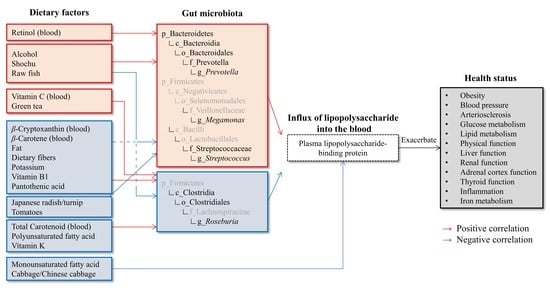
Association of Plasma Lipopolysaccharide-Binding Protein Concentration with Dietary Factors, Gut Microbiota, and Health Status in the Japanese General Adult Population: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval and Consent to Participate
2.2. Study Design and Population
2.3. Collection of Attribute Information
2.4. Blood Sampling and Measurement of Plasma LBP Concentrations
2.5. Clinical Markers and Clinical Scores
2.6. Dietary Factors
2.7. Analysis of Intestinal Bacteria by Next-Generation Sequencing
2.8. Statistics
3. Results
3.1. Characteristics of Participants
3.2. Distribution of Plasma LBP Concentration According to Participant Characteristics
3.3. Association of Plasma LBP Concentration and Clinical Markers or Clinical Scores
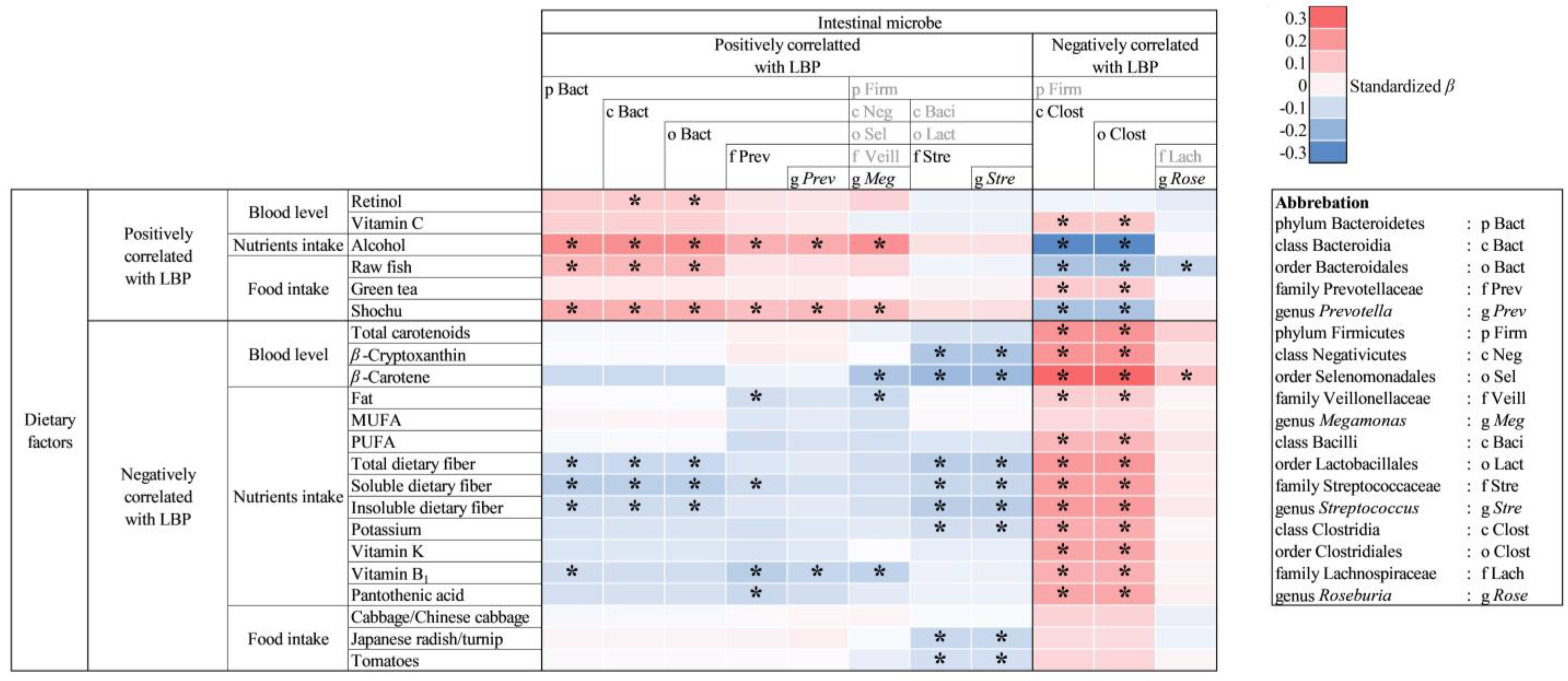
3.4. Association of Plasma LBP Concentration and Dietary Factors
| Category | Variable | β | Q Value |
|---|---|---|---|
| Blood antioxidant | Total carotenoids (μg/mL) 3 | −0.05 | 0.19 * |
| Lutein (μg/mL) | −0.04 | 0.33 | |
| Zeaxanthin (μg/mL) | −0.02 | 0.55 | |
| β-Cryptoxanthin (μg/mL) | −0.05 | 0.12 * | |
| α-Carotene (μg/mL) | −0.01 | 0.71 | |
| β-Carotene (μg/mL) | −0.04 | 0.08 * | |
| Lycopene (μg/mL) | −0.02 | 0.40 | |
| Retinol (μg/mL) | 0.09 | 0.08 * | |
| Vitamin C (μg/mL) | 0.06 | 0.16 * | |
| α-Tocopherol (μg/mL) | 0.06 | 0.36 | |
| Nutrition intake | Protein (100 g) | −0.11 | 0.36 |
| Fat (100 g) | −0.19 | 0.15 * | |
| SFA (100 g) | −0.48 | 0.26 | |
| MUFA (100 g) | −0.47 | 0.17 * | |
| PUFA (100 g) | −0.67 | 0.19 * | |
| Cholesterol (100 mg) | −0.01 | 0.43 | |
| Carbohydrate (100 g) | 0.00 | 0.80 | |
| Total dietary fiber (100 g) | −0.75 | 0.11 * | |
| Soluble dietary fiber (100 g) | −2.62 | 0.10 * | |
| Insoluble dietary fiber (100 g) | −1.09 | 0.11 * | |
| Sodium (100 mg) | 0.00 | 0.69 | |
| Potassium (100 mg) | −0.004 | 0.17 * | |
| Calcium (100 mg) | −0.01 | 0.42 | |
| Magnesium (100 mg) | −0.03 | 0.31 | |
| Phosphorus (100 mg) | −0.01 | 0.36 | |
| Iron (100 mg) | −0.86 | 0.32 | |
| Zinc (100 mg) | −1.63 | 0.21 | |
| Copper (100 mg) | −7.82 | 0.37 | |
| Manganese (100 mg) | 2.64 | 0.22 | |
| Retinol eq. (100 μg RE) | 0.00 | 0.79 | |
| Vitamin D (100 μg) | 0.01 | 0.79 | |
| α-Tocopherol (100 mg) | −1.05 | 0.22 | |
| Vitamin K (100 μg) | −0.02 | 0.16 * | |
| Vitamin B1 (100 mg) | −13.90 | 0.13 * | |
| Vitamin B2 (100 mg) | −3.41 | 0.51 | |
| Niacin (100 mg) | −0.22 | 0.49 | |
| Vitamin B6 (100 mg) | −5.37 | 0.28 | |
| Vitamin B12 (100 μg) | 0.07 | 0.72 | |
| Folate (100 μg) | −0.01 | 0.49 | |
| Pantothenic acid (100 mg) | −1.83 | 0.14 * | |
| Alcohol (100 g) | 0.14 | 0.12 * | |
| Food intake | |||
| Vegetables | Pickled green leaves vegetables (100 g) | −0.09 | 0.58 |
| Other pickled vegetables (100 g) | −0.09 | 0.54 | |
| Raw lettuces/cabbage (100 g) | −0.10 | 0.22 | |
| Green leaves vegetables (100 g) | −0.03 | 0.52 | |
| Cabbage/Chinese cabbage (100 g) | −0.10 | 0.11 * | |
| Carrots/pumpkin (100 g) | −0.10 | 0.39 | |
| Japanese radish/turnip (100 g) | −0.14 | 0.12 * | |
| Other root vegetables (100 g) | −0.07 | 0.30 | |
| Tomatoes (100 g) | −0.10 | 0.09 * | |
| Fish and shellfish | Squid/octopus/shrimp/shellfish (100 g) | 0.10 | 0.32 |
| Small fish with bones (100 g) | 0.06 | 0.60 | |
| Canned tuna (100 g) | −0.15 | 0.56 | |
| Dried fish/salted fish (100 g) | 0.00 | 0.80 | |
| Oily fish (100 g) | −0.02 | 0.72 | |
| Lean fish (100 g) | 0.01 | 0.78 | |
| Raw fish (100 g) | 0.11 | 0.08 * | |
| Grilled fish (100 g) | −0.03 | 0.51 | |
| Boiled fish (100 g) | 0.00 | 0.80 | |
| Fried fish (100 g) | 0.06 | 0.49 | |
| Alcoholic beverage | Sake (100 g) | 0.00 | 0.79 |
| Beer (100 g) | 0.00 | 0.56 | |
| Shochu (100 g) | 0.05 | 0.11 * | |
| Whiskey (100 g) | 0.05 | 0.55 | |
| Wine (100 g) | 0.03 | 0.54 | |
| Non-alcoholic beverage | Green tea (100 g) | 0.01 | 0.13 * |
| Black tea/oolong tea (100 g) | 0.02 | 0.31 | |
| Coffee (100 g) | −0.01 | 0.51 | |
| Cola drink/soft drink (100 g) | 0.00 | 0.79 |
3.5. Association of Plasma LBP Concentration and Intestinal Microbiota Composition
3.6. Association of Dietary Factors and Intestinal Microbiota Composition
4. Discussion
4.1. Characteristics of the Study Subjects
4.2. Health Statuses Correlated with Plasma LBP Concentration
4.3. Dietary Factors Correlated to Plasma LBP Concentration
4.4. Correlation between Plasma LBP Concentration and Intestinal Bacteria
4.5. Relationship between Dietary Factors and Intestinal Bacteria
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuke, N.; Nagata, N.; Suganuma, H.; Ota, T. Regulation of Gut Microbiota and Metabolic Endotoxemia with Dietary Factors. Nutrients 2019, 11, 2277. [Google Scholar] [CrossRef]
- Fougère, B.; Boulanger, E.; Nourhashémi, F.; Guyonnet, S.; Cesari, M. Chronic Inflammation: Accelerator of Biological Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1218–1225. [Google Scholar] [CrossRef]
- Liang, H.; Hussey, S.E.; Sanchez-Avila, A.; Tantiwong, P.; Musi, N. Effect of lipopolysaccharide on inflammation and insulin action in human muscle. PLoS ONE 2013, 8, e63983. [Google Scholar] [CrossRef]
- Mehta, N.N.; McGillicuddy, F.C.; Anderson, P.D.; Hinkle, C.C.; Shah, R.; Pruscino, L.; Tabita-Martinez, J.; Sellers, K.F.; Rickels, M.R.; Reilly, M.P. Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes 2010, 59, 172–181. [Google Scholar] [CrossRef]
- Pussinen, P.J.; Havulinna, A.S.; Lehto, M.; Sundvall, J.; Salomaa, V. Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care 2011, 34, 392–397. [Google Scholar] [CrossRef]
- Jin, R.; Willment, A.; Patel, S.S.; Sun, X.; Song, M.; Mannery, Y.O.; Kosters, A.; McClain, C.J.; Vos, M.B. Fructose induced endotoxemia in pediatric nonalcoholic Fatty liver disease. Int. J. Hepatol. 2014, 2014, 560620. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Madhulika, A.; Deepika, G.; Rao, G.V.; Reddy, D.N.; Subramanyam, C.; Sasikala, M.; Talukdar, R. Altered intestinal microbiota in patients with chronic pancreatitis: Implications in diabetes and metabolic abnormalities. Sci. Rep. 2017, 7, 43640. [Google Scholar] [CrossRef]
