Abstract
Gamma-hydroxybutyric acid (GHB) is a potent, short-acting central nervous system depressant as well as an inhibitory neurotransmitter or neuromodulator derived from gamma-aminobutyric acid (GABA), a major inhibitory neurotransmitter. The sodium salt of GHB, sodium oxybate, has been used for the treatment of narcolepsy and cataplexy, whereas GHB was termed as a date rape drug or a club drug in the 1990s. Ethanol is the most co-ingested drug in acute GHB intoxication. In this review, the latest findings on the combined effects of GHB and ethanol are summarized from toxicokinetic and toxicodynamic perspectives. For this purpose, we mainly discussed the pharmacology and toxicology of GHB, GHB intoxication under alcohol consumption, clinical cases of the combined intoxication of GHB and ethanol, and previous studies on the toxicokinetic and toxicodynamic interactions between GHB and ethanol in humans, animals, and an in vitro model. The combined administration of GHB and ethanol enhanced sedation and cardiovascular dysfunction, probably by the additive action of GABA receptors, while toxicokinetic changes of GHB were not significant. The findings of this review will contribute to clinical and forensic interpretation related to GHB intoxication. Furthermore, this review highlights the significance of studies aiming to further understand the enhanced inhibitory effects of GHB induced by the co-ingestion of ethanol.
1. Introduction
Gamma-hydroxybutyrate (GHB) is an endogenous short fatty acid, acting as an inhibitory neurotransmitter or neuromodulator in mammalian brains. Endogenous GHB is produced during the metabolic process of gamma-aminobutyric acid (GABA), an inhibitory neurotransmitter that is mainly derived from glutamic acid in neurons, and is again metabolized to GABA. The sodium salt of GHB, sodium oxybate (Xyrem®), was approved by the U.S. Food and Drug Administration as a central nervous system depressant for narcolepsy and cataplexy in 2002, while GHB was termed as a date rape drug or a club drug in the 1990s [1,2]. Subsequently, the precursors of GHB, 1,4-butanediol (1,4-BD) and gamma-butyrolactone (GBL), emerged as alternatives for illegal use. Exogenous GBL is converted into GHB by a lactonase that is present in the liver and serum but not in the brain, and exogenous 1,4-BD is oxidized via alcohol dehydrogenase (ADH) into gamma-hydroxybutyraldehyde, which is further metabolized by aldehyde dehydrogenase (ALDH) to GHB. Thus, both agents have the same psychoactive effects as GHB [3,4,5].
Alcohol, a neuro-inhibitor, is one of the most consumed and easily accessible psychoactive substances globally. In particular, certain alcohol consumption patterns such as binge drinking and drink spiking are serious social and public health issues and are often involved in crimes worldwide [6]. Ethanol is the most co-ingested drug, probably augmenting pleasure and euphoric effects of other psychoactive drugs, represented in emergency department visits of acute recreational drug intoxication in European countries [7,8]. The mechanism of action of acute ethanol exposure is complicated and involves multiple steps, i.e., directly inhibiting ADH as well as acting on a number of neurotransmitter receptors (e.g., GABAA, glycine, and glutamate receptors), ion channels (e.g., large-conductance Ca2+-activated K+ channel and G-protein-coupled inwardly rectifying K+ channel), and other non-ion-channel targets, including intracellular signaling molecules such as protein kinase C and adenylate cyclase in the brain [9]. Ethanol is metabolized by ADH, ALDH, and cytochrome P450 enzymes such as CYP2E1, CYP1A2, and CYP3A4 which are also involved in the metabolisms of other drugs. Acute alcohol exposure affects the neuro-stimulant or inhibitory effects of other psychoactive drugs. In particular, high doses of alcohol, which strongly induce inhibitory effects, reduced the stimulant effects or enhanced the inhibitory effects of concomitant drugs [8,10]. It was also reported that low doses of alcohol induced neuro-stimulant effects on behavioral changes caused by the administration of GHB [11]. Therefore, the combined effects of GHB and ethanol could be diverse; however, mechanisms underlying the GHB and ethanol interactions are not yet fully understood.
2. Search Methods and Results
In this review, the latest findings on the combined effects of GHB and ethanol are summarized from toxicokinetic and toxicodynamic perspectives. For this purpose, we mainly discussed the pharmacology and toxicology of GHB, GHB intoxication under alcohol consumption, clinical cases of the combined intoxication of GHB and ethanol, and previous studies on the toxicokinetic and toxicodynamic interactions between GHB and ethanol in humans and animals. We reviewed research papers published from January 1980 to March 2022 and listed them in PubMed. The search keywords were [“γ-hydroxybutyric acid”, “γ-hydroxybutyrate”, “GHB”, “1,4-butanediol”, “1,4-BD”, “γ-butyrolactone”, “GBL”] and [“Ethanol” or “Alcohol”].
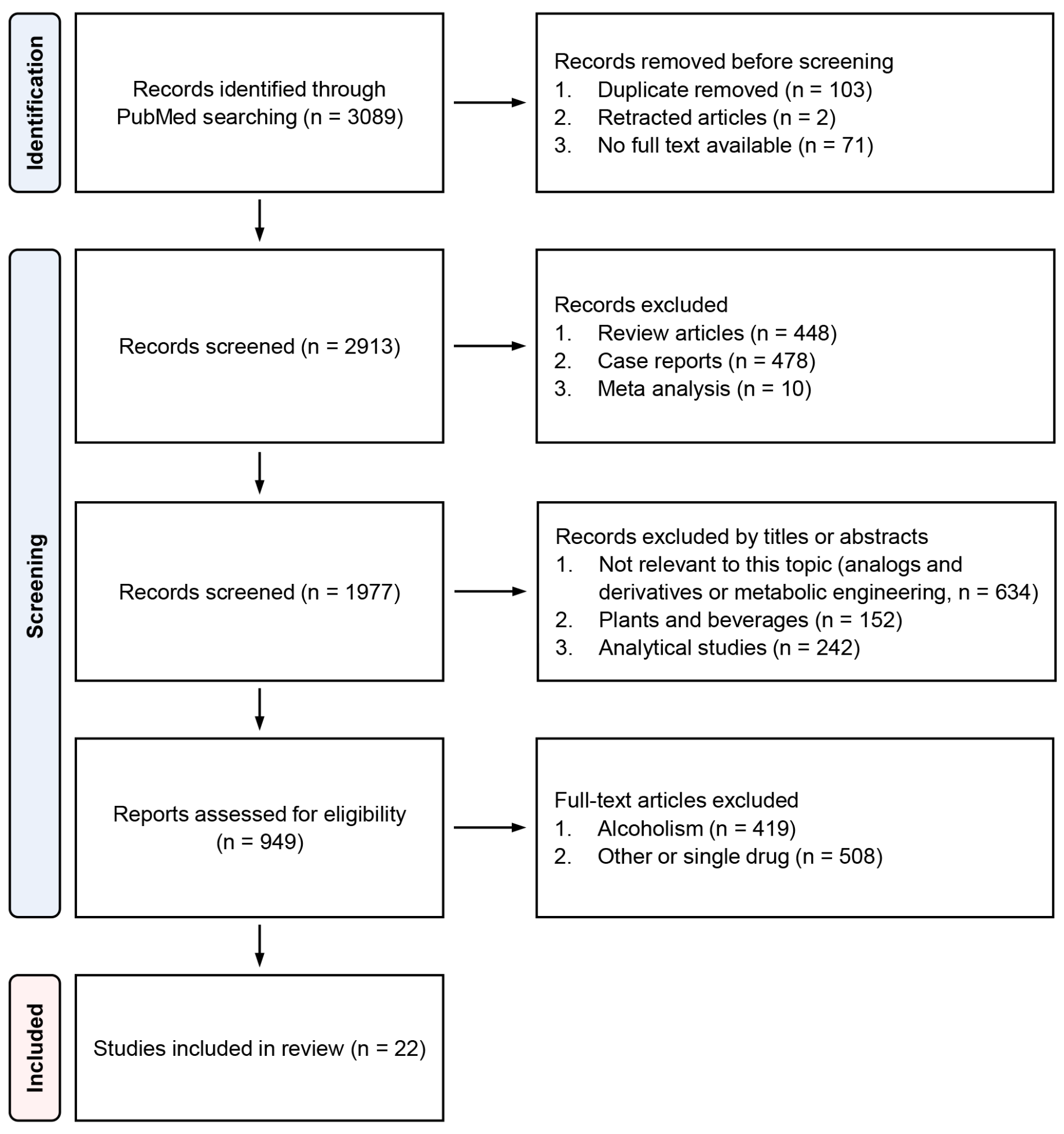
We found a total of 3089 papers with the aforementioned search keywords, and 22 of these papers, including three clinical case studies and human studies, 15 animal studies, and one in vitro study, were selected for the systematic review [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. The flowchart with details of paper selection steps is presented in Figure 1.

Figure 1.
Flow diagram of paper selection steps.
3. Pharmacology and Toxicology of GHB
GHB includes endogenous GHB synthesized in neurons and exogenous GHB acting through the blood–brain barrier (BBB) after ingestion. The physiological effects of GHB are mediated through binding to the GHB-specific receptor, identified as the α4β1δ subtype of the GABAA receptor, which is one of the high-affinity targets activated by nano-micromolar concentrations of GHB. The pharmacological and toxicological effects of GHB are mainly attributed to its action at the GABAB receptor, which is one of the low-affinity targets activated by millimolar concentrations of GHB [33]. Moreover, animal studies demonstrated that GHB administration up- or downregulates the extracellular concentrations of GABA, glutamic acid, and dopamine in the brain depending on drug doses or the specific action regions of the brain [33,34,35,36].
