Vitamin D and Bone Metabolism in Adult Patients with Neurofibromatosis Type 1
Abstract
1. Introduction
2. Experimental Design
2.1. Design and Setting
2.2. Clinical Assessment
2.3. Biochemical Assessment
2.4. Genetic Test for Germline NF1 Variants
2.5. Bone Evaluation
2.6. Statistical Analysis
3. Results
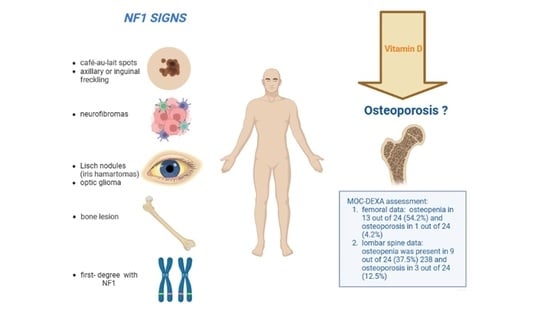
3.1. Diagnostic Criteria of NF1
3.2. Other NF1 Clinical Manifestations
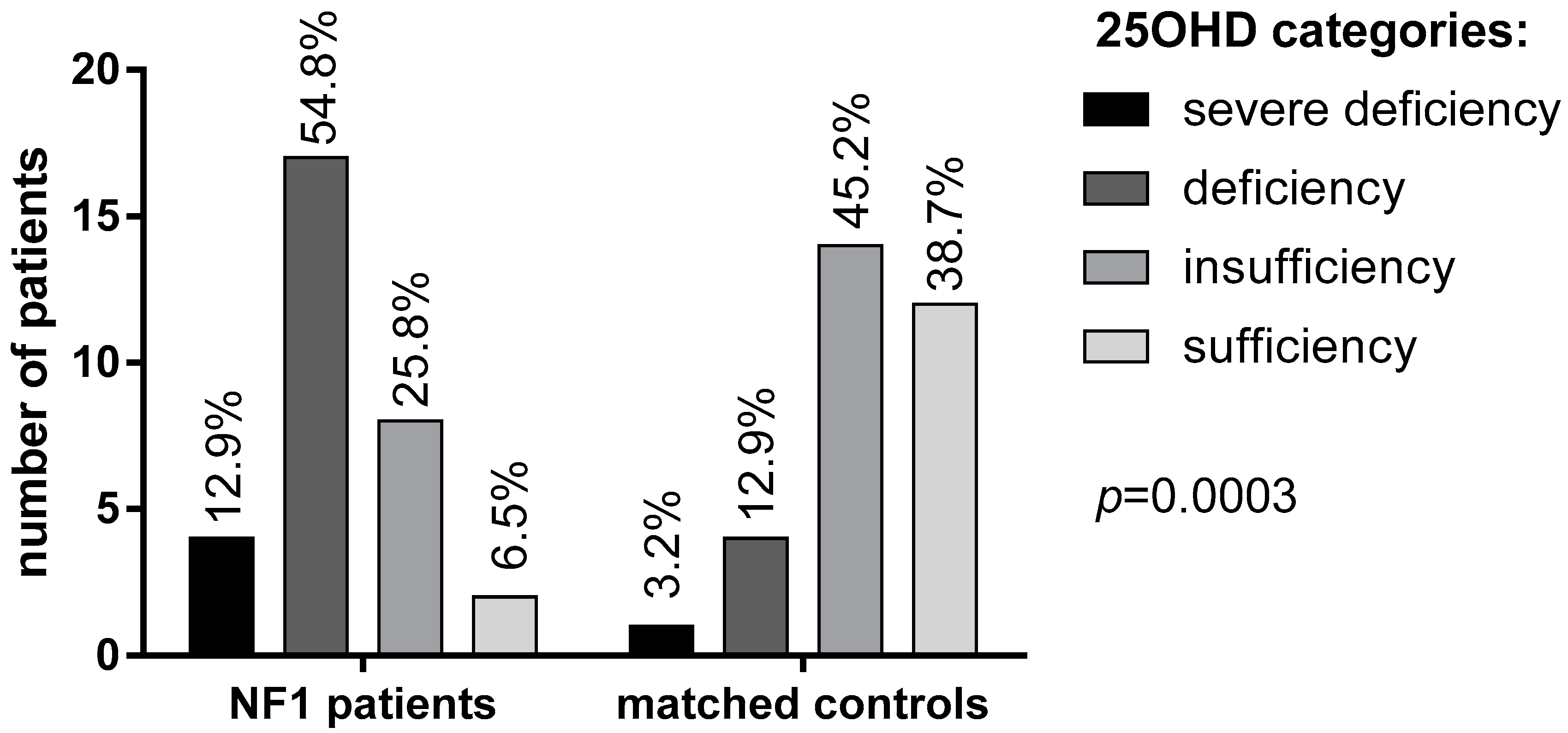
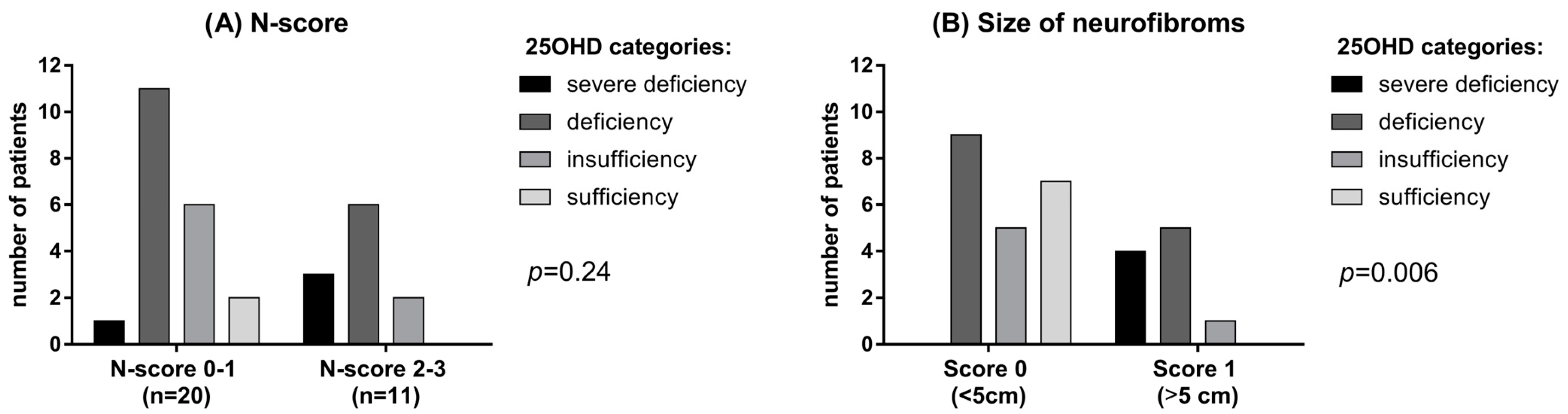
3.3. 25OHD and Bone Metabolism
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boyd, K.P.; Korf, B.R.; Theos, A. Neurofibromatosis type 1. J. Am. Acad. Dermatol. 2009, 61, 1–14. [Google Scholar] [CrossRef]
- Evans, D.G.; Howard, E.; Giblin, C.; Clancy, T.; Spencer, H.; Huson, S.M.; Lalloo, F. Birth incidence and prevalence of tumor-prone syndromes: Estimates from a UK family genetic register service. Am. J. Med. Genet. Part A 2010, 152, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Kallionpää, R.A.; Uusitalo, E.; Leppävirta, J.; Pöyhönen, M.; Peltonen, S.; Peltonen, J. Prevalence of neurofibromatosis type 1 in the Finnish population. Anesthesia Analg. 2018, 20, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, I.; Welti, S.; Bonneau, F.; Scheffzek, K. A novel bipartite phospholipid-binding module in the neurofibromatosis type 1 protein. EMBO Rep. 2006, 7, 174–179. [Google Scholar] [CrossRef]
- Peltonen, S.; Kallionpää, R.A.; Peltonen, J. Neurofibromatosis type 1 (NF1) gene: Beyond café au lait spots and dermal neurofibromas. Exp. Dermatol. 2017, 26, 645–648. [Google Scholar] [CrossRef]
- Ferner, R.E.; Huson, S.M.; Thomas, N.; Moss, C.; Willshaw, H.; Evans, D.G.; Upadhyaya, M.; Towers, R.; Gleeson, M.; Steiger, C.; et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J. Med. Genet. 2007, 44, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. National Institutes of Health Consensus Development Conference Statement: Neurofibromatosis. Bethesda, Md., USA, July 13–15, 1987. Neurofibromatosis 1988, 1, 172–178. [Google Scholar]
- Bergqvist, C.; Servy, A.; Valeyrie-Allanore, L.; Ferkal, S.; Combemale, P.; Wolkenstein, P.; NF France Network. Neurofibromatosis 1 French national guidelines based on an extensive literature review since 1966. Orphanet J. Rare Dis. 2020, 15, 37. [Google Scholar] [CrossRef]