- Zhang, R.; Miller, R.G.; Gascon, R.; Champion, S.; Katz, J.; Lancero, M.; Narvaez, A.; Honrada, R.; Ruvalcaba, D.; McGrath, M.S. Circulating endotoxin and systemic immune activation in sporadic amyotrophic lateral sclerosis (sALS). J. Neuroimmunol. 2009, 206, 121–124. [Google Scholar] [CrossRef]
- Röytiö, H.; Mokkala, K.; Vahlberg, T.; Laitinen, K. Dietary intake of fat and fibre according to reference values relates to higher gut microbiota richness in overweight pregnant women. Br. J. Nutr. 2017, 118, 343–352. [Google Scholar] [CrossRef]
- Ahola, A.J.; Lassenius, M.I.; Forsblom, C.; Harjutsalo, V.; Lehto, M.; Groop, P.-H. Dietary patterns reflecting healthy food choices are associated with lower serum LPS activity. Sci. Rep. 2017, 7, 6511. [Google Scholar] [CrossRef]
- Luthold, R.V.; Fernandes, G.R.; Franco-de-Moraes, A.C.; Folchetti, L.G.D.; Ferreira, S.R.G. Gut microbiota interactions with the immunomodulatory role of vitamin D in normal individuals. Metabolism 2017, 69, 76–86. [Google Scholar] [CrossRef]
- Dheda, S.; Min, H.; Vesey, D.; Hawley, C.; Johnson, D.W.; Fahim, M. Establishing a stable platform for the measurement of blood endotoxin levels in the dialysis population. Diagnosis 2021, 8, 249–256. [Google Scholar] [CrossRef]
- Geller, D.A.; Kispert, P.H.; Su, G.L.; Wang, S.C.; Di Silvio, M.; Tweardy, D.J.; Billiar, T.R.; Simmons, R.L. Induction of hepatocyte lipopolysaccharide binding protein in models of sepsis and the acute-phase response. Arch. Surg. 1993, 128, 22–27, discussion 27-8. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Ortega, F.; Serino, M.; Luche, E.; Waget, A.; Pardo, G.; Salvador, J.; Ricart, W.; Frühbeck, G.; Burcelin, R.; et al. Circulating lipopolysaccharide-binding protein (LBP) as a marker of obesity-related insulin resistance. Int. J. Obes. 2012, 36, 1442–1449. [Google Scholar] [CrossRef]
- Liu, X.; Lu, L.; Yao, P.; Ma, Y.; Wang, F.; Jin, Q.; Ye, X.; Li, H.; Hu, F.B.; Sun, L.; et al. Lipopolysaccharide binding protein, obesity status and incidence of metabolic syndrome: A prospective study among middle-aged and older Chinese. Diabetologia 2014, 57, 1834–1841. [Google Scholar] [CrossRef]
- Tilves, C.M.; Zmuda, J.M.; Kuipers, A.L.; Nestlerode, C.S.; Evans, R.W.; Bunker, C.H.; Patrick, A.L.; Miljkovic, I. Association of Lipopolysaccharide-Binding Protein With Aging-Related Adiposity Change and Prediabetes Among African Ancestry Men. Diabetes Care 2016, 39, 385–391. [Google Scholar] [CrossRef]
- Asada, M.; Oishi, E.; Sakata, S.; Hata, J.; Yoshida, D.; Honda, T.; Furuta, Y.; Shibata, M.; Suzuki, K.; Watanabe, H.; et al. Serum Lipopolysaccharide-Binding Protein Levels and the Incidence of Cardiovascular Disease in a General Japanese Population: The Hisayama Study. J. Am. Heart Assoc. 2019, 8, e013628. [Google Scholar] [CrossRef]
- Gonzalez-Quintela, A.; Alonso, M.; Campos, J.; Vizcaino, L.; Loidi, L.; Gude, F. Determinants of serum concentrations of lipopolysaccharide-binding protein (LBP) in the adult population: The role of obesity. PLoS ONE 2013, 8, e54600. [Google Scholar] [CrossRef]
- Onuma, H.; Tabara, Y.; Kawamoto, R.; Shimizu, I.; Kawamura, R.; Takata, Y.; Nishida, W.; Ohashi, J.; Miki, T.; Kohara, K.; et al. The GCKR rs780094 polymorphism is associated with susceptibility of type 2 diabetes, reduced fasting plasma glucose levels, increased triglycerides levels and lower HOMA-IR in Japanese population. J. Hum. Genet. 2010, 55, 600–604. [Google Scholar] [CrossRef]
- AKIZUKI, Y.; INOUE, Y. The Concept of Visual Acuity Ratio to the Maximum Level of Individual Visual Acuity. J. Light Vis. Environ. 2004, 28, 35–49. [Google Scholar] [CrossRef]
- Sasaki, S.; Yanagibori, R.; Amano, K. Self-administered diet history questionnaire developed for health education: A relative validation of the test-version by comparison with 3-day diet record in women. J. Epidemiol. 1998, 8, 203–215. [Google Scholar] [CrossRef]
- Sasaki, S.; Ushio, F.; Amano, K.; Morihara, M.; Todoriki, O.; Uehara, Y.; Toyooka, E. Serum biomarker-based validation of a self-administered diet history questionnaire for Japanese subjects. J. Nutr. Sci. Vitaminol. 2000, 46, 285–296. [Google Scholar] [CrossRef]
- Kobayashi, S.; Yuan, X.; Sasaki, S.; Osawa, Y.; Hirata, T.; Abe, Y.; Takayama, M.; Arai, Y.; Masui, Y.; Ishizaki, T. Relative validity of brief-type self-administered diet history questionnaire among very old Japanese aged 80 years or older. Public Health Nutr. 2019, 22, 212–222. [Google Scholar] [CrossRef]
- Kobayashi, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr. 2011, 14, 1200–1211. [Google Scholar] [CrossRef]
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both Comprehensive and Brief Self-Administered Diet History Questionnaires Satisfactorily Rank Nutrient Intakes in Japanese Adults. J. Epidemiol. 2012, 22, 151–159. [Google Scholar] [CrossRef]
- Oshima, S.; Sakamoto, H.; Ishiguro, Y.; Terao, J. Accumulation and clearance of capsanthin in blood plasma after the ingestion of paprika juice in men. J. Nutr. 1997, 127, 1475–1479. [Google Scholar] [CrossRef]
- Sugimura, Y.; Kanda, A.; Sawada, K.; Wai, K.M.; Tanabu, A.; Ozato, N.; Midorikawa, T.; Hisada, T.; Nakaji, S.; Ihara, K. Association between Gut Microbiota and Body Composition in Japanese General Population: A Focus on Gut Microbiota and Skeletal Muscle. Int. J. Environ. Res. Public Health 2022, 19, 7464. [Google Scholar] [CrossRef]
- Peters, B.A.; Wu, J.; Pei, Z.; Yang, L.; Purdue, M.P.; Freedman, N.D.; Jacobs, E.J.; Gapstur, S.M.; Hayes, R.B.; Ahn, J. Oral Microbiome Composition Reflects Prospective Risk for Esophageal Cancers. Cancer Res. 2017, 77, 6777–6787. [Google Scholar] [CrossRef]
- Willett, W.; Stampfer, M.J. Total energy intake: Implications for epidemiologic analyses. Am. J. Epidemiol. 1986, 124, 17–27. [Google Scholar] [CrossRef]
- J. Ministry of Health, Labour and Welfare, The National Health and Nutrition Survey, Japan. 2017. Available online: https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00450171&kikan=00450&tstat=000001041744&cycle=7&tclass1=000001123258&survey=健康&result_page=1&cycle_facet=cycle&tclass2val=0 (accessed on 6 February 2023).