GHB was first synthesized as an anesthetic in 1964 [37] but had not been commercialized because of its severe side effects, such as vomiting and convulsions. In the late 1980s, GHB was widely used by bodybuilders as a performance-enhancing agent for anabolic steroids and became readily available as an over-the-counter product in health food stores, gyms, and the Internet. Subsequently, since GHB was shown to elicit euphoria and sexual arousal, it was increasingly misused in dance clubs and ‘rave’ parties [38]. In 2000, GHB was classified as a Schedule I substance because it was frequently abused in sexual assaults and, when taken in excess, caused coma, bradycardia, agitation, hypotension, hypothermia, respiratory depression, and, in extreme cases, death [5,39]. However, increased GHB production is beneficial for protecting central and peripheral tissues during hypoxia, ischemia, or excessive metabolic demand [40]. GHB has a neuroprotective action against apoptosis and neuronal death caused by oxidative stress [41,42,43]. In addition, GHB interferes with sleep latency, promotes deep slow-wave non-rapid eye movement sleep [44,45], and has sedative effects [46,47]. GHB was also found to be effective in the treatment of narcolepsy [48] and alcohol and heroin dependence [49].
4. GHB Intoxication in Crimes under the Influence of Drugs
The acute intoxication of GHB, an illegal drug misused in crimes under the influence of drugs owing to its neuro-inhibitory property, has greatly increased recently and threatens public health report [50,51]. GHB is colorless, tasteless, and short-acting; thus, it can be surreptitiously administered to a victim’s drink, such as alcohol, to incapacitate victims and induce memory loss in crimes, such as robberies, sexual assaults, and fraudulent gambling. However, due to its short half-life (30–50 min), low urine excretion rate (less than 2%), endogenous presence in both the brain and other peripheral tissues and fluids, and post-mortem production, it is difficult to prove previous GHB administration through quantification of GHB in a victim’s biological specimens such as blood and urine [33,52,53]. To overcome this limitation, many previous studies have focused on the toxicokinetic evaluation of GHB to establish a detectable sampling time [54,55,56], reference value setup based on endogenous GHB concentrations to interpret the quantitative results [57,58] and biomarker discovery for GHB exposure by metabolomics [59,60].
A combination of GHB and alcohol is frequent in crimes, and acute alcohol intake is known to cause toxicokinetic and toxicodynamic perturbation on concomitant psychiatric drugs [61]. Both GHB and ethanol could affect the common neuronal systems such as the GABAergic system. Moreover, it was previously reported that acute alcohol exposure alters the concentrations of dopamine, glutamic acid, and GABA in rats’ brains [62,63]. GHB is metabolized to 2,4- or 3,4-dihydroxybutyric acid or succinic acid which goes through the Krebs cycle [64,65] and then rapidly eliminated from the body in the form of carbon dioxide and water. GHB was detected only within 6 h in the blood and 12 h in urine after a single exposure to 60 or 50 mg/kg of GHB [16,66,67]. Ethanol is first oxidized to acetaldehyde and then to acetate and is finally processed by the Krebs cycle. It is eliminated approximately 5 h after consumption stops, and ethanol oxidation is the largest carbon source for energy metabolism during this period [68]. The effect of ethanol on the toxicokinetic, as well as toxicodynamic, characteristics of GHB should be addressed. Further in-depth studies on the combined effect of GHB and ethanol, based on a variety of conditions in animal and clinical studies, are necessary.
5. Clinical Case Studies of GHB/GBL and Ethanol-Related Intoxication
Numerous clinical case studies have reported that GHB intoxication is the main cause of hospitalizations related to club drug use [69,70,71,72,73,74]. In European countries, hospitalization rates were significantly higher in patients intoxicated with GHB than in those intoxicated with heroin [3]. However, many cases of GHB intoxication are easily misdiagnosed because of unfamiliar symptoms and confusion with ethanol poisoning or other sedatives [75]. The most common symptom of GHB intoxication was central nervous system depression associated with bradycardia, hypotension, and hypothermia, as well as other additional symptoms such as aggression, exhaustion, memory loss, vomiting, drowsiness, apnea, seizures, suffocation, and coma [76,77]. Most of these symptoms disappear within 4–8 h owing to the short clearance time of GHB and its precursors and metabolites [15]. In the case of GHB toxicity, some of the reported symptoms may be related to multidrug use, and the use of ethanol in combination was more dangerous than the use of GHB alone [14]. Only a few clinical studies have reported details of the case characteristics of co-ingestion of GHB and alcohol (Table 1). A study of drug-related problems in patients who visited emergency departments within the Euro-DEN network over a 12-month period found that 71.7% of patients consumed GHB with other abusive substances, among which 50% consumed GHB with alcohol [12]. Similarly, among patients with GHB poisoning who visited emergency departments in 14 European countries over a period of 39 months, 426 patients reported ethanol consumption with GHB or GBL [13]. Patients with GHB intoxication, with or without co-ingestion of alcohol or other drugs, commonly showed altered behavior, reduced consciousness, anxiety, agitation or aggressiveness, bradycardia, hypotension, and hypothermia. The patients co-ingesting GHB and ethanol showed a higher frequency of vomiting, cardiovascular symptoms, decreased consciousness, and agitation than those ingesting GHB alone. In particular, a higher dose of GHB enhanced agitation or aggressive behavior [12,13,14]. Table 1 notes that ethanol is the most common agent among other psychoactive substances used in combination with GHB and exacerbates toxicity caused by the administration of GHB.

Table 1.
Clinical case studies of gamma-hydroxybutyric acid (GHB)/gamma-butyrolactone (GBL) and ethanol-related intoxication.
6. Toxicokinetic and Toxicodynamic Interactions between GHB and Ethanol in Humans
Table 2 summarizes toxicokinetic and toxicodynamic studies in healthy adults. Thai et al. reported that the mixed intake of GHB (50 mg/kg) with ethanol (0.6 g/kg) in 16 healthy adults increased maximal concentration (Cmax) by 16% and elimination half-life by 29%, compared with GHB ingestion alone, but there was no statistically significant difference. Ethanol has the potential to influence the bioavailability or clearance rate of GHB [15]. In another study by Haller et al. for the same clinical trial design, urine GHB concentrations were lower in the first 3 h following the co-ingestion of ethanol and GHB. However, co-ingestion of ethanol did not significantly affect renal clearance of GHB [16]. These results imply that it is necessary to investigate the toxicokinetic interaction between GHB and ethanol for more diverse clinical trial designs employing various dosages of GHB and ethanol. In contrast, toxicodynamic interactions between GHB and ethanol were clearly shown. The co-administration of ethanol significantly reduced oxygen saturation as well as systolic and diastolic blood pressure and significantly increased the heart rate, compared with GHB ingestion alone. Moreover, higher frequencies of abnormal symptoms such as hypotension and vomiting were observed upon co-ingestion of ethanol and GHB [15]. The toxicokinetic and toxicodynamic interactions of differently formulated GHB and ethanol were also studied. When a single dose of a solid immediate-release formulation of sodium oxybate and ethanol were co-administered in 24 healthy adults, no toxicokinetic interaction was observed. However, alertness and stimulation were significantly increased and sedation was decreased within 60 min after the co-administration [17]. As shown in Table 2, combined exposure to ethanol induced significant changes to the toxicodynamics of GHB, while minor toxicokinetic changes were observed. However, it should be noted that only the therapeutic doses of GHB were used in all the three human studies.

Table 2.
Human studies on toxicokinetic and toxicodynamic changes following gamma-hydroxybutyric acid (GHB) and ethanol co-administration.
7. Toxicokinetic and Toxicodynamic Interactions between GHB and Ethanol in Animals
Table 3 summarizes animal studies on toxicokinetic and toxicodynamic changes in GHB following co-administration of ethanol. In a previous study, behavioral changes following GHB and ethanol co-administration were investigated using the functional observational battery (FOB) [11]. FOB is a noninvasive procedure designed to detect and quantify gross functional deficits in young adult rats due to chemical exposure and is widely utilized as a neurobehavioral assessment tool to describe various behavioral and activity-related parameters in the rat strain [78]. Van Sassenbroeck et al. measured the whisker reflex (WR), startle reflex (SR), righting reflex (RR), and tail clamp reaction (TC) as FOB. This toxicodynamic interaction was studied using isobolographs and an interaction model in rats receiving a combination of steady state concentrations of ethanol (1000–3000 μg/mL) and GHB (200–1400 μg/mL). The results of this study showed that following GHB and ethanol co-administration, the effective concentration of GHB causing 50% of the maximal response (EC50) decreased in three reactions except WR. Additionally, RR showed a synergistic effect on GHB EC50 at higher ethanol concentrations (>2000 μg/mL) and additivity at lower ethanol concentrations. SR showed an antagonistic effect at ethanol concentrations of <1000 µg/mL and showed additivity at higher ethanol concentrations. The TC reaction was antagonistic at all ethanol concentrations. In addition, co-administration of 300 mg/kg GBL and 3 g/kg ethanol intraperitoneally significantly increased the sleep time compared with the administration of GBL alone (GBL, 66 ± 4 min; ethanol, 231 ± 9 min; GBL/ethanol, 389 ± 6 min) [11]. Cook et al. reported that the co-administration of GHB and ethanol significantly reduced locomotor activity and had a significant effect on the results of FOB compared to the administration of GHB alone. The co-administration of GHB and ethanol increased acute depressive behavioral responses by impairing RR and inverted screen performance (SIP), increasing hindlimb splay (HS), and decreasing forelimb grip strength (FGS) and body temperature [18]. This is similar to the results of another previous study where the co-administration of GHB and ethanol showed increased sedative effects [19]. However, other effects on the central nervous system observed in these two studies were inconsistent. In the former study, the co-administration of GHB (0.1–0.3 g/kg) and ethanol (3–4 g/kg) significantly reduced body temperature compared to the administration of GHB alone [18]. However, no difference in respiratory depression after co-administration of GHB and ethanol was observed in the latter study, despite the difference in the dosage of the drug (GHB, 1.5 g/kg) [19].
