1) and then diluted (1/50) in acetonitrile and injected [31]. The ion currents’ acquisition was carried out in single ion monitoring (MRM) mode in negative ESI ionization. The analyzed molecular adducts were, respectively, 579, 539, 317, and 271.2 for Naringin, Oleuropein, Myricetin, and Arbutin.
2.6. Phytochemical Analysis by GC-MS
Phytochemical identification of the hydro-ethanolic extract of P. sativum was carried out using GC-MS after silylation. This latter is based on dissolving 1 mg of the grind in 100 mL of HMDS-TMCS-Pyridine 3:1:9 (v/v/v) reagent. After 30 min incubation [32], the extract was injected into the GC-MS apparatus (Agilent Technologies MASS Selective Detector, 5973 Network) with a capillary column Agilent 19091S-433 model, 30 m in nominal length—0.25 mm in diameter and 0.25 μm thick. Helium served as the carrier gas, and the total flow of 31.4 mL/min and a split ratio of 30:1 was used. The temperature program was between 60 and 300 °C and maintained for 20 min of the run time. The detector temperature was set to 260 °C. Splitless injection was used [33].
2.7. Determination of Total Phenol and Flavonoid Contents
The quantification of total phenol content (TPC) and total flavonoid content (TFC) was carried out calorimetrically using the methods described by Slinkard and Singleton (1977) [34]. TPC content was expressed in milligrams (mg) of gallic acid equivalents per gram (g) of dry weight of extract (mg GAE/g DW) while the values of TFC were expressed in mg of quercetin equivalent per g of dry extract (mg EQ/g DW).
2.8. Assessment of Antioxidant Activity
2.8.1. Scavenging of the Free Radical (DPPH)
In this study, the DPPH test performed by Brand-Williams in 1995 [35] was followed. Methanol (100 μL) was mixed with 750 μL of DPPH solution, incubated for 30 min, and then absorbance was measured at OD517nm. BHT (butylated hydroxytoluene) was used as the standard antioxidant. To calculate the percentage of inhibition (IP) of DPPH, the following formula was used:
- IP: Inhibition Percentage.
- A0: OD of DPPH solution in the absence of the extract (negative control).
- A: OD of DPPH solution containing the extract.
2.8.2. Ferric Reducing Antioxidant Power (FRAP)
The FRAP assay was performed according to the method described by Oyazu (1986) [36]. Briefly, 200 µL of each extract was mixed with the buffer solution (0.5 mL) (0.2 M, pH = 6.6) and potassium ferricyanide [K3Fe (CN)6] at 1% (0.5 mL). The solution was kept at 50 °C in a water bath for 20 min. Next, to acidify to solution, 500 µL of trichloracetic acid at 10% was added to the solution and centrifuged for 10 min at 3000 rpm. Five hundred microliter of the top layer of the solution was mixed with distilled water (500 µL) and FeCl3 0.1% (100 µL). Ascorbic acid was used as standard, and the absorbance was read at OD700nm. The values were expressed as EC50 (mg/mL). The EC50 was calculated using the standard curve.
2.8.3. Total Antioxidant Capacity Test (TAC)
The TAC was evaluated by mixing 25 µL of every studied extract with 1 mL of liquid reactive solution (28 mM Na3PO4, 0.6 M H2SO4, and 4 mM (NH4)2MoO4). Following incubation (90 min, 95 °C), the absorbance values were read at OD695nm. The antioxidant potential was determined in mg of equivalent of ascorbic acid per gram of extracts (mg EAA/g of extracts) [37,38].
2.9. Antimicrobial Activity
The extracts were evaluated for their potential antimicrobial effect against three human pathogenic strains including one Gram-negative bacterium (Pseudomonas aeruginosa CECT118), one Gram-positive bacterium (Staphylococcus aureus CECT976), and one fungal species (Candida albicans ATCC 10231). The antimicrobial susceptibility of the three strains was evaluated using agar well diffusion and broth dilution methods.
2.9.1. Agar Well Diffusion Assay
The method of diffusion in agar wells was used to carry out the qualitative test, a widely-known assay to check the antimicrobial activity of herbal extracts [39,40]. From fresh overnight cultures, microbial suspensions were prepared and adjusted to 0.5 McFarland corresponding to 106 CFU/mL [41,42]. Afterwards, 5 mL of soft agar (agar 4 g/L) inoculated with 100 µL of the microbial suspension (106 CFU/mL) of each strain were poured over the surface of each plate. After solidification, wells of 8 mm diameter were aseptically punched using glass Pasteur pipets. Then, 50 μL of each extract was dissolved in the appropriate solvent (40 mg/mL) and introduced into the appropriate wells [43]. Under the same conditions, the controls were established using the solvent only. The Petri dishes were then incubated at 37 ± 2 °C for 48 h. The extracts diffuse through the agar medium and the formation of inhibition zones surrounding the wells indicate positive antimicrobial activities. Streptomycin (1 mg/mL) and fluconazole (5 mg/mL) served as positive controls for bacteria and fungi, respectively.
2.9.2. Determination of the Minimum Inhibitory Concentration (MIC)
The MIC of the hydro-ethanolic and polyphenolic extract was checked using microdilution assays according to the standards of the NCCLS [44] in 96-well microtiter microplates. First, 50 μL of the culture broth was introduced into each well of the microplates. Next, seven concentrations of the hydro-ethanolic extract and the polyphenolic fraction (0.78–50 mg/mL), streptomycin and fluconazole (0.078–5 mg/mL) were prepared in both LB and YPG in sterile haemolysis tubes. Each microplate well was inoculated by 100 µL of LB liquid culture medium for bacteria and YPG liquid culture medium for yeast strains, 50 µL of each extract, and then concentrations were carried out by successive two-fold dilutions. Afterwards, 50 μL of the microbial suspensions, whose turbidity was checked in the same way as described above, were inoculated into the microplate’s wells. The microplates were incubated at 37 °C for the bacterial strains and at 30 °C for Candida albicans ATCC 10231 under 150 agitation rpm for 24 h. By the end of the incubation, we added 20 µL of 2,3,5-triphenyltetrazolium chloride into each plate’s well and incubated the plate for 2 h. The formation of a pinkish coloration indicates that the growth is due to the activity of the dehydrogenases. The MIC corresponds to the lowest concentration that does not produce a red colour [45].
2.10. Analgesic Activity In Vivo
2.10.1. Abdominal Writhes
Five groups containing five mice each (25 mice) were prepared. The control mice were given 10 mL/kg of NaCl 0.9% and Tramadol (10 mg/kg) was used as a standard (reference drug) [46]. The other groups received two doses of the hydro-ethanolic extract (500–1000 mg/kg) and one dose from the polyphenolic fraction (200 mg/kg). One hour later, 1% acetic acid was injected by intraperitoneal route at 10 mL/kg rate. Ten minutes later, the number of writhes were determined over a duration of 20 min [47,48].
2.10.2. Formalin Induced Pain
First, we injected 10% formalin (20 mL) into the right posterior paw of each animal. With the help of a stopwatch, we recorded the licking and biting times. The initial nociceptive response is the sum of the seconds passed in licking and biting from 0 to 5 min after the injection of formalin while the second phase was from 15 to 30 min [49,50]. Half an hour beforehand, the animals were subjected to oral prior treatment by the test extract, NaCl or Tramadol [51].
2.11. Wound Healing Activity In Vivo
2.11.1. Ointments Preparation
The preparation of the ointment was carried out at 10% (w/w) by adding 1 g of the extract to 9 g of Vaseline, and melted using a bain-marie at a temperature of 50 °C. After homogenization, the preparations were kept at 4 °C in sealed containers [4].
2.11.2. Induction of Burn Injuries
In this experiment, 4 groups containing 5 rats each were prepared. The first group represents the negative control (Vaseline), the second group represents the positive control (Madecassol (1%)), and the third and fourth groups represent the groups treated with the hydro-ethanolic and polyphenolic extracts of P. sativum Hoffm., respectively. According to the protocol of Heidari et al., (2019), after anesthesia of rats with pentobarbital (50 mg/kg) and shaving the dorsal part with an electric clipper [52], the induction of the burn was carried out on the shaved part using an aluminum rod (1.7 cm) heated to 110 °C for 10 s. After 24 h, the treatment was started by applying the ointments to the burned zones for 25 days while photographing the healing progress using a digital camera and the ruler used as a scale. ImageJ® software was used to analyze the images and measure the rate of wound contraction using the following formula [53]:
- WC (%) = Rate of wound contraction.
- WS0 = Size of the wound at the first day.
- WSSD = Size of the wound at each specific day.
2.12. Statistical Analysis
Results obtained from each experiment were treated by using a one-way ANOVA followed by the post-hoc analysis with Tukey’s test in GraphPad Prism 6 software. Values were expressed as mean ± SD and the significance level was set at “p < 0.05”. The significant differences between treatments were shown using different superscript letters (a, b, c, etc.).
3. Results
3.1. Phytochemical Analysis by LC-MS/MS
The determination of parsley phytoconstituents was performed according to the molecular weight of the fragments generated. The analysis of the hydro-ethanolic extract revealed the presence of four molecules namely oleuropein, arbutin, myricetin, and naringin, all classified as polyphenols (Figure 1 and Table 1). For instance, negative ion ESI-MS/MS spectra of oleuropein resulted in the formation of m/z 539, a pseudomolecular ion, as the sole base peak of the ESI-MS spectra, while the MS/MS products were abundant (e.g., 307 and 275). Similarly, myricetin (m/z 317) was fragmented to four main products (150.8, 178.8, 270.9, and 286.9). The MS/MS spectra of identified compounds, annotations, and their characteristic fragmentation patterns are presented in Table 1. XIC chromatograms of identified compounds are provided in the Supplementary Material S1.
