Effects of a Maximal Exercise Followed by a Submaximal Exercise Performed in Normobaric Hypoxia (2500 m), on Blood Rheology, Red Blood Cell Senescence, and Coagulation in Well-Trained Cyclists
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Protocol
2.3. Maximal Incremental Test
2.4. Submaximal Exercise at VT1
2.5. Hemoglobin Saturation
2.6. Hematological Parameters, Fibrinogen, Lactate, and Glucose
2.7. Blood Rheological Parameters
2.7.1. Blood Viscosity and Hematocrit
2.7.2. Red Blood Cell Deformability
2.7.3. Red Blood Cell Aggregation
2.8. Red blood Cell Senescence Assessment
2.8.1. RBCs Preparation
2.8.2. Phosphatidylserine (PS) Exposure
2.8.3. Intracellular Reactive Oxygen Species (ROS)
2.8.4. Intracellular Calcium (Ca2+)
2.8.5. CD47 Exposure
2.9. Rotational Thromboelastometry
2.10. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. SpO2, Fibrinogen, Blood Lactate and Glucose Concentration, and Weight
3.3. Hematological Parameters
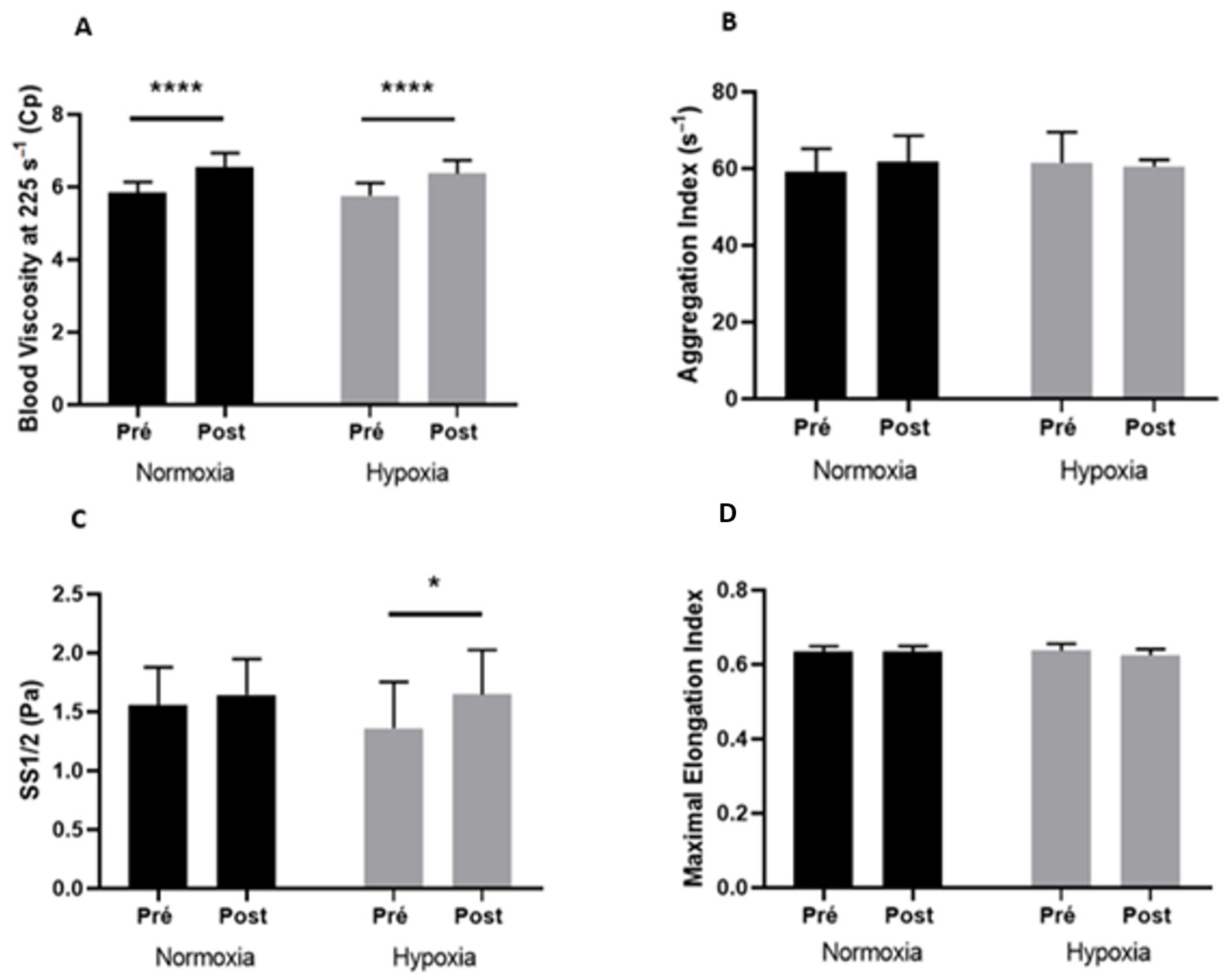
3.4. Blood Rheological Parameters
3.5. RBC Senescence
3.6. Coagulation Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Connes, P.; Suhr, F.; Martin, C.; Shin, S.; Aufradet, E.; Sunoo, S. New fundamental and applied mechanisms in exercisehemorheology. Clin. Hemorheol. Microcirc. 2010, 45, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Connes, P.; Simmonds, M.; Brun, J.; Baskurt, O. Exercise hemorheology: Classical data, recent findings and unresolved issues. Clin. Hemorheol. Microcirc. 2013, 53, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathi, K.; Lipowski, H.H. Capillary Recruitment in Response to Tissue Hypoxia and its Dependence on Red Blood Cell Deformability. 1999. Available online: https://journals.physiology.org/doi/epdf/10.1152/ajpheart.1999.277.6.H2145 (accessed on 28 April 2022).