- Nishijima, S.; Suda, W.; Oshima, K.; Kim, S.-W.; Hirose, Y.; Morita, H.; Hattori, M. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 2016, 23, 125–133. [Google Scholar] [CrossRef]
- Kim, S.; Goel, R.; Kumar, A.; Qi, Y.; Lobaton, G.; Hosaka, K.; Mohammed, M.; Handberg, E.M.; Richards, E.M.; Pepine, C.J.; et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin. Sci. 2018, 132, 701–718. [Google Scholar] [CrossRef]
- Sakura, T.; Morioka, T.; Shioi, A.; Kakutani, Y.; Miki, Y.; Yamazaki, Y.; Motoyama, K.; Mori, K.; Fukumoto, S.; Shoji, T.; et al. Lipopolysaccharide-binding protein is associated with arterial stiffness in patients with type 2 diabetes: A cross-sectional study. Cardiovasc. Diabetol. 2017, 16, 62. [Google Scholar] [CrossRef]
- Carpino, G.; Del Ben, M.; Pastori, D.; Carnevale, R.; Baratta, F.; Overi, D.; Francis, H.; Cardinale, V.; Onori, P.; Safarikia, S.; et al. Increased Liver Localization of Lipopolysaccharides in Human and Experimental NAFLD. Hepatology 2020, 72, 470–485. [Google Scholar] [CrossRef]
- Nier, A.; Brandt, A.; Conzelmann, I.B.; Özel, Y.; Bergheim, I. Non-Alcoholic Fatty Liver Disease in Overweight Children: Role of Fructose Intake and Dietary Pattern. Nutrients 2018, 10, 1329. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Piasecki, M.; Atherton, P.J. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res. Rev. 2018, 47, 123–132. [Google Scholar] [CrossRef]
- Tanimoto, Y.; Watanabe, M.; Kono, R.; Hirota, C.; Takasaki, K.; Kono, K. Aging changes in muscle mass of Japanese. Nihon Ronen Igakkai Zasshi. 2010, 47, 52–57. [Google Scholar] [CrossRef]
- Jin, B.; Li, Y.-P. Curcumin prevents lipopolysaccharide-induced atrogin-1/MAFbx upregulation and muscle mass loss. J. Cell. Biochem. 2007, 100, 960–969. [Google Scholar] [CrossRef]
- Doyle, A.; Zhang, G.; Abdel Fattah, E.A.; Eissa, N.T.; Li, Y.-P. Toll-like receptor 4 mediates lipopolysaccharide-induced muscle catabolism via coordinate activation of ubiquitin-proteasome and autophagy-lysosome pathways. FASEB J. 2011, 25, 99–110. [Google Scholar] [CrossRef]
- Albillos, A.; de la Hera, A.; González, M.; Moya, J.-L.; Calleja, J.-L.; Monserrat, J.; Ruiz-del-Arbol, L.; Alvarez-Mon, M. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology 2003, 37, 208–217. [Google Scholar] [CrossRef]
- Lavoie, J.L.; Sigmund, C.D. Minireview: Overview of the renin-angiotensin system—An endocrine and paracrine system. Endocrinology 2003, 144, 2179–2183. [Google Scholar] [CrossRef]
- Vakharia, K.; Hinson, J.P. Lipopolysaccharide directly stimulates cortisol secretion by human adrenal cells by a cyclooxygenase-dependent mechanism. Endocrinology 2005, 146, 1398–1402. [Google Scholar] [CrossRef]
- Kox, M.; van Eijk, L.T.; Zwaag, J.; van den Wildenberg, J.; Sweep, F.C.G.J.; van der Hoeven, J.G.; Pickkers, P. Voluntary activation of the sympathetic nervous system and attenuation of the innate immune response in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 7379–7384. [Google Scholar] [CrossRef]
- Yeager, M.P.; Rassias, A.J.; Pioli, P.A.; Beach, M.L.; Wardwell, K.; Collins, J.E.; Lee, H.-K.; Guyre, P.M. Pretreatment with stress cortisol enhances the human systemic inflammatory response to bacterial endotoxin. Crit. Care Med. 2009, 37, 2727–2732. [Google Scholar] [CrossRef]
- de Jongh, R.T.; Ijzerman, R.G.; Serné, E.H.; van Weissenbruch, M.M.; Voordouw, J.J.; Delemarre-van de Waal, H.A.; Stehouwer, C.D.A. Urinary cortisol is inversely associated with capillary recruitment in women: A potential explanation for the cortisol-blood pressure relationship. Clin. Sci. 2007, 113, 83–91. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, L.; Sheng, C.; Cheng, Z.; Cui, L.; Li, M.; Zhao, Y.; Shi, T.; Yau, T.O.; Li, F.; et al. Increased Serum Levels of Cortisol and Inflammatory Cytokines in People With Depression. J. Nerv. Ment. Dis. 2019, 207, 271–276. [Google Scholar] [CrossRef]
- Lin, X.; Shi, S.; Shi, S. Sepsis leads to thyroid impairment and dysfunction in rat model. Tissue Cell 2016, 48, 511–515. [Google Scholar] [CrossRef]
- Luo, B.; Yu, Z.; Li, Y. Thyroid hormone disorders and sepsis. Biomed. Mater. Eng. 2017, 28, S237–S241. [Google Scholar] [CrossRef]
- Mazzoccoli, G.; Carughi, S.; Sperandeo, M.; Pazienza, V.; Giuliani, F.; Tarquini, R. Neuro-endocrine correlations of hypothalamic-pituitary-thyroid axis in healthy humans. J. Biol. Regul. Homeost. Agents 2011, 25, 249–257. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21880214 (accessed on 6 February 2023).