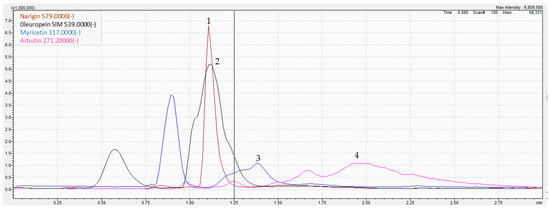
Figure 1.
LC-MS/MS chromatogram of the hydro-ethanolic extract of P. sativum. (1) Naringin, (2) Oleuropein, (3) Myricetin, (4) Arbutin.

Table 1.
LC-MS/MS identification composition of P. sativum Hoffm. hydro-ethanolic extract.
3.2. Phytochemical Analysis by GC-MS
Silylation is the appropriate method to check for thermolabile and non-volatile phytochemicals by GC-MS. It consists of replacing the active hydrogen in =NH, –NH2, –OH, –COOH, or –SH by a trimethylsilyl group. This showed that the hydro-ethanolic of P. sativum contained twenty compounds with a total of 99.9%. According to the area percentage, the most dominant compounds were malic acid (13.52%), D-glucose (13.529%), D-mannitol (10.957%), and talose (9.758%) (Table 2).

Table 2.
Identified compounds by GC-MS in the hydro-ethanolic extract of P. sativum Hoffm.
3.3. Estimation of Total Phenol and Flavonoid Contents
The total phenols and flavonoids contents contained in the hydro-ethanolic extract of P. sativum were 34.55 ± 3.74 mg GAE/g of extract and 16.46 ± 0.06 mg QE/g of extract, respectively.
3.4. Antioxidant Activity
3.4.1. DPPH and FRAP Assays
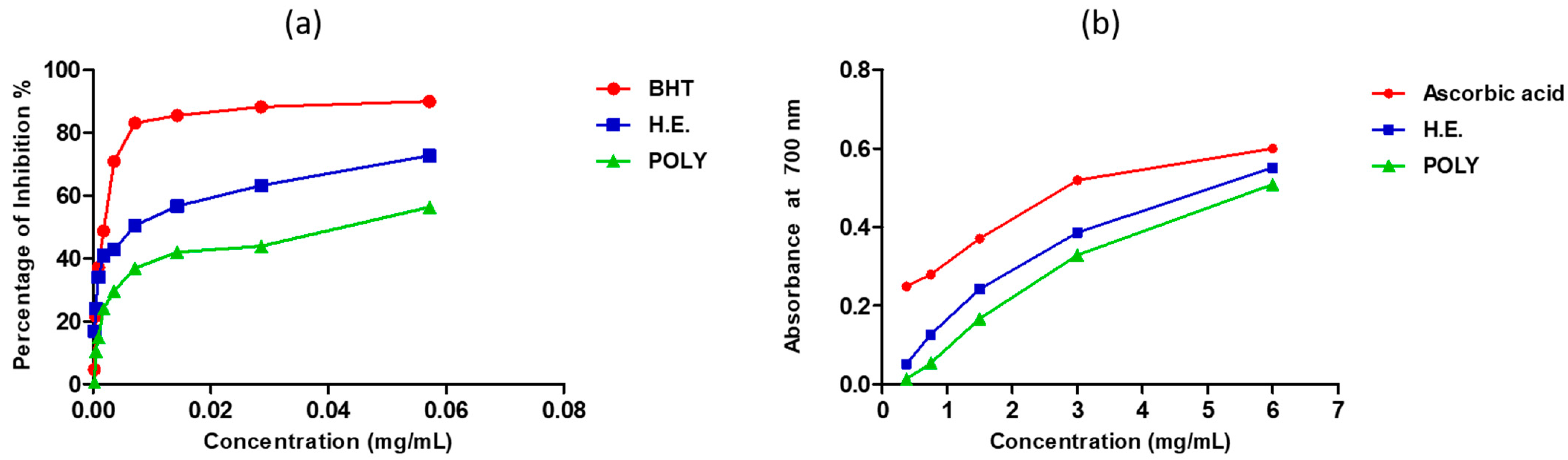
The antioxidant activity of our extracts was tested by DPPH and FRAP tests. The inhibition of the free radical DPPH by the hydro-ethanolic was greater than that of the polyphenols (Figure 2a). The 50% inhibition concentration (IC50) of the hydro-ethanolic extract was seen at 19.38 ± 0.15 µg/mL, and that of the polyphenols was obtained at 40.36 ± 1.47 µg/mL (Table 3). However, these results are significantly lower than those obtained using butylated hydroxytoluene (BHT) (IC50 = 1.97 ± 0.1 µg/mL) (Figure 2a and Table 3). The ferric reducing power of our extracts showed that hydro-ethanolic extract was more effective than polyphenols, but the potential of both extracts was slightly lower than that of the standard antioxidant ascorbic acid (Figure 2b & Table 3).
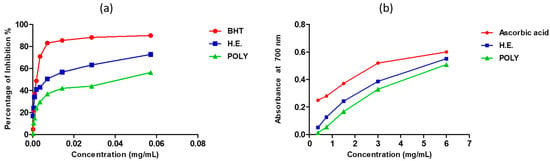
Figure 2.
Antioxidant activity of the hydro-ethanolic and polyphenols extracts of P. sativum Hoffm. using DPPH (a) and FRAP (b) assays.

Table 3.
Antioxidant activity of hydro-ethanolic and polyphenols of P. sativum Hoffm. using DPPH and FRAP methods.
3.4.2. Total Antioxidant Capacity (TAC)
TAC assay showed that the hydro-ethanolic has a greater total antioxidant capacity (175.2 ± 6.360 mg EAA/g) comparing to polyphenols (148.2 ± 13.86 mg EAA/g).
3.5. Antimicrobial Activity
The antimicrobial assay on the plate showed that the hydro-ethanolic extract elicited an antibacterial and anti-fungal activity against P. aeruginosa and C. albicans, respectively, while S. aureus was showed to be resistant. In contrast, the polyphenolic fraction exhibited a higher inhibition zone diameter against the three tested pathogens with a noticeable inhibitory effect toward P. aeruginosa (Table 4). Nevertheless, positive controls, streptomycin (1 mg/mL) and fluconazole (5 mg/mL), were relatively more potent comparatively to the tested extracts.

Table 4.
Inhibition zone diameters of P. sativum Hoffm. extracts tested against bacterial and fungal species.
The antimicrobial effects against the three species were noticed at MIC values ranging from 3.125 to 12.5 mg/mL. The polyphenols were the most active as they inhibited the three species with a prominent effect towards S. aureus (MIC = 3.125 mg/mL). Worth noting is that the broth dilution assay corroborated the non-toxic effect of the hydro-ethanolic extract against S. aureus even at the highest concentration tested, 50 mg/mL (Table 5).

Table 5.
MIC of P. sativum Hoffm. fractions tested against bacterial and fungal species.
3.6. Analgesic Activity
3.6.1. Abdominal Writhes
Analgesic activity was evaluated using acetic acid method. Although less efficient than the standard drug (Tramadol), the hydro-ethanolic (1000 mg/kg) and polyphenols (200 mg/kg) induced a significant decrease in the number of writes by 38.96 and 29.23%, respectively, followed by the hydro-ethanolic extract at 500 mg/kg by 23.07% as compared to the negative control animals which we have taken as reference to calculate the percent of inhibition (Table 6 and Figure 3).

Table 6.
Effect of P. sativum Hoffm. on acetic acid-induced writhing in mice (n = 5).
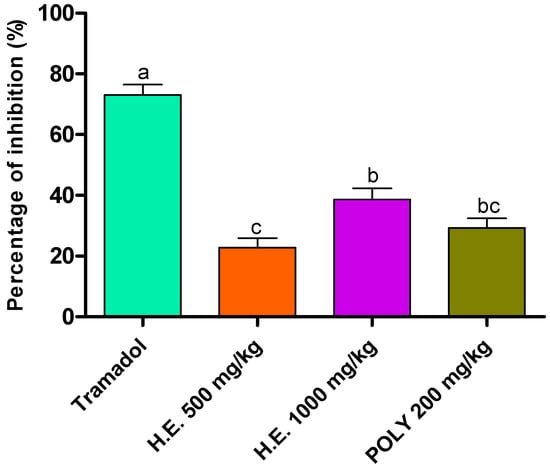
Figure 3.
Inhibitory effect of the hydro-ethanolic (H.E.) and polyphenolic (Ploy) extracts of P. sativum Hoffm. and Tramadol (positive control) on contortions in mice. The different letters in superscript (a, b, c) indicate the significant difference between treatments at p < 0.05.
