Effect of Maturity Stage on Cereal and Leguminous Seeds’ Metabolome as Analyzed Using Gas Chromatography Mass-Spectrometry (GC-MS) and Chemometric Tools
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Samples
2.2. Gas Chromatography-Mass Spectrometry (GC-MS) Primary Metabolite Profiling
2.2.1. Samples’ Preparation and GC-MS Analysis Post Silylation
2.2.2. Identification of Metabolites and Multivariate Data Analysis of GC-MS Dataset
2.3. Total Protein Content
3. Results
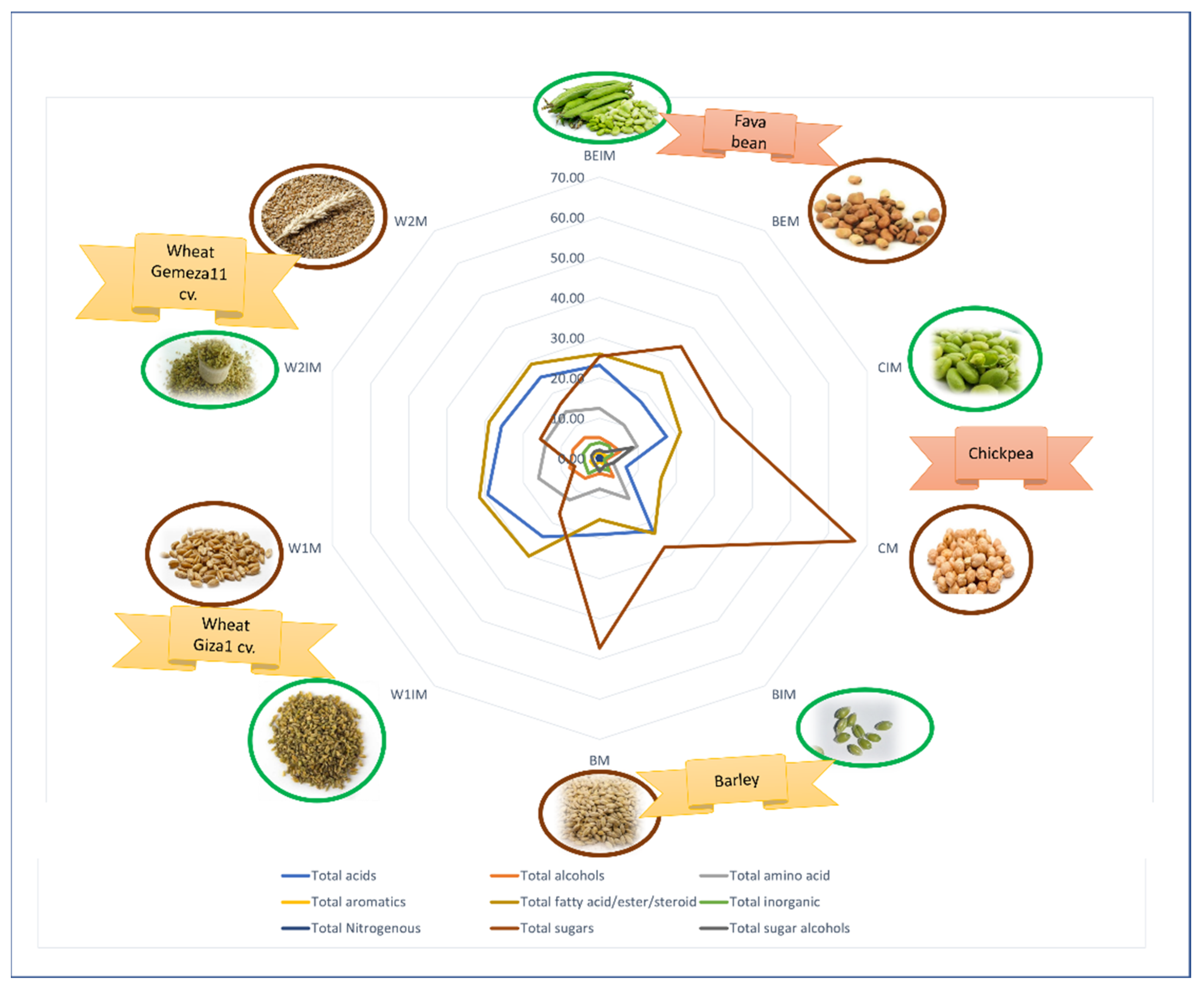
3.1. Primary Metabolite Profiling viz. Sugars, Fatty and Organic Acids via GC-MS Post Silylation
3.1.1. Sugars and Sugar Alcohols
3.1.2. Fatty Acids/Esters and Steroids
3.1.3. Organic Acids
3.1.4. Amino Acids
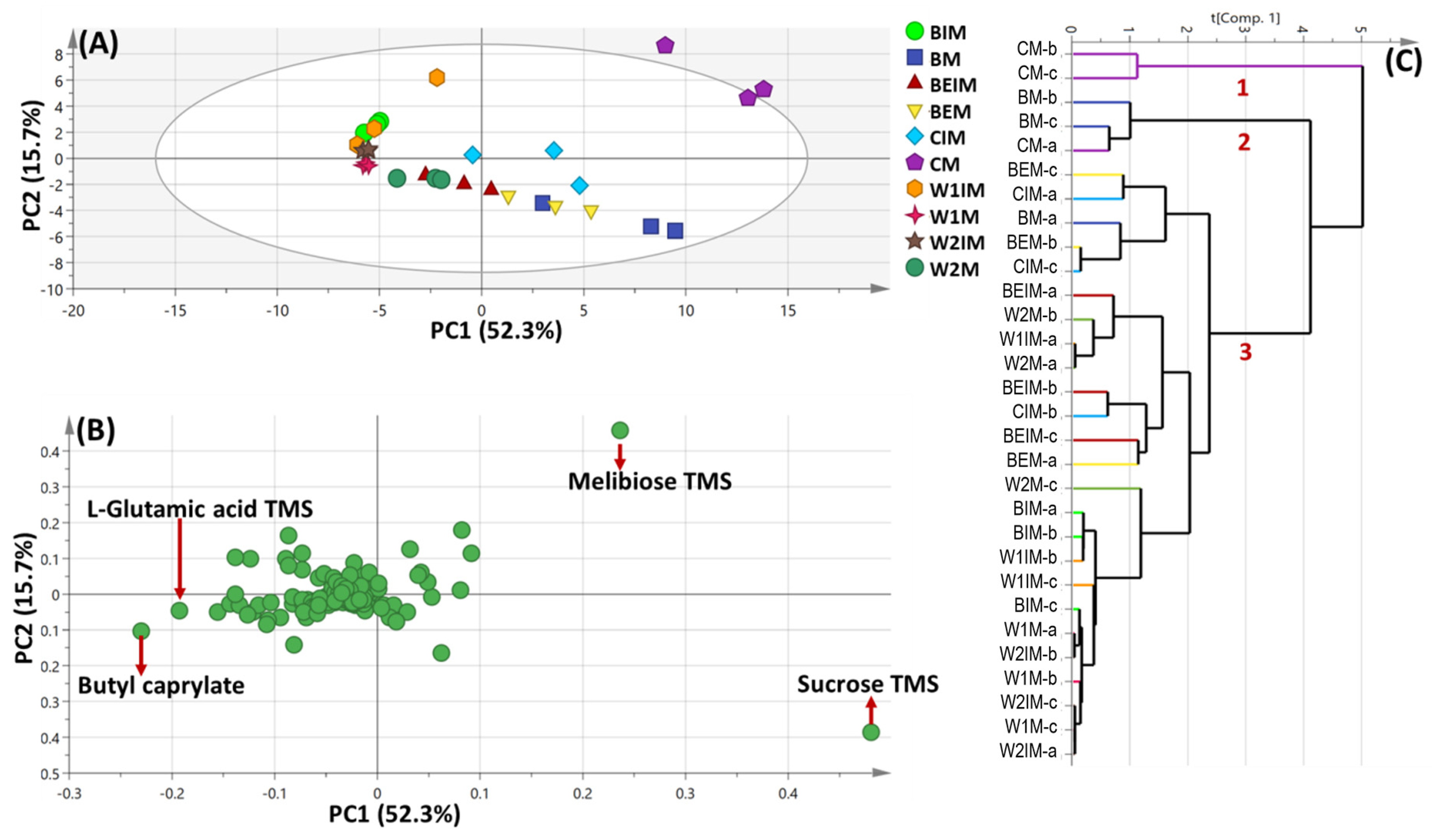
3.2. PCA and HCA Multivariate Data Analyses of Primary Silylated Metabolites of Mature and Immature Seeds of Different Cultivars
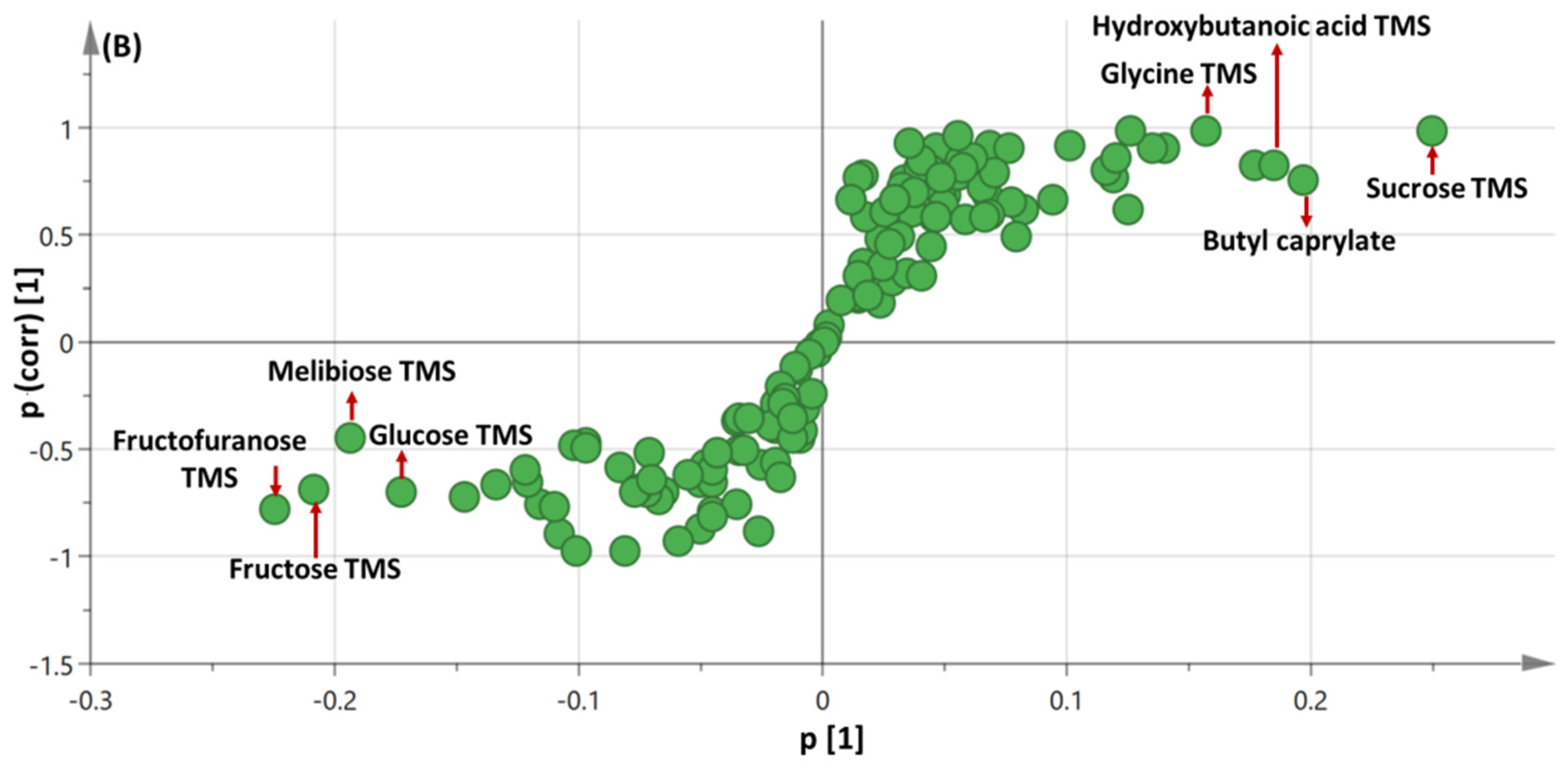
3.3. OPLS-DA Analysis of Immature versus Mature Seeds Primary Silylated Metabolites in All Seed Specimens Dataset
3.4. Multivariate Data Analyses of the Primary Silylated Metabolites in Cereals (Wheat and Barley) Models
3.4.1. PCA and OPLS Multivariate Data Analyses of the Primary Silylated Metabolites in Barley Specimens
3.4.2. OPLS Multivariate Data Analyses of the Primary Silylated Metabolites in Mature vs. Immature Wheat Specimens
OPLS Multivariate Data Analysis of the Primary Silylated Metabolites in Mature vs. Immature Triticum aestivum (cv. Giza)
OPLS Multivariate Data Analysis of the Primary Silylated Metabolites in Mature vs. Immature T. aestivum (cv. Gemeza 11)
3.5. Multivariate Data Analyses of The Primary Silylated Metabolites in Legumes Models
3.5.1. PCA and OPLS Multivariate Data Analyses of the Primary Silylated Metabolites in Chickpea Specimens
3.5.2. OPLS Multivariate Data Analyses of the Primary Silylated Metabolites in Mature vs. Immature Bean Specimens
3.6. Seeds Total Protein Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erenstein, O.; Poole, N.; Donovan, J. Role of Staple Cereals in Human Nutrition: Separating the Wheat from the Chaff in the Infodemics Age. Trends Food Sci. Technol. 2022, 119, 508–513. [Google Scholar] [CrossRef]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted Grains: A Comprehensive Review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Saa, D.L.T.; Nissen, L.; Gianotti, A. Metabolomic Approach to Study the Impact of Flour Type and Fermentation Process on Volatile Profile of Bakery Products. Food Res. Int. 2019, 119, 510–516. [Google Scholar] [CrossRef]
- Vongsak, B.; Kongkiatpaiboon, S.; Jaisamut, S.; Konsap, K. Comparison of Active Constituents, Antioxidant Capacity, and α-Glucosidase Inhibition in Pluchea Indica Leaf Extracts at Different Maturity Stages. Food Biosci. 2018, 25, 68–73. [Google Scholar] [CrossRef]
- Farag, M.A.; El Senousy, A.S.; El-Ahmady, S.H.; Porzel, A.; Wessjohann, L.A. Comparative Metabolome-Based Classification of Senna Drugs: A Prospect for Phyto-Equivalency of Its Different Commercial Products. Metabolomics 2019, 15, 80. [Google Scholar] [CrossRef]
- Suo, J.; Ma, Z.; Zhao, B.; Ma, S.; Zhang, Z.; Hu, Y.; Yang, B.; Yu, W.; Wu, J.; Song, L. Metabolomics Reveal Changes in Flavor Quality and Bioactive Components in Post-Ripening Torreya Grandis Nuts and the Underlying Mechanism. Food Chem. 2023, 406, 134987. [Google Scholar] [CrossRef]
- Medendorp, J.; DeYoung, D.; Thiagarajan, D.G.; Duckworth, R.; Pittendrigh, B. A Systems Perspective of the Role of Dry Beans and Pulses in the Future of Global Food Security: Opportunities and Challenges. In Dry Beans and Pulses: Production, Processing, and Nutrition; Wiley Online Library: New York, NY, USA, 2022; pp. 531–550. [Google Scholar] [CrossRef]
- Merga, B.; Haji, J. Economic Importance of Chickpea: Production, Value, and World Trade. Cogent Food Agric. 2019, 5, 1615718. [Google Scholar] [CrossRef]