- Waltz, X.; Hardy-Dessources, M.D.; Lemonne, N.; Mougenel, D.; Lalanne-Mistrih, M.L.; Lamarre, Y. Is there a relationship between the hematocrit-to-viscosity ratio and microvascular oxygenation in brain and muscle? Clin. Hemorheol. Microcirc. 2015, 59, 37–43. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Meiselman, H.J. Blood rheology and hemodynamics. Semin. Thromb. Hemost. 2003, 29, 435–450. [Google Scholar]
- Nader, E.; Guillot, N.; Lavorel, L.; Hancco, I.; Fort, R.; Stauffer, E. Eryptosis and hemorheological responses to maximal exercise in athletes: Comparison between running and cycling. Scand. J. Med. Sci. Sport. 2018, 28, 1532–1540. [Google Scholar] [CrossRef]
- Simmonds, M.J.; Connes, P.; Sabapathy, S. Exercise-Induced Blood Lactate Increase Does Not Change Red Blood Cell Deformability in Cyclists. PLoS ONE 2013, 8, e71219. [Google Scholar] [CrossRef] [PubMed]
- Brun, J.; Khaled, S.; Raynaud, E.; Bouix, D.; Jean-Paul, M.; Orsetti, A. The triphasic effects of exercise on blood rheology: Which relevance to physiology and pathophysiology? Clin. Hemorheol. Microcirc. 1998, 19, 89–104. [Google Scholar]
- Isbister, J.P. Blood Volume, Hematocrit and Hemorheology: The Interrelationships-IOS Press. 1994. Available online: https://content.iospress.com/articles/clinical-hemorheology-and-microcirculation/ch14-3-01 (accessed on 29 April 2022).