- Barnum-Huckins, K.M.; Martinez, A.O.; Rivera, E.V.; Adrian, E.K.; Herbert, D.C.; Weaker, F.J.; Walter, C.A.; Adrian, G.S. A comparison of the suppression of human transferrin synthesis by lead and lipopolysaccharide. Toxicology 1997, 118, 11–22. [Google Scholar] [CrossRef]
- DeGregorio-Rocasolano, N.; Martí-Sistac, O.; Gasull, T. Deciphering the Iron Side of Stroke: Neurodegeneration at the Crossroads Between Iron Dyshomeostasis, Excitotoxicity, and Ferroptosis. Front. Neurosci. 2019, 13, 85. [Google Scholar] [CrossRef]
- Hare, D.J.; Doecke, J.D.; Faux, N.G.; Rembach, A.; Volitakis, I.; Fowler, C.J.; Grimm, R.; Doble, P.A.; Cherny, R.A.; Masters, C.L.; et al. Decreased plasma iron in Alzheimer’s disease is due to transferrin desaturation. ACS Chem. Neurosci. 2015, 6, 398–402. [Google Scholar] [CrossRef]
- López-Moreno, J.; García-Carpintero, S.; Jimenez-Lucena, R.; Haro, C.; Rangel-Zúñiga, O.A.; Blanco-Rojo, R.; Yubero-Serrano, E.M.; Tinahones, F.J.; Delgado-Lista, J.; Pérez-Martínez, P.; et al. Effect of Dietary Lipids on Endotoxemia Influences Postprandial Inflammatory Response. J. Agric. Food Chem. 2017, 65, 7756–7763. [Google Scholar] [CrossRef]
- Umoh, F.I.; Kato, I.; Ren, J.; Wachowiak, P.L.; Ruffin, M.T.; Turgeon, D.K.; Sen, A.; Brenner, D.E.; Djuric, Z. Markers of systemic exposures to products of intestinal bacteria in a dietary intervention study. Eur. J. Nutr. 2016, 55, 793–798. [Google Scholar] [CrossRef]
- Nagata, N.; Xu, L.; Kohno, S.; Ushida, Y.; Aoki, Y.; Umeda, R.; Fuke, N.; Zhuge, F.; Ni, Y.; Nagashimada, M.; et al. Glucoraphanin Ameliorates Obesity and Insulin Resistance Through Adipose Tissue Browning and Reduction of Metabolic Endotoxemia in Mice. Diabetes 2017, 66, 1222–1236. [Google Scholar] [CrossRef]
- He, C.; Huang, L.; Lei, P.; Liu, X.; Li, B.; Shan, Y. Sulforaphane Normalizes Intestinal Flora and Enhances Gut Barrier in Mice with BBN-Induced Bladder Cancer. Mol. Nutr. Food Res. 2018, 62, e1800427. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, J.; Li, J.; Bai, Y.; Luo, Y.; Ji, B.; Xia, B.; Liu, Z.; Tan, X.; Lv, J.; et al. Lycopene Alleviates DSS-Induced Colitis and Behavioral Disorders via Mediating Microbes-Gut-Brain Axis Balance. J. Agric. Food Chem. 2020, 68, 3963–3975. [Google Scholar] [CrossRef]
- Kopec, R.E.; Gleize, B.; Borel, P.; Desmarchelier, C.; Caris-Veyrat, C. Are lutein, lycopene, and β-carotene lost through the digestive process? Food Funct. 2017, 8, 1494–1503. [Google Scholar] [CrossRef]
- Ara, T.; Sakurai, N.; Takahashi, S.; Waki, N.; Suganuma, H.; Aizawa, K.; Matsumura, Y.; Kawada, T.; Shibata, D. TOMATOMET: A metabolome database consists of 7118 accurate mass values detected in mature fruits of 25 tomato cultivars. Plant Direct 2021, 5, e00318. [Google Scholar] [CrossRef]
- Mohri, S.; Takahashi, H.; Sakai, M.; Takahashi, S.; Waki, N.; Aizawa, K.; Suganuma, H.; Ara, T.; Matsumura, Y.; Shibata, D.; et al. Wide-range screening of anti-inflammatory compounds in tomato using LC-MS and elucidating the mechanism of their functions. PLoS ONE 2018, 13, e0191203. [Google Scholar] [CrossRef]
- Xiao, M.-L.; Chen, G.-D.; Zeng, F.-F.; Qiu, R.; Shi, W.-Q.; Lin, J.-S.; Cao, Y.; Li, H.-B.; Ling, W.-H.; Chen, Y.-M. Higher serum carotenoids associated with improvement of non-alcoholic fatty liver disease in adults: A prospective study. Eur. J. Nutr. 2019, 58, 721–730. [Google Scholar] [CrossRef]
- Sugiura, M.; Nakamura, M.; Ikoma, Y.; Yano, M.; Ogawa, K.; Matsumoto, H.; Kato, M.; Ohshima, M.; Nagao, A. The homeostasis model assessment-insulin resistance index is inversely associated with serum carotenoids in non-diabetic subjects. J. Epidemiol. 2006, 16, 71–78. [Google Scholar] [CrossRef]
- Matsumoto, M.; Waki, N.; Suganuma, H.; Takahashi, I.; Kurauchi, S.; Sawada, K.; Tokuda, I.; Misawa, M.; Ando, M.; Itoh, K.; et al. Association between Biomarkers of Cardiovascular Diseases and the Blood Concentration of Carotenoids among the General Population without Apparent Illness. Nutrients 2020, 12, 2310. [Google Scholar] [CrossRef]
- Vieira, M.M.; Paik, J.; Blaner, W.S.; Soares, A.M.; Mota, R.M.S.; Guerrant, R.L.; Lima, A.A.M. Carotenoids, retinol, and intestinal barrier function in children from northeastern Brazil. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 652–659. [Google Scholar] [CrossRef]
- dela Seña, C.; Narayanasamy, S.; Riedl, K.M.; Curley, R.W.; Schwartz, S.J.; Harrison, E.H. Substrate specificity of purified recombinant human β-carotene 15,15’-oxygenase (BCO1). J. Biol. Chem. 2013, 288, 37094–37103. [Google Scholar] [CrossRef]
- He, C.; Deng, J.; Hu, X.; Zhou, S.; Wu, J.; Xiao, D.; Darko, K.O.; Huang, Y.; Tao, T.; Peng, M.; et al. Vitamin A inhibits the action of LPS on the intestinal epithelial barrier function and tight junction proteins. Food Funct. 2019, 10, 1235–1242. [Google Scholar] [CrossRef]
- Morseth, M.S.; Strand, T.A.; Torheim, L.E.; Chandyo, R.K.; Ulak, M.; Shrestha, S.K.; Shrestha, B.; Henjum, S. Nutrient intake and environmental enteric dysfunction among Nepalese children 9-24 months old-the MAL-ED birth cohort study. Pediatr. Res. 2018, 84, 509–515. [Google Scholar] [CrossRef]
- Sabui, S.; Kapadia, R.; Ghosal, A.; Schneider, M.; Lambrecht, N.W.G.; Said, H.M. Biotin and pantothenic acid oversupplementation to conditional SLC5A6 KO mice prevents the development of intestinal mucosal abnormalities and growth defects. Am. J. Physiol. Cell Physiol. 2018, 315, C73–C79. [Google Scholar] [CrossRef]
- Greenspon, J.; Perrone, E.E.; Alaish, S.M. Shoshin beriberi mimicking central line sepsis in a child with short bowel syndrome. World J. Pediatr. 2010, 6, 366–368. [Google Scholar] [CrossRef]
- Liangpunsakul, S.; Toh, E.; Ross, R.A.; Heathers, L.E.; Chandler, K.; Oshodi, A.; McGee, B.; Modlik, E.; Linton, T.; Mangiacarne, D.; et al. Quantity of alcohol drinking positively correlates with serum levels of endotoxin and markers of monocyte activation. Sci. Rep. 2017, 7, 4462. [Google Scholar] [CrossRef]
- Butt, M.S.; Sultan, M.T. Green tea: Nature’s defense against malignancies. Crit. Rev. Food Sci. Nutr. 2009, 49, 463–473. [Google Scholar] [CrossRef]
- Dey, P.; Sasaki, G.Y.; Wei, P.; Li, J.; Wang, L.; Zhu, J.; McTigue, D.; Yu, Z.; Bruno, R.S. Green tea extract prevents obesity in male mice by alleviating gut dysbiosis in association with improved intestinal barrier function that limits endotoxin translocation and adipose inflammation. J. Nutr. Biochem. 2019, 67, 78–89. [Google Scholar] [CrossRef]
- Li, J.; Sasaki, G.Y.; Dey, P.; Chitchumroonchokchai, C.; Labyk, A.N.; McDonald, J.D.; Kim, J.B.; Bruno, R.S. Green tea extract protects against hepatic NFκB activation along the gut-liver axis in diet-induced obese mice with nonalcoholic steatohepatitis by reducing endotoxin and TLR4/MyD88 signaling. J. Nutr. Biochem. 2018, 53, 58–65. [Google Scholar] [CrossRef]