- Bueno, P.C.D.S.; Barbalho, S.M.; Guiguer, É.L.; Souza, M.D.S.S.D.; Medeiros, I.R.A.D.; Zattiti, I.V.; Bueno, M.D.S.; Nutels, G.S.; Goulart, R.D.A.; Araújo, A.C. Effects of Green Wheat (Triticum turgidum) and Common Wheat (Triticum aestivum) on the Metabolic Profile of Wistar Rats. J. Med. Food 2019, 22, 1222–1225. [Google Scholar] [CrossRef]
- Călinoiu, L.F.; Vodnar, D.C. Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef]
- Swarnalakshmi, K.; Yadav, V.; Tyagi, D.; Dhar, D.W.; Kannepalli, A.; Kumar, S. Significance of Plant Growth Promoting Rhizobacteria in Grain Legumes: Growth Promotion and Crop Production. Plants 2020, 9, 1596. [Google Scholar] [CrossRef]
- Pasqualone, A.; Abdallah, A.; Summo, C. Symbolic Meaning and Use of Broad Beans in Traditional Foods of the Mediterranean Basin and the Middle East. J. Ethn. Foods 2020, 7, 1–13. [Google Scholar] [CrossRef]
- Xie, Z.L.; Zhang, T.F.; Chen, X.Z.; Li, G.D.; Zhang, J.G. Effects of Maturity Stages on the Nutritive Composition and Silage Quality of Whole Crop Wheat. Asian-Australas. J. Anim. Sci. 2012, 25, 1374. [Google Scholar] [CrossRef] [PubMed]
- Mefleh, M. Cereals of the Mediterranean Region: Their Origin, Breeding History and Grain Quality Traits. In Cereal-Based Foodstuffs: The Backbone of Mediterranean Cuisine; Boukid, F., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–18. ISBN 978-3-030-69228-5. [Google Scholar]
- Sarhan, M.G.; El-Hamed, A. Effect of Potassium Application Methods on some Wheat Varieties (Triticum aestivum L.) Production under Middle Egypt Conditions. Alex. J. Soil Water Sci. 2018, 2, 48–65. [Google Scholar] [CrossRef]
- Özdemir, E.; Topal, A.; Çakir, İ. The Effects of Different Harvest Periods to Bio-Active Compounds in Wheat. Mediterr. Agric. Sci. 2022, 35, 33–38. [Google Scholar] [CrossRef]
- Iametti, S.; Bonomi, F.; Pagani, M.A.; Zardi, M.; Cecchini, C.; D’Egidio, M.G. Properties of the Protein and Carbohydrate Fractions in Immature Wheat Kernels. J. Agric. Food Chem. 2006, 54, 10239–10244. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, S.S. Utilisation of Immature Wheat Flour as an Alternative Flour with Antioxidant Activity and Consumer Perception on Its Baked Product. Food Chem. 2017, 232, 237–244. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, S.S. Antioxidant and Antiproliferative Activities in Immature and Mature Wheat Kernels. Food Chem. 2016, 196, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Ranathunga, R.A.A.; Suwannaporn, P. Young Cereal Grains as a New Source of Healthy and Hypoallergenic Foods: A Review. J. Food Sci. Technol. 2022, 59, 3336–3348. [Google Scholar] [CrossRef]
- Farag, M.A.; Xiao, J.; Abdallah, H.M. Nutritional Value of Barley Cereal and Better Opportunities for Its Processing as a Value-Added Food: A Comprehensive Review. Crit Rev. Food Sci. Nutr. 2022, 62, 1092–1104. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, M.J.; Ryu, H.W.; Yuk, H.J.; Choi, S.-W.; Lee, K.-S.; Kim, S.-L.; Seo, W.D. Elucidation of Phenolic Antioxidants in Barley Seedlings (Hordeum vulgare L.) by UPLC-PDA-ESI/MS and Screening for Their Contents at Different Harvest Times. J. Funct. Foods 2016, 26, 667–680. [Google Scholar] [CrossRef]
- Rahate, K.A.; Madhumita, M.; Prabhakar, P.K. Nutritional Composition, Anti-Nutritional Factors, Pretreatments-Cum-Processing Impact and Food Formulation Potential of Faba Bean (Vicia faba L.): A Comprehensive Review. LWT 2021, 138, 110796. [Google Scholar] [CrossRef]
- Boukhanouf, S.; Louaileche, H.; Perrin, D. Phytochemical Content and in Vitro Antioxidant Activity of Faba Bean (Vicia faba L.) as Affected by Maturity Stage and Cooking Practice. Int. Food Res. J. 2016, 23, 954–961. [Google Scholar]