- Connes, P.; Bouix, D.; Durand, F.; Kippelen, P.; Mercier, J.; Préfaut, C. Is Hemoglobin Desaturation Related to Blood Viscosity in Athletes During Exercise? Int. J. Sport. Med. 2004, 25, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Brun, J.F.; Supparo, I.; Fons, C.; El Bouhmadi, A.; Orsetti, A. Low values of blood viscosity and erythrocyte aggregation are associated with lower increases in blood lactate during submaximal exercise. Clin. Hemorheol. Microcirc. 1994, 14, 105–116. [Google Scholar] [CrossRef]
- Brun, J.F.; Micallef, J.P.; Supparo, I.; Rama, D.; Benezis, C.; Orsetti, A. Maximal oxygen uptake and lactate thresholds during exercise are related to blood viscosity and erythrocyte aggregation in professional football players. Clin. Hemorheol. Microcirc. 1995, 15, 201–212. [Google Scholar] [CrossRef]
- Smith, J.A.; Telford, R.D.; Kolbuch-Braddon, M.; Weidemann, M.J. Lactate/H + uptake by red blood cells during exercise alters their physical properties. Eur. J. Appl. Physiol. 1996, 75, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Van Beaumont, W.; Underkofler, S.; van Beaumont, S. Erythrocyte volume, plasma volume, and acid-base changes in exercise and heat dehydration. J. Appl. Physiol. 1981, 50, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Baskurt, O.K.; Temiz, A.; Meiselman, H.J. Effect of Superoxide Anions on Red Blood Cell Rheologic Properties. Free Radic. Biol. Med. 1998, 24, 102–110. [Google Scholar] [CrossRef]
- Hierso, R.; Waltz, X.; Mora, P.; Romana, M.; Lemonne, N.; Connes, P. Effects of oxidative stress on red blood cell rheology in sickle cell patients. Br. J. Haematol. 2014, 166, 601–606. [Google Scholar] [CrossRef]
- Lang, D.F.; Lang, K.S.; Lang, P.A.; Huber, S.M.; Wieder, T. Mechanisms and Significance of Eryptosis. Antioxid. Redox Signal. 2006, 8, 1183–1192. [Google Scholar] [CrossRef]
- Lang, E.; Lang, F. Mechanisms and pathophysiological significance of eryptosis, the suicidal erythrocyte death. Semin. Cell Dev. Biol. 2015, 39, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Nader, E.; Monedero, D.; Robert, M.; Skinner, S.; Stauffer, E.; Cibiel, A.; Germain, M.; Hugonnet, J.; Scheer, A.; Joly, P.; et al. Impact of a 10 km running trial on eryptosis, red blood cell rheology, and electrophysiology in endurance trained athletes: A pilot study. Eur. J. Appl. Physiol. 2019, 120, 255–266. [Google Scholar] [CrossRef]
- Robert, M.; Stauffer, E.; Nader, E.; Skinner, S.; Boisson, C.; Cibiel, A.; Feasson, L.; Renoux, C.; Robach, P.; Joly, P.; et al. Impact of Trail Running Races on Blood Viscosity and Its Determinants: Effects of Distance. Int. J. Mol. Sci. 2020, 21, 8531. [Google Scholar] [CrossRef]
- Nader, E. Modulateurs de la Rhéologie Erythrocytaire et de L’éryptose Dans la Drépanocytose: Pierre Angulaire Entre Microparticules Erythrocytaires et Dysfonction Vasculaire? Ph.D. Thesis, Université de Lyon, Lyon, France, 2019. Available online: http://www.theses.fr/2019LYSE1236 (accessed on 4 May 2022).
- Kalafatis, M.; Swords, N.A.; Rand, M.D.; Mann, K.G. Membrane-dependent reactions in blood coagulation: Role of the vitamin K-dependent enzyme complexes. Biochim. Et Biophys. Acta BBA-Mol. Basis Dis. 1994, 1227, 113–129. [Google Scholar] [CrossRef]