- Uusitalo, E.; Leppävirta, J.; Koffert, A.; Suominen, S.B.; Vahtera, J.; Vahlberg, T.; Pöyhönen, M.; Peltonen, J.; Peltonen, S. Incidence and Mortality of Neurofibromatosis: A Total Population Study in Finland. J. Investig. Dermatol. 2015, 135, 904–906. [Google Scholar] [CrossRef]
- Elefteriou, F.; Kolanczyk, M.; Schindeler, A.; Viskochil, D.H.; Hock, J.M.; Schorry, E.K.; Crawford, A.H.; Friedman, J.M.; Little, D.; Peltonen, J.; et al. Skeletal abnormalities in neurofibromatosis type 1: Approaches to therapeutic options. Am. J. Med. Genet. Part A 2009, 149A, 2327–2338. [Google Scholar] [CrossRef]
- Ferrara, U.P.; Tortora, C.; Rosano, C.; Assunto, A.; Rossi, A.; Pagano, S.; Falco, M.; Simeoli, C.; Ferrigno, R.; D’Amico, A.; et al. Bone metabolism in patients with type 1 neurofibromatosis: Key role of sun exposure and physical activity. Sci. Rep. 2022, 12, 4368. [Google Scholar] [CrossRef] [PubMed]
- Jalabert, M.; Ferkal, S.; Souberbielle, J.-C.; Sbidian, E.; Mageau, A.; Eymard, F.; Le Corvoisier, P.; Allanore, L.; Chevalier, X.; Wolkenstein, P.; et al. Bone Status According to Neurofibromatosis Type 1 Phenotype: A Descriptive Study of 60 Women in France. Calcif. Tissue Int. 2021, 108, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Heervä, E.; Leinonen, P.; Kuorilehto, T.; Peltonen, S.; Pöyhönen, M.; Väänänen, K.; Peltonen, J. Neurofibromatosis 1-Related Osteopenia Often Progresses to Osteoporosis in 12 Years. Calcif. Tissue Int. 2013, 92, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Petramala, L.; Giustini, S.; Zinnamosca, L.; Marinelli, C.; Colangelo, L.; Cilenti, G.; Formicuccia, M.C.; D’Erasmo, E.; Calvieri, S.; Letizia, C. Bone mineral metabolism in patients with neurofibromatosis type 1 (von Recklingausen disease). Arch. Dermatol. Res. 2012, 304, 325–331. [Google Scholar] [CrossRef]
- Allaire, E.; Forough, R.; Clowes, M.; Starcher, B.; Clowes, A.W. Local overexpression of TIMP-1 prevents aortic aneurysm degeneration and rupture in a rat model. J. Clin. Investig. 1998, 102, 1413–1420. [Google Scholar] [CrossRef]
- Fowlkes, J.L.; Thrailkill, K.M.; Bunn, R.C. RASopathies: The Musculoskeletal Consequences and their Etiology and Pathogenesis. Bone 2021, 152, 116060. [Google Scholar] [CrossRef]
- Riccardi, C.; Perrone, L.; Napolitano, F.; Sampaolo, S.; Melone, M.A.B. Understanding the Biological Activities of Vitamin D in Type 1 Neurofibromatosis: New Insights into Disease Pathogenesis and Therapeutic Design. Cancers 2020, 12, 2965. [Google Scholar] [CrossRef]
