Effectiveness of Intermittent Hypoxia–Hyperoxia Therapy in Different Pathologies with Possible Metabolic Implications
Abstract
1. Introduction
2. Experimental Design
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burtscher, M.; Gatterer, H.; Szubski, C.; Pierantozzi, E.; Faulhaber, M. Effects of interval hypoxia on exercise tolerance: Special focus on patients with CAD or COPD. Sleep Breath. 2009, 14, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Serebrovskaya, T.V.; Swanson, R.J.; E Kolesnikova, E. Intermittent hypoxia: Mechanisms of action and some applications to bronchial asthma treatment. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2003, 54, 35–41. [Google Scholar]
- Bao, X.; Liu, H.; Liu, H.-Y.; Long, Y.; Tan, J.-W.; Zhu, Z.-M. The effect of intermittent hypoxia training on migraine: A randomized controlled trial. Am. J. Transl. Res. 2020, 12, 4059–4065. [Google Scholar] [PubMed]
- Serebrovska, T.; Portnychenko, A.; I Drevytska, T.; Portnichenko, V.; Xi, L.; Egorov, E.; Gavalko, A.V.; Naskalova, S.; Chizhova, V.; Shatylo, V.B. Intermittent hypoxia training in prediabetes patients: Beneficial effects on glucose homeostasis, hypoxia tolerance and gene expression. Exp. Biol. Med. 2017, 242, 1542–1552. [Google Scholar] [CrossRef] [PubMed]
- Bayer, U.; Glazachev, O.S.; Likar, R.; Burtscher, M.; Kofler, W.; Pinter, G.; Stettner, H.; Demschar, S.; Trummer, B.; Neuwersch, S. Adaptation to intermittent hypoxia-hyperoxia improves cognitive performance and exercise tolerance in the elderly. Adv. Gerontol. 2017, 7, 214–220. [Google Scholar] [CrossRef]
- Iliescu, M.G.; Ionescu, E.V.; Tica, I.; Prazaru, M.D.; Popa, F.L.; Popescu, M.N.; Berteanu, M.; Irsay, L.; Ciortea, V.M.; Voinea, F.; et al. The role of natural biotherapeutic factors in pain and functional management of knee osteoarthritis. Farmacia 2022, 70, 65–69. [Google Scholar] [CrossRef]
- Serebrovska, T.; Grib, O.N.; Portnichenko, V.; Serebrovska, Z.O.; Egorov, E.; Shatylo, V.B. Intermittent Hypoxia/Hyperoxia Versus Intermittent Hypoxia/Normoxia: Comparative Study in Prediabetes. High Alt. Med. Biol. 2019, 20, 383–391. [Google Scholar] [CrossRef]
- Glazachev, O.S. Optimization of Clinical Application of Interval Hypoxic Training. Biomed. Eng. 2013, 47, 134–137. [Google Scholar] [CrossRef]