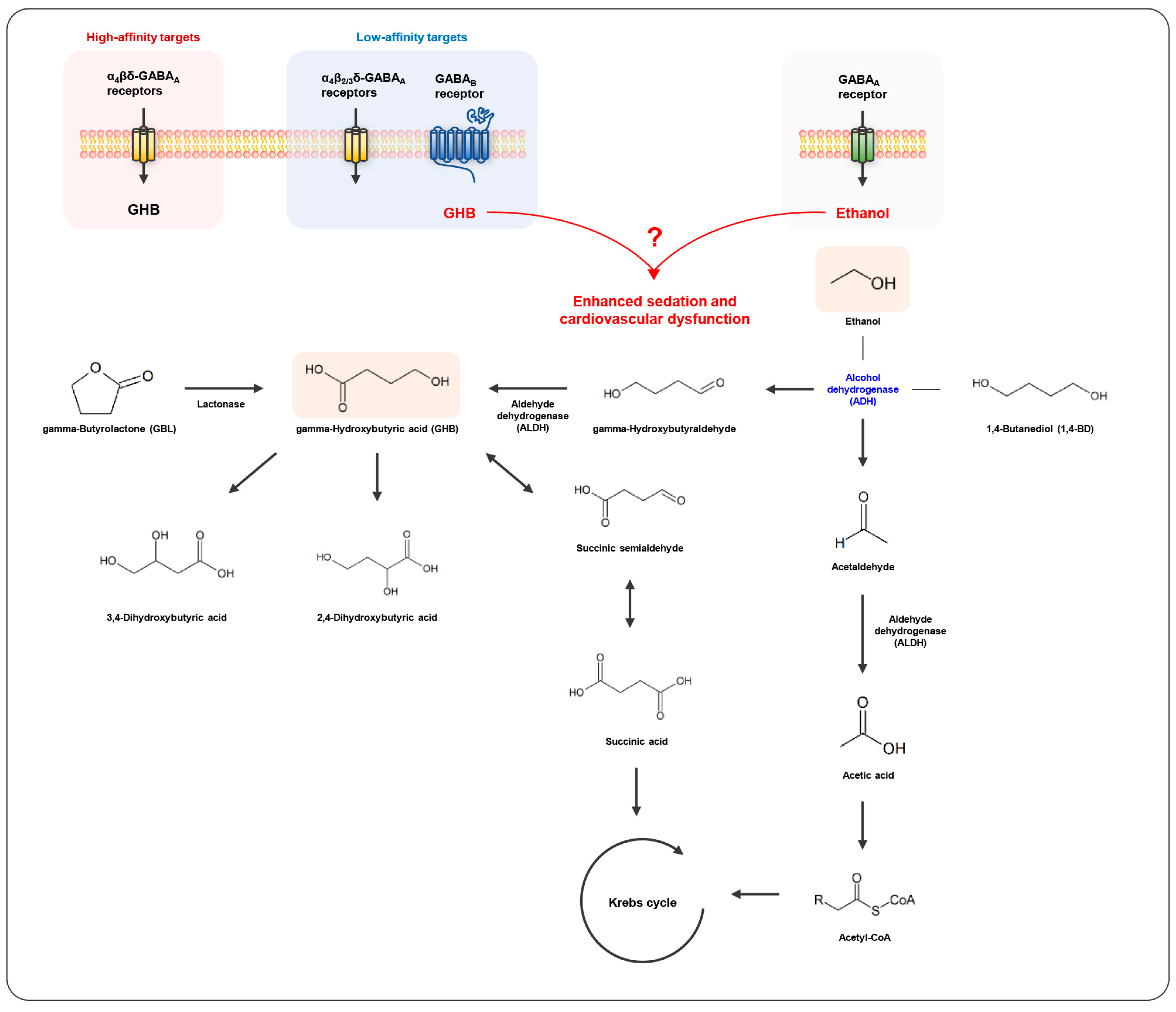
Mechanistic studies were conducted to elucidate the toxicological effects of GHB and ethanol. GHB-induced physiological and behavioral changes are caused by interactions with GHB-specific receptors, the GABAA receptor, and GABAB receptors in various regions of the brain [79,80,81]. It has been previously suggested that GHB is a substrate of the monocarboxylate transporter (MCT) family (SLC16A) [82,83] and sodium-binding MCT (SMCT) family (SLC5A) [84]. MCT1, a ubiquitous protein encoded by the human SLC16A1 gene, is expressed in the BBB and plays an important role in transporting substrates into and out of the brain [85]. MCT mediates renal reuptake of GHB [82,86], and GABAA and GABAB receptors mainly regulate the sedative effects of ethanol and GHB, respectively [87,88,89]. As shown in Table 3, Rodriguez-Cruz and Morris reported that MCT1 inhibitors (AR-C155858, AZD-3965) significantly reduced respiratory depression and sedative effects caused by the co-administration of GHB and ethanol and decreased the brain concentration and brain-to-plasma concentration ratio of GHB at return of righting reflex (RRR) [19]. Morse and Morris investigated the effects of the MCT inhibitor (L-lactate), GABAA receptor antagonist (bicuculline), and GABAB receptor antagonists (SGS742, SCH50911) on the toxicological interactions of GHB, and ethanol L-lactate significantly reduced sleep time, mortality, and brain-to-plasma ratio of GHB at RRR following the co-ingestion of GHB and ethanol. However, there was no significant difference in the brain’s GHB concentrations at the measured time point. Additionally, the administration of bicuculline did not affect respiratory depression following co-administration of GHB and ethanol while that of SCH50911 decreased the respiratory rate and completely prevented death from the administration of high concentrations of ethanol (0.3–0.4%) [20]. These previous studies demonstrated that the combined administration of GHB and ethanol increased inhibitory effects, but MCT1 inhibition could alleviate toxicity by inhibiting GHB brain uptake [19,20]. The inhibition of MCT has been paid attention to as a potential treatment strategy for GHB intoxication [90,91,92,93].
Fung et al. reported that 1,4-BD showed a more potent CNS inhibitory effect than GHB. Nevertheless, it was observed that the total duration of loss of righting reflex (LRR) due to 1,4-BD showed a tendency to decrease when administered with ethanol, while that due to GHB increased significantly [21]. Another study demonstrated that there were no significant differences in reinforcing effects and demand functions between ethanol administration alone and the co-administration of ethanol and GHB in the self-administration experiment in Rhesus monkeys [22]. Hicks and Varner reported that 1,4-BD is potentially more dangerous because it is approximately 10 times more potent as a cardiovascular stimulant than GHB when administered intragastrically [25]. Carai et al. also demonstrated that 1,4-BD traverses the BBB more quickly than GHB and has a stronger efficacy [24]. In both studies, ethanol reduced the risks associated with 1,4-BD [24,25].
Table 4 summarizes animal and in vitro studies on toxicokinetic and toxicodynamic changes in 1,4-BD following co-administration of ethanol. Since the inhibitory effects of 1,4-BD are mediated by its conversion to GHB by ADH [4], a major metabolic enzyme of ethanol, the co-administration of 1,4-BD with ethanol is considered to provoke significant metabolic interactions. The ethanol co-administration decreased the mean arterial pressure (MAP) and heart rate (HR) enhanced by 1,4-BD [23], inhibited the sedative effect [24,26], and reduced the mortality rate [22], which implies that competitive inhibition of ethanol in the conversion of 1,4-BD to GHB may alter the physiological effects attributed to 1,4-BD. Poldrugo and Snead reported that ethanol blocked the electroencephalogram activity of 1,4-BD when administered before 1,4-BD. Moreover, ethanol inhibited the elevation of GHB concentrations in the brain and liver of 1,4-BD-administered animals. From these results, the authors concluded that acute administration of ethanol recovered the inhibitory effects of 1,4-BD by blocking the degradation of 1,4-BD to its active metabolite GHB through competition for ADH [26]. When the ADH inhibitor, pyrazole, was administered to rats, the conversion rate of 1,4-BD to GHB in the liver was significantly reduced [27]. Treatment with another ADH inhibitor, fomepizole, in human-derived hepatocytes strongly inhibited the conversion of 1,4-BD to GHB [28]. On the other hand, Poldrugo et al. also reported that the co-administration of 1,4-BD and ethanol increased the mortality rate, compared with the administration of 1,4-BD alone, in rats and caused microscopic pathological changes in the liver and kidneys [29]. Further studies are necessary to investigate the toxicodynamic interactions between 1,4-BD and ethanol for more diverse animal study designs employing various dosages of 1,4-BD and ethanol.