- Altieri, B.; Muscogiuri, G.; Barrea, L.; Mathieu, C.; Vallone, C.V.; Mascitelli, L.; Bizzaro, G.; Altieri, V.M.; Tirabassi, G.; Balercia, G.; et al. Does vitamin D play a role in autoimmune endocrine disorders? A proof of concept. Rev. Endocr. Metab. Disord. 2017, 18, 335–346. [Google Scholar] [CrossRef]
- Altieri, B.; Di Dato, C.; Modica, R.; Bottiglieri, F.; Di Sarno, A.; Pittaway, J.F.; Martini, C.; Faggiano, A.; Colao, A. Bone Metabolism and Vitamin D Implication in Gastroenteropancreatic Neuroendocrine Tumors. Nutrients 2020, 12, 1021. [Google Scholar] [CrossRef]
- Filopanti, M.; Verga, U.; Ulivieri, F.M.; Giavoli, C.; Rodari, G.; Arosio, M.; Natacci, F.; Spada, A. Trabecular Bone Score (TBS) and Bone Metabolism in Patients Affected with Type 1 Neurofibromatosis (NF1). Calcif. Tissue Int. 2019, 104, 207–213. [Google Scholar] [CrossRef]
- Schnabel, C.; Jett, K.; Friedman, J.; Frieling, I.; Kruse, H.-P.; Mautner, V. Effect of vitamin D3 treatment on bone density in neurofibromatosis 1 patients: A retrospective clinical study. Jt. Bone Spine 2013, 80, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Kluwe, L.; Hagel, C.; Friedrich, R.E.; Schnabel, C.; Schön, G.; Mautner, V. Vitamin D receptor expression and serum 25(OH)D concentration inversely associates with burden of neurofibromas. Eur. J. Cancer Prev. 2019, 28, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. Med. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Altieri, B.; Muscogiuri, G.; Laudisio, D.; Annunziata, G.; Colao, A.; Faggiano, A.; Savastano, S. Impact of Nutritional Status on Gastroenteropancreatic Neuroendocrine Tumors (GEP-NET) Aggressiveness. Nutrients 2018, 10, 1854. [Google Scholar] [CrossRef] [PubMed]
- Altieri, B.; Barrea, L.; Modica, R.; Bottiglieri, F.; de Cicco, F.; Muscogiuri, G.; Circelli, L.; Savarese, G.; Di Somma, C.; Savastano, S.; et al. Vitamin D deficiency and tumor aggressiveness in gastroenteropancreatic neuroendocrine tumors. Endocrine 2022, 75, 623–634. [Google Scholar] [CrossRef]
- Altieri, B.; Cavalier, E.; Bhattoa, H.P.; Pérez-López, F.R.; López-Baena, M.T.; Pérez-Roncero, G.R.; Chedraui, P.; Annweiler, C.; Della Casa, S.; Zelzer, S.; et al. Vitamin D testing: Advantages and limits of the current assays. Eur. J. Clin. Nutr. 2020, 74, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Glaser, D.L.; Kaplan, F.S. Osteoporosis: Definition and clinical presentation. Spine 1997, 22, 12S–16S. [Google Scholar] [CrossRef] [PubMed]