- Yu, C.; Tang, H.; Guo, Y.; Bian, Z.; Yang, L.; Chen, Y.; Tang, A.; Zhou, X.; Yang, X.; Chen, J.; et al. Hot Tea Consumption and Its Interactions With Alcohol and Tobacco Use on the Risk for Esophageal Cancer: A Population-Based Cohort Study. Ann. Intern. Med. 2018, 168, 489–497. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Protective Effect of Green Tea Consumption on Colorectal Cancer Varies by Lifestyle Factors. Nutrients 2019, 11, 2612. [Google Scholar] [CrossRef]
- Miwa, N.; Kashiwagi, M.; Kawamori, F.; Masuda, T.; Sano, Y.; Hiroi, M.; Kurashige, H. Levels of Vibrio parahaemolyticus and thermostable direct hemolysin gene-positive organisms in retail seafood determined by the most probable number-polymerase chain reaction (MPN-PCR) method. Shokuhin Eiseigaku Zasshi. 2006, 47, 41–45. [Google Scholar] [CrossRef]
- Hiyoshi, H.; Kodama, T.; Iida, T.; Honda, T. Contribution of Vibrio parahaemolyticus virulence factors to cytotoxicity, enterotoxicity, and lethality in mice. Infect. Immun. 2010, 78, 1772–1780. [Google Scholar] [CrossRef]
- Hayakawa, R.; Kobayashi, N.; Kato, N.; Hara-Kudo, Y.; Araki, E. Histamine formation in Japanese marine fish species and the effect of frozen storage. Shokuhin Eiseigaku Zasshi. 2013, 54, 402–409. [Google Scholar] [CrossRef]
- Kanny, G.; Grignon, G.; Dauca, M.; Guedenet, J.C.; Moneret-Vautrin, D.A. Ultrastructural changes in the duodenal mucosa induced by ingested histamine in patients with chronic urticaria. Allergy 1996, 51, 935–939. [Google Scholar] [CrossRef]
- Ito, T.; Kawakami, R.; Tanisawa, K.; Miyawaki, R.; Ishii, K.; Torii, S.; Suzuki, K.; Sakamoto, S.; Muraoka, I.; Oka, K.; et al. Dietary patterns and abdominal obesity in middle-aged and elderly Japanese adults: Waseda Alumni’s Sports, Exercise, Daily Activity, Sedentariness and Health Study (WASEDA’S Health Study). Nutrition 2019, 58, 149–155. [Google Scholar] [CrossRef]
- Tanisawa, K.; Ito, T.; Kawakami, R.; Usui, C.; Kawamura, T.; Suzuki, K.; Sakamoto, S.; Ishii, K.; Muraoka, I.; Oka, K.; et al. Association between alcohol dietary pattern and prevalence of dyslipidaemia: WASEDA’S Health Study. Br. J. Nutr. 2021, 127, 1712–1722. [Google Scholar] [CrossRef]
- Schoultz, I.; McKay, C.M.; Graepel, R.; Phan, V.C.; Wang, A.; Söderholm, J.; McKay, D.M. Indomethacin-induced translocation of bacteria across enteric epithelia is reactive oxygen species-dependent and reduced by vitamin C. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G536–G545. [Google Scholar] [CrossRef]
- Baltes, S.; Nau, H.; Lampen, A. All-trans retinoic acid enhances differentiation and influences permeability of intestinal Caco-2 cells under serum-free conditions. Dev. Growth Differ. 2004, 46, 503–514. [Google Scholar] [CrossRef]
- Kato, Y.; Ikehara, S.; Maruyama, K.; Inagawa, M.; Oshima, M.; Yokota, K.; Yamazaki, T.; Kishi, M.; Murai, S.; Umesawa, M.; et al. Trends in dietary intakes of vitamins A, C and E among Japanese men and women from 1974 to 2001. Public Health Nutr. 2009, 12, 1343–1350. [Google Scholar] [CrossRef]
- Medina-Vera, I.; Sanchez-Tapia, M.; Noriega-López, L.; Granados-Portillo, O.; Guevara-Cruz, M.; Flores-López, A.; Avila-Nava, A.; Fernández, M.L.; Tovar, A.R.; Torres, N. A dietary intervention with functional foods reduces metabolic endotoxaemia and attenuates biochemical abnormalities by modifying faecal microbiota in people with type 2 diabetes. Diabetes Metab. 2019, 45, 122–131. [Google Scholar] [CrossRef]
- Leite, A.Z.; Rodrigues, N.d.C.; Gonzaga, M.I.; Paiolo, J.C.C.; de Souza, C.A.; Stefanutto, N.A.V.; Omori, W.P.; Pinheiro, D.G.; Brisotti, J.L.; Matheucci Junior, E.; et al. Detection of Increased Plasma Interleukin-6 Levels and Prevalence of Prevotella copri and Bacteroides vulgatus in the Feces of Type 2 Diabetes Patients. Front. Immunol. 2017, 8, 1107. [Google Scholar] [CrossRef]
- Franke, T.; Deppenmeier, U. Physiology and central carbon metabolism of the gut bacterium Prevotella copri. Mol. Microbiol. 2018, 109, 528–540. [Google Scholar] [CrossRef]
- Spiga, L.; Winter, M.G.; Furtado de Carvalho, T.; Zhu, W.; Hughes, E.R.; Gillis, C.C.; Behrendt, C.L.; Kim, J.; Chessa, D.; Andrews-Polymenis, H.L.; et al. An Oxidative Central Metabolism Enables Salmonella to Utilize Microbiota-Derived Succinate. Cell Host Microbe 2017, 22, 291–301.e6. [Google Scholar] [CrossRef]
- Ferreyra, J.A.; Wu, K.J.; Hryckowian, A.J.; Bouley, D.M.; Weimer, B.C.; Sonnenburg, J.L. Gut microbiota-produced succinate promotes C. difficile infection after antibiotic treatment or motility disturbance. Cell Host Microbe 2014, 16, 770–777. [Google Scholar] [CrossRef]
- Macias-Ceja, D.C.; Ortiz-Masiá, D.; Salvador, P.; Gisbert-Ferrándiz, L.; Hernández, C.; Hausmann, M.; Rogler, G.; Esplugues, J.V.; Hinojosa, J.; Alós, R.; et al. Succinate receptor mediates intestinal inflammation and fibrosis. Mucosal Immunol. 2019, 12, 178–187. [Google Scholar] [CrossRef]
- Wright, D.P.; Rosendale, D.I.; Robertson, A.M. Prevotella enzymes involved in mucin oligosaccharide degradation and evidence for a small operon of genes expressed during growth on mucin. FEMS Microbiol. Lett. 2000, 190, 73–79. [Google Scholar] [CrossRef]
- Rho, J.; Wright, D.P.; Christie, D.L.; Clinch, K.; Furneaux, R.H.; Roberton, A.M. A novel mechanism for desulfation of mucin: Identification and cloning of a mucin-desulfating glycosidase (sulfoglycosidase) from Prevotella strain RS2. J. Bacteriol. 2005, 187, 1543–1551. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef]
- Sakon, H.; Nagai, F.; Morotomi, M.; Tanaka, R. Sutterella parvirubra sp. nov. and Megamonas funiformis sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2008, 58, 970–975. [Google Scholar] [CrossRef]
- Park, S.-H.; Kim, K.-A.; Ahn, Y.-T.; Jeong, J.-J.; Huh, C.-S.; Kim, D.-H. Comparative analysis of gut microbiota in elderly people of urbanized towns and longevity villages. BMC Microbiol. 2015, 15, 49. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.-X.; Xie, C.-Y.; Fan, J.; Lv, J.; Xu, X.-J.; Lv, J.; Kuai, W.-T.; Jia, Y.-T. Gegen Qinlian decoction enhances immunity and protects intestinal barrier function in colorectal cancer patients via gut microbiota. World J. Gastroenterol. 2020, 26, 7633–7651. [Google Scholar] [CrossRef]
- Maccioni, L.; Gao, B.; Leclercq, S.; Pirlot, B.; Horsmans, Y.; De Timary, P.; Leclercq, I.; Fouts, D.; Schnabl, B.; Stärkel, P. Intestinal permeability, microbial translocation, changes in duodenal and fecal microbiota, and their associations with alcoholic liver disease progression in humans. Gut Microbes 2020, 12, 1782157. [Google Scholar] [CrossRef]