- Lu, Y.; Tian, C.; Gao, C.; Wang, B.; Yang, W.; Kong, X.; Chai, L.; Chen, G.; Yin, X.; He, Y. Phenolic Composition, Antioxidant Capacity and Inhibitory Effects on α-Glucosidase and Lipase of Immature Faba Bean Seeds. Int. J. Food Prop. 2018, 21, 2366–2377. [Google Scholar] [CrossRef]
- Segev, A.; Badani, H.; Galili, L.; Hovav, R.; Kapulnik, Y.; Shomer, I.; Galili, S. Total Phenolic Content and Antioxidant Activity of Chickpea (Cicer arietinum L.) as Affected by Soaking and Cooking Conditions. Food Nutr. Sci. 2011, 2, 724–730. [Google Scholar] [CrossRef]
- Millar, K.A.; Gallagher, E.; Burke, R.; McCarthy, S.; Barry-Ryan, C. Proximate Composition and Anti-Nutritional Factors of Fava-Bean (Vicia Faba), Green-Pea and Yellow-Pea (Pisum Sativum) Flour. J. Food Compos. Anal. 2019, 82, 103233. [Google Scholar] [CrossRef]
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.L.L.; Chibbar, R.N. Nutritional Quality and Health Benefits of Chickpea (Cicer arietinum L.): A Review. Br. J. Nutr. 2012, 108, S11–S26. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, N.S.; Wessjohann, L.A.; Mocan, A.; Vodnar, D.C.; ElSayed, N.H.; ElToumy, S.A.; Mohamed, D.A.; Aziz, Z.A.; Ehrlich, A.; Farag, M.A. Nutrient and Sensory Metabolites Profiling of Averrhoa carambola L. (Starfruit) in the Context of Its Origin and Ripening Stage by GC/MS and Chemometric Analysis. Molecules 2020, 25, 2423. [Google Scholar] [CrossRef]
- Farag, M.A.; El-Kersh, D.M.; Rasheed, D.M.; Heiss, A.G. Volatiles Distribution in Nigella Species (Black cumin Seeds) and in Response to Roasting as Analyzed via Solid-Phase Microextraction (SPME) Coupled to Chemometrics. Ind. Crops Prod. 2017, 108, 564–571. [Google Scholar] [CrossRef]
- Farag, M.A.; Mohsen, M.; Heinke, R.; Wessjohann, L.A. Metabolomic Fingerprints of 21 Date Palm Fruit Varieties from Egypt Using UPLC/PDA/ESI-QTOF-MS and GC-MS Analyzed by Chemometrics. Food Res. Int. 2014, 64, 218–226. [Google Scholar] [CrossRef]
- Sáez-Plaza, P.; Navas, M.J.; Wybraniec, S.; Michałowski, T.; Asuero, A.G. An Overview of the Kjeldahl Method of Nitrogen Determination. Part II: Sample Preparation, Working Scale, Instrumental Finish, and Quality Control. Crit. Rev. Anal. Chem. 2013, 43, 224–272. [Google Scholar] [CrossRef]
- ElMasry, G.; Mandour, N.; Ejeez, Y.; Demilly, D.; Al-Rejaie, S.; Verdier, J.; Belin, E.; Rousseau, D. Multichannel Imaging for Monitoring Chemical Composition and Germination Capacity of Cowpea (Vigna unguiculata) Seeds during Development and Maturation. Crop J. 2021, 10, 1399–1411. [Google Scholar] [CrossRef]
- Chen, T.; Li, G.; Islam, M.R.; Fu, W.; Feng, B.; Tao, L.; Fu, G. Abscisic Acid Synergizes with Sucrose to Enhance Grain Yield and Quality of Rice by Improving the Source-Sink Relationship. BMC Plant Biol. 2019, 19, 525. [Google Scholar] [CrossRef] [PubMed]
- Cheema, A.; Garg, N. Differential Effectiveness of Arbuscular Mycorrhizae in Improving Rhizobial Symbiosis by Modulating Sucrose Metabolism and Antioxidant Defense in Chickpea under As Stress. Symbiosis 2022, 86, 49–69. [Google Scholar] [CrossRef]
- Li, Y.; Yu, G.; Lv, Y.; Long, T.; Li, P.; Hu, Y.; Liu, H.; Zhang, J.; Liu, Y.; Li, W.-C.; et al. Combinatorial Interaction of Two Adjacent Cis-Active Promoter Regions Mediates the Synergistic Induction of Bt2 Gene by Sucrose and ABA in Maize Endosperm. Plant Sci. 2018, 274, 332–340. [Google Scholar] [CrossRef]
- Farag, M.A.; Khattab, A.R.; Ehrlich, A.; Kropf, M.; Heiss, A.G.; Wessjohann, L.A. Gas Chromatography/Mass Spectrometry-Based Metabolite Profiling of Nutrients and Antinutrients in Eight Lens and Lupinus Seeds (Fabaceae). J. Agric. Food Chem. 2018, 66, 4267–4280. [Google Scholar] [CrossRef]
- Christou, C.; Poulli, E.; Yiannopoulos, S.; Agapiou, A. GC–MS Analysis of D-Pinitol in Carob: Syrup and Fruit (Flesh and Seed). J. Chromatogr. B 2019, 1116, 60–64. [Google Scholar] [CrossRef]