- Sumann, G.; Fries, D.; Griesmacher, A.; Falkensammer, G.; Klingler, A.; Koller, A.; Streif, W.; Greie, S.; Schobersberger, B.; Schobersberger, W. Blood coagulation activation and fibrinolysis during a downhill marathon run. Blood Coagul. Fibrinolysis 2007, 18, 435–440. [Google Scholar] [CrossRef]
- Millet, G.; Schmitt, L. S’entraîner en Altitude: Mécanismes, Méthodes, Exemples, Conseils Pratiques; De Boeck: Brussel, Belgium, 2011. [Google Scholar]
- Dosek, A.; Ohno, H.; Acs, Z.; Taylor, A.; Radak, Z. High altitude and oxidative stress. Respir. Physiol. Neurobiol. 2007, 158, 128–131. [Google Scholar] [CrossRef]
- Pialoux, V.; Hanly, P.J.; Foster, G.E.; Brugniaux, J.V.; Beaudin, A.E.; Hartmann, S.E.; Pun, M.; Duggan, C.T.; Poulin, M.J. Effects of Exposure to Intermittent Hypoxia on Oxidative Stress and Acute Hypoxic Ventilatory Response in Humans. Am. J. Respir. Crit. Care Med. 2009, 180, 1002–1009. [Google Scholar] [CrossRef]
- Pialoux, V.; Mounier, R.; Ponsot, E.; Rock, E.; Mazur, A.; Dufour, S.P.; Richard, R.L.; Richalet, J.-P.; Coudert, J.D.; Fellmann, N. Effects of exercise and training in hypoxia on antioxidant/pro-oxidant balance. Eur. J. Clin. Nutr. 2006, 60, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Hughson, R.L.; Green, H.J.; Sharratt, M.T. Gas exchange, blood lactate, and plasma catecholamines during incremental exercise in hypoxia and normoxia. J. Appl. Physiol. 1995, 79, 1134–1141. [Google Scholar] [CrossRef]
- Płoszczyca, K.; Czuba, M.; Chalimoniuk, M.; Gajda, R.; Baranowski, M. Red Blood Cell 2,3-Diphosphoglycerate Decreases in Response to a 30 km Time Trial Under Hypoxia in Cyclists. Front. Physiol. 2021, 12, 670977. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Wang, R.; Chen, D.; Noviana, M.; Zhu, H. Nitric oxide inhibits hypoxia-induced impairment of human RBC deformability through reducing the cross-linking of membrane protein band 3. J. Cell Biochem. 2019, 120, 305–320. [Google Scholar] [CrossRef]
- Dias, G.F.; Tozoni, S.S.; Bohnen, G.; Grobe, N.; Rodrigues, S.D.; Meireles, T.; Nakao, L.S.; Pecoits-Filho, R.; Kotanko, P.; Moreno-Amaral, A.N. Uremia and Inadequate Oxygen Supply Induce Eryptosis and Intracellular Hypoxia in Red Blood Cells. Cell Physiol. Biochem. 2021, 55, 449–459. [Google Scholar]
- Tozoni, S.S.; Dias, G.F.; Bohnen, G.; Grobe, N.; Pecoits-Filho, R.; Kotanko, P. Uremia and Hypoxia Independently Induce Eryptosis and Erythrocyte Redox Imbalance|Cell Physiol Biochem. Cell Physiol. Biochem. 2019, 53, 794–804. [Google Scholar] [PubMed]
- Hinkelbein, J.; Jansen, S.; Iovino, I.; Kruse, S.; Meyer, M.; Cirillo, F.; Drinhaus, H.; Hohn, A.; Klein, C.; De Robertis, E.; et al. Thirty Minutes of Hypobaric Hypoxia Provokes Alterations of Immune Response, Haemostasis, and Metabolism Proteins in Human Serum. Int. J. Mol. Sci. 2017, 18, 1882. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Fahey, T.D.; Baldwin, K.M. Obesity, body composition, and exercise. In Exercise Physiology–Human Bioenergetics and Its Application, 4th ed.; McGraw Hill: New York, NY, USA, 2005; pp. 617–648. [Google Scholar]
- Wasserman, K.; Whipp, B.J.; Koyl, S.N.; Beaver, W.L. Anaerobic threshold and respiratory gas exchange during exercise. J. Appl. Physiol. 1973, 35, 236–243. [Google Scholar] [CrossRef]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. A new method for detecting anaerobic threshold by gas exchange. J. Appl. Physiol. 1986, 60, 2020–2027. [Google Scholar] [CrossRef]