- Imhann, F.; Bonder, M.J.; Vich Vila, A.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.M.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2016, 65, 740–748. [Google Scholar] [CrossRef]
- Rengarajan, S.; Vivio, E.E.; Parkes, M.; Peterson, D.A.; Roberson, E.D.O.; Newberry, R.D.; Ciorba, M.A.; Hsieh, C.-S. Dynamic immunoglobulin responses to gut bacteria during inflammatory bowel disease. Gut Microbes 2020, 11, 405–420. [Google Scholar] [CrossRef]
- Leiva-Gea, I.; Sánchez-Alcoholado, L.; Martín-Tejedor, B.; Castellano-Castillo, D.; Moreno-Indias, I.; Urda-Cardona, A.; Tinahones, F.J.; Fernández-García, J.C.; Queipo-Ortuño, M.I. Gut Microbiota Differs in Composition and Functionality Between Children With Type 1 Diabetes and MODY2 and Healthy Control Subjects: A Case-Control Study. Diabetes Care 2018, 41, 2385–2395. [Google Scholar] [CrossRef]
- Kasahara, K.; Krautkramer, K.A.; Org, E.; Romano, K.A.; Kerby, R.L.; Vivas, E.I.; Mehrabian, M.; Denu, J.M.; Bäckhed, F.; Lusis, A.J.; et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat. Microbiol. 2018, 3, 1461–1471. [Google Scholar] [CrossRef]
- Kisuse, J.; La-Ongkham, O.; Nakphaichit, M.; Therdtatha, P.; Momoda, R.; Tanaka, M.; Fukuda, S.; Popluechai, S.; Kespechara, K.; Sonomoto, K.; et al. Urban Diets Linked to Gut Microbiome and Metabolome Alterations in Children: A Comparative Cross-Sectional Study in Thailand. Front. Microbiol. 2018, 9, 1345. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Xiong, X.-Q.; Yang, T.; Cui, T.; Hou, N.-L.; Lai, X.; Liu, S.; Guo, M.; Liang, X.-H.; et al. Effect of vitamin A supplementation on gut microbiota in children with autism spectrum disorders—A pilot study. BMC Microbiol. 2017, 17, 204. [Google Scholar] [CrossRef]
- Dubinkina, V.B.; Tyakht, A.V.; Odintsova, V.Y.; Yarygin, K.S.; Kovarsky, B.A.; Pavlenko, A.V.; Ischenko, D.S.; Popenko, A.S.; Alexeev, D.G.; Taraskina, A.Y.; et al. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome 2017, 5, 141. [Google Scholar] [CrossRef]
- Deng, Y.-D.; Peng, X.-B.; Zhao, R.-R.; Ma, C.-Q.; Li, J.-N.; Yao, L.-Q. The intestinal microbial community dissimilarity in hepatitis B virus-related liver cirrhosis patients with and without at alcohol consumption. Gut Pathog. 2019, 11, 58. [Google Scholar] [CrossRef]
- Moritani, K.; Takeshita, T.; Shibata, Y.; Ninomiya, T.; Kiyohara, Y.; Yamashita, Y. Acetaldehyde production by major oral microbes. Oral Dis. 2015, 21, 748–754. [Google Scholar] [CrossRef]
- Brown, S.D.; Guss, A.M.; Karpinets, T.V.; Parks, J.M.; Smolin, N.; Yang, S.; Land, M.L.; Klingeman, D.M.; Bhandiwad, A.; Rodriguez, M.; et al. Mutant alcohol dehydrogenase leads to improved ethanol tolerance in Clostridium thermocellum. Proc. Natl. Acad. Sci. USA 2011, 108, 13752–13757. [Google Scholar] [CrossRef]
- Berding, K.; Holscher, H.D.; Arthur, A.E.; Donovan, S.M. Fecal microbiome composition and stability in 4- to 8-year old children is associated with dietary patterns and nutrient intake. J. Nutr. Biochem. 2018, 56, 165–174. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Fåk, F.; Nookaew, I.; Tremaroli, V.; Fagerberg, B.; Petranovic, D.; Bäckhed, F.; Nielsen, J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012, 3, 1245. [Google Scholar] [CrossRef]
- Fenn, K.; Strandwitz, P.; Stewart, E.J.; Dimise, E.; Rubin, S.; Gurubacharya, S.; Clardy, J.; Lewis, K. Quinones are growth factors for the human gut microbiota. Microbiome 2017, 5, 161. [Google Scholar] [CrossRef]
- Ahn, S.-J.; Desai, S.; Blanco, L.; Lin, M.; Rice, K.C. Acetate and Potassium Modulate the Stationary-Phase Activation of lrgAB in Streptococcus mutans. Front. Microbiol. 2020, 11, 401. [Google Scholar] [CrossRef]
- Laverde Gomez, J.A.; Mukhopadhya, I.; Duncan, S.H.; Louis, P.; Shaw, S.; Collie-Duguid, E.; Crost, E.; Juge, N.; Flint, H.J. Formate cross-feeding and cooperative metabolic interactions revealed by transcriptomics in co-cultures of acetogenic and amylolytic human colonic bacteria. Environ. Microbiol. 2019, 21, 259–271. [Google Scholar] [CrossRef]
- Duncan, S.H.; Barcenilla, A.; Stewart, C.S.; Pryde, S.E.; Flint, H.J. Acetate utilization and butyryl coenzyme A (CoA):acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl. Environ. Microbiol. 2002, 68, 5186–5190. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Belzer, C.; Goossens, M.; Kleerebezem, M.; De Vos, W.M.; Thas, O.; De Weirdt, R.; Kerckhof, F.-M.; Van de Wiele, T. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 2013, 7, 949–961. [Google Scholar] [CrossRef]
- Jung, T.-H.; Park, J.H.; Jeon, W.-M.; Han, K.-S. Butyrate modulates bacterial adherence on LS174T human colorectal cells by stimulating mucin secretion and MAPK signaling pathway. Nutr. Res. Pract. 2015, 9, 343–349. [Google Scholar] [CrossRef]
- Carrothers, J.M.; York, M.A.; Brooker, S.L.; Lackey, K.A.; Williams, J.E.; Shafii, B.; Price, W.J.; Settles, M.L.; McGuire, M.A.; McGuire, M.K. Fecal Microbial Community Structure Is Stable over Time and Related to Variation in Macronutrient and Micronutrient Intakes in Lactating Women. J. Nutr. 2015, 145, 2379–2388. [Google Scholar] [CrossRef]
- Chen, T.; Long, W.; Zhang, C.; Liu, S.; Zhao, L.; Hamaker, B.R. Fiber-utilizing capacity varies in Prevotella- versus Bacteroides-dominated gut microbiota. Sci. Rep. 2017, 7, 2594. [Google Scholar] [CrossRef]
- French, S.J.; Read, N.W. Effect of guar gum on hunger and satiety after meals of differing fat content: Relationship with gastric emptying. Am. J. Clin. Nutr. 1994, 59, 87–91. [Google Scholar] [CrossRef]
- Zwe, Y.H.; Goh, Z.H.E.; Chau, M.L.; Aung, K.T.; Yuk, H.-G. Survival of an emerging foodborne pathogen: Group B Streptococcus (GBS) serotype III sequence type (ST) 283-under simulated partial cooking and gastric fluid conditions. Food Sci. Biotechnol. 2019, 28, 939–944. [Google Scholar] [CrossRef]
- Leth, M.L.; Ejby, M.; Workman, C.; Ewald, D.A.; Pedersen, S.S.; Sternberg, C.; Bahl, M.I.; Licht, T.R.; Aachmann, F.L.; Westereng, B.; et al. Differential bacterial capture and transport preferences facilitate co-growth on dietary xylan in the human gut. Nat. Microbiol. 2018, 3, 570–580. [Google Scholar] [CrossRef]
- Nakajima, N.; Ishihara, K.; Matsuura, Y. Dietary-fiber-degrading enzymes from a human intestinal Clostridium and their application to oligosaccharide production from nonstarchy polysaccharides using immobilized cells. Appl. Microbiol. Biotechnol. 2002, 59, 182–189. [Google Scholar] [CrossRef]
- Wei, X.; Fu, X.; Xiao, M.; Liu, Z.; Zhang, L.; Mou, H. Dietary galactosyl and mannosyl carbohydrates: In-vitro assessment of prebiotic effects. Food Chem. 2020, 329, 127179. [Google Scholar] [CrossRef]