3.6.2. Formalin Induced Pain
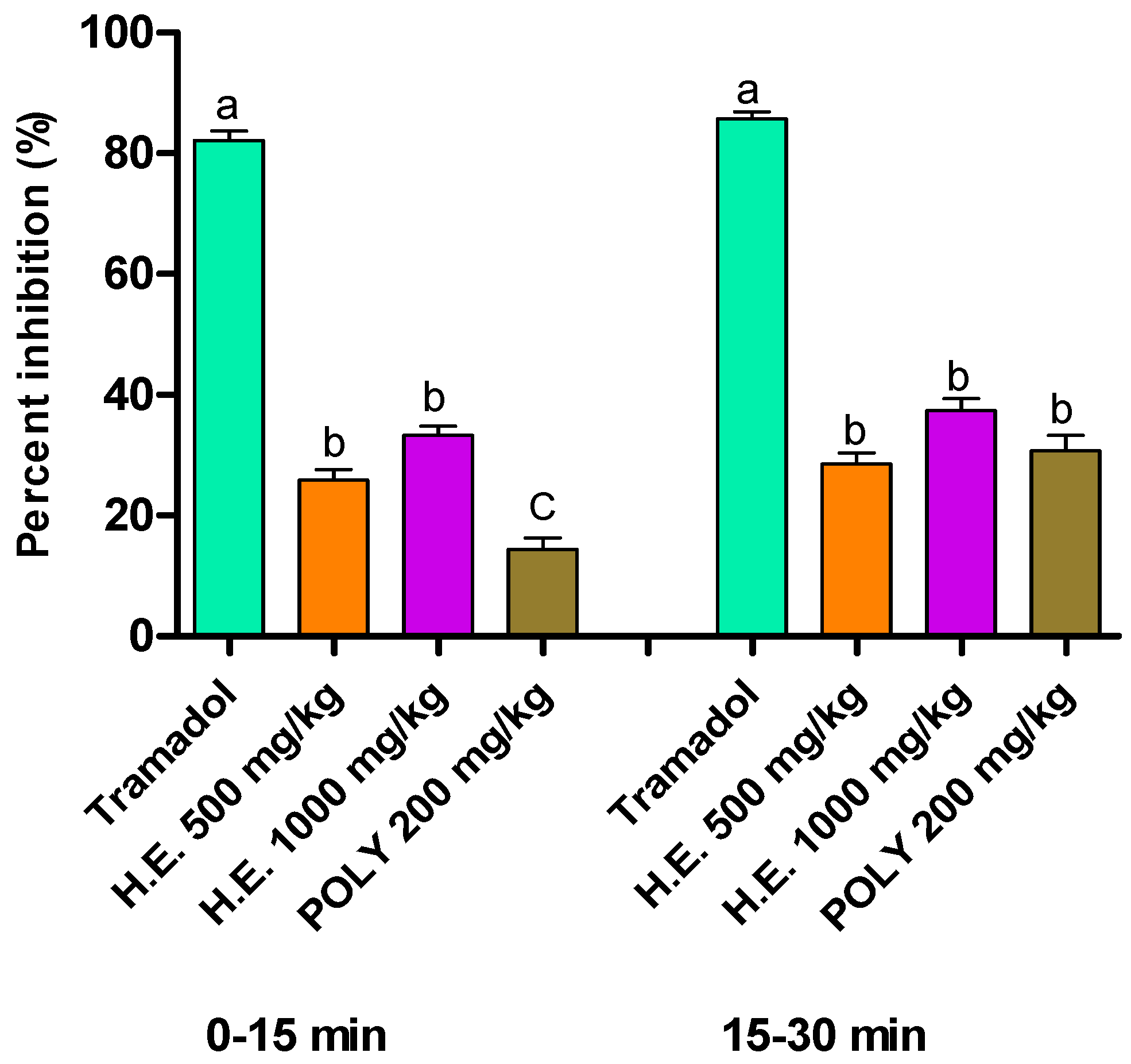
In the formalin test, the hydro-ethanolic extract at the dose 500 and 1000 mg/kg of P. sativum Hoffm. elicited a significant reduction in response to nociception during the first phases (0–5 min) and the second phases at the doses 500 and 1000 mg/kg of the hydro-ethanolic extract and polyphenols comparatively to the control mice. Worth noting is that Tramadol was the most potent in both phases by up to 85.7% reduction in response time. During the first phase, hydro-ethanolic extract reduced the response time by 33.32% and 25.86% using 1000 and 500 mg/kg, respectively. In the second phase, both hydro-ethanolic and polyphenols extracts showed a significant reduction in response time by 28.55% using hydro-ethanolic at 500 mg/kg, 37.35% using hydro-ethanolic at 1000 mg/kg, and 30.76% using polyphenols at 200 mg/kg (Table 7 and Figure 4).

Table 7.
Effect of the hydro-ethanolic and polyphenolic extracts of P. sativum Hoffm. on the response of mice upon the formalin-induced pain.
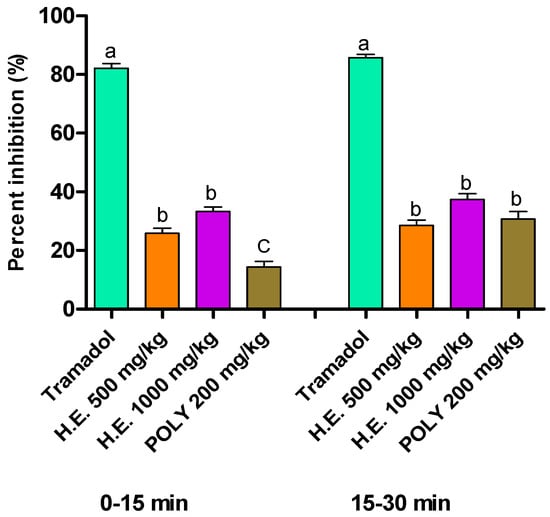
Figure 4.
Percent of inhibition of formalin-induced pain in mice following the application of hydro-ethanolic (H.E.) and polyphenolic (Ploy) extracts of P. sativum Hoffm. and Tramadol (positive control). Inhibition rates were determined compared to the non-treated animals. The different letters in superscript (a, b, c) indicate the significant difference between treatments at p < 0.05.
3.7. Wound Healing Activity
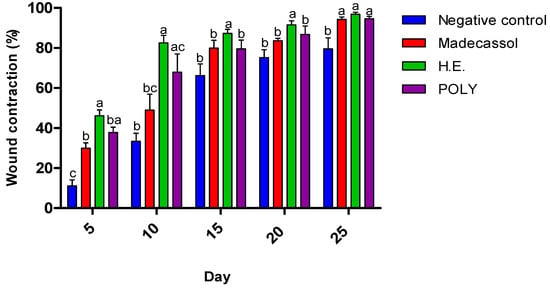
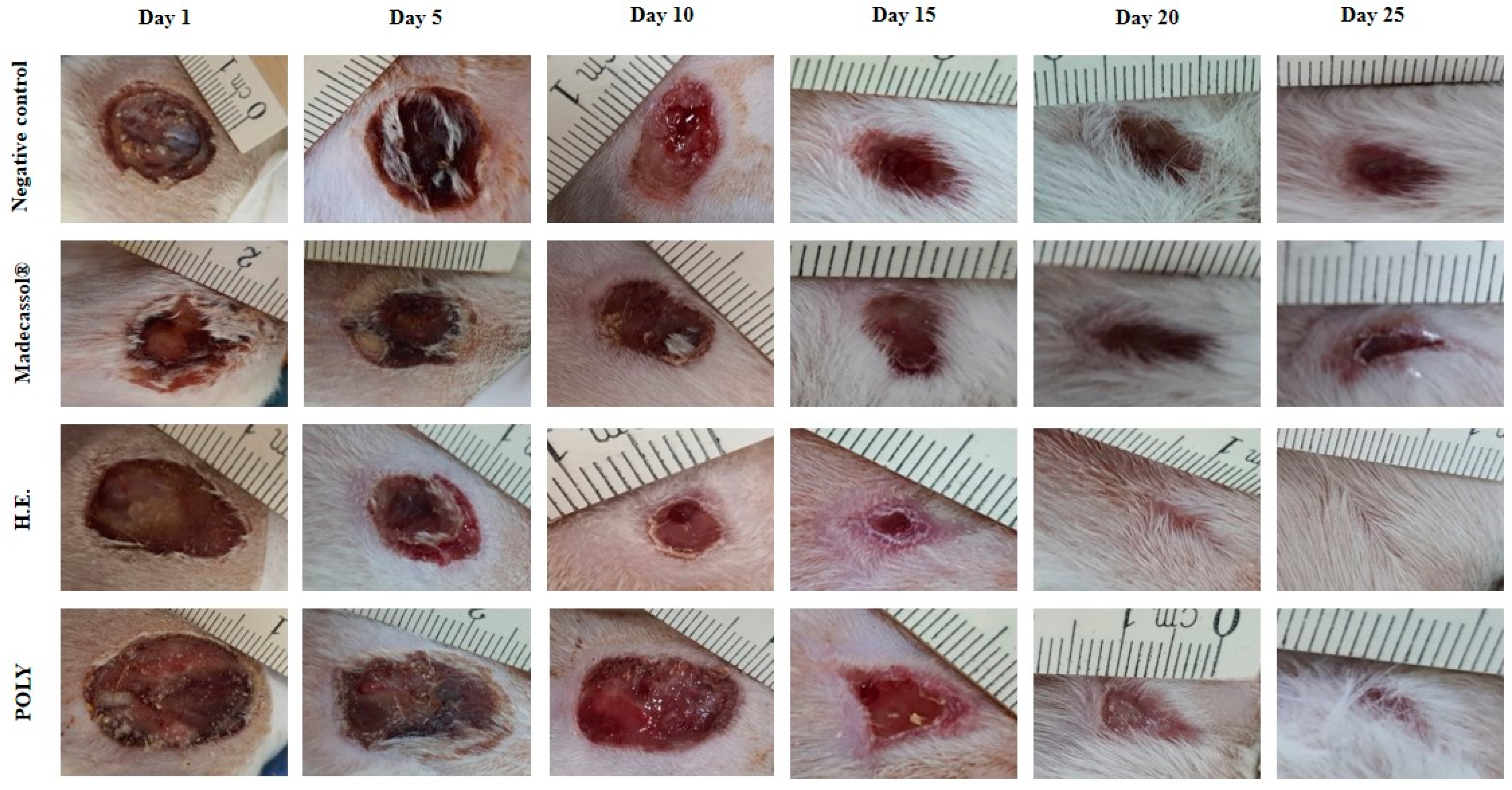
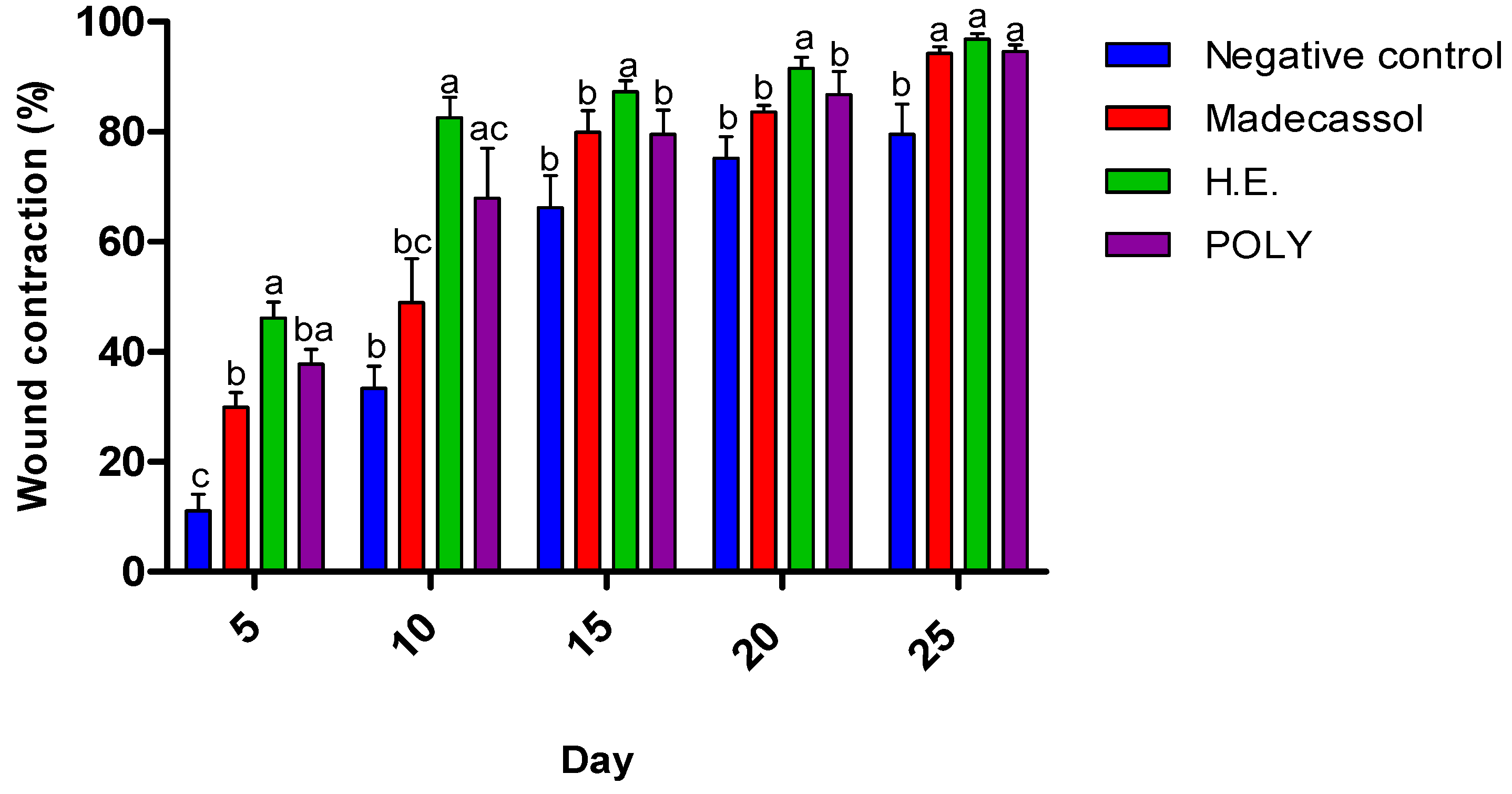
The effects of the ointments prepared from hydro-ethanolic and polyphenols extracts are presented in Table 8. We showed that both applied extracts induced a significant healing activity as compared the untreated animals. Figure 5 illustrates the healing progression using the extracts and the controls from the 1st to the 25th day. The negative control group (Vaseline®), positive control group (Madecassol®), and polyphenols did not induce a complete wound closure. In contrast, the hydro-ethanolic extract induced a complete cicatrization of the wound at the 20th day.

Table 8.
Wound size (cm2) of the hydro-ethanolic and polyphenols extracts of P. sativum Hoffm. from day 1 till day 25.
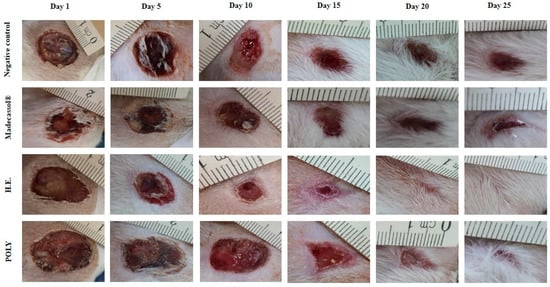
Figure 5.
Morphological aspects of cutaneous burn healing process upon application of the hydro-ethanolic (H.E) and Poly (Polyphenols) extracts of P. sativum Hoffm. and the control groups during 25 days of treatment. The rate of the healing activities induced by each application were compared to each other. As shown in Figure 6, the hydro-ethanolic and polyphenols extracts elicited a high wound contraction at the 5th day by 46.45% and 38.7%, respectively. At the 25th day, the hydro-ethanolic extract elicited the highest healing (up to 97.17%), followed by the polyphenols and Madecassol ointment with almost the same percentage of inhibition (94.98% and 94.27%, respectively) while the negative control group was improved by only 80.62%.
4. Discussion