- Rebuck, A.; Chapman, K.; D’Urzo, A. The Accuracy and Response Characteristics of a Simplified Ear Oximeter. Chest 1983, 83, 860–864. [Google Scholar] [CrossRef]
- Prefaut, C.; Durand, F.; Mucci, P.; Caillaud, C. Exercise-Induced Arterial Hypoxaemia in Athletes. Sport. Med. 2000, 30, 47–61. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Boynard, M.; Cokelet, G.C.; Connes, P.; Cooke, B.M.; Forconi, S.; Liao, F.; Hardeman, M.R.; Jung, F.; Meiselman, H.J.; et al. New guidelines for hemorheological laboratory techniques. Clin. Hemorheol. Microcirc. 2009, 23, 75–97. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Meiselman, H.J. Analyzing shear stress–elongation index curves: Comparison of two approaches to simplify data presentation. Clin. Hemorheol. Microcirc. 2004, 31, 23–30. [Google Scholar] [PubMed]
- Nader, E.; Skinner, S.; Romana, M.; Fort, R.; Lemonne, N.; Guillot, N.; Gauthier, A.; Antoine-Jonville, S.; Renoux, C.; Hardy-Dessources, M.-D.; et al. Blood Rheology: Key Parameters, Impact on Blood Flow, Role in Sickle Cell Disease and Effects of Exercise. Front. Physiol. 2019, 10, 1329. [Google Scholar] [CrossRef] [PubMed]
- Raberin, A.; Nader, E.; Ayerbe, J.L.; Mucci, P.; Pialoux, V.; Meric, H.; Connes, P.; Durand, F. Implication of Blood Rheology and Pulmonary Hemodynamics on Exercise-Induced Hypoxemia at Sea Level and Altitude in Athletes. Int. J. Sport Nutr. Exerc. Metab. 2021, 31, 397–405. [Google Scholar] [CrossRef]
- Salazar Vázquez, B.Y.; Martini, J.; Chávez Negrete, A.; Tsai, A.G.; Forconi, S.; Cabrales, P. Cardiovascular benefits in moderate increases of blood and plasma viscosity surpass those associated with lowering viscosity: Experimental and clinical evidence. Clin. Hemorheol. Microcirc. 2010, 44, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Salazar Vázquez, B.Y.; Cabrales, P.; Tsai, A.G.; Intaglietta, M. Nonlinear cardiovascular regulation consequent to changes in blood viscosity. Clin. Hemorheol. Microcirc. 2011, 49, 29–36. [Google Scholar] [CrossRef]
- Salazar Vázquez, B.Y.; Wettstein, R.; Cabrales, P.; Tsai, A.G.; Intaglietta, M. Microvascular experimental evidence on the relative significance of restoring oxygen carrying capacity vs. blood viscosity in shock resuscitation. Biochim. Biophys. Acta 2008, 1784, 1421–1427. [Google Scholar] [CrossRef]
- Connes, P.; Pichon, A.; Hardy-Dessources, M.-D.; Waltz, X.; Lamarre, Y.; Simmonds, M.J.; Tripette, J. Blood viscosity and hemodynamics during exercise. Clin. Hemorheol. Microcirc. 2012, 51, 101–109. [Google Scholar] [CrossRef]
- Chien, S. Red Cell Deformability and its Relevance to Blood Flow. Annu. Rev. Physiol. 1987, 49, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Késmárky, G.; Kenyeres, P.; Rabai, M.; Toth, K. Plasma viscosity: A forgotten variable. Clin. Hemorheol. Microcirc. 2008, 39, 243–246. [Google Scholar] [CrossRef]
- Brust, M.; Aouane, O.; Thiébaud, M.; Flormann, D.; Verdier, C.; Kaestner, L.; Laschke, M.W.; Selmi, H.; Benyoussef, A.; Podgorski, T.; et al. The plasma protein fibrinogen stabilizes clusters of red blood cells in microcapillary flows. Sci. Rep. 2014, 4, 4348. [Google Scholar] [CrossRef] [PubMed]
- Tripette, J.; Hardy-Dessources, M.-D.; Beltan, E.; Sanouiller, A.; Bangou, J.; Chalabi, T.; Chout, R.; Hedreville, M.; Broquere, C.; Nebor, D.; et al. Blood rheological responses to running and cycling: A potential effect on the arterial hypoxemia of highly trained triathletes? Int. J. Sport. Med. 2005, 26, 15. [Google Scholar]