- Peck, W.A. Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [Google Scholar] [CrossRef]
- Cacciari, E.; Milani, S.; Balsamo, A.; Spada, E.; Bona, G.; Cavallo, L.; Cerutti, F.; Gargantini, L.; Greggio, N.; Tonini, G.; et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J. Endocrinol. Investig. 2006, 29, 581–593. [Google Scholar] [CrossRef]
- Krishnatry, R.; Zhukova, N.; Guerreiro Stucklin, A.S.; Pole, J.D.; Mistry, M.; Fried, I.; Ramaswamy, V.; Bartels, U.; Huang, A.; Laperriere, N.; et al. Clinical and treatment factors determining long-term outcomes for adult survivors of childhood low-grade glioma: A population-based study. Cancer 2016, 122, 1261–1269. [Google Scholar] [CrossRef]
- Madden, J.R.; Rush, S.Z.; Stence, N.; Foreman, N.; Liu, A.K. Radiation-induced Gliomas in 2 Pediatric Patients with Neurofibromatosis Type 1. J. Pediatr. Hematol. 2014, 36, e105–e108. [Google Scholar] [CrossRef]
- Tucker, T.; Schnabel, C.; Hartmann, M.; Friedrich, R.E.; Frieling, I.; Kruse, H.-P.; Mautner, V.-F.; Friedman, J.M. Bone health and fracture rate in individuals with neurofibromatosis 1 (NF1). J. Med. Genet. 2009, 46, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Hockett, C.W.; Eelloo, J.; Huson, S.M.; Roberts, S.A.; Berry, J.L.; Chaloner, C.; Rawer, R.; Mughal, M.Z. Vitamin D status and muscle function in children with neurofibromatosis type 1 (NF1). J. Musculoskelet. Neuronal Interact. 2013, 13, 111–119. [Google Scholar] [PubMed]
- Lammert, M.; Friedman, J.M.; Roth, H.J.; Friedrich, R.E.; Kluwe, L.; Atkins, D.; Schooler, T.; Mautner, V.-F. Vitamin D deficiency associated with number of neurofibromas in neurofibromatosis 1. J. Med. Genet. 2006, 43, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, D.H.; Ferner, R.E.; Listernick, R.H.; Korf, B.R.; Wolters, P.L.; Johnson, K.J. Neurofibromatosis type 1. Nat. Rev. Dis. Prim. 2017, 3, 17004. [Google Scholar] [CrossRef]
- Arrington, D.K.; Danehy, A.R.; Peleggi, A.; Proctor, M.R.; Irons, M.B.; Ullrich, N.J. Calvarial defects and skeletal dysplasia in patients with neurofibromatosis Type 1. J. Neurosurg. Pediatr. 2013, 11, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Soucy, E.A.; Van Oppen, D.; Nejedly, N.L.; Gao, F.; Gutmann, D.H.; Hollander, A.S. Height assessments in children with neurofibromatosis type 1. J. Child Neurol. 2013, 28, 303–307. [Google Scholar] [CrossRef]
- Souza, M.; Jansen, A.; Martins, A.; Rodrigues, L.; Rezende, N. Body composition in adults with neurofibromatosis type 1. Rev. Assoc. Med. Bras. 2016, 62, 831–836. [Google Scholar] [CrossRef]