- Nematbakhsh, M. Re: D-Pinitol Attenuates Cisplatin-Induced Nephrotoxicity in Rats: Impact on Proinflammatory Cytokines. Chem. Biol. Interact. 2018, 296, 43–44. [Google Scholar] [CrossRef]
- Ibrahim, N.; Taleb, M.; Heiss, A.G.; Kropf, M.; Farag, M.A. GC-MS Based Metabolites Profiling of Nutrients and Anti-Nutrients in 10 Lathyrus Seed Genotypes: A Prospect for Phyto-Equivalency and Chemotaxonomy. Food Biosci. 2021, 42, 101183. [Google Scholar] [CrossRef]
- Sose, M.T.; Rathod, V.K. Ultrasound Assisted Enzyme Catalysed Synthesis of Butyl Caprylate in Solvent Free System. Indian Chem. Eng. 2021, 63, 402–413. [Google Scholar] [CrossRef]
- van Rooijen, M.A.; Mensink, R.P. Palmitic Acid versus Stearic Acid: Effects of Interesterification and Intakes on Cardiometabolic Risk Markers—A Systematic Review. Nutrients 2020, 12, 615. [Google Scholar] [CrossRef]
- Fatima, S.; Hu, X.; Gong, R.-H.; Huang, C.; Chen, M.; Wong, H.L.X.; Bian, Z.; Kwan, H.Y. Palmitic Acid Is an Intracellular Signaling Molecule Involved in Disease Development. Cell. Mol. Life Sci. 2019, 76, 2547–2557. [Google Scholar] [CrossRef] [PubMed]
- Urugo, M.M.; Teka, T.A.; Teshome, P.G.; Tringo, T.T. Palm Oil Processing and Controversies over Its Health Effect: Overview of Positive and Negative Consequences. J. Oleo Sci. 2021, 70, 1693–1706. [Google Scholar] [CrossRef] [PubMed]
- Hozzein, W.N.; Abuelsoud, W.; Wadaan, M.A.M.; Shuikan, A.M.; Selim, S.; Al Jaouni, S.; AbdElgawad, H. Exploring the Potential of Actinomycetes in Improving Soil Fertility and Grain Quality of Economically Important Cereals. Sci. Total Environ. 2019, 651, 2787–2798. [Google Scholar] [CrossRef] [PubMed]
- Moghei, M.; Tavajohi-Fini, P.; Beatty, B.; Adegoke, O.A.J. Ketoisocaproic Acid, a Metabolite of Leucine, Suppresses Insulin-Stimulated Glucose Transport in Skeletal Muscle Cells in a BCAT2-Dependent Manner. Am. J. Physiol.-Cell Physiol. 2016, 311, C518–C527. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Khattab, A.R.; Shamma, S.; Afifi, S.M. Profiling of Primary Metabolites and Volatile Determinants in Mahlab Cherry (Prunus mahaleb L.) Seeds in the Context of Its Different Varieties and Roasting as Analyzed Using Chemometric Tools. Foods 2021, 10, 728. [Google Scholar] [CrossRef]
- Molfetta, M.; Morais, E.G.; Barreira, L.; Bruno, G.L.; Porcelli, F.; Dugat-Bony, E.; Bonnarme, P.; Minervini, F. Protein Sources Alternative to Meat: State of the Art and Involvement of Fermentation. Foods 2022, 11, 2065. [Google Scholar] [CrossRef]
- Rustgi, S.; Shewry, P.; Brouns, F.; Deleu, L.J.; Delcour, J.A. Wheat Seed Proteins: Factors Influencing Their Content, Composition, and Technological Properties, and Strategies to Reduce Adverse Reactions. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1751–1769. [Google Scholar] [CrossRef]
- Kumari, P.V.; Sangeetha, N. Nutritional Significance of Cereals and Legumes Based Food Mix-A Review. Int. J. Agric. Life Sci. 2017, 3, 115–122. [Google Scholar]




| Name | Species | Family | Cultivar (cv.) | Source | Maturity Stage | Code |
|---|---|---|---|---|---|---|
| Wheat | Triticum aestivum L. | Poaceae | Giza | El Qanater ElKhayreya, Egypt | Immature | W1IM |
| Mature | W1M | |||||
| Gemeza 11 | Immature | W2IM | ||||
| Mature | W2M | |||||
| Barley | Hordeum vulgare L. | Giza 3 | Agricultural Experiment and Research Station, Faculty of Agriculture, Cairo University | Immature | BIM | |
| Mature | BM | |||||
| Bean | Vicia faba L. | Fabaceae | Sakha 3 | Agricultural Research station, Itay El-Barud, Egypt | Immature | BEIM |
| Mature | BEM | |||||
| Chickpea | Cicer arietinum L. | Giza 1 | Immature | CIM | ||
| Mature | CM |
| Peak No. | Rt (min) | KI | Name | Barley | Bean | Chickpea | Wheat (cv. Giza) | Wheat (cv. Gemeza) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IM | M | IM | M | IM | M | IM | M | IM | M | ||||
| Organic Acids | |||||||||||||