Parsley is a medicinal plant largely used a garnish and food-flavoring agent but also in traditional pharmacopoeia to treat several diseases such as inflammation, diabetes, cancer, digestive disorders, and kidney stones [14,54]. Nevertheless, many of these traditional applications remain to be discovered and proven. Here, we annotated the phytoconstituents of its hydro-ethanolic extract and studied the analgesic, antioxidant, and antimicrobial of the hydro-ethanolic and polyphenol extracts of parsley (P. sativum Hoffm.). Most importantly, we believe that this is the first study to assess the wound healing properties of P. sativum Hoffm. extracts using animal models.
The LC-MS/MS analysis of P. sativum corroborated the presence of some polyphenols known for their biological effects such as oleuropein (antioxidant, anti-atherogenic, antimicrobial, anti-cancer, antiviral activity, anti-inflammatory, hypoglycemic, and hypolipidemic activities) [55], arbutin (wound healing, anti-inflammatory, antioxidant, analgesic, anticancer, antiparkinsonic, and hypoglycemic effects) [56], myricetin (antimutagen, anti-ulcer, anticarcinogen, antioxidant, antibacterial, anti-diabetic, cardioprotective, anti-amyloidogenic, anti-inflammatory, and antiviral activities) [57,58], and naringin (anti-atherosclerosis, anti-diabetic, neuroprotective, cardioprotective, rheumatologic, and osteoporosis disorders). Moreover, the GC-MS analysis of the hydro-ethanolic extract of P. sativum Hoffm. showed the presence of some compounds renowned for their pharmacological activities like malic acid (anti-thrombotic) [59], D-glucose (anti-cancer, anti-diabetic) [60,61], carbazoles (antifungal) [62], and myo-inositol (against both male and female infertilities) [54]. The chemical compounds identified here are likely to be behind the biological and pharmacological activities demonstrated in this study. In line with other works, our results show the presence of phytochemicals in parsley such as alkaloids, polyphenols, and sugars that contribute to the structure of the flavonoid glycosides and amino acids [9,14,62].
The analgesic activity was tested by two protocols: abdominal writhes and formalin induced pain as described by Ganguly et al., 2016 [63]. Our findings suggest that the analgesic activity of P. sativum Hoffm. could be related to its effect on prostaglandin biosynthesis [64]. In fact, acetic acid induces the secretion of prostaglandins (PGE2 and PGE2α), partly involving peritoneal receptors and inflammatory discomfort [65]. Other studies showed the analgesic effect of the ethanolic extract of parsley prepared at 100, 150, and 200 mg/kg acts by decreasing both phases of pain using the formalin test [19]. Using this assay, there is two distinct biphasic nociceptive responses known as early and late phases [66]. Molecules that target precisely the central nervous system (CNS) can inhibit both phases by a similar mechanism. However, drugs acting on the peripheric nervous system (PNS) inhibit the late phases only [67,68]. The early phase could be triggered by the nociceptors induction in the paw reflecting the centrally mediated pain, while the late phase is activated by the release of pro-inflammatory agents such as serotonin, bradykinin, histamine, and prostaglandins [68,69]. However, this could be also due to the central nociceptive neuron’s activation [69,70].