- Tripette, J.; Hardy-Dessources, M.D.; Beltan, E.; Sanouiller, A.; Bangou, J.; Chalabi, T. Endurance running trial in tropical environment: A blood rheological study. Clin. Hemorheol. Microcirc. 2011, 47, 261–268. [Google Scholar] [CrossRef]
- Chou, S.-L.; Huang, Y.-C.; Fu, T.-C.; Hsu, C.-C.; Wang, J.-S. Cycling Exercise Training Alleviates Hypoxia-Impaired Erythrocyte Rheology. Med. Sci. Sport Exerc. 2016, 48, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.R.; Mohandas, N.; Shohet, S.B. Osmotic Gradient Ektacytometry: Comprehensive Characterization of Red Cell Volume and Surface Maintenance. Blood 1983, 61, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Renoux, C.; Faivre, M.; Bessaa, A.; Da Costa, L.; Joly, P.; Gauthier, A.; Connes, P. Impact of surface-area-to-volume ratio, internal viscosity and membrane viscoelasticity on red blood cell deformability measured in isotonic condition. Sci. Rep. 2019, 9, 6771. [Google Scholar] [CrossRef]
- Simmonds, M.J.; Tripette, J.; Sabapathy, S.; Marshall-Gradisnik, S.M.; Connes, P. Cardiovascular dynamics during exercise are related to blood rheology. Clin. Hemorheol. Microcirc. 2011, 49, 231–241. [Google Scholar] [CrossRef]
- Lipowsky, H.; Cram, L.; Justice, W.; Eppihimer, M. Effect of erythrocyte deformability on in vivo red cell transit time and hematocrit and their correlation with in vitro filterability. Microvasc. Res. 1993, 46, 43–64. [Google Scholar] [CrossRef]
- Driessen, G.K.; Haest, C.W.M.; Heidtmann, H.; Kamp, D.; Schmid-Schönbein, H. Effect of reduced red cell ‘deformability’ on flow velocity in capillaries of rat mesentery. Pflugers Arch. 1980, 388, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Brugnara, C.; de Franceschi, L.; Alper, S.L. Inhibition of Ca(2+)-dependent K+ transport and cell dehydration in sickle erythrocytes by clotrimazole and other imidazole derivatives. J. Clin. Investig. 1993, 92, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Rock, G.; Tittley, P.; Pipe, A. Coagulation factor changes following endurance exercise. Clin. J. Sport Med. 1997, 7, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; El-Sayed, M.S.; Waterhouse, J.; Reilly, T. Activation and Disturbance of Blood Haemostasis Following Strenuous Physical Exercise. Int. J. Sports Med. 1999, 20, 149–153. [Google Scholar] [CrossRef]
- Dickson, B.C. Virchow’s triad. Br. J. Haematol. 2009, 145, 433. [Google Scholar] [CrossRef]

| Normoxia | Hypoxia | ||
|---|---|---|---|
| VO2max (mL/min/kg) | 65.5 ± 9.4 | 56.1 ± 4.7 | * |
| MAP (W) | 408.0 ± 40.5 | 363.8 ± 37.4 | ** |
| VO2 at VT1 (mL/min/kg) | 51.2 ± 9.9 | 40.6 ± 7.3 | * |
| Power at VT1 (W) | 297.0 ± 35.9 | 228.8 ± 22.3 | ** |
| VT1 (% of VO2max) | 77.7 ± 7.8 | 72.1 ± 7.7 | ns |
| Normoxia | Hypoxia | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Fibrinogen (g/L) | 2.5 ± 0.4 | 2.6 ± 0.4 | 2.4 ± 0.2 | 2.6 ± 0.4 |
| Blood Lactate (mM) | 2.2 ± 0.7 | 5.3 ± 1.4 ** | 1.7 ± 0.3 | 5.6 ± 2.0 ** |
| Blood Glucose (g/L) | 5.2 ± 0.5 | 6.0 ± 1.2 | 4.9 ± 0.5 | 5.4 ± 0.4 |
| Weight (kg) | 66.2 ± 7.6 | 65.1 ± 7.3 **** | 66.2 ± 7.5 | 65.2 ± 7.4 **** |
| Normoxia | Hypoxia | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Hct (%) | 44.9 ± 1.8 | 46.6 ± 1.7 * | 45,4 ± 2,3 | 46.6 ± 1.9 * |
| RBC (1012/L) | 4.4 ± 0.2 | 4.6 ± 0.2 ** | 4.5 ± 0.2 | 4.6 ± 0.2 ** |