| 1 | 6.74 | 1048 | Lactic Acid (2 TMS) | 1.55 ± 1.01 | 0.86 ± 0.30 | 0.89 ± 0.10 | 1.80 ± 0.66 | 0.79 ± 0.29 | 0.37 ± 0.02 | 1.16 ± 0.41 | 1.63 ± 0.22 | 2.06 ± 0.88 | 1.34 ± 0.31 |
| 6 | 7.01 | 1072 | Glycolic acid (2 TMS) | 0.35 ± 0.04 | 0.21 ± 0.09 | 0.35 ± 0.06 | 0.26 ± 0.05 | 0.26 ± 0.07 | 0.09 ± 0.01 | 0.36 ± 0.04 | 0.41 ± 0.06 | 0.37 ± 0.01 | 0.38 ± 0.06 |
| 7 | 7.50 | 1110 | Oxalic acid (2 TMS) | 0.52 ± 0.24 | 0.29 ± 0.09 | 0.72 ± 0.17 | 0.40 ± 0.18 | 0.36 ± 0.08 | 0.20 ± 0.04 | 0.54 ± 0.26 | 0.51 ± 0.03 | 0.42 ± 0.02 | 0.57 ± 0.18 |
| 8 | 8.2 | 1150 | Hydracrylic acid (2 TMS) | 0.23 ± 0.02 | 0.14 ± 0.03 | 0.24 ± 0.02 | 0.20 ± 0.03 | 0.19 ± 0.04 | 0.06 ± 0.01 | 0.25 ± 0.03 | 0.32 ± 0.02 | 0.26 ± 0.03 | 0.26 ± 0.03 |
| 10 | 9.74 | 1241 | Hydroxybutanoic acid (2 TMS) | 4.12 ± 0.46 | 2.47 ± 0.83 | 4.53 ± 0.21 | 3.10 ± 0.49 | 3.45 ± 1.09 | 1.11 ± 0.13 | 4.51 ± 0.79 | 6.18 ± 0.07 | 4.81 ± 0.33 | 4.92 ± 0.37 |
| 11 | 9.78 | 1245 | Hydroxybutanoic acid isomer (2 TMS) | 3.25 ± 2.31 | 2.11 ± 1.64 | 4.89 ± 0.27 | 0.73 ± 0.11 | 2.85 ± 2.27 | 0.85 ± 0.64 | 4.74 ± 0.85 | 6.54 ± 0.02 | 3.78 ± 2.39 | 5.26 ± 0.41 |
| 13 | 10.19 | 1270 | Octanoic acid (2 TMS) | 3.61 ± 0.87 | 2.24 ± 0.65 | 3.99 ± 0.49 | 3.22 ± 0.34 | 2.89 ± 1.12 | 1.37 ± 0.17 | 4.13 ± 0.35 | 4.86 ± 0.28 | 4.36 ± 0.28 | 3.97 ± 0.50 |
| 17 | 10.95 | 1320 | Succinic acid (2 TMS) | 1.27 ± 0.12 | 1.26 ± 0.12 | 1.35 ± 0.14 | 1.20 ± 0.23 | 1.27 ± 0.21 | 0.37 ± 0.05 | 1.39 ± 0.24 | 1.59 ± 0.12 | 1.59 ± 0.03 | 1.49 ± 0.07 |
| 18 | 11.31 | 1343 | Glyceric acid (3 TMS) | 1.13 ± 0.09 | 0.61 ± 0.17 | 1.14 ± 0.49 | 0.84 ± 0.04 | 1.05 ± 0.17 | 0.33 ± 0.07 | 1.22 ± 0.10 | 1.39 ± 0.04 | 1.29 ± 0.08 | 1.29 ± 0.10 |
| 22 | 13.25 | 1481 | Ketoisocaproic acid (TMS) | 2.66 ± 0.45 | 1.87 ± 0.44 | 2.96 ± 0.17 | 2.57 ± 0.27 | 2.32 ± 0.32 | 0.87 ± 0.09 | 2.40 ± 0.21 | 3.34 ± 0.12 | 3.13 ± 0.19 | 3.02 ± 0.07 |
| 23 | 13.51 | 1500 | Malic acid (3 TMS) | 0.62 ± 0.17 | 5.50 ± 1.65 | 0.33 ± 0.02 | 0.82 ± 0.16 | 0.40 ± 0.12 | 0.14 ± 0.03 | 0.53 ± 0.19 | 0.28 ± 0.04 | 0.77 ± 0.08 | 0.30 ± 0.05 |
| 24 | 13.74 | 1518 | Unknown | 0.73 ± 0.19 | 0.48 ± 0.13 | 0.69 ± 0.06 | 0.84 ± 0.13 | 0.63 ± 0.29 | 0.20 ± 0.02 | 0.79 ± 0.05 | 1.06 ± 0.15 | 0.95 ± 0.15 | 0.94 ± 0.18 |
| 26 | 16.05 | 1703 | Suberic acid (2 TMS) | 0.21 ± 0.03 | 0.12 ± 0.05 | 0.19 ± 0.09 | 0.20 ± 0.05 | 0.18 ± 0.07 | 0.06 ± 0.00 | 0.27 ± 0.04 | 0.27 ± 0.06 | 0.24 ± 0.05 | 0.20 ± 0.04 |
| 31 | 17.19 | 1800 | Azelaic acid (2 TMS) | 0.32 ± 0.06 | 0.17 ± 0.03 | 0.31 ± 0.02 | 0.25 ± 0.03 | 0.29 ± 0.07 | 0.17 ± 0.10 | 0.51 ± 0.19 | 0.35 ± 0.04 | 0.37 ± 0.03 | 0.39 ± 0.02 |
| 32 | 17.2 | 1801 | Azelaic acid isomer (2 TMS) | 0.48 ± 0.04 | 0.26 ± 0.07 | 0.42 ± 0.04 | 0.34 ± 0.07 | 0.40 ± 0.05 | 0.23 ± 0.11 | 0.64 ± 0.15 | 0.51 ± 0.07 | 0.49 ± 0.04 | 0.53 ± 0.06 |
| 36 | 17.58 | 1837 | Citric acid (4 TMS) | 1.41 ± 0.49 | 0.38 ± 0.11 | 0.16 ± 0.02 | 0.61 ± 0.17 | 0.09 ± 0.02 | 0.51 ± 0.22 | 0.74 ± 0.45 | 0.08 ± 0.02 | 0.85 ± 0.09 | 0.10 ± 0.02 |
| Total organic acids | 22.44 | 18.98 | 23.17 | 17.39 | 17.43 | 6.92 | 24.18 | 29.32 | 25.73 | 24.96 | |||
| Alcohols | |||||||||||||
| 3 | 5.31 | 918 | Ethylene glycol (2 TMS) | 2.61 ± 0.10 | 1.61 ± 0.50 | 2.80 ± 0.20 | 2.32 ± 0.40 | 2.13 ± 0.64 | 0.71 ± 0.08 | 2.80 ± 0.45 | 3.61 ± 0.06 | 3.03 ± 0.11 | 3.17 ± 0.26 |
| 5 | 6.63 | 1037 | Propanediol (2 TMS) | 0.47 ± 0.04 | 0.27 ± 0.09 | 0.49 ± 0.04 | 0.38 ± 0.05 | 0.39 ± 0.10 | 0.12 ± 0.01 | 0.48 ± 0.06 | 0.70 ± 0.03 | 0.50 ± 0.02 | 0.54 ± 0.07 |
| 14 | 10.42 | 1286 | Glycerol (3 TMS) | 2.63 ± 0.04 | 1.93 ± 0.38 | 1.89 ± 0.34 | 1.72 ± 0.52 | 2.85 ± 1.21 | 0.64 ± 0.14 | 2.90 ± 0.59 | 3.48 ± 0.36 | 3.46 ± 0.64 | 2.70 ± 0.17 |
| Total alcohols | 5.71 | 3.80 | 5.18 | 4.42 | 5.36 | 1.46 | 6.18 | 7.79 | 7.00 | 6.41 | |||
| Amino Acids | |||||||||||||