The mechanism of the sedative effect of 1,4-BD was studied using ADH inhibitors (4-methylpyrazole, disulfiram, and ethanol), the GHB receptor antagonist (NCS-382), and GABAB receptor antagonists (SCH 50911 and CGP 46381). 4-Methylpyrazole and ethanol completely prevented the sedative effect of 1,4-BD, and disulfiram partly blocked it. In addition, the sedative effects of 1,4-BD were antagonized by the GABAB receptor antagonists, SCH 50911 and CGP 46381, but not by the GHB receptor antagonist, NCS-382. Thus, the authors concluded that the sedative effects of 1,4-BD were mediated by GABAB, but not by the GHB receptor [24].
In summary, the co-administration of GHB and ethanol induced strong toxicodynamic interactions probably due to the combined action of the GABA receptors, while toxicokinetic changes of GHB were not significant in animal studies. Since ethanol inhibits the conversion of 1,4-BD to GHB, the concentration of 1,4-BD in the body is increased by the co-administration of GHB with ethanol. The toxicodynamic changes from ethanol co-ingestion could vary depending on the use of GHB or its precursors.

Table 3.
Animal studies on toxicokinetic and toxicodynamic changes in gamma-hydroxybutyric acid (GHB) following co-administration of ethanol.
Table 3.
Animal studies on toxicokinetic and toxicodynamic changes in gamma-hydroxybutyric acid (GHB) following co-administration of ethanol.
| Subject | Method | Result | Ref. | |||
|---|---|---|---|---|---|---|
| Male Wistar rats | Group | Saline, GHB (GBL), EtOH, GBL/EtOH | Toxicokinetics | GHB/EtOH → Vmax (↓), VT (↑), Vdss (↑) | [11] | |
| Protocol | Toxicokinetics: EtOH (infusion to steady-state EtOH target conc. 300–3000 µg/mL) followed by GHB (a single bolus, 400 mg/kg) Toxicodynamics: 20 min after target conc. of GHB (infusion to steady state GHB target conc. 200–1400 µg/mL) or EtOH (infusion to steady-state EtOH target conc. 1000–3000 µg/mL) Sedation test (RR): EtOH (3 g/kg, i.p.), GBL (0.3 g/kg, i.p.), GBL/EtOH | Toxicodynamics | RR: GHB/EtOH (>2000 µg/mL) → synergy; GHB/EtOH (lower conc.) → additivity | |||
| SR: GHB/EtOH (<1000 µg/mL) → antagonism; GHB/EtOH (higher conc.) → additivity | ||||||
| TC: GHB/EtOH (all conc.) → antagonism | ||||||
| Sedation (RR) | GBL/EtOH > EtOH > GBL | |||||
| Male Swiss-Webster mice | Group | Vehicle, GHB, EtOH, GHB/EtOH | Locomotor activity | Vehicle, EtOH > GHB > GHB/EtOH | [18] | |
| Protocol | Behavior test: GHB (0.1–1.0 g/kg, i.g.), EtOH (2.0–5.0 g/kg, i.g.) | |||||
| RR | Vehicle, GHB, EtOH < GHB/EtOH | |||||
| FGS | Vehicle > GHB, EtOH > GHB/EtOH | |||||
| ISP | Vehicle > GHB, EtOH > GHB/EtOH | |||||
| HS | Vehicle < GHB, EtOH < GHB/EtOH | |||||
| Body temperature | Vehicle > GHB, EtOH > GHB/EtOH | |||||
| Male SD rats | Group | GHB, GHB/EtOH, GHB/EtOH/AZD or ARC | Toxicokinetics | GHB ≃ GHB/EtOH (oral) GHB/EtOH (i.v.) → terminal T1/2 (↑) | [19] | |
| Protocol | EtOH (2 g/kg, i.v.), GHB (0.6 g/kg, i.v. or 1.5 g/kg, i.g.), AR-C 155858 (MCT1 inhibitors, 5 mg/kg, i.v.), AZD-3965 (MCT1 inhibitors, 5 mg/kg, i.v.) | |||||
| Sedation | EtOH < GHB/EtOH/AZD-3965 ≃ GHB < GHB/EtOH | |||||
| MCT1 inhibition by AR-C 155858 | GHB/EtOH → respiratory depression (↓) GHB/EtOH → CLNR (↓) GHB → T1/2 (↑), CLNR (↓), Vss (↓) | |||||
| MCT1 inhibition by AZD-3965 | GHB conc. in brain and brain-to-plasma ratio at RRR (↓) GHB/EtOH → respiratory depression (↓) GHB/EtOH → CLR (↑), Vss/F (↑), Cmax (↓) GHB → CL/F (↑), CLNR/F (↑), Vss/F (↑), CLR (↑), AUC (↓), Cmax (↓), Tmax (↓) | |||||
| Male SD rats | Group | GHB, EtOH, GHB/EtOH, GHB/EtOH/inhibitors or antagonists | Toxicokinetics | GHB ≃ GHB/0.1–0.4% EtOH | [20] | |
| Protocol | Sedation: EtOH (2.0 g/kg, i.v.), GHB (600 mg/kg, i.v.), L-lactate (MCT inhibitor, 66 mg/kg + 302.5 mg/kg/h), Bicuculline (bic, GABAAR antagonist, 1 mg/kg), SGS742 (SGS, GABABR antagonist, 500, 1000 mg/kg), SCH50911 (SCH, GABABR antagonist, 100, 200 mg/kg) Respiratory depression/fatality and toxicokinetics: GHB (600, 1500 mg/kg, i.v.), GHB/EtOH (steady-state conc. 0.1–0.2% or 0.3–0.4%), GHB/EtOH (steady-state conc. 0.1–0.2% or 0.3–0.4%)/inhibitors or antagonists Oral toxicokinetics:GHB (1.5 g/kg, i.g.), EtOH (2.5 g/kg, i.g.) | RR | GHB/EtOH > GHB, GHB/EtOH/L-lactate | |||
| Sleep time | GHB/EtOH≃GHB/EtOH/bic > GHB > GHB/EtOH/SGS or SCH > EtOH | |||||
| GHB conc. in brain and brain-to-plasma ratio at RRR | GHB ≃ GHB/EtOH > GHB/EtOH/L-lactate | |||||
| Respiratory Depression | Frequency: | GHB/EtOH/SCH → completely prevented GHB ≃ GHB/EtOH ≃ GHB/EtOH/bic | ||||
| Tidal volume: | GHB/EtOH/SCH → completely prevented GHB > GHB/EtOH ≃ GHB/EtOH/bic | |||||
| Fatality: | GHB/EtOH > GHB/EtOH/L-lactate > GHB, GHB/EtOH/SCH | |||||
| Male SD rats | Group | 1,4-BD, GHB, EtOH, 1,4-BD/EtOH, GHB/EtOH | Mutual metabolic inhibition | EtOH/1,4-BD → significant; EtOH/GHB → not significant | [21] | |
| Protocol | Toxicokinetics: 1,4-BD (1.58, 6.34 mmol/kg, i.v. or oral), GHB (1.58, 1.79, 6.34 mmol/kg, i.v.), EtOH (6.34, 12.7 mmol/kg, i.v.) LRR test: 6.34 mmol/kg (i.v.) | |||||
| Oral absorption of 1,4-BD | Rapid and complete | |||||
| Total duration of LRR | 1,4-BD > 1,4-BD/EtOH > GHB/EtOH > GHB | |||||
| Rhesus monkeys | Group | EtOH, GHB/EtOH | Reinforcing effects in self-administration | EtOH ≃ GHB/EtOH | [22] | |
| Protocol | Self-administration: EtOH (50, 100, 200 mg/kg/inj, i.v.), GHB (1.0, 3.2 mg/kg/inj, i.v.) | Demand functions in self-administration | EtOH ≃ GHB/EtOH | |||
AUC, area under the plasma concentration-time curve; Cmax, maximal concentration; CL, total clearance; CLR, renal clearance; CLNR, nonrenal clearance; conc., concentration; EtOH, ethanol; F, bioavailability; FGS, Forelimb grip strength; GBL, gamma-butyrolactone; GHB, gamma-hydroxybutyric acid; GABABR, GABAB receptor; GABAAR, GABAA receptor; HS, Hindlimb splay; i.p., intraperitoneal injection; i.v., intravenous injection; i.g., intragastric administration; inj., injection; ISP, Inverted screen performance; LRR, loss of righting reflex; MCT, monocarboxylate transporter; RR, righting reflex; RRR, return of righting reflex; SD, Sprague-Dawley; SR, startle reflex to a hand clap; TC, tail clamp reaction; Tmax, time of maximal concentration; T1/2, half-life; Vmax, maximal metabolic rate; VT, peripheral volume of distribution; Vdss, steady-state volume of distribution; Vss, steady-state volume of distribution; 1,4-BD, 1,4-butanediol; ≃, no significantly different; >, significantly different; <, significantly different; /, co-ingestion; ↓, decrease; ↑, increase.

Table 4.
Animal and in vitro studies on toxicokinetic and toxicodynamic changes in 1,4-butanediol following co-administration of ethanol.
Table 4.