- Miraglia, E.; Moliterni, E.; Iacovino, C.; Roberti, V.; Laghi, A.; Moramarco, A.; Giustini, S. Cutaneous manifestations in neurofibromatosis type 1. Clin. Ter. 2020, 171, e371–e377. [Google Scholar] [CrossRef]
- Ahlawat, S.; Ly, K.I.; Fayad, L.M.; Fisher, M.J.; Lessing, A.J.; Berg, D.J.; Salamon, J.M.; Mautner, V.-F.; Babovic-Vuksanovic, D.; Dombi, E.; et al. Imaging Evaluation of Plexiform Neurofibromas in Neurofibromatosis Type 1. Neurology 2021, 97, S111–S119. [Google Scholar] [CrossRef]
- Ly, K.I.; Blakeley, J.O. The Diagnosis and Management of Neurofibromatosis Type 1. Med. Clin. N. Am. 2019, 103, 1035–1054. [Google Scholar] [CrossRef] [PubMed]
- Kordić, R.; Sabol, Z.; Cerovski, B.; Katušić, D.; Jukić, T. Eye disorders in neurofibromatosis (NF1). Coll. Antropol. 2005, 29 (Suppl. S1), 29–31. [Google Scholar] [PubMed]
- Campen, C.J.; Gutmann, D.H. Optic Pathway Gliomas in Neurofibromatosis Type 1. J. Child Neurol. 2018, 33, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Sellmer, L.; Farschtschi, S.; Marangoni, M.; Heran, M.K.S.; Birch, P.; Wenzel, R.; Friedman, J.M.; Mautner, V.-F. Non-optic glioma in adults and children with neurofibromatosis 1. Orphanet J. Rare Dis. 2017, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.G.R.; Salvador, H.; Chang, V.Y.; Erez, A.; Voss, S.D.; Schneider, K.W.; Scott, H.S.; Plon, S.E.; Tabori, U. Cancer and Central Nervous System Tumor Surveillance in Pediatric Neurofibromatosis 1. Clin. Cancer Res. 2017, 23, e46–e53. [Google Scholar] [CrossRef]
| Parameters | NF1 Patients | Controls | p-Value |
|---|---|---|---|
| Age at time of the study, years | 41 ± 11.4 | 43.8 ± 7.2 | 0.106 |
| BMI, kg/m2 | 27 ± 5.8 | 25.5 ± 4.2 | 0.406 |
| 25OHD, ng/mL | 16.4 ± 6 | 27.2 ± 9.2 | <0.0001 |
| Albumin-corrected serum calcium, mg/dL | 9.4 ± 0.4 | 9.3 ± 0.4 | 0.423 |
| Phosphorus, mg/dL | 3.6 ± 0.5 | 3.9 ± 0.5 | 0.035 |
| PTH, pg/mL | 42.3 ± 31.9 | 23.8 ± 15.5 | 0.077 |
| Clinical Signs | Patients (n.) |
|---|---|
| Café-au-lait spots (CLS) | 28 (90.3%) |
| Neurofibromas | 29 (93.5%) |
| Surgically treated neurofibromas | 12 (38.7%) |
| Plexiform neurofibromas | 3 (9.7%) |
| Freckling | 13 (41.9%) |
| Lisch nodules | 17 (54.8%) |
| Optic pathway glioma | 7 (22.6%) |
| Bone signs | 17 (54.8%) |
| Scoliosis | 13 (41.9%) |
| Tibial pseudoarthrosis | 3 (9.7%) |
| Sphenoidal dysplasia | 1 (3.2%) |