Balance between the release of anti and pro-inflammatory cytokines and analgesic mediators induces the chronicity of pain [71,72]. In a recent study, we demonstrated the anti-inflammatory effect of the hydro-ethanolic and polyphenolic extracts from P. sativum Hoffm. [26]. As the healing process is strongly associated with the inflammatory response by the intervention phase of monocytes and neutrophils, the proliferation of epithelial cells and fibroblasts, the synthesis of collagen, and the action of keratinocytes fibroblasts [73]. We evaluated the potential healing activity of our extracts in rats and showed that application of ointments prepared from the hydro-ethanolic extract and polyphenolics induced a significant cicatrizing effect as compared the untreated animals. This is most likely due to the presence of bioactive compounds that support inflammation to repair lesions and accelerate cell regeneration in damaged tissues. In fact, several phytochemicals such as polysaccharides, alkaloids, and saponins have been demonstrated to have wound healing properties [74]. For instance, triterpenes isolated from Centella asiatica stimulated glycosaminoglycans synthesis and ameliorated collagen remodeling [75]. In addition, madecassoside from this plant administered orally improved both collagen synthesis and angiogenesis. Worth noting is that Arctium lappa L. was able to monitor adhesion of dermal fibroblasts and regulate their gene expression by targeting the Wnt/β-catenin signaling pathway which is well documented as a major wound regulator [76]. Other molecules including apigenin are known for controlling the wound healing process [77].
As nutrients are important factors in wound healing, many studies showed that nutrient shortage is likely linked to the delayed healing of wounds [78]. For instance, vitamin K is mandatory during the first phase (hemostasis). Its deficiency alters wound repair, hemorrhage, and infection [79]. Interestingly, parsley is known as one of the leafy green vegetables to be rich in vitamin K [78]. In addition, as malic acid is one of the most abundant molecules identified in this study, its action as a wound healing agent could corroborate a previous study that showed that purified fractions from the leaves of Sempervivum tectorum L. harboring high contents of malic acid promote cellular proliferation and migration [80,81]. Other studies have investigated the role of malic acid derived polymers on muscle regeneration and bone repair [82].
Flavonoid contents of P. sativum Hoffm. were different from those obtained by Pereira et al., (2014) [83] who showed a low TFC in the hydro-ethanolic extract of parsley. This can be explained by the difference in the extraction method used, geographical regions of growth, seasonal variations, harvesting time, and postharvest treatment [5]. Previous studies have shown similar results regarding the TFC of parsley (27.2 mg QE/g) [84,85]. Other works revealed many factors that can influence the content of TPC such as genetic and extrinsic factors namely climatic and geographic ones [86]. There is also the duration of storage, chemotype, and the degree of maturation of the plant which have a strong influence on the polyphenols contents [87].
Here, the antioxidant activity was monitored using DPPH, FRAP and TAC tests. The difference seen between the hydro-ethanolic extract and the polyphenolics could be related to the presence of other chemicals having antioxidant activity other than the polyphenols. As previously reported, this antioxidant ability can be due to the presence of malic acid in P. sativum Hoffm. [88,89]. Other studies have shown the significant antioxidant capacity of the aerial part of parsley due to the presence of flavonoid [90,91]. For instance, Marin et al. (2016) demonstrated that the water extract of parsley exhibited a low oxidation inhibitory effect using FRAP test, with an EC50 of 0.93 mmol/L [92,93]. Moreover, it has been proposed that a high TAC may be closely related to the presence of a high content of polyphenolics [94].
The antimicrobial activity of P. sativum Hoffm. polyphenolic fraction towards S. aureus, P. aeruginosa, and C. albicans highlights the potential use of this plant’s extract in treating bacterial and fungal wound infections. In fact, these pathogenic species along with others such as S. epidermidis, Escherichia coli, Klebsiella pneumonia, and Proteus are commonly isolated from infected wounds. Therefore, our findings provide useful information on the wound healing properties of plant-based chemicals through controlling infectious agents [95].
To explain the mechanisms of action of plant-derived antimicrobial compounds, several studies have attempted to correlate their antibacterial effect with their phytochemical composition [96]. Some scientists have suggested that the antibacterial action is associated with high concentrations of phenols, monoterpenes, aldehydes, and ketones that perturb the integrity of microbial membranes [97]. This may be due to the hydrophobic nature of some phytocompounds, allowing their accumulation on the cell membranes and disrupt their structure and function. This also weakens the microbial enzyme machinery, allows intracellular components to leak, and leads to apoptosis [98,99]. Other investigations have reported that these products can coagulate the microbial cytoplasm and bring down lipids and proteins [100]. Mostafa et al., (2020) recently reported that the stem bark extract from Salix tetrasperma impaired the virulence of P. aeruginosa by hindering its swimming and swarming on plates and by inhibiting its hemolytic and proteolytic activities [101]. Similarly, Ben bakrim et al., (2022) showed that the leaf extract from Ximenia americana var. caffra has the ability to inhibit the biofilm formation by the skin pathogen P. aeruginosa and reduced its mobilities in a dose-dependent manner [13]. Overall, the profile of a plant chemical composition may influence its targets, mechanisms of action, and consequently, its antibacterial activity.
5. Conclusions
This study profiled the phytochemical composition of the hydro-ethanolic extract of P. sativum Hoffm. and highlighted some biological and pharmacological activities of its hydro-ethanolic extract and polyphenolic fraction. Our findings corroborate many previous investigations on the role of parsley-based phytochemicals such as polyphenols as analgesic, antioxidant, and antimicrobial agents. However, as far as we know, this is the first study to deliver proof that parsley could serve as a source of wound healing bioactive principles. Nevertheless, more evidence is needed to prove their direct influence using fractionation and guided bioassays and individual compounds isolation. In addition, further in-depth assessments should address the underpinning molecular and physiological mechanisms of observed analgesic and healing activities in vivo. Lastly, our study shed light on the potential and promising role of P. sativum Hoffm. as a source of analgesic, antimicrobial, and wound healing plant-based agents.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/metabo13020260/s1, Figure S1: XIC chromatograms of identified compounds.
Author Contributions
Conceptualization: D.B. and M.S.; methodology: A.D. and F.Z.M.; software: A.I.H.; validation: A.B.; formal analysis: R.C.; investigation: O.A.K.; data curation: F.E.-z.A. and A.S.; writing—original draft preparation: M.S.; writing—review and editing: M.S. and I.M.; supervision: D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R141). Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
The institutional ethical committee of care and use of the laboratory animals at the Faculty of Sciences Dhar El Mehraz, Sidi Mohamed Ben Abdallah Fez University, Morocco, reviewed and approved the present study #04/2019/LBEAS.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the main article and the supplementary materials.
Acknowledgments
The authors extend their appreciation to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R141), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors have not declared any conflict of interests.