- Behrendt, T.; Bielitzki, R.; Behrens, M.; Herold, F.; Schega, L. Effects of Intermittent Hypoxia–Hyperoxia on Performance- and Health-Related Outcomes in Humans: A Systematic Review. Sports Med. Open 2022, 8, 1–28. [Google Scholar] [CrossRef]
- Glazachev, O.; Kopylov, P.; Susta, D.; Dudnik, E.; Zagaynaya, E. Adaptations following an intermittent hypoxia-hyperoxia training in coronary artery disease patients: A controlled study. Clin. Cardiol. 2017, 40, 370–376. [Google Scholar] [CrossRef]
- Bayer, U.; Likar, R.; Pinter, G.; Stettner, H.; Demschar, S.; Trummer, B.; Neuwersch, S.; Glazachev, O.; Burtscher, M. Intermittent hypoxic-hyperoxic training on cognitive performance in geriatric patients. Alzheimer’s Dementia Transl. Res. Clin. Interv. 2017, 3, 114–122. [Google Scholar] [CrossRef]
- Dudnik, E.; Zagaynaya, E.; Glazachev, O.S.; Susta, D. Intermittent Hypoxia–Hyperoxia Conditioning Improves Cardiorespiratory Fitness in Older Comorbid Cardiac Outpatients without Hematological Changes: A Randomized Controlled Trial. High Alt. Med. Biol. 2018, 19, 339–343. [Google Scholar] [CrossRef]
- Melesse, D.Y.; Mutua, M.K.; Choudhury, A.; Wado, Y.D.; Faye, C.M.; Neal, S.; Boerma, T. Adolescent sexual and reproductive health in sub-Saharan Africa: Who is left behind? BMJ Glob. Health 2020, 5, e002231. [Google Scholar] [CrossRef]
- Serebrovska, Z.O.; Serebrovska, T.V.; Kholin, V.A.; Tumanovska, L.V.; Shysh, A.M.; Pashevin, D.A.; Goncharov, S.V.; Stroy, D.; Grib, O.N.; Shatylo, V.B.; et al. Intermittent Hypoxia-Hyperoxia Training Improves Cognitive Function and Decreases Circulating Biomarkers of Alzheimer’s Disease in Patients with Mild Cognitive Impairment: A Pilot Study. Int. J. Mol. Sci. 2019, 20, 5405. [Google Scholar] [CrossRef]
- Bayer, U.; Likar, R.; Pinter, G.; Stettner, H.; Demschar, S.; Trummer, B.; Neuwersch, S.; Glazachev, O.; Burtscher, M. Efects of intermittent hypoxia–hyperoxia on mobility and perceived health in geriatric patients performing a multimodal training intervention: A randomized controlled trial. BMC Geriatr. 2019, 19, 167. [Google Scholar] [CrossRef]
- Afina, A.B.; Oleg, S.G.; Alexander, A.B.; Ines, D.; Yu, S.A.; Nikita, V.V.; Denis, S.T.; Daria, G.G.; Zhang, Y.; Chavdar, S.P.; et al. The Effects of Intermittent Hypoxic–Hyperoxic Exposures on Lipid Profile and Inflammation in Patients with Metabolic Syndrome. Front. Cardiovasc. Med. 2021, 8, 700826. [Google Scholar] [CrossRef]