- Nakayama, J.; Yamamoto, A.; Palermo-Conde, L.A.; Higashi, K.; Sonomoto, K.; Tan, J.; Lee, Y.-K. Impact of Westernized Diet on Gut Microbiota in Children on Leyte Island. Front. Microbiol. 2017, 8, 197. [Google Scholar] [CrossRef]
- Wan, Y.; Tong, W.; Zhou, R.; Li, J.; Yuan, J.; Wang, F.; Li, D. Habitual animal fat consumption in shaping gut microbiota and microbial metabolites. Food Funct. 2019, 10, 7973–7982. [Google Scholar] [CrossRef]
- De Weirdt, R.; Hernandez-Sanabria, E.; Fievez, V.; Mees, E.; Geirnaert, A.; Van Herreweghen, F.; Vilchez-Vargas, R.; Van den Abbeele, P.; Jauregui, R.; Pieper, D.H.; et al. Mucosa-associated biohydrogenating microbes protect the simulated colon microbiome from stress associated with high concentrations of poly-unsaturated fat. Environ. Microbiol. 2017, 19, 722–739. [Google Scholar] [CrossRef]
- Huws, S.A.; Kim, E.J.; Lee, M.R.F.; Scott, M.B.; Tweed, J.K.S.; Pinloche, E.; Wallace, R.J.; Scollan, N.D. As yet uncultured bacteria phylogenetically classified as Prevotella, Lachnospiraceae incertae sedis and unclassified Bacteroidales, Clostridiales and Ruminococcaceae may play a predominant role in ruminal biohydrogenation. Environ. Microbiol. 2011, 13, 1500–1512. [Google Scholar] [CrossRef]
- Jadoun, J.; Yazbak, A.; Rushrush, S.; Rudy, A.; Azaizeh, H. Identification of a New Antibacterial Sulfur Compound from Raphanus sativus Seeds. Evid. Based. Complement. Alternat. Med. 2016, 2016, 9271285. [Google Scholar] [CrossRef]
- Kurepina, N.; Kreiswirth, B.N.; Mustaev, A. Growth-inhibitory activity of natural and synthetic isothiocyanates against representative human microbial pathogens. J. Appl. Microbiol. 2013, 115, 943–954. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Escoté, X.; Ortega, F.; Serino, M.; Campbell, M.; Michalski, M.-C.; Laville, M.; Xifra, G.; Luche, E.; Domingo, P.; et al. A role for adipocyte-derived lipopolysaccharide-binding protein in inflammation- and obesity-associated adipose tissue dysfunction. Diabetologia 2013, 56, 2524–2537. [Google Scholar] [CrossRef]
- Hamamura, H.; Adachi, H.; Enomoto, M.; Fukami, A.; Nakamura, S.; Nohara, Y.; Morikawa, N.; Sakaue, A.; Toyomasu, K.; Yamamoto, M.; et al. Serum Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) is Independently Associated with Insulin Resistance, Triglycerides, Lipoprotein(a) Levels but not Low-Density Lipoprotein Cholesterol Levels in a General Population. J. Atheroscler. Thromb. 2021, 28, 329–337. [Google Scholar] [CrossRef]
- Hoshino, Y.; Seichi, A. Locomo 25—A screening tool for risk of locomotive syndrome. Nihon Rinsho. Jpn. J. Clin. Med. 2014, 72, 1839–1843. [Google Scholar]
- Ikeda, K.; Noda, K.; Yamaguchi, S. A Relation between Adaptation Luminance and Visual Acuity for the Landort Ring under the Uniform Background. J. Illum. Eng. Inst. Jpn. 1980, 64, 591–597. [Google Scholar] [CrossRef]
- Inagaki, H.; Ito, K.; Sakuma, N.; Sugiyama, M.; Okamura, T.; Awata, S. Reliability and validity of the simplified Japanese version of the WHO-Five Well-being Index (S-WHO-5-J). Nihon Koshu Eisei Zasshi 2013, 60, 294–301. [Google Scholar]
- Radloff, L.S. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
| All Subjects | Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Mean ± SD or n (%) | Median | IQR | Mean ± SD or n (%) | Median | IQR | Mean ± SD or n (%) | Median | IQR |
| Sex (male/female) | 375 (41.9)/521 (58.1) | NA | NA | NA | NA | NA | NA | NA | NA |
| Age (years) | 54.7 ± 15.0 | 56.0 | 43.0–67.0 | 54.2 ± 15.3 | 55.0 | 41.0–67.0 | 55.0 ± 14.9 | 57.0 | 43.0–66.0 |
| Current smoker | 128 (14.3) | NA | NA | 91 (24.3) | NA | NA | 37 (7.1) | NA | NA |
| Current drinker | 435 (48.5) | NA | NA | 261 (69.6) | NA | NA | 174 (33.4) | NA | NA |
| Body mass index (kg/m2) | 23.1 ± 3.6 | 22.8 | 20.5–25.1 | 23.9 ± 3.4 | 23.5 | 21.6–25.8 | 22.5 ± 3.6 | 22.2 | 19.8–24.4 |
| Abdominal circumference (cm) | 85.0 ± 10.0 | 84.7 | 78.6–90.6 | 89.0 ± 9.3 | 87.8 | 83.2–93.6 | 82.2 ± 9.5 | 81.6 | 75.4–87.8 |
| Systolic blood pressure (mmHg) | 124 ± 18 | 122 | 111–134 | 127 ± 18 | 125 | 114–137 | 121 ± 17 | 120 | 109–131 |
| Diastolic blood pressure (mmHg) | 72 ± 12 | 71 | 63–79 | 74 ± 12 | 73 | 66–82 | 70 ± 11 | 69 | 62–78 |
| baPWV (cm/s) | 1500 ± 382 | 1440 | 1220–1690 | 1567 ± 404 | 1498 | 1285–1730 | 1445 ± 358 | 1386 | 1170–1668 |
| HOMA-IR | 1.32 ± 1.27 | 1.03 | 0.78–1.50 | 1.41 ± 1.72 | 1.03 | 0.74–1.52 | 1.25 ± 0.81 | 1.02 | 0.80–1.49 |
| Blood glucose (mg/dL) | 95.1 ± 16.2 | 91.5 | 86.0–99.0 | 98.6 ± 19.3 | 94.0 | 88.0–103.0 | 92.5 ± 12.8 | 90.0 | 84.0–97.0 |
| HbA1c (%) | 5.68 ± 0.58 | 5.60 | 5.40–5.80 | 5.71 ± 0.66 | 5.60 | 5.40–5.80 | 5.66 ± 0.52 | 5.60 | 5.40–5.80 |
| Blood insulin (μU/mL) | 5.42 ± 5.05 | 4.50 | 3.50–6.20 | 5.59 ± 7.14 | 4.40 | 3.20–6.25 | 5.29 ± 2.68 | 4.60 | 3.60–6.20 |
| Triglyceride (mg/dL) | 100 ± 73 | 80 | 58–116 | 127 ± 94 | 96 | 71–150 | 81 ± 44 | 70 | 53–97 |
| Total cholesterol (mg/dL) | 207 ± 34 | 204 | 185–231 | 204 ± 33 | 202 | 182–227 | 210 ± 34 | 207 | 187–234 |
| HDL-cholesterol (mg/dL) | 65 ± 17 | 63 | 53–76 | 59 ± 16 | 55 | 48–68 | 70 ± 16 | 69 | 58–79 |
| LDL-cholesterol (mg/dL) | 116 ± 28 | 115 | 97–133 | 114 ± 28 | 113 | 95–132 | 117 ± 29 | 116 | 98–134 |
| Variable | n | Mean ± SD | Median | IQR | p |
|---|---|---|---|---|---|
| All subjects | 896 | 5.77 ± 1.68 | 5.66 | 4.63–6.70 | |
| Age strata (years) | |||||
| 20–29 | 46 | 5.46 ± 1.85 | 5.15 | 4.25–6.81 | <0.001 1 |
| 30–39 | 141 | 5.29 ± 1.73 | 5.12 | 4.09–6.17 | |
| 40–49 | 140 | 5.73 ± 1.69 | 5.53 | 4.58–6.52 | |
| 50–59 | 183 | 5.83 ± 1.65 | 5.75 | 4.73–6.81 | |
| 60–69 | 239 | 5.85 ± 1.62 | 5.87 | 4.85–6.74 | |
| 70–79 | 125 | 6.12 ± 1.59 | 6.06 | 4.96–6.99 | |
| ≥80 | 22 | 6.23 ± 1.62 | 6.37 | 5.14–7.37 | |
| BMI strata (kg/m2) | |||||
| <18.5 | 62 | 5.06 ± 1.53 | 4.84 | 4.09–5.82 | |
| 18.5–24.9 | 605 | 5.64 ± 1.67 | 5.54 | 4.52–6.57 | 0.01 (vs. <18.5) 2 |
| ≥25.0 | 229 | 6.29 ± 1.62 | 6.17 | 5.24–7.12 | <0.001 (vs. other 2 groups) 2 |
| Sex | |||||
| Male | 375 | 5.93 ± 1.62 | 5.88 | 4.95–6.86 | 0.002 3 |
| Female | 521 | 5.65 ± 1.71 | 5.48 | 4.47–6.55 | |
| Smoking habit | |||||
| Non-smoker | 768 | 5.72 ± 1.64 | 5.63 | 4.57–6.64 | 0.04 3 |
| Current smoker | 128 | 6.06 ± 1.88 | 5.91 | 4.97–6.89 | |
| Drinking habit | |||||
| Non-drinker | 461 | 5.77 ± 1.67 | 5.67 | 4.67–6.73 | 0.96 3 |
| Current drinker | 435 | 5.76 ± 1.69 | 5.66 | 4.62–6.67 |
| Category | Variable | β | Q Value |
|---|---|---|---|
| Obesity | Abdominal circumference (cm) 2 | 0.02 | 0.05 * |
| Body fat percentage (%) | 0.03 | 0.32 | |
| Body water content (kg) | −0.02 | 0.21 | |
| Visceral fat level (levels) | 0.09 | 0.03 * | |
| Basal metabolic rate (kcal/day) | −0.01 | 0.53 | |
| Basal metabolic rate level (levels) | −0.08 | 0.03 * | |