Animal and in vitro studies on toxicokinetic and toxicodynamic changes in 1,4-butanediol following co-administration of ethanol.
| Subject | Method | Result | Ref. | |||
|---|---|---|---|---|---|---|
| Male LE rats (behavioral study), Male SD rats cardiovascular study) | Group | Saline, 1,4-BD, EtOH, 1,4-BD/EtOH | Behavioral study (response rate, % of control) | 1,4-BD, EtOH → dose-dependently decrease, 1,4-BD/EtOH > 1,4-BD | [23] | |
| Protocol | Behavioral study (fixed-ratio 20 schedule of food presentation): EtOH (0.25–2 g/kg), 1,4-BD (0.18–0.56 g/kg) Cardiovascular study: Saline (1.0 mL, i.p. or i.v.), EtOH (2.0 g/kg, i.p.), 1,4-BD (0.18–1.0 g/kg, i.p. or i.v.), 1,4-BD (0.56 g/kg, i.v.)/EtOH (2.0 g/kg, i.p.) | |||||
| Mean arterial blood pressure | 1,4-BD > Saline 1,4-BD > 1,4-BD/EtOH | |||||
| Heart rate | 1,4-BD > Saline EtOH > Saline 1,4-BD > 1,4-BD/EtOH | |||||
| Male DBA/2JIco mice | Group | 1,4-BD, GHB, EtOH/1,4-BD, 1,4-BD/inhibitors,1,4-BD/antagonists | LRR | 4MP/1,4-BD, EtOH/1,4-BD, DS/1,4-BD < 1,4-BD NCS-382/1,4-BD ≃ 1,4-BD SCH50911/1,4-BD < 1,4-BD CGP 46381/1,4-BD < 1,4-BD | [24] | |
| Protocol | GHB and 1,4-BD (0.2–1 g/kg, i.p.), EtOH (1 g/kg, i.p.), 4-methylpyrazole (4MP, ADH inhibitor, 0.1 mg/kg, i.p.), disulfiram (DS, ALDH inhibitor, 1–30 mg/kg, i.p.), NCS-382 (GHB receptor antagonist, 0.25 g/kg, i.p.), SCH50911, CGP46381 (GABAB receptor antagonist, 0.1 g/kg, i.p.) | |||||
| Male SD rats | Group | Saline, GHB, 1,4-BD, EtOH/1,4-BD | Mean arterial blood pressure | 1,4-BD > GHB | [25] | |
| Protocol | Cardiovascular study: GHB (0.56–10 g/kg, i.g.), 1,4-BD (0.18–1.0 g/kg, i.g.), 1,4-BD (1.8 g/kg, i.g.) Mortality: EtOH (2.0 g/kg, i.p.)/1,4-BD (1.8 g/kg, i.g.) | Heart rate | 1,4-BD > GHB | |||
| Mortality | 1,4-BD > EtOH/1,4-BD | |||||
| Male SD rats | Group | Vehicle, EtOH, 1,4-BD, 1,4-BD/EtOH, GBL | Electroencephalogram activity | 1,4-BD > GBL, EtOH followed by 1,4-BD < GBL | [26] | |
| Protocol | 1,4-BD (1 g/kg, i.g.), EtOH (3 g/kg, i.g.), GBL (400 mg/kg, i.g.) | LRR | EtOH < EtOH/1,4-BD | |||
| GHB conc. in brain and liver | 1,4-BD > EtOH/1,4-BD | |||||
| EtOH conc. (blood) | EtOH ≃ EtOH/1,4-BD | |||||
| Male SD rats (in vitro) | Group | 1,4-BD, EtOH, Pyrazole, Disulfiram | Conversion rate of 1,4-BD to GHB | EtOH in brain and liver (↓) Pyrazole, Disulfiram in liver (↓) | [27] | |
| Protocol | Conversion rate of 1,4-BD to GHB in brain and liver and oxidation rate of 1,4-BD to GHB: 1,4-BD (8 mM), EtOH (10, 20 mM), pyrazole (ADH inhibitor, 1 mM), disulfiram (ALDH inhibitor, 1 mM) | |||||
| Oxidation of 1,4-BD to GHB | Competitively inhibited by EtOH | |||||
| Human livers (autopsy within 72 h after death) | Group | 1,4-BD, 1,4-BD/EtOH, 1,4-BD/AL, 1,4-BD/inhibitors | Conversion of 1,4-BD to GHB | 1,4-BD/EtOH (↓), 1,4-BD/AL (↑) | [28] | |
| Protocol | 10 human livers (5 men, 5 women, 43–79 years old) Conversion of 1,4-BD to GHB: 1,4-BD (3–80 mM), EtOH (0–2 mM), acetaldehyde (AL, ADH inhibitor, 0–2 mM) Inhibitors efficiency: 1,4-BD (0.5–5 mM) + ADH inhibitors (fomepizole, pyrazole) or ALDH inhibitors (disulfiram, cimetidine) | Inhibitors efficiency | Fomepizole: | GHB formation (↓), most potent inhibitor | ||
| Pyrazole: | GHB formation (↓) | |||||
| Disulfiram: | GHB formation (↓) | |||||
| Cimetidine: | GHB formation (↓), weakest inhibitor | |||||
| Male SD rats | Group | 1,4-BD, EtOH, 1,4-BD/EtOH | EtOH conc. (blood) | EtOH ≃ 1,4-BD/EtOH | [29] | |
| Protocol | Measurement of EtOH and 1,4-BD levels, mortality rate and histochemistry (brain. Liver, kidney): 1,4-BD (1 g/kg, i.g.), EtOH (3 g/kg, i.p.) | 1,4-BD conc. (brain, liver, kidney) | 1,4-BD/EtOH > 1,4-BD | |||
| Mortality rate | 1,4-BD/EtOH > 1,4-BD | |||||
| Histological alterations | EtOH → no change; 1,4-BD → hyperemia in all organs (↑); 1,4-BD/EtOH → tissue damage (↑), fatty infiltration and necrosis in liver, extensive medullary necrosis in kidney) | |||||
ADH, alcohol dehydrogenase; AL, acetaldehyde; ALDH, aldehyde dehydrogenase; conc., concentration; EtOH, ethanol; GBL, gamma-butyrolactone; GHB, gamma-hydroxybutyric acid; i.p., intraperitoneal injection; i.v., intravenous injection; i.g., intragastric administration; LE, Long–Evans; LRR, loss-of-righting reflex; SD, Sprague-Dawley; 1,4-BD, 1,4-butanediol; ≃, no significantly different; >, significantly different; <, significantly different; /, co-ingestion; ↓, decrease; ↑, increase.
8. Drug Discrimination or Responding Following GHB and Ethanol Co-Administration
The effects of the co-ingestion of GHB and ethanol on drug discrimination or response in animals are summarized in Table 5. Drug discrimination is a series of procedures found to be particularly useful for characterizing similarities and differences among drugs and their abuse liability [94,95,96,97]. Cook et al. reported that ethanol produced less than 50% of GHB-like discriminative stimulus effects, but GHB did not affect the GHB-like discriminative stimulus effects of ethanol [18]. Metcalf et al. found that GHB and ethanol were not cross-generalized in the ethanol- and GHB-trained rats, respectively. However, there was a synergistic interaction between GHB and ethanol when administered as a mixture in the GHB- and ethanol-trained rats. When the GHB- and ethanol-trained rats were tested with the mixed dose of 225 mg/kg GHB and 750 mg/kg ethanol, complete generalization was produced in both groups [30]. Regarding the response in rats under a fixed-ratio (FR) 10 schedule of sugar solution presentation, GHB decreased the drug response rate in a dose-dependent manner, and the co-administration of GHB and ethanol further decreased the response rate. However, the GHB antagonist, NCS 382, did not appear to interfere with the effects of GHB. The authors of this study suggest that many of the behavioral actions induced by exogenous GHB are due to GHB action at sites other than the GHB receptor [31]. The effects of reinforcement types, such as food or water, and administration routes, such as intraperitoneal (IP) or intragastric (IG) administration on stimulus generalization, were also studied. Food maintained significantly higher response rates than water. The IG-water group was most sensitive to a lower dose of GHB, and only the IP-water group failed to generalize to orally-administered GHB. GBL and 1,4-BD were fully substituted, except for in the IP-food group, while ethanol was partially substituted in all groups. The combination of GHB (150 mg/kg) and ethanol did not show any additive effects in all groups [32]. The finding that animals can partially or fully generalize among GHB, ethanol, and their mixture is further evidence that the effects of GHB and ethanol are not completely identical but may be involved in each different subtype of GABA receptors for enhanced inhibitory effects.

Table 5.
Drug discrimination or response following gamma-hydroxybutyric acid (GHB) and ethanol co-administration.
9. Future Directions and Conclusions
Clinical cases reported that the combined exposure to GHB and ethanol can cause severe sedation and respiratory depression, potentially resulting in death more frequently, compared with the administration of GHB alone. Previous animal studies also demonstrated that the combined administration of GHB and ethanol enhanced sedation and cardiovascular dysfunction. However, the toxicodynamic alterations caused by the co-administration of GHB and ethanol poorly correlate with toxicokinetic changes in GHB. Both GHB and ethanol are metabolized to inactive metabolites through the Krebs cycle. Since the toxicological effects of both GHB and ethanol are mainly caused by the action of the GABA receptors, i.e., the GABAB and the GABAA receptors, respectively, it is assumed that the enhanced inhibitory effects of GHB caused by ethanol could be more or less additive. 1,4-BD is rapidly and extensively converted to GHB, and has stronger and more prolonged sedative effects than GHB [24,98]. However, the co-administration of 1,4-BD with ethanol results in a decrease in the sedative effects since 1,4-BD is converted to GHB by ADH, where ethanol acts as an inhibitor (Figure 2). There is still insufficient evidence to understand the action mechanism of the interactions between GHB or its precursors and ethanol, which could be an obstacle for the diagnosis and treatment of GHB intoxication in the future. Therefore, toxicokinetic investigations of not only GHB but also other metabolites related with GHB and ethanol, as well as toxicodynamic interactions, need to be performed using a variety of exposure doses or durations in animal and clinical settings.

Figure 2.
Interactions between gamma-hydroxybutyric acid (GHB) and ethanol.