- Chen, P.-W.; Hsu, C.-C.; Lai, L.-F.; Chi, C.-P.; Yu, S.-H. Effects of Hypoxia–Hyperoxia Preconditioning on Indicators of Muscle Damage After Acute Resistance Exercise in Male Athletes. Front. Physiol. 2022, 13, 444. [Google Scholar] [CrossRef]
- Behrendt, T.; Bielitzki, R.; Behrens, M.; Glazachev, O.S.; Schega, L. Effects of Intermittent Hypoxia-Hyperoxia Exposure Prior to Aerobic Cycling Exercise on Physical and Cognitive Performance in Geriatric Patients—A Randomized Controlled Trial. Front. Physiol. 2022, 13, 1048. [Google Scholar] [CrossRef]
- Burtscher, M.; Pachinger, O.; Ehrenbourg, I.; Mitterbauer, G.; Faulhaber, M.; Pühringer, R.; Tkatchouk, E. Intermittent hypoxia increases exercise tolerance in elderly men with and without coronary artery disease. Int. J. Cardiol. 2004, 96, 247–254. [Google Scholar] [CrossRef]
- Haider, T.; Casucci, G.; Linser, T.; Faulhaber, M.; Gatterer, H.; Ott, G.; Linser, A.; Ehrenbourg, I.; Tkatchouk, E.; Burtscher, M.; et al. Interval hypoxic training improves autonomic cardiovascular and respiratory control in patients with mild chronic obstructive pulmonary disease. J. Hypertens. 2009, 27, 1648–1654. [Google Scholar] [CrossRef]
- Burtscher, M.; Haider, T.; Domej, W.; Linser, T.; Gatterer, H.; Faulhaber, M.; Pocecco, E.; Ehrenburg, I.; Tkatchuk, E.; Koch, R.; et al. Intermittent hypoxia increases exercise tolerance in patients at risk for or with mild COPD. Respir. Physiol. Neurobiol. 2009, 165, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Mekjavic, I.B.; Debevec, T.; Amon, M.; Keramidas, M.E.; Kounalakis, S.N. Intermittent Normobaric Hypoxic Exposures at Rest: Effects on Performance in Normoxia and Hypoxia. Aviat. Space Environ. Med. 2012, 83, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Kon, M.; Ohiwa, N.; Honda, A.; Matsubayashi, T.; Ikeda, T.; Akimoto, T.; Suzuki, Y.; Hirano, Y.; Russell, A.P. Effects of systemic hypoxia on human muscular adaptations to resistance exercise training. Physiol. Rep. 2014, 2, e12033. [Google Scholar] [CrossRef] [PubMed]
- Lizamore, C.; Kathiravel, Y.; Elliott, J.; Hellemans, J.; Hamlin, M.; Information, R. The effect of short-term intermittent hypoxic exposure on heart rate variability in a sedentary population. Acta Physiol. Hung. 2016, 103, 75–85. [Google Scholar] [CrossRef]
- Irsay, L.; Checicheș, A.; Perja, D.; Borda, I.M.; Dogaru, G.; Onac, I.; Ungur, R.; Ciortea, V. Pharmacological pain management in patients with chronic kidney disease. Balneo Res. J. 2019, 10, 12–16. [Google Scholar] [CrossRef]