References
- Zhang, Y.; Li, X.; Zou, D.; Liu, W.; Yang, J.; Zhu, N.; Huo, L.; Wang, M.; Hong, J.; Wu, P.; et al. Treatment of Type 2 Diabetes and Dyslipidemia with the Natural Plant Alkaloid Berberine. J. Clin. Endocrinol. Metab. 2008, 93, 2559–2565. [Google Scholar] [CrossRef]
- Zeilhofer, H.U. Prostanoids in Nociception and Pain. Biochem. Pharmacol. 2007, 73, 165–174. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, I.; Bakrim, W.B.; Bitchagno, G.T.M.; Annaz, H.; Mahmoud, M.F.; Sobeh, M. Unraveling the Phytochemistry, Traditional Uses, and Biological and Pharmacological Activities of Thymus Algeriensis Boiss. & Reut. Oxid. Med. Cell. Longev. 2022, 2022, 6487430. [Google Scholar]
- Rojkind, M.; Dominguez-Rosales, J.-A.; Nieto, N.; Greenwel, P. Role of Hydrogen Peroxide and Oxidative Stress in Healing Responses. Cell. Mol. Life Sci. CMLS 2002, 59, 1872–1891. [Google Scholar] [CrossRef] [PubMed]
- López, M.G.; Sánchez-Mendoza, I.R.; Ochoa-Alejo, N. Compartive Study of Volatile Components and Fatty Acids of Plants and in Vitro Cultures of Parsley (Petroselinum Crispum (Mill) Nym Ex Hill). J. Agric. Food Chem. 1999, 47, 3292–3296. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Kim, M.-Y.; Cho, J.Y. Anti-Inflammatory Effects of Luteolin: A Review of in Vitro, in Vivo, and in Silico Studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef]
- Chaves, D.S.A.; Frattani, F.S.; Assafim, M.; de Almeida, A.P.; Zingali, R.B.; Costa, S.S. Composition Chimique Phénolique de l’ Extrait de Petroselinum Crispum et Son Effet Sur l’hémostase. Nat. Prod. Commun. 2011, 6, 1934578X1100600709. [Google Scholar] [CrossRef]
- Haque, A.; Pant, A.B. Mitigating Covid-19 in the Face of Emerging Virus Variants, Breakthrough Infections and Vaccine Hesitancy. J. Autoimmun. 2022, 102792. [Google Scholar] [CrossRef]
- Slighoua, M.; Mahdi, I.; Amrati, F.E.; Boukhira, S.; Youbi, A.E.H.E.; Bari, A.; Bousta, D. Ethnopharmacological Survey of Medicinal Plants Used in the Traditional Treatment of Female Infertility in Fez Region, Morocco. Phytothérapie 2020, 18, 321–339. [Google Scholar] [CrossRef]
- Tümen, G.; Malyer, H.; Başer, K.H.C.; Öz Aydın, S. Plants Used in Anatolia for Wound Healing. In Proceedings of the IVth International Congress of Ethnobotany (ICEB 2005), Istanbul, Turkey, 21–26 August 2005; Volume 217, p. 221. [Google Scholar]
- Bakrim, W.B.; Nurcahyanti, A.D.R.; Dmirieh, M.; Mahdi, I.; Elgamal, A.M.; El Raey, M.A.; Wink, M.; Sobeh, M. Phytochemical Profiling of the Leaf Extract of Ximenia Americana Var. Caffra and Its Antioxidant, Antibacterial, and Antiaging Activities In Vitro and in Caenorhabditis Elegans: A Cosmeceutical and Dermatological Approach. Oxid. Med. Cell. Longev. 2022, 2022, e3486257. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Abbasabadi, Z.; Ardekani, M.R.S.; Rahimi, R.; Farzaei, F. Parsley: A Review of Ethnopharmacology, Phytochemistry and Biological Activities. J. Tradit. Chin. Med. 2013, 33, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Thangavelu, S.; Balasubramanian, B.; Palanisamy, S.; Shanmugam, V.; Natchiappan, S.; Kalibulla, S.I.; Rathinasamy, B.; Arumugam, V.A. Characterization and Phytoconstituents of Petroselinum Crispum (Mill) and Coriandrum Sativum (Linn) and Their Impacts on Inflammation—An in Vitro Analysis against Human Adenocarcinoma Cells with Molecular Docking. S. Afr. J. Bot. 2022, 146, 776–788. [Google Scholar] [CrossRef]
- AbdelKhalek, A.S.; Youssef, H.A.; Ali, M.F.; Ali, M.M.; Abdel-Hakiem, M.A.H.; Mahmoud, H.F.F. An Assessment of Clinical, Biometric, Cosmetic and Microscopic Outcomes of Four Suture Techniques for Cutaneous Closure of Laparotomy Wounds: An Experimental Study in Rabbits. J. Dairy Vet. Anim. Res. 2019, 8, 42–53. [Google Scholar] [CrossRef]
- Esther, T.L.H. Investigation of the Antioxidant, Anticancer, Wound Healing, Immunomodulatory and Dna Protective Activities of Coriandrum Sativum and Petroselinum Crispum/Esther Tang Lai Har. Ph.D. Thesis, University of Malaya, Kuala Lumpur, Malaysia, 2014. [Google Scholar]
- Behtash, N.; Kargarzadeh, F.; Shafaroudi, H. Analgesic Effects of Seed Extract from Petroselinum Crispum (Tagetes Minuta) in Animal Models. Toxicol. Lett. 2008, 180, S127–S128. [Google Scholar] [CrossRef]
- Eidi, A.; Eidi, M.; Badiei, L. Antinociceptive Effects of Ethanolic Extract of Parsley (Petroselinum Crispum L.) Leaves in Mice. Med. Sci. J. Islam. Azad Univesity Tehran Med. Branch 2009, 19, 181–186. [Google Scholar]
- Moazedi, A.A.; Mirzaie, D.N.; Seyyednejad, S.M.; Zadkarami, M.R.; Amirzargar, A. Spasmolytic Effect of Petroselinum Crispum (Parsley) on Rat’s Ileum at Different Calcium Chloride Concentrations. Pak. J. Biol. Sci. PJBS 2007, 10, 4036–4042. [Google Scholar] [CrossRef] [PubMed]
- Annaz, H.; Sane, Y.; Bitchagno, G.T.M.; Ben Bakrim, W.; Drissi, B.; Mahdi, I.; El Bouhssini, M.; Sobeh, M. Caper (Capparis Spinosa L.): An Updated Review on Its Phytochemistry, Nutritional Value, Traditional Uses, and Therapeutic Potential. Front. Pharmacol. 2022, 13. [Google Scholar] [CrossRef]
- Ngaffo, C.M.; Tankeo, S.B.; Guefack, M.-G.F.; Nayim, P.; Wamba, B.E.; Kuete, V.; Mbaveng, A.T. Phytochemical Analysis and Antibiotic-Modulating Activity of Cocos Nucifera, Glycine Max and Musa Sapientum Methanol Extracts against Multidrug Resistant Gram-Negative Bacteria. Investig. Med. Chem. Pharmacol. 2021, 4, 53. [Google Scholar]
- Terekhov, R.P.; Selivanova, I.A.; Anurova, M.N.; Zhevlakova, A.K.; Nikitin, I.D.; Cong, Z.; Ma, S.; Yang, F.; Dong, Z.; Liao, Y. Comparative Study of Wound-Healing Activity of Dihydroquercetin Pseudopolymorphic Modifications. Bull. Exp. Biol. Med. 2021, 170, 444–447. [Google Scholar]
- Dahm, H. Silver Nanoparticles in Wound Infections: Present Status and Future Prospects. In Nanotechnology in Skin, Soft Tissue, and Bone Infections; Rai, M., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 151–168. ISBN 978-3-030-35147-2. [Google Scholar]
- Sawyer, R.G.; Pruett, T.L. Wound Infections. Surg. Clin. N. Am. 1994, 74, 519–536. [Google Scholar] [CrossRef]
- Slighoua, M.; Mahdi, I.; Amrati, F.; Di Cristo, F.; Amaghnouje, A.; Grafov, A.; Boucetta, N.; Bari, A.; Bousta, D. Assessment of in Vivo Estrogenic and Anti-Inflammatory Activities of the Hydro-Ethanolic Extract and Polyphenolic Fraction of Parsley (Petroselinum Sativum Hoffm.). J. Ethnopharmacol. 2021, 265, 113290. [Google Scholar] [CrossRef] [PubMed]
- Lilienblum, W.; Dekant, W.; Foth, H.; Gebel, T.; Hengstler, J.G.; Kahl, R.; Kramer, P.-J.; Schweinfurth, H.; Wollin, K.-M. Alternative Methods to Safety Studies in Experimental Animals: Role in the Risk Assessment of Chemicals under the New European Chemicals Legislation (REACH). Arch. Toxicol. 2008, 82, 211–236. [Google Scholar] [CrossRef] [PubMed]
- Wafa, G.; Amadou, D.; Larbi, K.M.; Héla, E.F.O. Larvicidal Activity, Phytochemical Composition, and Antioxidant Properties of Different Parts of Five Populations of Ricinus Communis L. Ind. Crops Prod. 2014, 56, 43–51. [Google Scholar] [CrossRef]
- Amrati, F.E.-Z.; Bourhia, M.; Saghrouchni, H.; Slighoua, M.; Grafov, A.; Ullah, R.; Ezzeldin, E.; Mostafa, G.A.; Bari, A.; Ibenmoussa, S.; et al. Caralluma Europaea (Guss.) N.E.Br.: Anti-Inflammatory, Antifungal, and Antibacterial Activities against Nosocomial Antibiotic-Resistant Microbes of Chemically Characterized Fractions. Molecules 2021, 26, 636. [Google Scholar] [CrossRef] [PubMed]
- Slighoua, M.; Mahdi, I.; ez-zahra Amrati, F.; Boucetta, N.; Cristo, F.D.; Boukhira, S.; El youbi el Hamsas, A.; Tattou, M.I.; Grafov, A.; Bari, A.; et al. Pharmacological Effects of Lavandula Officinalis Chaix and Its Polyphenols: Focus on Their in Vivo Estrogenic and Anti-Inflammatory Properties. South Afr. J. Bot. 2022, 146, 354–364. [Google Scholar] [CrossRef]
- Amaghnouje, A.; Mechchate, H.; Es-safi, I.; Boukhira, S.; Aliqahtani, A.S.; Noman, O.M.; Nasr, F.A.; Conte, R.; Calarco, A.; Bousta, D. Subacute Assessment of the Toxicity and Antidepressant-Like Effects of Origanum Majorana L. Polyphenols in Swiss Albino Mice. Molecules 2020, 25, 5653. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Wang, Y. Analysis of Neurosterols by GC–MS and LC–MS/MS. J. Chromatogr. B 2009, 877, 2778–2805. [Google Scholar] [CrossRef]