| 9 | 9.40 | 1219 | Glycine (3 TMS) | 2.03 ± 0.12 | 1.10 ± 0.46 | 2.23 ± 0.20 | 1.53± 0.37 | 1.49 ± 0.77 | 0.44 ± 0.03 | 2.35 ± 0.36 | 3.09 ± 0.15 | 2.41 ± 0.11 | 2.51 ± 0.27 |
| 16 | 10.88 | 1315 | Glutamic acid (TMS) | 6.50 ± 0.51 | 3.77 ± 1.25 | 6.67 ± 0.67 | 5.34 ± 0.79 | 5.41 ± 1.57 | 1.89 ± 0.21 | 7.64 ± 0.79 | 8.78 ± 0.47 | 7.46 ± 0.37 | 7.93 ± 0.63 |
| 20 | 12.19 | 1401 | Glycine (3 TMS)/3-Aminoisobutyric acid (3 TMS) | 3.01 ± 0.61 | 2.09 ± 0.48 | 2.55 ± 0.34 | 2.53 ± 0.37 | 2.21 ± 0.34 | 0.90 ± 0.13 | 1.97 ± 0.18 | 3.04 ± 0.12 | 3.17 ± 0.19 | 2.81 ± 0.21 |
| 21 | 12.63 | 1434 | β-Alanine (3 TMS) | 0.75 ± 0.04 | 0.48 ± 0.13 | 0.88 ± 0.13 | 0.68 ± 0.11 | 0.59 ± 0.09 | 0.20 ± 0.02 | 0.76 ± 0.07 | 1.04 ± 0.03 | 0.86 ± 0.05 | 1.01 ± 0.05 |
| 25 | 13.99 | 1536 | Oxoproline (2 TMS) | 0.16 ± 0.02 | 0.23 ± 0.06 | 0.07 ± 0.01 | 0.13 ± 0.02 | 0.08 ± 0.02 | 0.04 ± 0.01 | 0.13 ± 0.02 | 0.10 ± 0.03 | 0.16 ± 0.05 | 0.10 ± 0.00 |
| Total amino acids | 12.44 | 7.67 | 12.40 | 10.21 | 9.77 | 3.48 | 12.84 | 16.04 | 14.07 | 14.36 | |||
| Aromatics | |||||||||||||
| 4 | 6.56 | 1032 | Phenol (TMS) | 1.03 ± 0.06 | 0.63 ± 0.25 | 1.04 ± 0.11 | 0.85 ± 0.14 | 0.85 ± 0.22 | 0.19 ± 0.15 | 0.83 ± 0.52 | 1.43 ± 0.06 | 1.14 ± 0.08 | 1.22 ± 0.10 |
| 12 | 9.94 | 1254 | Benzoic Acid (TMS) | 0.99 ± 0.04 | 0.21 ± 0.08 | 0.30 ± 0.06 | 0.28 ± 0.05 | 1.01 ± 0.32 | 0.13 ± 0.05 | 1.48 ± 0.17 | 0.94 ± 0.07 | 0.47 ± 0.04 | 0.59 ± 0.04 |
| Total aromatics | 2.01 | 0.84 | 1.34 | 1.13 | 1.86 | 0.32 | 2.31 | 2.37 | 1.61 | 1.82 | |||
| Fatty acid/ester/steroid | |||||||||||||
| 19 | 11.58 | 1361 | Butyl caprylate | 9.28 ± 0.61 | 6.24 ± 2.10 | 10.74 ± 0.92 | 8.79 ± 1.45 | 7.57 ± 2.31 | 2.82 ± 0.36 | 10.63 ± 1.09 | 12.89 ± 0.52 | 11.94 ± 0.65 | 12.42 ± 1.01 |
| 38 | 17.72 | 1850 | Myristic acid (TMS) | 0.35 ± 0.03 | 0.18 ± 0.05 | 0.27 ± 0.01 | 0.26 ± 0.01 | 0.24 ± 0.07 | 0.09 ± 0.02 | 0.43 ± 0.08 | 0.36 ± 0.01 | 0.36 ± 0.01 | 0.34 ± 0.02 |
| 48 | 19.76 | 2045 | Palmitic Acid (TMS) | 5.15 ± 0.59 | 2.66 ± 1.00 | 3.99 ± 0.39 | 3.95 ± 0.29 | 3.75 ± 0.87 | 2.85 ± 3.07 | 6.62 ± 1.85 | 5.68 ± 0.42 | 5.35 ± 0.17 | 4.67 ± 0.11 |
| 50 | 20.72 | 2145 | Margaric acid (TMS) | 0.22 ± 0.02 | 0.15 ± 0.05 | 0.21 ± 0.03 | 0.20 ± 0.03 | 0.18 ± 0.05 | 0.12 ± 0.11 | 0.24 ± 0.02 | 0.30 ± 0.01 | 0.26 ± 0.03 | 0.25 ± 0.01 |
| 51 | 21.39 | 2215 | Linoleic acid (TMS) | 0.31 ± 0.04 | 0.49 ± 0.18 | 0.63 ± 0.23 | 0.90 ± 0.19 | 0.55 ± 0.31 | 0.82 ± 0.91 | 0.92 ± 0.09 | 0.93 ± 0.22 | 0.96 ± 0.03 | 0.92 ± 0.24 |
| 52 | 21.42 | 2218 | Oleic Acid (TMS) | 1.40 ± 0.17 | 1.08 ± 0.40 | 1.78 ± 0.33 | 2.38 ± 0.32 | 1.21 ± 0.04 | 2.53 ± 3.23 | 2.54 ± 0.59 | 2.12 ± 0.40 | 1.62 ± 0.36 | 1.97 ± 0.36 |
| 53 | 21.47 | 2224 | Linoleic acid (TMS) | 0.36 ± 0.02 | 0.11 ± 0.09 | 0.24 ± 0.35 | 0.41 ± 0.34 | 0.33 ± 0.21 | 0.39 ± 0.65 | 0.52 ± 0.35 | 0.15 ± 0.09 | 0.31 ± 0.05 | 0.13 ± 0.12 |
| 54 | 21.48 | 2225 | Oleic Acid (TMS) | 0.38 ± 0.04 | 0.20 ± 0.07 | 0.99 ± 0.58 | 1.33 ± 0.23 | 0.33 ± 0.08 | 0.87 ± 1.28 | 0.61 ± 0.20 | 0.83 ± 0.57 | 0.43 ± 0.04 | 0.41 ± 0.00 |
| 55 | 21.64 | 2243 | Stearic acid (TMS) | 4.23 ± 0.41 | 2.80 ± 0.89 | 4.68 ± 0.41 | 4.20 ± 0.31 | 3.59 ± 1.09 | 2.62 ± 2.32 | 4.89 ± 0.45 | 5.76 ± 0.17 | 5.29 ± 0.22 | 5.36 ± 0.28 |
| 56 | 24.36 | 2568 | Palmitoylglycerol (2 TMS) | 0.08 ± 0.02 | 0.06 ± 0.04 | 0.05 ± 0.04 | 0.07 ± 0.04 | 0.12 ± 0.04 | 0.11 ± 0.11 | 0.11 ± 0.01 | 0.10 ± 0.01 | 0.05 ± 0.03 | 0.07 ± 0.03 |
| 57 | 24.62 | 2601 | 1-Monopalmitin (2 TMS) | 0.33 ± 0.09 | 0.38 ± 0.15 | 0.65 ± 0.09 | 0.42 ± 0.12 | 0.43 ± 0.14 | 0.56 ± 0.71 | 0.71 ± 0.12 | 0.76 ± 0.08 | 0.73 ± 0.05 | 0.71 ± 0.07 |
| 60 | 25.95 | 2768 | Monooleoylglycerol (2 TMS) | 0.26 ± 0.03 | 0.23 ± 0.05 | 0.43 ± 0.37 | 2.09 ± 0.34 | 0.25 ± 0.15 | 0.24 ± 0.28 | 0.30 ± 0.05 | 0.33 ± 0.06 | 0.32 ± 0.05 | 0.34 ± 0.11 |
| 61 | 26.09 | 2787 | Glycerol monostearate (2 TMS) | 0.22 ± 0.08 | 0.26 ± 0.10 | 0.44 ± 0.08 | 0.30 ± 0.10 | 0.29 ± 0.09 | 0.26 ± 0.25 | 0.47 ± 0.11 | 0.54 ± 0.10 | 0.48 ± 0.01 | 0.51 ± 0.05 |