- Irsay, L.; Checiches, A.; Perja, D.; Borda, I.M.; Dogaru, G.; Ungur, R.; Ciubean, A.; Ciortea, V. Pharmacological pain management in patients with chronic hepatic disease. Balneo Res. J. 2019, 10, 119–123. [Google Scholar] [CrossRef]
- Ungur, R.A.; Ciortea, V.M.; Irsay, L.; Ciubean, A.D.; Năsui, B.A.; Codea, R.A.; Singurean, V.E.; Groza, O.B.; Căinap, S.; Martiș Petruț, G.S.; et al. Can Ultrasound Therapy Be an Environmental-Friendly Alternative to Non-Steroidal Anti-Inflammatory Drugs in Knee Osteoarthritis Treatment? Materials 2021, 14, 2715. [Google Scholar] [CrossRef]
- Rybnikova, E.A.; Nalivaeva, N.N.; Zenko, M.Y.; Baranova, K.A. Intermittent Hypoxic Training as an Effective Tool for Increasing the Adaptive Potential, Endurance and Working Capacity of the Brain. Front. Neurosci. 2022, 16, 941740. [Google Scholar] [CrossRef]
- Mankovska, I.M.; Serebrovska, T.V. Mitochondria as a Target of Intermittent Hypoxia. Int. J. Physiol. Pathophysiol. 2015, 6, 347–362. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, Q.; Yang, H.; Wei, W. Oxygen sensing and adaptability won the 2019 Nobel Prize in Physiology or medicine. Genes Dis. 2019, 6, 328–332. [Google Scholar] [CrossRef]
- Serebrovska, T.; Xi, L. Intermittent hypoxia training as non-pharmacologic therapy for cardiovascular diseases: Practical analysis on methods and equipment. Exp. Biol. Med. 2016, 241, 1708–1723. [Google Scholar] [CrossRef]
- Dale, E.A.; Ben Mabrouk, F.; Mitchell, G.S. Unexpected Benefits of Intermittent Hypoxia: Enhanced Respiratory and Nonrespiratory Motor Function. Physiology 2014, 29, 39–48. [Google Scholar] [CrossRef]
- Serebrovskaya, T.V.; Exi, L. Intermittent Hypoxia in Childhood: The Harmful Consequences Versus Potential Benefits of Therapeutic Uses. Front. Pediatr. 2015, 3, 44. [Google Scholar] [CrossRef]
- Dempsey, J.A.; Morgan, B.J. Humans In Hypoxia: A Conspiracy Of Maladaptation?! Physiology 2015, 30, 304–316. [Google Scholar] [CrossRef]
- Eltzschig, H.K. Extracellular adenosine signaling in molecular medicine. J. Mol. Med. 2013, 91, 141–146. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Weissmüller, T.; Mager, A.; Eckle, T. Nucleotide Metabolism and Cell-Cell Interactions; Humana Press: Totowa, NJ, USA, 2006; Volume 341, pp. 73–88. [Google Scholar] [CrossRef]