- Kabran, G.R.; Mamyrbekova-Bekro, J.A.; Pirat, J.-L.; Bekro, Y.-A.; Sommerer, N.; Verbaere, A.; Meudec, E. Identification de composés phénoliques extraits de deux plantes de la pharmacopée ivoirienne */Identification of phenolic compounds from two plants of ivorian pharmacopeia *. J. Société Ouest-Afr. Chim. 2014, 38, 57–63. [Google Scholar]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on Products of Browning Reaction Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Baali, F.; Boumerfeg, S.; Napoli, E.; Boudjelal, A.; Righi, N.; Deghima, A.; Baghiani, A.; Ruberto, G. Chemical Composition and Biological Activities of Essential Oils from Two Wild Algerian Medicinal Plants: Mentha Pulegium L. and Lavandula Stoechas L. J. Essent. Oil Bear. Plants 2019, 22, 821–837. [Google Scholar] [CrossRef]
- Mašković, P.Z.; Manojlović, N.T.; Mandić, A.I.; Mišan, A.Č.; Milovanović, I.L.; Radojković, M.M.; Cvijović, M.S.; Solujić, S.R. Phytochemical Screening and Biological Activity of Extracts of Plant Species Halacsya Sendtneri (Boiss.) Dörfl. Hem. Ind. 2012, 66, 43–51. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Mabadahanye, K. Isolation and Analysing Chemical Profiles of Bioactive Compounds from South Africa Medicinal Plants with Activity against Pathogenic Organisms; University of Johannesburg: Johannesburg, South Africa, 2020; ISBN 9798544213154. [Google Scholar]
- Athanassiadis, B.; Abbott, P.V.; George, N.; Walsh, L.J. An in Vitro Study of the Antimicrobial Activity of Some Endodontic Medicaments and Their Bases Using an Agar Well Diffusion Assay. Aust. Dent. J. 2009, 54, 141–146. [Google Scholar] [CrossRef]
- Nalawade, T.M.; Bhat, K.G.; Sogi, S. Antimicrobial Activity of Endodontic Medicaments and Vehicles Using Agar Well Diffusion Method on Facultative and Obligate Anaerobes. Int. J. Clin. Pediatr. Dent. 2016, 9, 335. [Google Scholar]
- Hayet, E.; Maha, M.; Samia, A.; Mata, M.; Gros, P.; Raida, H.; Ali, M.M.; Mohamed, A.S.; Gutmann, L.; Mighri, Z. Antimicrobial, Antioxidant, and Antiviral Activities of Retama Raetam (Forssk.) Webb Flowers Growing in Tunisia. World J. Microbiol. Biotechnol. 2008, 24, 2933–2940. [Google Scholar] [CrossRef]
- Wayne, P.A. Clinical and Laboratory Standards Institute: Performance Standards for Antimicrobial Susceptibility Testing: 20th Informational Supplement. CLSI Doc. M100-S20 2010. [Google Scholar]
- Adeoyo, O.R.; Pletschke, B.I.; Dames, J.F. Molecular Identification and Antibacterial Properties of an Ericoid Associated Mycorrhizal Fungus. BMC Microbiol. 2019, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J.; Perry, C.M. Tramadol. Drugs 2000, 60, 139–176. [Google Scholar] [CrossRef]
- França, D.S.; Souza, A.L.; Almeida, K.R.; Dolabella, S.S.; Martinelli, C.; Coelho, M.M. B Vitamins Induce an Antinociceptive Effect in the Acetic Acid and Formaldehyde Models of Nociception in Mice. Eur. J. Pharmacol. 2001, 421, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Hajhashemi, V.; Ghannadi, A.; Sharif, B. Anti-Inflammatory and Analgesic Properties of the Leaf Extracts and Essential Oil of Lavandula Angustifolia Mill. J. Ethnopharmacol. 2003, 89, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Manglik, A.; Lin, H.; Aryal, D.K.; McCorvy, J.D.; Dengler, D.; Corder, G.; Levit, A.; Kling, R.C.; Bernat, V.; Hübner, H. Structure-Based Discovery of Opioid Analgesics with Reduced Side Effects. Nature 2016, 537, 185–190. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, X.; Huang, H.; Jin, Z.; Gao, J.; Guo, Y.; Zhong, Y.; Li, Z.; Zong, X.; Wang, K. Optimization of 4-Arylthiophene-3-Carboxylic Acid Derivatives as Inhibitors of ANO1: Lead Optimization Studies toward Their Analgesic Efficacy for Inflammatory Pain. Eur. J. Med. Chem. 2022, 237, 114413. [Google Scholar] [CrossRef]
- De Miranda, F.G.G.; Vilar, J.C.; Alves, I.A.N.; de Holanda Cavalcanti, S.C.; Antoniolli, Â.R. Antinociceptive and Antiedematogenic Properties and Acute Toxicity of Tabebuia Avellanedae Lor. Ex Griseb. Inner Bark Aqueous Extract. BMC Pharmacol. 2001, 1, 1–5. [Google Scholar] [CrossRef]
- Heidari, M.; Bahramsoltani, R.; Abdolghaffari, A.H.; Rahimi, R.; Esfandyari, M.; Baeeri, M.; Hassanzadeh, G.; Abdollahi, M.; Farzaei, M.H. Efficacy of Topical Application of Standardized Extract of Tragopogon Graminifolius in the Healing Process of Experimental Burn Wounds. J. Tradit. Complement. Med. 2019, 9, 54–59. [Google Scholar] [CrossRef]
- Slighoua, M.; Chebaibi, M.; Mahdi, I.; Amrati, F.E.; Conte, R.; Cordero, M.A.W.; Alotaibi, A.; Saghrouchni, H.; Agour, A.; Zair, T.; et al. The LC-MS/MS Identification and Analgesic and Wound Healing Activities of Lavandula Officinalis Chaix: In Vivo and In Silico Approaches. Plants 2022, 11, 3222. [Google Scholar] [CrossRef]
- Charles, D.J. Antioxidant Properties of Spices, Herbs and Other Sources [Electronic Resource]; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Omar, S.H. Oleuropein in Olive and Its Pharmacological Effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef]
- Bhalla, M. Pharmacological Aspects of a Bioactive Compound Arbutin: A Comprehensive Review. Biointerface Res. Appl. Chem. 2022, 13, 119. [Google Scholar] [CrossRef]
- Gupta, G.; Siddiqui, M.A.; Khan, M.M.; Ajmal, M.; Ahsan, R.; Rahaman, M.A.; Ahmad, M.A.; Arshad, M.; Khushtar, M. Current Pharmacological Trends on Myricetin. Drug Res. 2020, 70, 448–454. [Google Scholar] [CrossRef]
- Ong, K.C.; Khoo, H.-E. Biological Effects of Myricetin. Gen. Pharmacol. Vasc. Syst. 1997, 29, 121–126. [Google Scholar] [CrossRef]
- Zhang, Q.-C.; Zhao, Y.; Bian, H.-M. Anti-Thrombotic Effect of a Novel Formula from Corni Fructus with Malic Acid, Succinic Acid and Citric Acid. Phytother. Res. 2014, 28, 722–727. [Google Scholar] [CrossRef]
- Wokoun, U.; Hellriegel, M.; Emons, G.; Gründker, C. Co-Treatment of Breast Cancer Cells with Pharmacologic Doses of 2-Deoxy-D-Glucose and Metformin: Starving Tumors. Oncol. Rep. 2017, 37, 2418–2424. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Kim, S.-H.; Hagerman, A.E.; Lü, J. Anti-Cancer, Anti-Diabetic and Other Pharmacologic and Biological Activities of Penta-Galloyl-Glucose. Pharm. Res. 2009, 26, 2066–2080. [Google Scholar] [CrossRef] [PubMed]
- Thevissen, K.; Marchand, A.; Chaltin, P.; Meert, E.M.K.; Cammue, B.P.A. Antifungal Carbazoles. Curr. Med. Chem. 2009, 16, 2205–2211. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.; Al Mahmud, Z.; Kumar Saha, S.; Abdur Rahman, S.M. Evaluation of Antinociceptive and Antidiarrhoeal Properties of Manilkara Zapota Leaves in Swiss Albino Mice. Pharm. Biol. 2016, 54, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- de Fátima Arrigoni-Blank, M.; Dmitrieva, E.G.; Franzotti, E.M.; Antoniolli, A.R.; Andrade, M.R.; Marchioro, M. Anti-Inflammatory and Analgesic Activity of Peperomia Pellucida (L.) HBK (Piperaceae). J. Ethnopharmacol. 2004, 91, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.G.; Silva, R.O.; Damasceno, S.R.; Carvalho, N.S.; Prudêncio, R.S.; Aragão, K.S.; Guimarães, M.A.; Campos, S.A.; Véras, L.M.; Godejohann, M. Anti-Inflammatory and Antinociceptive Activity of Epiisopiloturine, an Imidazole Alkaloid Isolated from Pilocarpus Microphyllus. J. Nat. Prod. 2013, 76, 1071–1077. [Google Scholar] [CrossRef]
- Fischer, L.G.; Leitão, R.; Etcheverry, S.R.; de Campos-Buzzi, F.; Vãzquez, A.A.; Heinzen, H.A.; Filho, V.C. Analgesic Properties of Extracts and Fractions from Erythrina Crista-Galli (Fabaceae) Leaves. Nat. Prod. Res. 2007, 21, 759–766. [Google Scholar] [CrossRef]
- Ahmadiani, A.; Hosseiny, J.; Semnanian, S.; Javan, M.; Saeedi, F.; Kamalinejad, M.; Saremi, S. Antinociceptive and Anti-Inflammatory Effects of Elaeagnus Angustifolia Fruit Extract. J. Ethnopharmacol. 2000, 72, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Daud, A.; Habib, N.; Riera, A.S. Anti-Inflammatory, Anti-Nociceptive and Antipyretic Effects of Extracts of Phrygilanthus Acutifolius Flowers. J. Ethnopharmacol. 2006, 108, 198–203. [Google Scholar] [CrossRef] [PubMed]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M. TRPA1 Mediates Formalin-Induced Pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef]