| Blood pressure | Systolic blood pressure (mmHg) | 0.07 | <0.001 * |
| Diastolic blood pressure (mmHg) | 0.05 | 0.05 * | |
| Arteriosclerosis | baPWV (cm/s) | 0.08 | <0.001 * |
| Glucose metabolism | HOMA-IR | 0.18 | 0.04 * |
| Blood glucose (mg/dL) | 0.02 | 0.41 | |
| HbA1c (%) | 0.04 | 0.009 * | |
| Blood insulin (μU/mL) | 0.16 | 0.04 * | |
| C peptide (ng/mL) | 0.14 | 0.009 * | |
| Glycoalbumin (%) | 0.02 | 0.37 | |
| Lipid metabolism | Triglyceride (mg/dL) | 0.13 | 0.14 * |
| Total cholesterol (mg/dL) | 0.01 | 0.63 | |
| HDL-cholesterol (mg/dL) | −0.12 | <0.001 * | |
| LDL-cholesterol (mg/dL) | 0.04 | 0.40 | |
| Physical function | Estimated bone mass (kg) | −0.01 | 0.63 |
| Total muscle mass (kg) | −0.01 | 0.52 | |
| Right leg muscle mass (kg) | −0.02 | 0.27 | |
| Left leg muscle mass (kg) | −0.02 | 0.29 | |
| Right arm muscle mass (kg) | −0.02 | 0.19 * | |
| Left arm muscle mass (kg) | −0.03 | 0.13 * | |
| Trunk muscle mass (kg) | 0.00 | 0.80 | |
| Locomo 25 score (points) | −1.84 | 0.24 | |
| Eyesight | Far-sightedness | −0.05 | 0.49 |
| Near-sightedness | −0.02 | 0.73 | |
| Liver function | Albumin (g/dL) | −0.01 | 0.16 * |
| AST (U/L) | 0.16 | <0.001 * | |
| ALT (U/L) | 0.17 | 0.02 * | |
| γ-GTP (U/L) | 0.31 | <0.001 * | |
| Total bilirubin (mg/dL) | −0.11 | 0.07 * | |
| Total Protein (g/dL) | 0.03 | <0.001 * | |
| Renal function | Creatinine (mg/dL) | 0.02 | 0.48 |
| Blood urea nitrogen (mg/dL) | −0.01 | 0.75 | |
| Plasma renin activity (ng/mL/h) | 0.27 | 0.16 * | |
| Urine albumin creatinine ratio (mg/gCr) | 0.50 | 0.004 * | |
| Adrenal cortex function | Aldosterone (pg/mL) | −0.01 | 0.77 |
| Cortisol (μg/dL) | 0.14 | 0.02 * | |
| Thyroid function | Free thyroxine (ng/dL) | 0.05 | 0.02 * |
| Thyroid stimulating hormone (μIU/mL) | −0.07 | 0.58 | |
| Inflammation | Neutrophil (%) | 0.11 | <0.001 * |
| Stab neutrophil (%) | 0.44 | <0.001 * | |
| Segmented neutrophil (%) | 0.09 | <0.001 * | |
| Lymphocyte (%) | −0.16 | <0.001 * | |
| Monocyte (%) | 0.09 | 0.20 | |
| Eosinophil (%) | −0.67 | 0.06 * | |
| Basophil (%) | −0.18 | 0.10 * | |
| IgG (mg/dL) | 0.06 | 0.10 * | |
| IgA (mg/dL) | 0.17 | 0.009 * | |
| IgM (mg/dL) | 0.06 | 0.52 | |
| Complement C3 (mg/dL) | 0.16 | <0.001 * | |
| Complement C4 (mg/dL) | 0.25 | <0.001 * | |
| hs-CRP (mg/dL) | 1.52 | <0.001 * | |
| IL-6 (pg/mL) | 0.43 | <0.001 * | |
| Hematological test | White blood cell (cells/μL) | 0.16 | <0.001 * |
| Red blood cell (104 cells/μL) | −0.01 | 0.54 | |
| Hemoglobin (g/dL) | −0.01 | 0.54 | |
| Hematocrit (%) | −0.54 | 0.32 | |
| Mean cell volume (fL) | 0.00 | 0.60 | |
| Mean corpuscular hemoglobin (pg) | 0.00 | 0.80 | |
| Mean cell hemoglobin concentration (g/dL) | 0.00 | 0.46 | |
| Platelet (104 cells/μL) | 0.05 | 0.23 | |
| Iron metabolism | Ferritin (ng/mL) | −0.02 | 0.77 |
| Serum iron (mmol/L) | −0.14 | 0.07 * |
| Variable 2 | β | Q Value |
|---|---|---|
| phylum Actinobacteria | −0.07 | 0.65 |
| class Actinobacteria | −0.07 | 0.65 |
| order Bifidobacteriales | −0.12 | 0.56 |
| family Bifidobacteriaceae | −0.12 | 0.56 |
| genus Bifidobacterium | −0.12 | 0.56 |
| class Coriobacteriia | NA | NA |
| order Coriobacteriales | 0.03 | 0.79 |
| family Coriobacteriaceae | 0.03 | 0.79 |
| genus Collinsella | 0.01 | 0.80 |
| phylum Bacteroidetes | 0.26 | 0.16 * |
| class Bacteroidia | 0.25 | 0.17 * |
| order Bacteroidales | 0.25 | 0.17 * |
| family Prevotellaceae | 0.30 | 0.04 * |
| genus Prevotella | 0.32 | 0.04 * |
| family Bacteroidaceae | −0.17 | 0.49 |
| genus Bacteroides | −0.17 | 0.49 |
| family Rikenellaceae | −0.31 | 0.57 |
| genus Alistipes | −0.28 | 0.60 |
| family Porphyromonadaceae | −0.59 | 0.54 |
| genus Parabacteroides | 0.06 | 0.79 |
| phylum Firmicutes | −0.16 | 0.44 |
| class Clostridia | −0.21 | 0.17 * |
| order Clostridiales | −0.21 | 0.17 * |
| family Lachnospiraceae | −0.12 | 0.49 |
| genus Blautia | 0.03 | 0.79 |
| genus Anaerostipes | −0.20 | 0.52 |
| genus Roseburia | −0.57 | 0.18 * |
| genus Fusicatenibacter | −0.76 | 0.26 |
| genus Lachnospiraceae incertae sedis | −0.28 | 0.73 |
| family Ruminococcaceae | −0.17 | 0.32 |
| genus Faecalibacterium | −0.32 | 0.29 |
| genus Ruminococcus | −0.25 | 0.50 |
| genus Ruminococcus 2 | 0.05 | 0.76 |
| genus Gemmiger | −0.36 | 0.56 |
| family Clostridiaceae | NA | NA |
| genus Clostridium IV | −0.14 | 0.68 |
| class Negativicutes | 0.50 | 0.21 |
| order Selenomonadales | 0.50 | 0.21 |
| family Veillonellaceae | 0.46 | 0.26 |
| genus Megamonas | 0.56 | 0.1998 * |
| class Erysipelotrichia | −0.07 | 0.77 |
| order Erysipelotrichales | −0.07 | 0.77 |
| family Erysipelotrichaceae | −0.07 | 0.77 |
| class Bacilli | 0.43 | 0.26 |
| order Lactobacillales | 0.45 | 0.23 |
| family Streptococcaceae | 0.64 | 0.16 * |
| genus Streptococcus | 0.65 | 0.15 * |
| phylum Proteobacteria | 0.14 | 0.73 |
| class Betaproteobacteria | −1.10 | 0.37 |
| order Burkholderiales | −1.10 | 0.37 |
| family Sutterellaceae | −1.06 | 0.38 |
| family Unclassified | 0.56 | 0.59 |
| genus Unclassified | 0.31 | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuke, N.; Yamashita, T.; Shimizu, S.; Matsumoto, M.; Sawada, K.; Jung, S.; Tokuda, I.; Misawa, M.; Suzuki, S.; Ushida, Y.; et al. Association of Plasma Lipopolysaccharide-Binding Protein Concentration with Dietary Factors, Gut Microbiota, and Health Status in the Japanese General Adult Population: A Cross-Sectional Study. Metabolites 2023, 13, 250. https://doi.org/10.3390/metabo13020250
Fuke N, Yamashita T, Shimizu S, Matsumoto M, Sawada K, Jung S, Tokuda I, Misawa M, Suzuki S, Ushida Y, et al. Association of Plasma Lipopolysaccharide-Binding Protein Concentration with Dietary Factors, Gut Microbiota, and Health Status in the Japanese General Adult Population: A Cross-Sectional Study. Metabolites. 2023; 13(2):250. https://doi.org/10.3390/metabo13020250
Chicago/Turabian StyleFuke, Nobuo, Takahiro Yamashita, Sunao Shimizu, Mai Matsumoto, Kaori Sawada, Songee Jung, Itoyo Tokuda, Mina Misawa, Shigenori Suzuki, Yusuke Ushida, and et al. 2023. "Association of Plasma Lipopolysaccharide-Binding Protein Concentration with Dietary Factors, Gut Microbiota, and Health Status in the Japanese General Adult Population: A Cross-Sectional Study" Metabolites 13, no. 2: 250. https://doi.org/10.3390/metabo13020250
APA StyleFuke, N., Yamashita, T., Shimizu, S., Matsumoto, M., Sawada, K., Jung, S., Tokuda, I., Misawa, M., Suzuki, S., Ushida, Y., Mikami, T., Itoh, K., & Suganuma, H. (2023). Association of Plasma Lipopolysaccharide-Binding Protein Concentration with Dietary Factors, Gut Microbiota, and Health Status in the Japanese General Adult Population: A Cross-Sectional Study. Metabolites, 13(2), 250. https://doi.org/10.3390/metabo13020250