| Hb (g/L) | 133.3 ± 5.1 | 139.4 ± 5.5 ** | 135.7 ± 5.9 | 140.3 ± 5.6 ** |
| WBC (109/L) | 4.5 ± 0.7 | 5.8 ± 0.9 ** | 4.8 ± 1.6 | 6.5 ± 2.1 ** |
| PLT (109/L) | 146.6 ± 28.8 | 180.5 ± 53.7 ** | 158.3 ± 24.9 | 204.1 ± 35.2 ** |
| MCV (fL) | 88.4 ± 2.3 | 88.0 ± 2.4 | 89.3 ± 1.7 | 88.6 ± 2.8 |
| MCH (pg) | 30.2 ± 1.0 | 30.0 ± 0.8 | 30.3 ± 0.6 | 30.4 ± 0.9 |
| MCHC (g/L) | 342.1 ± 10.5 | 340.1 ± 8.5 | 339.3 ± 7.3 | 343.1 ± 7.8 ** |
| Normoxia | Hypoxia | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| NATEM Mode | ||||
| CT (s) | 385.0 ± 88.9 | 347.8 ± 68.1 | 385.0 ± 59.5 | 344.8 ± 63.9 |
| A5 (mm) | 33.67 ± 4.8 | 34.11 ± 4.8 | 34.78 ± 2.9 | 34.67 ± 3.9 |
| A10 (mm) | 45.22 ± 4.6 | 46.56 ± 4.9 | 46.67 ± 2.6 | 47.33 ± 3.1 |
| A20 (mm) | 52.11 ± 4.5 | 53.89 ± 4.9 * | 53.78 ± 2.7 | 55.0 ± 3.3 * |
| A30 (mm) | 52.44 ± 4.6 | 54.33 ± 5.1 ** | 54.11 ± 3.3 | 55.67 ± 3.6 ** |
| α (°) | 62.22 ± 5.3 | 62.11 ± 5.7 | 63.56 ± 4.6 | 62.11 ± 5.6 |
| CFT (s) | 146.3 ± 34.0 | 149.7 ± 42.0 | 137.1 ± 26.3 | 147.8 ± 37.5 |
| MCF (mm) | 52.78 ± 4.6 | 54.56 ± 4.9 ** | 54.22 ± 2.7 | 55.89 ± 3.3 ** |
| EXTEM Mode | ||||
| CT (s) | 65.50 ± 11.0 | 68.88 ± 8.0 | 67.75 ± 6.0 | 67.75 ± 9.1 |
| A5 (mm) | 36.63 ± 5.5 | 38.50 ± 6.2 | 38.25 ± 4.2 | 38.88 ± 4.5 |
| A10 (mm) | 46.50 ± 5.4 | 48.63 ± 5.3 * | 48.13 ± 4.2 | 49.13 ± 4.6 * |
| A20 (mm) | 53.38 ± 5.4 | 55.75 ± 4.9 ** | 54.50 ± 4.2 | 56.25 ± 4.4 ** |
| A30 (mm) | 54.79 ± 5.2 | 57.04 ± 5.0 ** | 54.88 ± 4.2 | 57.25 ± 4.6 ** |
| α (°) | 67.63 ± 5.7 | 67.88 ± 5.2 | 68.88 ± 3.0 | 68.25 ± 3.1 |
| CFT (s) | 122.1 ± 30.0 | 113.0 ± 31.4 | 106.6 ± 16.1 | 109.3 ± 16.4 |
| MCF (mm) | 54.50 ± 5.0 | 56.63 ± 4.7 | 57.13 ± 5.2 | 57.38 ± 4.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carin, R.; Deglicourt, G.; Rezigue, H.; Martin, M.; Nougier, C.; Boisson, C.; Dargaud, Y.; Joly, P.; Renoux, C.; Connes, P.; et al. Effects of a Maximal Exercise Followed by a Submaximal Exercise Performed in Normobaric Hypoxia (2500 m), on Blood Rheology, Red Blood Cell Senescence, and Coagulation in Well-Trained Cyclists. Metabolites 2023, 13, 179. https://doi.org/10.3390/metabo13020179
Carin R, Deglicourt G, Rezigue H, Martin M, Nougier C, Boisson C, Dargaud Y, Joly P, Renoux C, Connes P, et al. Effects of a Maximal Exercise Followed by a Submaximal Exercise Performed in Normobaric Hypoxia (2500 m), on Blood Rheology, Red Blood Cell Senescence, and Coagulation in Well-Trained Cyclists. Metabolites. 2023; 13(2):179. https://doi.org/10.3390/metabo13020179
Chicago/Turabian StyleCarin, Romain, Gabriel Deglicourt, Hamdi Rezigue, Marie Martin, Christophe Nougier, Camille Boisson, Yesim Dargaud, Philippe Joly, Céline Renoux, Philippe Connes, and et al. 2023. "Effects of a Maximal Exercise Followed by a Submaximal Exercise Performed in Normobaric Hypoxia (2500 m), on Blood Rheology, Red Blood Cell Senescence, and Coagulation in Well-Trained Cyclists" Metabolites 13, no. 2: 179. https://doi.org/10.3390/metabo13020179
APA StyleCarin, R., Deglicourt, G., Rezigue, H., Martin, M., Nougier, C., Boisson, C., Dargaud, Y., Joly, P., Renoux, C., Connes, P., Stauffer, E., & Nader, E. (2023). Effects of a Maximal Exercise Followed by a Submaximal Exercise Performed in Normobaric Hypoxia (2500 m), on Blood Rheology, Red Blood Cell Senescence, and Coagulation in Well-Trained Cyclists. Metabolites, 13(2), 179. https://doi.org/10.3390/metabo13020179