- Parvizpur, A.; Ahmadiani, A.; Kamalinejad, M. Probable Role of Spinal Purinoceptors in the Analgesic Effect of Trigonella Foenum (TFG) Leaves Extract. J. Ethnopharmacol. 2006, 104, 108–112. [Google Scholar] [CrossRef]
- Crovetti, G.; Martinelli, G.; Issi, M.; Barone, M.; Guizzardi, M.; Campanati, B.; Moroni, M.; Carabelli, A. Platelet Gel for Healing Cutaneous Chronic Wounds. Transfus. Apher. Sci. 2004, 30, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.J.; Heo, S.-I.; Wang, M.-H. Free Radical Scavenging and Total Phenolic Contents from Methanolic Extracts of Ulmus Davidiana. Food Chem. 2008, 108, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Haney, E.F.; Mansour, S.C.; Hancock, R.E. Antimicrobial Peptides: An Introduction. Antimicrob. Pept. 2017, 3–22. [Google Scholar]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound Healing and the Use of Medicinal Plants. Evid. Based Complement. Alternat. Med. 2019, 2019. [Google Scholar] [CrossRef]
- Babu, M.K.; Prasad, O.S.; Murthy, T.E. Comparison of the Dermal Wound Healing of Centella Asiatica Extract Impregnated Collagen and Cross Linked Collagen Scaffolds. J. Chem. Pharm. Res. 2011, 3, 353–362. [Google Scholar]
- Ramnath, V.; Sekar, S.; Sankar, S.; Sastry, T.P.; Mandal, A.B. In Vivo Evaluation of Composite Wound Dressing Material Containing Soya Protein and Sago Starch. Int. J. Pharm. Sci. 2012, 4, 414–419. [Google Scholar]
- Motealleh, B.; Zahedi, P.; Rezaeian, I.; Moghimi, M.; Abdolghaffari, A.H.; Zarandi, M.A. Morphology, Drug Release, Antibacterial, Cell Proliferation, and Histology Studies of Chamomile-loaded Wound Dressing Mats Based on Electrospun Nanofibrous Poly (ε-caprolactone)/Polystyrene Blends. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 977–987. [Google Scholar] [CrossRef]
- Brown, K.L.; Phillips, T.J. Nutrition and Wound Healing. Clin. Dermatol. 2010, 28, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.E.; Gottschlich, M.M.; Kopcha, R.; Khoury, J.; Warden, G.D. A Prospective Analysis of Serum Vitamin K in Severely Burned Pediatric Patients. J. Burn Care Rehabil. 1998, 19, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Caruelle, J.-P.; Barritault, D.; Jeanbat-Mimaud, V.; Cammas-Marion, S.; Langlois, V.; Guerin, P.; Barbaud, C. Bioactive Functionalized Polymer of Malic Acid for Bone Repair and Muscle Regeneration. J. Biomater. Sci. Polym. Ed. 2000, 11, 979–991. [Google Scholar] [CrossRef]
- Jeanbat-Mimaud, V.; Barbaud, C.; Caruelle, J.-P.; Barritault, D.; Cammas-Marion, S.; Guérin, P. Functionalized Polymers of Malic Acid Stimulate Tissue Repair Presumably by Regulating Heparin Growth Factors Bioavailability. In Biomedical Polymers and Polymer Therapeutics; Springer: Berlin/Heidelberg, Germany, 2002; pp. 243–251. [Google Scholar]
- Cattaneo, F.; De Marino, S.; Parisi, M.; Festa, C.; Castaldo, M.; Finamore, C.; Duraturo, F.; Zollo, C.; Ammendola, R.; Zollo, F. Wound Healing Activity and Phytochemical Screening of Purified Fractions of Sempervivum Tectorum L. Leaves on HCT 116. Phytochem. Anal. 2019, 30, 524–534. [Google Scholar] [CrossRef]
- Pereira, M.P.; Tavano, O.L. Use of Different Spices as Potential Natural Antioxidant Additives on Cooked Beans (Phaseolus Vulgaris). Increase of DPPH Radical Scavenging Activity and Total Phenolic Content. Plant Foods Hum. Nutr. 2014, 69, 337–343. [Google Scholar] [CrossRef]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and total flavonoids in bulgarian fruits and vegetables. JU Chem. Metal 2005, 40, 255–260. [Google Scholar]
- Papuc, C.; Predescu, C.; Nicorescu, V.; Stefan, G.; Nicorescu, I. Antioxidant Properties of a Parsley (Petroselinum crispum) Juice Rich in Polyphenols and Nitrites. Curr. Res. Nutr. Food Sci. J. 2016, 4, 114–118. [Google Scholar] [CrossRef]
- Lafraxo, H.; Bakour, M.; Laaroussi, H.; El Ghouizi, A.; Ousaaid, D.; Aboulghazi, A.; Lyoussi, B. The Synergistic Beneficial Effect of Thyme Honey and Olive Oil against Diabetes and Its Complications Induced by Alloxan in Wistar Rats. Evid. Based Complement. Alternat. Med. 2021, 2021. [Google Scholar] [CrossRef]
- Lee, C.E.; Petersen, C.H. Effects of Developmental Acclimation on Adult Salinity Tolerance in the Freshwater-Invading Copepod Eurytemora Affinis. Physiol. Biochem. Zool. 2003, 76, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Hanachi, P.; Golkho, S.H. Using HPLC to Determination the Composition and Antioxidant Activity of Berberis Vulgaris. Eur. J. Sci. Res. 2009, 29, 47–54. [Google Scholar]
- Kazemi, M.; Hadavi, E.; Hekmati, J. Effect of Salicylic Acid, Malic Acid, Citric Acid and Sucrose on Antioxidant Activity, Membrane Stability and ACC-Oxidase Activity in Relation to Vase Life of Carnation Cut Flowers. J. Plant Sci. 2012, 7, 78–84. [Google Scholar] [CrossRef]
- Al-Juhaimi, F.; Ghafoor, K. Total Phenols and Antioxidant Activities of Leaf and Stem Extracts from Coriander, Mint and Parsley Grown in Saudi Arabia. Pak J Bot 2011, 43, 2235–2237. [Google Scholar]
- Wong, P.Y.Y.; Kitts, D.D. Studies on the Dual Antioxidant and Antibacterial Properties of Parsley (Petroselinum Crispum) and Cilantro (Coriandrum Sativum) Extracts. Food Chem. 2006, 97, 505–515. [Google Scholar] [CrossRef]
- Agyare, C.; Appiah, T.; Boakye, Y.D.; Apenteng, J.A. Chapter 25 - Petroselinum Crispum: A Review. In Medicinal Spices and Vegetables from Africa; Kuete, V., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 527–547. ISBN 978-0-12-809286-6. [Google Scholar]
- Marín, I.; Sayas-Barberá, E.; Viuda-Martos, M.; Navarro, C.; Sendra, E. Chemical Composition, Antioxidant and Antimicrobial Activity of Essential Oils from Organic Fennel, Parsley, and Lavender from Spain. Foods 2016, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Herken, E.N.; Guzel, S. Total Antioxidant Capacity and Total Phenol Contents of Selected Commercial Fruit Juices in Turkey. Int. J. Food Prop. 2010, 13, 1373–1379. [Google Scholar] [CrossRef]
- Atef, N.M.; Shanab, S.M.; Negm, S.I.; Abbas, Y.A. Evaluation of Antimicrobial Activity of Some Plant Extracts against Antibiotic Susceptible and Resistant Bacterial Strains Causing Wound Infection. Bull. Natl. Res. Cent. 2019, 43, 144. [Google Scholar] [CrossRef]
- Keita, K.; Darkoh, C.; Okafor, F. Secondary Plant Metabolites as Potent Drug Candidates against Antimicrobial-Resistant Pathogens. SN Appl. Sci. 2022, 4, 1–10. [Google Scholar] [CrossRef]
- Chaillot, J.; Tebbji, F.; Remmal, A.; Boone, C.; Brown, G.W.; Bellaoui, M.; Sellam, A. The Monoterpene Carvacrol Generates Endoplasmic Reticulum Stress in the Pathogenic Fungus Candida Albicans. Antimicrob. Agents Chemother. 2015, 59, 4584–4592. [Google Scholar] [CrossRef]
- Lv, F.; Liang, H.; Yuan, Q.; Li, C. In Vitro Antimicrobial Effects and Mechanism of Action of Selected Plant Essential Oil Combinations against Four Food-Related Microorganisms. Food Res. Int. 2011, 44, 3057–3064. [Google Scholar] [CrossRef]
- Moussii, I.M.; Nayme, K.; Timinouni, M.; Jamaleddine, J.; Filali, H.; Hakkou, F. Synergistic Antibacterial Effects of Moroccan Artemisia Herba Alba, Lavandula Angustifolia and Rosmarinus Officinalis Essential Oils. Synergy 2020, 10, 100057. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Mohamady, M.A.; Fernández-López, J.; Abd ElRazik, K.A.; Omer, E.A.; Pérez-Alvarez, J.A.; Sendra, E. In Vitro Antioxidant and Antibacterial Activities of Essentials Oils Obtained from Egyptian Aromatic Plants. Food Control 2011, 22, 1715–1722. [Google Scholar] [CrossRef]
- Mostafa, I.; Abbas, H.A.; Ashour, M.L.; Yasri, A.; El-Shazly, A.M.; Wink, M.; Sobeh, M. Polyphenols from Salix Tetrasperma Impair Virulence and Inhibit Quorum Sensing of Pseudomonas Aeruginosa. Molecules 2020, 25, 1341. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).