| 65 | 26.33 | 2813 | Sebacic acid (TMS) | 0.34 ± 0.18 | 0.24 ± 0.13 | 0.58 ± 0.41 | 0.33 ± 0.17 | 2.02 ± 1.74 | 1.66 ± 0.44 | 0.61 ± 0.27 | 0.30 ± 0.18 | 0.42 ± 0.29 | 0.56 ± 0.07 |
| 66 | 26.46 | 2823 | Lignoceric acid (TMS) | 0.11 ± 0.01 | 0.08 ± 0.05 | 0.08 ± 0.01 | 0.09 ± 0.02 | 0.07 ± 0.01 | 0.08 ± 0.10 | 0.23 ± 0.14 | 0.14 ± 0.05 | 0.14 ± 0.03 | 0.09 ± 0.05 |
| 67 | 26.48 | 2826 | Squalene | 0.21 ± 0.05 | 0.13 ± 0.04 | 0.22 ± 0.08 | 0.47 ± 0.32 | 0.16 ± 0.04 | 0.08 ± 0.07 | 0.26 ± 0.05 | 0.29 ± 0.07 | 0.36 ± 0.12 | 0.20 ± 0.02 |
| Total fatty acid/ester/steroid | 23.21 | 15.29 | 25.99 | 26.18 | 21.09 | 16.10 | 30.09 | 31.47 | 29.00 | 28.96 | |||
| Total inorganic compounds | |||||||||||||
| 15 | 10.44 | 1286 | Phosphoric acid (3 TMS) | 3.81 ± 0.35 | 2.50 ± 0.40 | 3.90 ± 0.64 | 3.85 ± 1.06 | 3.26 ± 0.37 | 1.14 ± 0.23 | 4.72 ± 1.16 | 3.94 ± 0.15 | 4.23 ± 0.08 | 4.07 ± 0.05 |
| Total inorganic | 3.81 | 2.50 | 3.90 | 3.85 | 3.26 | 1.14 | 4.72 | 3.94 | 4.23 | 4.07 | |||
| Nitrogenous compounds | |||||||||||||
| 2 | 5.24 | 913 | Unknown | 0.72 ± 0.10 | 0.41 ± 0.20 | 0.64 ± 0.14 | 0.53 ± 0.12 | 0.54 ± 0.14 | 0.16 ± 0.01 | 0.66 ± 0.14 | 0.64 ± 0.41 | 0.67 ± 0.07 | 0.68 ± 0.07 |
| Total Nitrogenous compounds | 0.72 | 0.41 | 0.64 | 0.53 | 0.54 | 0.16 | 0.66 | 0.64 | 0.67 | 0.68 | |||
| Sugars | |||||||||||||
| 33 | 17.37 | 1816 | Methyl glucofuranoside (4 TMS) | 0.20 ± 0.10 | 0.12 ± 0.08 | 0.22 ± 0.20 | 0.42 ± 0.25 | 0.32 ± 0.27 | 0.03 ± 0.02 | 0.39 ± 0.08 | 0.55 ± 0.39 | 0.50 ± 0.38 | 0.49 ± 0.20 |
| 34 | 17.47 | 1826 | Fructofuranose (5 TMS) | 1.86 ± 0.60 | 0.34 ± 0.14 | 0.15 ± 0.04 | 0.06 ± 0.02 | 0.11 ± 0.03 | 0.03 ± 0.01 | 0.84 ± 0.52 | 0.10 ± 0.01 | 1.04 ± 0.15 | 0.13 ± 0.02 |
| 35 | 17.56 | 1834 | Fructofuranose isomer (5 TMS) | 5.71 ± 1.92 | 0.96 ± 0.36 | 0.50 ± 0.03 | 0.99 ± 0.26 | 0.25 ± 0.08 | 0.85 ± 0.39 | 2.85 ± 1.71 | 0.19 ± 0.04 | 3.22 ± 0.36 | 0.25 ± 0.04 |
| 40 | 17.89 | 1865 | Galactofuranose (5 TMS) | 0.45 ± 0.16 | 0.07 ± 0.01 | 0.20 ± 0.04 | 0.04 ± 0.01 | 0.09 ± 0.04 | 0.04 ± 0.01 | 0.23 ± 0.06 | 0.07 ± 0.02 | 0.23 ± 0.02 | 0.07 ± 0.01 |
| 41 | 18.08 | 1883 | Mannose (5 TMS) | 0.13 ± 0.02 | 0.10 ± 0.01 | 0.46 ± 0.09 | 0.13 ± 0.08 | 0.09 ± 0.03 | 0.02 ± 0.01 | 0.12 ± 0.03 | 0.27 ± 0.18 | 0.20 ± 0.05 | 0.27 ± 0.18 |
| 42 | 18.4 | 1913 | Fructose (5 TMS) | 0.94 ± 0.15 | 0.33 ± 0.22 | 1.15 ± 0.08 | 0.04 ± 0.02 | 0.25 ± 0.16 | 0.02 ± 0.01 | 0.40 ± 0.26 | 0.09 ± 0.03 | 0.53 ± 0.11 | 0.20 ± 0.22 |
| 43 | 18.45 | 1917 | Glucose (5 TMS) | 4.57 ± 1.06 | 0.78 ± 0.27 | 1.29 ± 0.14 | 0.14 ± 0.04 | 0.34 ± 0.12 | 0.06 ± 0.02 | 1.51 ± 0.97 | 0.27 ± 0.14 | 1.74 ± 0.27 | 0.35 ± 0.06 |
| 44 | 18.57 | 1929 | Galactopyranose, Mannose (5 TMS) | 0.21 ± 0.06 | 0.13 ± 0.02 | 0.90 ± 0.23 | 0.07 ± 0.01 | 0.17 ± 0.05 | 0.02 ± 0.01 | 0.15 ± 0.06 | 0.05 ± 0.01 | 0.20 ± 0.01 | 0.06 ± 0.02 |
| 47 | 19.34 | 2002 | Glucose isomer (5 TMS) | 6.37 ± 1.57 | 1.14 ± 0.36 | 1.92 ± 0.20 | 0.21 ± 0.05 | 0.45 ± 0.16 | 0.08 ± 0.03 | 2.10 ± 1.44 | 0.35 ± 0.23 | 2.45 ± 0.34 | 0.47 ± 0.08 |
| 58 | 25.35 | 2693 | Sucrose (8 TMS) | 0.49 ± 0.21 | 41.21 ± 10.34 | 16.86 ± 4.92 | 31.18 ± 7.04 | 21.74 ± 10.20 | 35.22 ± 8.32 | 0.58 ± 0.21 | 3.26 ± 0.26 | 1.01 ± 0.16 | 12.54 ± 3.88 |
| 59 | 25.69 | 2737 | Cellobiose, (isomer 2) (8 TMS) | 0.05 ± 0.01 | 0.11 ± 0.10 | 0.19 ± 0.06 | 0.17 ± 0.13 | 0.10 ± 0.05 | 0.07 ± 0.03 | 0.35 ± 0.14 | 0.19 ± 0.14 | 0.20 ± 0.14 | 0.20 ± 0.11 |
| 62 | 26.24 | 2804 | Lactose (8 TMS) | 0.29 ± 0.3 | 0.31 ± 0.01 | 0.14 ± 0.13 | 0.13 ± 0.16 | 0.30 ± 0.49 | 0.01 ± 0.01 | 0.21 ± 0.22 | 0.11 ± 0.10 | 0.13 ± 0.14 | 0.21 ± 0.25 |
| 63 | 26.31 | 2810 | Sucrose (8 TMS) | 0.62 ± 0.05 | 0.43 ± 0.16 | 0.74 ± 0.07 | 0.61 ± 0.10 | 0.52 ± 0.13 | 0.21 ± 0.02 | 0.64 ± 0.07 | 0.82 ± 0.06 | 0.87 ± 0.08 | 0.89 ± 0.04 |