Author Contributions
Conceptualization, S.L.; investigation, S.J. and M.K.; data curation, M.K.; writing—original draft preparation, S.J. and M.K.; writing—review and editing, S.L.; visualization, S.K.; supervision, S.L.; funding acquisition, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from the Ministry of Food and Drug Safety (22212MFDS253) and the Basic Science Research Program (NRF-2016R1A6A1A03011325 and NRF-2018R1D1A1B07048159) through the National Research Foundation funded by the Ministry of Education in Korea.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Bay, T.; Eghorn, L.F.; Klein, A.B.; Wellendorph, P. GHB receptor targets in the CNS: Focus on high-affinity binding sites. Biochem. Pharmacol. 2014, 87, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Trombley, T.A.; Capstick, R.A.; Lindsley, C.W. DARK Classics in Chemical Neuroscience: Gamma-Hydroxybutyrate (GHB). ACS Chem. Neurosci. 2019, 11, 3850–3859. [Google Scholar] [CrossRef]
- Brunt, T.M.; van Amsterdam, J.G.; van den Brink, W. GHB, GBL and 1,4-BD addiction. Curr. Pharm. Des. 2014, 20, 4076–4085. [Google Scholar] [CrossRef] [PubMed]
- Irwin, R.D. NTP summary report on the metabolism, disposition, and toxicity of 1,4-butanediol (CAS No. 110-63-4). Toxic. Rep. Ser. 1996, 54, 1–28. [Google Scholar]
- Zvosec, D.L.; Smith, S.W.; McCutcheon, J.R.; Spillane, J.; Hall, B.J.; Peacock, E.A. Adverse events, including death, associated with the use of 1,4-butanediol. N. Engl. J. Med. 2001, 344, 87–94. [Google Scholar] [CrossRef]
- Busardò, F.P.; Varì, M.R.; di Trana, A.; Malaca, S.; Carlier, J.; di Luca, N.M. Drug-facilitated sexual assaults (DFSA): A serious underestimated issue. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10577–10587. [Google Scholar] [CrossRef]
- Miró, Ò.; Waring, W.S.; Dargan, P.I.; Wood, D.M.; Dines, A.M.; Yates, C.; Giraudon, I.; Moughty, A.; O’Connor, N.; Heyerdahl, F.; et al. Variation of drugs involved in acute drug toxicity presentations based on age and sex: An epidemiological approach based on European emergency departments. Clin. Toxicol. 2021, 59, 896–904. [Google Scholar] [CrossRef]
- Singh, A.K. Alcohol Interaction with Cocaine, Methamphetamine, Opioids, Nicotine, Cannabis, and γ-Hydroxybutyric Acid. Biomedicines 2019, 7, 16. [Google Scholar] [CrossRef]
- Abrahao, K.P.; Salinas, A.G.; Lovinger, D.M. Alcohol and the Brain: Neuronal Molecular Targets, Synapses, and Circuits. Neuron 2017, 96, 1223–1238. [Google Scholar] [CrossRef]
- Hendler, R.A.; Ramchandani, V.A.; Gilman, J.; Hommer, D.W. Stimulant and sedative effects of alcohol. Curr. Top. Behav. Neurosci. 2013, 13, 489–509. [Google Scholar] [CrossRef]
- Van Sassenbroeck, D.K.; De Paepe, P.; Belpaire, F.M.; Buylaert, W.A. Characterization of the pharmacokinetic and pharmacodynamic interaction between gamma-hydroxybutyrate and ethanol in the rat. Toxicol. Sci. 2003, 73, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Miró, Ò.; Galicia, M.; Dargan, P.; Dines, A.M.; Giraudon, I.; Heyerdahl, F.; Hovda, K.E.; Yates, C.; Wood, D.M.; Liakoni, E.; et al. Intoxication by gamma hydroxybutyrate and related analogues: Clinical characteristics and comparison between pure intoxication and that combined with other substances of abuse. Toxicol. Lett. 2017, 277, 84–91. [Google Scholar] [CrossRef]
- Galicia, M.; Dargan, P.I.; Dines, A.M.; Yates, C.; Heyerdahl, F.; Hovda, K.E.; Giraudon, I.; Wood, D.M.; Miró, Ò. Clinical relevance of ethanol coingestion in patients with GHB/GBL intoxication. Toxicol. Lett. 2019, 314, 37–42. [Google Scholar] [CrossRef]
- Liechti, M.E.; Kunz, I.; Greminger, P.; Speich, R.; Kupferschmidt, H. Clinical features of gamma-hydroxybutyrate and gamma-butyrolactone toxicity and concomitant drug and alcohol use. Drug Alcohol Depend. 2006, 81, 323–326. [Google Scholar] [CrossRef]
- Thai, D.; Dyer, J.E.; Benowitz, N.L.; Haller, C.A. Gamma-hydroxybutyrate and ethanol effects and interactions in humans. J. Clin. Psychopharmacol. 2006, 26, 524–529. [Google Scholar] [CrossRef]
- Haller, C.; Thai, D.; Jacob, P., 3rd; Dyer, J.E. GHB urine concentrations after single-dose administration in humans. J. Anal. Toxicol. 2006, 30, 360–364. [Google Scholar] [CrossRef]
- Pross, N.; Patat, A.; Vivet, P.; Bidaut, M.; Fauchoux, N. Pharmacodynamic interactions of a solid formulation of sodium oxybate and ethanol in healthy volunteers. Br. J. Clin. Pharmacol. 2015, 80, 480–492. [Google Scholar] [CrossRef]
- Cook, C.D.; Biddlestone, L.; Coop, A.; Beardsley, P.M. Effects of combining ethanol (EtOH) with gamma-hydroxybutyrate (GHB) on the discriminative stimulus, locomotor, and motor-impairing functions of GHB in mice. Psychopharmacology 2006, 185, 112–122. [Google Scholar] [CrossRef]
- Rodriguez-Cruz, V.; Morris, M.E. γ-Hydroxybutyric Acid-Ethanol Drug-Drug Interaction: Reversal of Toxicity with Monocarboxylate Transporter 1 Inhibitors. J. Pharmacol. Exp. Ther. 2021, 378, 42–50. [Google Scholar] [CrossRef]
- Morse, B.L.; Morris, M.E. Toxicokinetics/Toxicodynamics of γ-hydroxybutyrate-ethanol intoxication: Evaluation of potential treatment strategies. J. Pharmacol. Exp. Ther. 2013, 346, 504–513. [Google Scholar] [CrossRef]
- Fung, H.L.; Tsou, P.S.; Bulitta, J.B.; Tran, D.C.; Page, N.A.; Soda, D.; Mi Fung, S. Pharmacokinetics of 1,4-butanediol in rats: Bioactivation to gamma-hydroxybutyric acid, interaction with ethanol, and oral bioavailability. AAPS J. 2008, 10, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Winger, G.; Galuska, C.M.; Hursh, S.R. Modification of ethanol’s reinforcing effectiveness in rhesus monkeys by cocaine, flunitrazepam, or gamma-hydroxybutyrate. Psychopharmacology 2007, 193, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Gerak, L.R.; Hicks, A.R.; Winsauer, P.J.; Varner, K.J. Interaction between 1,4-butanediol and ethanol on operant responding and the cardiovascular system. Eur. J. Pharmacol. 2004, 506, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Carai, M.A.; Colombo, G.; Reali, R.; Serra, S.; Mocci, I.; Castelli, M.P.; Cignarella, G.; Gessa, G.L. Central effects of 1,4-butanediol are mediated by GABA(B) receptors via its conversion into gamma-hydroxybutyric acid. Eur. J. Pharmacol. 2002, 441, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Hicks, A.R.; Varner, K.J. Cardiovascular responses elicited by intragastric administration of BDL and GHB. J. Recept. Signal Transduct. Res. 2008, 28, 429–436. [Google Scholar] [CrossRef]
- Poldrugo, F.; Snead, O.C., 3rd. 1,4 Butanediol, gamma-hydroxybutyric acid and ethanol: Relationships and interactions. Neuropharmacology 1984, 23, 109–113. [Google Scholar] [CrossRef]
- Poldrugo, F.; Snead, O.C., 3rd. 1,4-Butanediol and ethanol compete for degradation in rat brain and liver in vitro. Alcohol 1986, 3, 367–370. [Google Scholar] [CrossRef]
- Lenz, D.; Jübner, M.; Bender, K.; Wintermeyer, A.; Beike, J.; Rothschild, M.A.; Käferstein, H. Inhibition of 1,4-butanediol metabolism in human liver in vitro. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 647–654. [Google Scholar] [CrossRef]
- Poldrugo, F.; Barker, S.; Basa, M.; Mallardi, F.; Snead, O.C. Ethanol potentiates the toxic effects of 1,4-butanediol. Alcohol. Clin. Exp. Res. 1985, 9, 493–497. [Google Scholar] [CrossRef]
- Metcalf, B.R.; Stahl, J.M.; Allen, J.D.; Woolfolk, D.R.; Soto, P.L. Discrimination of gamma-hydroxybutyrate and ethanol administered separately and as a mixture in rats. Pharmacol. Biochem. Behav. 2001, 70, 31–41. [Google Scholar] [CrossRef]
- Lamb, R.J.; Munn, J.; Duiker, N.J.; Coop, A.; Wu, H.; Koek, W.; France, C.P. Interactions of gamma-hydroxy butyrate with ethanol and NCS 382. Eur. J. Pharmacol. 2003, 470, 157–162. [Google Scholar] [CrossRef]