- Bowser, J.L.; Lee, J.W.; Yuan, X.; Eltzschig, H.K. The hypoxia-adenosine link during inflammation. J. Appl. Physiol. 2017, 123, 1303–1320. [Google Scholar] [CrossRef]
- Neudecker, M.V.; Brodsky, M.K.S.; Kreth, M.S.; Ginde, M.A.A.; Eltzschig, M.H.K. Emerging Roles for MicroRNAs in Perioperative Medicine. Anesthesiology 2016, 124, 489–506. [Google Scholar] [CrossRef]
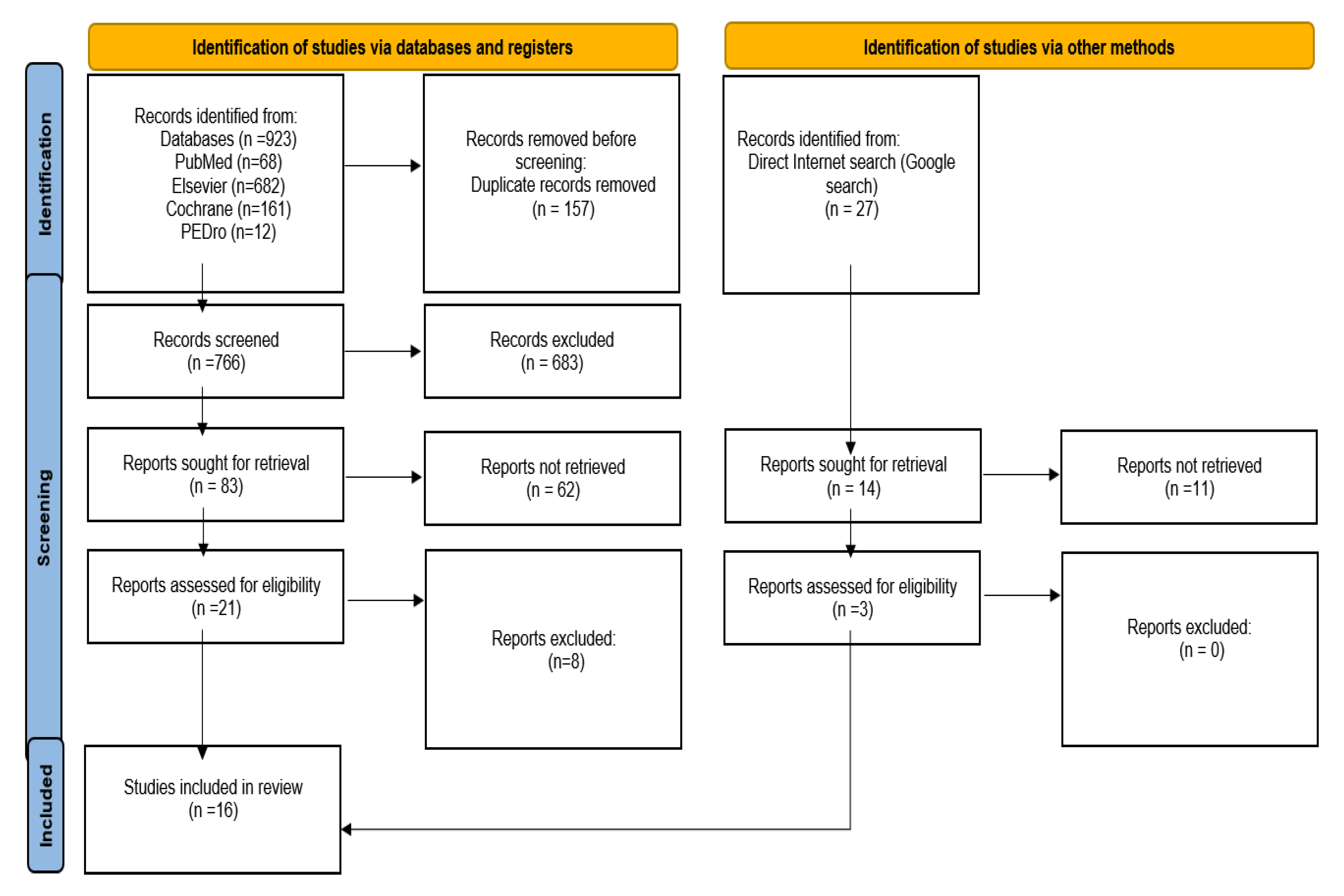
| PubMed | Elsevier | Cochrane | PEDro | Total | |
|---|---|---|---|---|---|
| Intermittent hypoxia–hyperoxia | 29 | 85 | 33 | 3 | 150 |
| Intermittent hypoxia–hyperoxia training | 5 | 110 | 21 | 3 | 139 |
| Intermittent hypoxic training | 34 | 487 | 107 | 6 | 634 |
| Total | 68 | 682 | 161 | 12 | 923 |
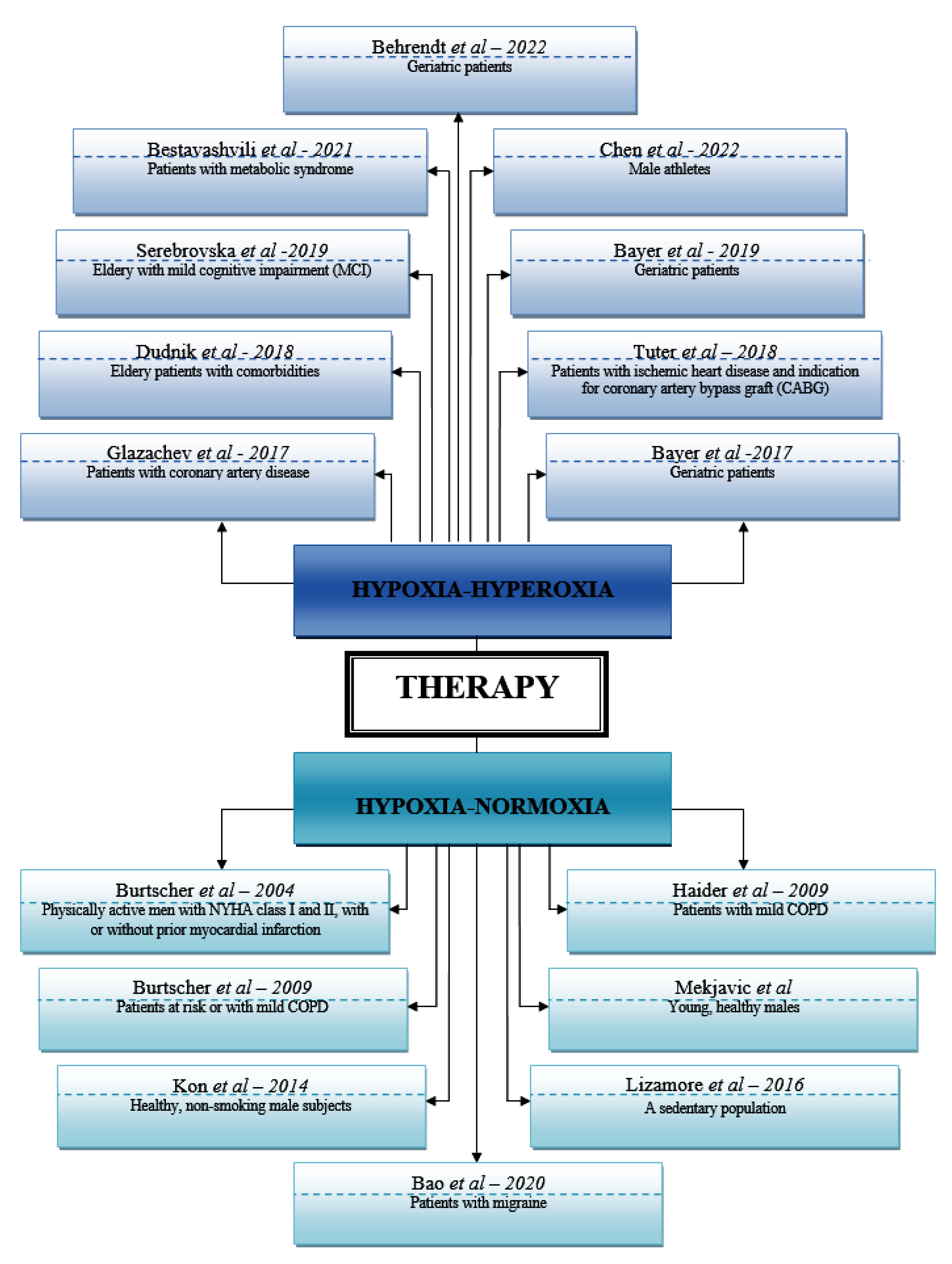
| Authors | Country | Year | Study Design | No. pac incl | References |
|---|---|---|---|---|---|
| Glazachev et al. | Russia | 2017 | non-randomized controlled before-and-after trial | 46 | 21 |
| Bayer et al. | Austria | 2017 | double-blind, randomized, stratified and placebo-controlled study | 34 | 46 |
| Dudnik et al. | Russia | 2018 | randomized controlled trial | 28 | 16 |
| Tuter et al. | Russia | 2018 | single-center, randomized controlled trial | 120 | 29 |
| Serebrovska et al. | Ukraine | 2019 | a pilot study | 21 | 73 |
| Bayer et al. | Austria | 2019 | double-blind, randomized controlled clinical trial | 34 | 46 |
| Bestavashvili et al. | Russia | 2021 | single-center, single-blind, randomized controlled trial | 65 | 36 |
| Chen et al. | Taiwan | 2022 | randomized, double-blind study | 11 | 53 |
| Behrendt et al. | Germany | 2022 | randomized, two-armed, controlled and single-blinded trial | 28 | 164 |
| Authors | Country | Year | Study Design | No. pac incl | References |
|---|---|---|---|---|---|
| Burtscher et al. | Austria | 2004 | randomized, double-blind | 16 | 37 |
| Haider et al. | Austria | 2009 | randomized, double-blind, placebo-controlled study | 18 | 43 |
| Burtscher et al. | Austria | 2009 | randomized, double-blind | 18 | 42 |
| Mekjavic et al. | Slovenia | 2012 | not mentioned | 18 | 36 |
| Kon et al. | Japan | 2014 | a pilot study | 16 | 34 |
| Lizamore et al. | New Zealand | 2016 | Single-blind study | 16 | 30 |
| Bao et al. | China | 2020 | prospective, assessor-blinded randomized controlled trial | 48 | 30 |
| Pathology | Effect Observed |
|---|---|