| 64 | 26.33 | 2812 | Melibiose (8 TMS) | 0.06 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 4.10 ± 5.24 | 4.06 ± 1.03 | 0.06 ± 0.01 | 0.03 ± 0.00 | 0.05 ± 0.03 | 0.07 ± 0.02 |
| 68 | 26.89 | 2863 | Melibiose isomer I (8 TMS) | 0.03 ± 0.02 | 0.06 ± 0.00 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.12 ± 0.05 | 0.61 ± 0.20 | 0.04 ± 0.03 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.01 |
| 69 | 27.50 | 2918 | Melibiose isomer II (8 TMS) | 0.03 ± 0.00 | 0.15 ± 0.04 | 0.04 ± 0.01 | 0.07 ± 0.03 | 2.95 ± 1.66 | 25.33 ± 1.17 | 3.84 ± 6.59 | 0.05 ± 0.02 | 0.07 ± 0.01 | 0.23 ± 0.11 |
| 37 | 17.66 | 1843 | Fructose (5 TMS) | 5.38 ± 1.00 | 0.97 ± 0.40 | 0.51 ± 0.03 | 0.10 ± 0.01 | 0.19 ± 0.06 | 0.06 ± 0.01 | 2.76 ± 2.26 | 0.16 ± 0.03 | 3.04 ± 0.36 | 0.21 ± 0.08 |
| Total sugars | 27.39 | 47.23 | 25.35 | 34.42 | 32.10 | 66.73 | 17.07 | 6.59 | 15.52 | 16.66 | |||
| Sugar alcohols | |||||||||||||
| 27 | 16.36 | 1729 | Ribitol (5 TMS) | 0.30 ± 0.48 | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.04 ± 0.04 | (-) | 0.02 ± 0.02 | 0.01 ± 0.00 | 0.02 ± 0.02 | 0.01 ± 0.00 |
| 28 | 16.49 | 1741 | Unknown (5 TMS) | 0.09 ± 0.02 | 0.50 ± 0.10 | 0.07 ± 0.03 | 0.07 ± 0.01 | 0.11 ± 0.04 | 0.03 ± 0.01 | 0.17 ± 0.03 | 0.16 ± 0.03 | 0.13 ± 0.02 | 0.16 ± 0.04 |
| 29 | 16.56 | 1746 | Arabitol (5 TMS) | 0.02 ± 0.01 | 0.39 ± 0.10 | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.04 ± 0.03 | 0.02 ± 0.02 | 0.03 ± 0.01 |
| 30 | 16.87 | 1772 | meso-Erythritol (4 TMS) | 0.59 ± 0.03 | 0.37 ± 0.09 | 0.48 ± 0.06 | 0.44 ± 0.06 | 0.46 ± 0.13 | 0.16 ± 0.03 | 0.64 ± 0.07 | 0.74 ± 0.08 | 0.65 ± 0.04 | 0.76 ± 0.14 |
| 39 | 17.81 | 1857 | Pinitol (5 TMS) | 0.07 ± 0.01 | 0.15 ± 0.16 | 0.15 ± 0.22 | 0.15 ± 0.18 | 6.11 ± 8.08 | 1.34 ± 0.36 | 0.06 ± 0.02 | 0.04 ± 0.00 | 0.05 ± 0.00 | 0.07 ± 0.04 |
| 45 | 18.87 | 1957 | Sorbitol (6 TMS) | 0.05 ± 0.02 | 0.27 ± 0.04 | 0.04 ± 0.03 | 0.05 ± 0.01 | 0.11 ± 0.07 | 0.03 ± 0.00 | 0.09 ± 0.01 | 0.09 ± 0.02 | 0.11 ± 0.03 | 0.06 ± 0.02 |
| 46 | 19.13 | 1982 | Pinitol isomer (5 TMS) | 0.23 ± 0.31 | 0.13 ± 0.14 | 0.43 ± 0.14 | 0.30 ± 0.35 | 0.08 ± 0.04 | 0.09 ± 0.05 | 0.23 ± 0.30 | 0.10 ± 0.03 | 0.16 ± 0.02 | 0.20 ± 0.17 |
| 49 | 20.49 | 2122 | Myo-Inositol (6 TMS) | 0.86 ± 0.08 | 0.52 ± 0.05 | 0.68 ± 0.35 | 0.56 ± 0.31 | 1.07 ± 0.24 | 0.58 ± 0.09 | 0.64 ± 0.18 | 0.51 ± 0.22 | 0.88 ± 0.22 | 0.55 ± 0.06 |
| 70 | 28 | 2963 | Galactinol (9 TMS) | 0.06 ± 0.02 | 0.94 ± 0.25 | 0.17 ± 0.04 | 0.30 ± 0.04 | 0.59 ± 0.22 | 1.45 ± 0.37 | 0.07 ± 0.01 | 0.14 ± 0.12 | 0.13 ± 0.01 | 0.26 ± 0.11 |
| Total sugar alcohols | 2.26 | 3.28 | 2.05 | 1.89 | 8.59 | 3.69 | 1.94 | 1.82 | 2.16 | 2.08 | |||
| Total 100% | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saied, D.B.; Ramadan, N.S.; El-Sayed, M.M.; Farag, M.A. Effect of Maturity Stage on Cereal and Leguminous Seeds’ Metabolome as Analyzed Using Gas Chromatography Mass-Spectrometry (GC-MS) and Chemometric Tools. Metabolites 2023, 13, 163. https://doi.org/10.3390/metabo13020163
Saied DB, Ramadan NS, El-Sayed MM, Farag MA. Effect of Maturity Stage on Cereal and Leguminous Seeds’ Metabolome as Analyzed Using Gas Chromatography Mass-Spectrometry (GC-MS) and Chemometric Tools. Metabolites. 2023; 13(2):163. https://doi.org/10.3390/metabo13020163
Chicago/Turabian StyleSaied, Doaa B., Nehal S. Ramadan, Magdy M. El-Sayed, and Mohamed A. Farag. 2023. "Effect of Maturity Stage on Cereal and Leguminous Seeds’ Metabolome as Analyzed Using Gas Chromatography Mass-Spectrometry (GC-MS) and Chemometric Tools" Metabolites 13, no. 2: 163. https://doi.org/10.3390/metabo13020163
APA StyleSaied, D. B., Ramadan, N. S., El-Sayed, M. M., & Farag, M. A. (2023). Effect of Maturity Stage on Cereal and Leguminous Seeds’ Metabolome as Analyzed Using Gas Chromatography Mass-Spectrometry (GC-MS) and Chemometric Tools. Metabolites, 13(2), 163. https://doi.org/10.3390/metabo13020163