- Baker, L.E.; Pynnonen, D.; Poling, A. Influence of reinforcer type and route of administration on gamma-hydroxybutyrate discrimination in rats. Psychopharmacology 2004, 174, 220–227. [Google Scholar] [CrossRef]
- Felmlee, M.A.; Morse, B.L.; Morris, M.E. γ-Hydroxybutyric Acid: Pharmacokinetics, Pharmacodynamics, and Toxicology. AAPS J. 2021, 23, 22. [Google Scholar] [CrossRef]
- Castelli, M.P.; Ferraro, L.; Mocci, I.; Carta, F.; Carai, M.A.; Antonelli, T.; Tanganelli, S.; Cignarella, G.; Gessa, G.L. Selective gamma-hydroxybutyric acid receptor ligands increase extracellular glutamate in the hippocampus, but fail to activate G protein and to produce the sedative/hypnotic effect of gamma-hydroxybutyric acid. J. Neurochem. 2003, 87, 722–732. [Google Scholar] [CrossRef]
- Gobaille, S.; Hechler, V.; Andriamampandry, C.; Kemmel, V.; Maitre, M. gamma-Hydroxybutyrate modulates synthesis and extracellular concentration of gamma-aminobutyric acid in discrete rat brain regions in vivo. J. Pharmacol. Exp. Ther. 1999, 290, 303–309. [Google Scholar]
- Hechler, V.; Gobaille, S.; Bourguignon, J.J.; Maitre, M. Extracellular events induced by gamma-hydroxybutyrate in striatum: A microdialysis study. J. Neurochem. 1991, 56, 938–944. [Google Scholar] [CrossRef]
- Cash, C.D. Gamma-hydroxybutyrate: An overview of the pros and cons for it being a neurotransmitter and/or a useful therapeutic agent. Neurosci. Biobehav. Rev. 1994, 18, 291–304. [Google Scholar] [CrossRef]
- Duenas, M.R. Tobacco Smoke and Atherosclerosis Progression. JAMA 1998, 280, 32–33. [Google Scholar] [CrossRef]
- Chin, R.L.; Sporer, K.A.; Cullison, B.; Dyer, J.E.; Wu, T.D. Clinical Course of γ-Hydroxybutyrate Overdose. Ann. Emerg. Med. 1998, 31, 716–722. [Google Scholar] [CrossRef]
- Mamelak, M. Gammahydroxybutyrate: An endogenous regulator of energy metabolism. Neurosci. Biobehav. Rev. 1989, 13, 187–198. [Google Scholar] [CrossRef]
- Kemmel, V.; Klein, C.; Dembélé, D.; Jost, B.; Taleb, O.; Aunis, D.; Mensah-Nyagan, A.G.; Maitre, M. A single acute pharmacological dose of γ-hydroxybutyrate modifies multiple gene expression patterns in rat hippocampus and frontal cortex. Physiol. Genom. 2010, 41, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Ottani, A.; Saltini, S.; Bartiromo, M.; Zaffe, D.; Renzo Botticelli, A.; Ferrari, A.; Bertolini, A.; Genedani, S. Effect of gamma-hydroxybutyrate in two rat models of focal cerebral damage. Brain Res. 2003, 986, 181–190. [Google Scholar] [CrossRef]
- Wendt, G.; Kemmel, V.; Patte-Mensah, C.; Uring-Lambert, B.; Eckert, A.; Schmitt, M.J.; Mensah-Nyagan, A.G. Gamma-hydroxybutyrate, acting through an anti-apoptotic mechanism, protects native and amyloid-precursor-protein-transfected neuroblastoma cells against oxidative stress-induced death. Neuroscience 2014, 263, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, O.; Montplaisir, J.; Lamarre, M.; Bedard, M.A. The effect of gamma-hydroxybutyrate on nocturnal and diurnal sleep of normal subjects: Further considerations on REM sleep-triggering mechanisms. Sleep 1990, 13, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Mamelak, M. Narcolepsy and depression and the neurobiology of gammahydroxybutyrate. Prog. Neurobiol. 2009, 89, 193–219. [Google Scholar] [CrossRef]
- Carter, L.P.; Wu, H.; Chen, W.; Matthews, M.M.; Mehta, A.K.; Hernandez, R.J.; Thomson, J.A.; Ticku, M.K.; Coop, A.; Koek, W.; et al. Novel gamma-hydroxybutyric acid (GHB) analogs share some, but not all, of the behavioral effects of GHB and GABAB receptor agonists. J. Pharmacol. Exp. Ther. 2005, 313, 1314–1323. [Google Scholar] [CrossRef]
- Connelly, W.M.; Errington, A.C.; Crunelli, V. γ-Hydroxybutyric acid (GHB) is not an agonist of extrasynaptic GABAA receptors. PLoS ONE 2013, 8, e79062. [Google Scholar] [CrossRef]
- Scrima, L.; Hartman, P.G.; Johnson, F.H., Jr.; Thomas, E.E.; Hiller, F.C. The effects of gamma-hydroxybutyrate on the sleep of narcolepsy patients: A double-blind study. Sleep 1990, 13, 479–490. [Google Scholar] [CrossRef]
- Gallimberti, L.; Spella, M.R.; Soncini, C.A.; Gessa, G.L. Gamma-hydroxybutyric acid in the treatment of alcohol and heroin dependence. Alcohol 2000, 20, 257–262. [Google Scholar] [CrossRef]
- United National Office of Drugs and Crime. World Drug Report 2021. Available online: https://www.unodc.org/unodc/en/data-and-analysis/wdr2021.html (accessed on 20 December 2021).
- Supreme Prosecutor’s Office, Republic of Korea. 2020 Narcotic Crime White Paper. pp. 162–174. Available online: https://www.spo.go.kr/preview/skin/doc.html?fn=0ac5769a-3e62-4808-9336-9895cb28b407.pdf&rs=/preview/result/board/1204/ (accessed on 10 June 2021).
- Busardò, F.P.; Jones, A.W. Interpreting γ-hydroxybutyrate concentrations for clinical and forensic purposes. Clin. Toxicol. 2019, 57, 149–163. [Google Scholar] [CrossRef]
- Busardò, F.P.; Bertol, E.; Vaiano, F.; Baglio, G.; Montana, A.; Barbera, N.; Zaami, S.; Romano, G. Post mortem concentrations of endogenous gamma hydroxybutyric acid (GHB) and in vitro formation in stored blood and urine samples. Forensic Sci. Int. 2014, 243, 144–148. [Google Scholar] [CrossRef]
- Årnes, M.; Bachs, L.; Sammarai, M.A.; Jones, A.W.; Høiseth, G. Rate of elimination of γ-hydroxybutyrate from blood determined by analysis of two consecutive samples from apprehended drivers in Norway. Forensic Sci. Int. 2020, 314, 110374. [Google Scholar] [CrossRef]
- Liechti, M.E.; Quednow, B.B.; Liakoni, E.; Dornbierer, D.; von Rotz, R.; Gachet, M.S.; Gertsch, J.; Seifritz, E.; Bosch, O.G. Pharmacokinetics and pharmacodynamics of γ-hydroxybutyrate in healthy subjects. Br. J. Clin. Pharmacol. 2016, 81, 980–988. [Google Scholar] [CrossRef]
- Schröck, A.; Hari, Y.; König, S.; Auwärter, V.; Schürch, S.; Weinmann, W. Pharmacokinetics of GHB and detection window in serum and urine after single uptake of a low dose of GBL—An experiment with two volunteers. Drug Test. Anal. 2014, 6, 363–366. [Google Scholar] [CrossRef]
- Brailsford, A.D.; Cowan, D.A.; Kicman, A.T. Urinary γ-hydroxybutyrate concentrations in 1126 female subjects. J. Anal. Toxicol. 2010, 34, 555–561. [Google Scholar] [CrossRef]
- Vaiano, F.; Serpelloni, G.; Furlanetto, S.; Palumbo, D.; Mari, F.; Fioravanti, A.; Bertol, E. Determination of endogenous concentration of γ-hydroxybutyric acid (GHB) in hair through an ad hoc GC-MS analysis: A study on a wide population and influence of gender and age. J. Pharm. Biomed. Anal. 2016, 118, 161–166. [Google Scholar] [CrossRef]
- Jung, S.; Kim, S.; Seo, Y.; Lee, S. Metabolic Alterations Associated with γ-Hydroxybutyric Acid and the Potential of Metabolites as Biomarkers of Its Exposure. Metabolites 2021, 11, 101. [Google Scholar] [CrossRef]
- Steuer, A.E.; Raeber, J.; Simbuerger, F.; Dornbierer, D.A.; Bosch, O.G.; Quednow, B.B.; Seifritz, E.; Kraemer, T. Towards Extending the Detection Window of Gamma-Hydroxybutyric Acid-An Untargeted Metabolomics Study in Serum and Urine Following Controlled Administration in Healthy Men. Metabolites 2021, 11, 166. [Google Scholar] [CrossRef]
- Tanaka, E. Toxicological interactions involving psychiatric drugs and alcohol: An update. J. Clin. Pharm. Ther. 2003, 28, 81–95. [Google Scholar] [CrossRef]
- Ameeta Rani, V.; Nadiger, H.A.; Marcus, S.R.; Chandrakala, M.V.; Sadasivudu, B. Acute and short term effects of ethanol on the metabolism of glutamic acid and GABA in rat brain. Neurochem. Res. 1985, 10, 297–306. [Google Scholar] [CrossRef]