| Chronic obstructive pulmonary disease | In randomized, double-blind, controlled clinical studies it was demonstrated that mild repetitive acute IH (12–15% O2 for 3–5 min, followed by intervals of 3–5 min of normoxia, 5–9 episodes per day, for 15 days) can produce the following increases: exercise time, baroreflex sensitivity, total hemoglobin, hypercapnic ventilatory response, forced vital capacity and forced expiratory volume in 1 s. |
| Arterial hypertension | In 56 known patients with stage I-II hypertension, moderate IH reduced heart rate, systolic and diastolic blood pressure and peripheral resistance. IH reduces the symptoms of angina, normalizes microcirculation and lipid metabolism and increases maximal oxygen consumption and exercise tolerance, being proven to be a safe therapy for elderly patients. Increased endothelial NO production that produces the opening of reserve capillaries and vasodilatation can determine the antihypertensive effects of moderate IH (reduced peripheral resistance), reduced sympathetic activity, minimized calcium overload of vascular smooth muscles, improved water and salt metabolism, increased activity of antioxidant enzymes and increased synthesis of angiogenic growth factors, including VEGF and FGF. |
| Myocardial Infarction | In humans, moderate IH increases maximal oxygen consumption in older men (50–70 years), both with and without coronary artery disease. During submaximal exercise (cycling at 1 W/kg), systolic blood pressure, heart rate, perceived exertion and blood lactate concentration are diminished by IH. Myocardial protection is correlated with the ability of moderate IH to increase coronary blood flow, myocardial vascularity, cardiomyoglobin and antioxidant enzyme expression. IH increases erythropoietin (EPO) concentrations, stimulating erythropoiesis and increasing hematocrit, blood viscosity and platelet count. |
| Inflammatory/immune responses to IH | Some studies suggest that moderate IH protocols enhance the innate immune system while having a general anti-inflammatory effect. For example, in healthy humans, exposure to the 4 5 min episodes of 10% O2 (5 min interval in room air, 14 days) increases the phagocytic and bactericidal activities of neutrophils, while suppressing the pro-inflammatory mediators TNF-α and IL-4 by more than 90%. These responses, which persisted at least 7 days after IH, may increase the body’s immune defenses without associated inflammation. |