| Patients ID | CLS ≥ 6 | Neurofibromas ≥ 2 or 1 Plexiform | Axillary or Inguinal Freckling | Optic Glioma | Lisch Nodule | Osseous Lesions | SIS Score |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 0 | 1 | 1 | 0 | 4 |
| 2 | 1 | 1 | 1 | 0 | 0 | 0 | 3 |
| 3 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| 4 | 1 | 1 | 1 | 0 | 1 | 1 | 5 |
| 5 | 1 | 0 | 1 | 0 | 0 | 0 | 2 |
| 6 | 1 | 1 | 1 | 0 | 1 | 1 | 5 |
| 7 | 1 | 1 | 0 | 0 | 1 | 1 | 4 |
| 8 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| 9 | 1 | 1 | 1 | 0 | 1 | 1 | 5 |
| 10 | 1 | 1 | 0 | 0 | 0 | 1 | 3 |
| 11 | 0 | 1 | 0 | 0 | 1 | 1 | 3 |
| 12 | 1 | 1 | 1 | 0 | 1 | 0 | 4 |
| 13 | 1 | 1 | 0 | 1 | 1 | 1 | 5 |
| 14 | 1 | 1 | 0 | 1 | 0 | 0 | 3 |
| 15 | 1 | 1 | 1 | 0 | 1 | 1 | 5 |
| 16 | 1 | 1 | 1 | 1 | 0 | 0 | 4 |
| 17 | 1 | 1 | 0 | 0 | 1 | 0 | 3 |
| 18 | 1 | 1 | 0 | 0 | 1 | 0 | 3 |
| 19 | 1 | 1 | 0 | 0 | 0 | 0 | 2 |
| 20 | 1 | 1 | 0 | 0 | 0 | 0 | 2 |
| 21 | 1 | 1 | 1 | 1 | 0 | 0 | 4 |
| 22 | 1 | 1 | 0 | 0 | 0 | 0 | 2 |
| 23 | 1 | 1 | 1 | 0 | 1 | 1 | 5 |
| 24 | 1 | 1 | 1 | 0 | 1 | 1 | 5 |
| 25 | 1 | 1 | 1 | 0 | 1 | 1 | 5 |
| 26 | 1 | 1 | 1 | 0 | 0 | 1 | 4 |
| 27 | 1 | 1 | 0 | 0 | 0 | 1 | 3 |
| 28 | 1 | 1 | 0 | 1 | 1 | 1 | 5 |
| 29 | 1 | 1 | 0 | 0 | 1 | 1 | 4 |
| 30 | 1 | 1 | 0 | 1 | 1 | 1 | 5 |
| 31 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modica, R.; Altieri, B.; D’Aniello, F.; Benevento, E.; Cannavale, G.; Minotta, R.; Liccardi, A.; Colao, A.; Faggiano, A. Vitamin D and Bone Metabolism in Adult Patients with Neurofibromatosis Type 1. Metabolites 2023, 13, 255. https://doi.org/10.3390/metabo13020255
Modica R, Altieri B, D’Aniello F, Benevento E, Cannavale G, Minotta R, Liccardi A, Colao A, Faggiano A. Vitamin D and Bone Metabolism in Adult Patients with Neurofibromatosis Type 1. Metabolites. 2023; 13(2):255. https://doi.org/10.3390/metabo13020255
Chicago/Turabian StyleModica, Roberta, Barbara Altieri, Francesco D’Aniello, Elio Benevento, Giuseppe Cannavale, Roberto Minotta, Alessia Liccardi, Annamaria Colao, and Antongiulio Faggiano. 2023. "Vitamin D and Bone Metabolism in Adult Patients with Neurofibromatosis Type 1" Metabolites 13, no. 2: 255. https://doi.org/10.3390/metabo13020255
APA StyleModica, R., Altieri, B., D’Aniello, F., Benevento, E., Cannavale, G., Minotta, R., Liccardi, A., Colao, A., & Faggiano, A. (2023). Vitamin D and Bone Metabolism in Adult Patients with Neurofibromatosis Type 1. Metabolites, 13(2), 255. https://doi.org/10.3390/metabo13020255