- Piepponen, T.P.; Kiianmaa, K.; Ahtee, L. Effects of ethanol on the accumbal output of dopamine, GABA and glutamate in alcohol-tolerant and alcohol-nontolerant rats. Pharmacol. Biochem. Behav. 2002, 74, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, S.; Lee, M.S.; Kim, M.; Park, M.; Han, S.; Han, S.; Lee, H.S.; Lee, S. Urinary Profile of Endogenous Gamma-Hydroxybutyric Acid and its Biomarker Metabolites in Healthy Korean Females: Determination of Age-Dependent and Intra-Individual Variability and Identification of Metabolites Correlated With Gamma-Hydroxybutyric Acid. Front. Pharmacol. 2022, 13, 853971. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, M.S.; Kim, M.; Ko, B.J.; Lee, H.S.; Lee, S. Derivatization-assisted LC-MS/MS method for simultaneous quantification of endogenous gamma-hydroxybutyric acid and its metabolic precursors and products in human urine. Anal. Chim. Acta 2022, 1194, 339401. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Nutt, D.J. Gamma hydroxy butyrate abuse and dependency. J. Psychopharmacol. 2005, 19, 195–204. [Google Scholar] [CrossRef]
- Kintz, P.; Goullé, J.P.; Cirimele, V.; Ludes, B. Window of detection of gamma-hydroxybutyrate in blood and saliva. Clin. Chem. 2001, 47, 2033–2034. [Google Scholar] [CrossRef]
- Wilson, D.F.; Matschinsky, F.M. Ethanol metabolism: The good, the bad, and the ugly. Med. Hypotheses 2020, 140, 109638. [Google Scholar] [CrossRef]
- Galicia, M.; Nogue, S.; Miró, O. Liquid ecstasy intoxication: Clinical features of 505 consecutive emergency department patients. Emerg. Med. J. 2011, 28, 462–466. [Google Scholar] [CrossRef]
- Knudsen, K.; Jonsson, U.; Abrahamsson, J. Twenty-three deaths with gamma-hydroxybutyrate overdose in western Sweden between 2000 and 2007. Acta Anaesthesiol. Scand. 2010, 54, 987–992. [Google Scholar] [CrossRef]
- Krul, J.; Girbes, A.R. γ-Hydroxybutyrate: Experience of 9 years of γ-hydroxybutyrate (GHB)-related incidents during rave parties in The Netherlands. Clin. Toxicol. 2011, 49, 311–315. [Google Scholar] [CrossRef]
- Munir, V.L.; Hutton, J.E.; Harney, J.P.; Buykx, P.; Weiland, T.J.; Dent, A.W. Gamma-hydroxybutyrate: A 30 month emergency department review. Emerg. Med. Australas. 2008, 20, 521–530. [Google Scholar] [CrossRef]
- Van Sassenbroeck, D.K.; De Neve, N.; De Paepe, P.; Belpaire, F.M.; Verstraete, A.G.; Calle, P.A.; Buylaert, W.A. Abrupt awakening phenomenon associated with gamma-hydroxybutyrate use: A case series. Clin. Toxicol. 2007, 45, 533–538. [Google Scholar] [CrossRef]
- Zvosec, D.L.; Smith, S.W.; Porrata, T.; Strobl, A.Q.; Dyer, J.E. Case series of 226 γ-hydroxybutyrate-associated deaths: Lethal toxicity and trauma. Am. J. Emerg. Med. 2011, 29, 319–332. [Google Scholar] [CrossRef]
- Wood, D.M.; Brailsford, A.D.; Dargan, P.I. Acute toxicity and withdrawal syndromes related to γ-hydroxybutyrate (GHB) and its analogues γ-butyrolactone (GBL) and 1,4-butanediol (1,4-BD). Drug Test. Anal. 2011, 3, 417–425. [Google Scholar] [CrossRef]
- Devlin, R.J.; Henry, J.A. Clinical review: Major consequences of illicit drug consumption. Crit. Care 2008, 12, 202. [Google Scholar] [CrossRef]
- Snead, O.C., 3rd; Gibson, K.M. Gamma-hydroxybutyric acid. N. Engl. J. Med. 2005, 352, 2721–2732. [Google Scholar] [CrossRef]
- Tegeris, J.S.; Balster, R.L. A comparison of the acute behavioral effects of alkylbenzenes using a functional observational battery in mice. Fundam. Appl. Toxicol. 1994, 22, 240–250. [Google Scholar] [CrossRef]
- Lingenhoehl, K.; Brom, R.; Heid, J.; Beck, P.; Froestl, W.; Kaupmann, K.; Bettler, B.; Mosbacher, J. Gamma-hydroxybutyrate is a weak agonist at recombinant GABA(B) receptors. Neuropharmacology 1999, 38, 1667–1673. [Google Scholar] [CrossRef]
- Mathivet, P.; Bernasconi, R.; De Barry, J.; Marescaux, C.; Bittiger, H. Binding characteristics of gamma-hydroxybutyric acid as a weak but selective GABAB receptor agonist. Eur. J. Pharmacol. 1997, 321, 67–75. [Google Scholar] [CrossRef]
- Nicholson, K.L.; Balster, R.L. GHB: A new and novel drug of abuse. Drug Alcohol Depend. 2001, 63, 1–22. [Google Scholar] [CrossRef]
- Wang, Q.; Darling, I.M.; Morris, M.E. Transport of gamma-hydroxybutyrate in rat kidney membrane vesicles: Role of monocarboxylate transporters. J. Pharmacol. Exp. Ther. 2006, 318, 751–761. [Google Scholar] [CrossRef]
- Wang, Q.; Morris, M.E. Flavonoids modulate monocarboxylate transporter-1-mediated transport of gamma-hydroxybutyrate in vitro and in vivo. Drug Metab. Dispos. 2007, 35, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Morris, M.E. The drug of abuse gamma-hydroxybutyrate is a substrate for sodium-coupled monocarboxylate transporter (SMCT) 1 (SLC5A8): Characterization of SMCT-mediated uptake and inhibition. Drug Metab. Dispos. 2009, 37, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Vijay, N.; Morris, M.E. Role of monocarboxylate transporters in drug delivery to the brain. Curr. Pharm. Des. 2014, 20, 1487–1498. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.E.; Hu, K.; Wang, Q. Renal clearance of gamma-hydroxybutyric acid in rats: Increasing renal elimination as a detoxification strategy. J. Pharmacol. Exp. Ther. 2005, 313, 1194–1202. [Google Scholar] [CrossRef]
- Beleslin, D.B.; Djokanović, N.; Jovanović Mićić, D.; Samardzić, R. Opposite effects of GABAA and NMDA receptor antagonists on ethanol-induced behavioral sleep in rats. Alcohol 1997, 14, 167–173. [Google Scholar] [CrossRef]
- Carai, M.A.; Colombo, G.; Brunetti, G.; Melis, S.; Serra, S.; Vacca, G.; Mastinu, S.; Pistuddi, A.M.; Solinas, C.; Cignarella, G.; et al. Role of GABA(B) receptors in the sedative/hypnotic effect of gamma-hydroxybutyric acid. Eur. J. Pharmacol. 2001, 428, 315–321. [Google Scholar] [CrossRef]
- Liljequist, S.; Engel, J. Effects of GABAergic agonists and antagonists on various ethanol-induced behavioral changes. Psychopharmacology 1982, 78, 71–75. [Google Scholar] [CrossRef]
- Morse, B.L.; Vijay, N.; Morris, M.E. γ-Hydroxybutyrate (GHB)-induced respiratory depression: Combined receptor-transporter inhibition therapy for treatment in GHB overdose. Mol. Pharmacol. 2012, 82, 226–235. [Google Scholar] [CrossRef]
- Morse, B.L.; Vijay, N.; Morris, M.E. Mechanistic modeling of monocarboxylate transporter-mediated toxicokinetic/toxicodynamic interactions between γ-hydroxybutyrate and L-lactate. AAPS J. 2014, 16, 756–770. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Morris, M.E. Effects of L-lactate and D-mannitol on gamma-hydroxybutyrate toxicokinetics and toxicodynamics in rats. Drug Metab. Dispos. 2008, 36, 2244–2251. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Morris, M.E. Pharmacokinetic interaction between the flavonoid luteolin and gamma-hydroxybutyrate in rats: Potential involvement of monocarboxylate transporters. AAPS J. 2008, 10, 47–55. [Google Scholar] [CrossRef]
- Colpaert, F.C.; Janssen, P.A. Agonist and antagonist effects of prototype opiate drugs in fentanyl dose-dose discrimination. Psychopharmacology 1986, 90, 222–228. [Google Scholar] [CrossRef]
- Goudie, A.J.; Leathley, M.J. Actions of ritanserin, a 5-HT(2/1C) antagonist, in benzodiazepine-dependent rats. Behav. Pharmacol. 1993, 4, 247–255. [Google Scholar] [CrossRef]
- Holtzman, S.G. Caffeine as a model drug of abuse. Trends Pharmacol. Sci. 1990, 11, 355–356. [Google Scholar] [CrossRef]
- Overton, D.A. Multiple drug training as a method for increasing the specificity of the drug discrimination procedure. J. Pharmacol. Exp. Ther. 1982, 221, 166–172. [Google Scholar]
- Goodwin, A.K.; Brown, P.R.; Jansen, E.E.; Jakobs, C.; Gibson, K.M.; Weerts, E.M. Behavioral effects and pharmacokinetics of gamma-hydroxybutyrate (GHB) precursors gamma-butyrolactone (GBL) and 1,4-butanediol (1,4-BD) in baboons. Psychopharmacology 2009, 204, 465–476. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).