| Metabolic responses to IH | IH protocols have beneficial effects on metabolism, including decreased body weight, cholesterol, and blood sugar levels, as well as increased insulin sensitivity. Mechanisms of moderate IH-induced weight loss may include increased serotonin and/or leptin levels. Body weight is reduced with moderate IH by increasing hepatic leptin expression and increasing blood leptin concentration. Moderate hypoxia (14.6% O2) reduces cholesterol and blood glucose and increases insulin sensitivity in patients with type 2 diabetes. Hypoxia also increases glycolysis and fatty acid oxidation and mitochondrial enzyme activity and reduces cholesterol synthesis. |
| Bone | IH has positive effects on bone tissue remodeling. Exposure of rats to IH determined high alkaline phosphatase activity in bone tissue, thus suggesting increased osteoblast activity and new bone formation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uzun, A.-B.; Iliescu, M.G.; Stanciu, L.-E.; Ionescu, E.-V.; Ungur, R.A.; Ciortea, V.M.; Irsay, L.; Motoașcă, I.; Popescu, M.N.; Popa, F.L.; et al. Effectiveness of Intermittent Hypoxia–Hyperoxia Therapy in Different Pathologies with Possible Metabolic Implications. Metabolites 2023, 13, 181. https://doi.org/10.3390/metabo13020181
Uzun A-B, Iliescu MG, Stanciu L-E, Ionescu E-V, Ungur RA, Ciortea VM, Irsay L, Motoașcă I, Popescu MN, Popa FL, et al. Effectiveness of Intermittent Hypoxia–Hyperoxia Therapy in Different Pathologies with Possible Metabolic Implications. Metabolites. 2023; 13(2):181. https://doi.org/10.3390/metabo13020181
Chicago/Turabian StyleUzun, Andreea-Bianca, Mădălina Gabriela Iliescu, Liliana-Elena Stanciu, Elena-Valentina Ionescu, Rodica Ana Ungur, Viorela Mihaela Ciortea, Laszlo Irsay, Irina Motoașcă, Marius Nicolae Popescu, Florina Ligia Popa, and et al. 2023. "Effectiveness of Intermittent Hypoxia–Hyperoxia Therapy in Different Pathologies with Possible Metabolic Implications" Metabolites 13, no. 2: 181. https://doi.org/10.3390/metabo13020181
APA StyleUzun, A.-B., Iliescu, M. G., Stanciu, L.-E., Ionescu, E.-V., Ungur, R. A., Ciortea, V. M., Irsay, L., Motoașcă, I., Popescu, M. N., Popa, F. L., Pazara, L., & Tofolean, D.-E. (2023). Effectiveness of Intermittent Hypoxia–Hyperoxia Therapy in Different Pathologies with Possible Metabolic Implications. Metabolites, 13(2), 181. https://doi.org/10.3390/metabo13020181








