Meibomian Gland Assessment in Routine Ophthalmology Practice
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Schein Questionnaire
2.3. Objective Dry Eye Signs
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Skin Type
3.2. Objective Dry Eye Signs and Meibomian Gland Function
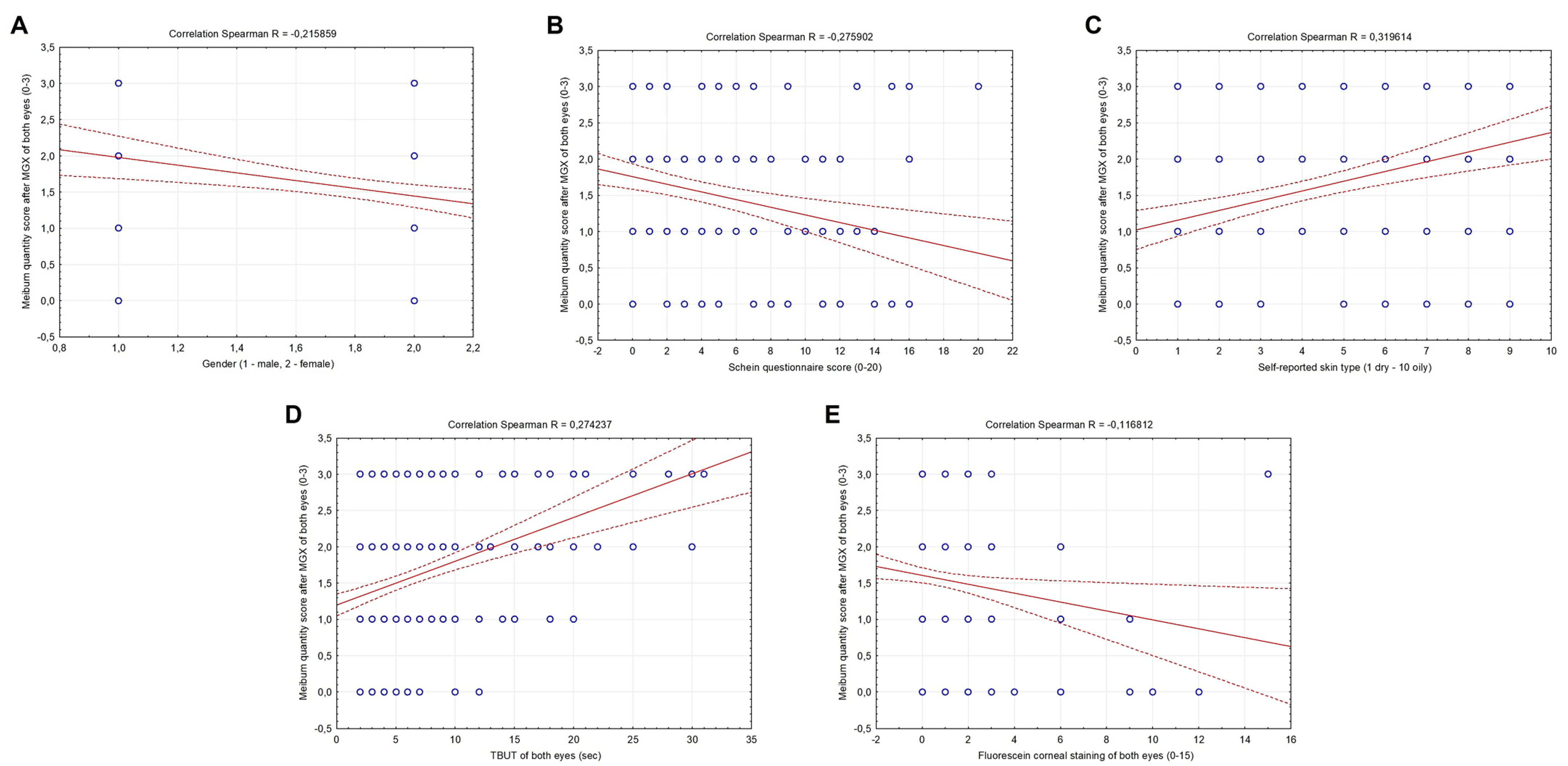
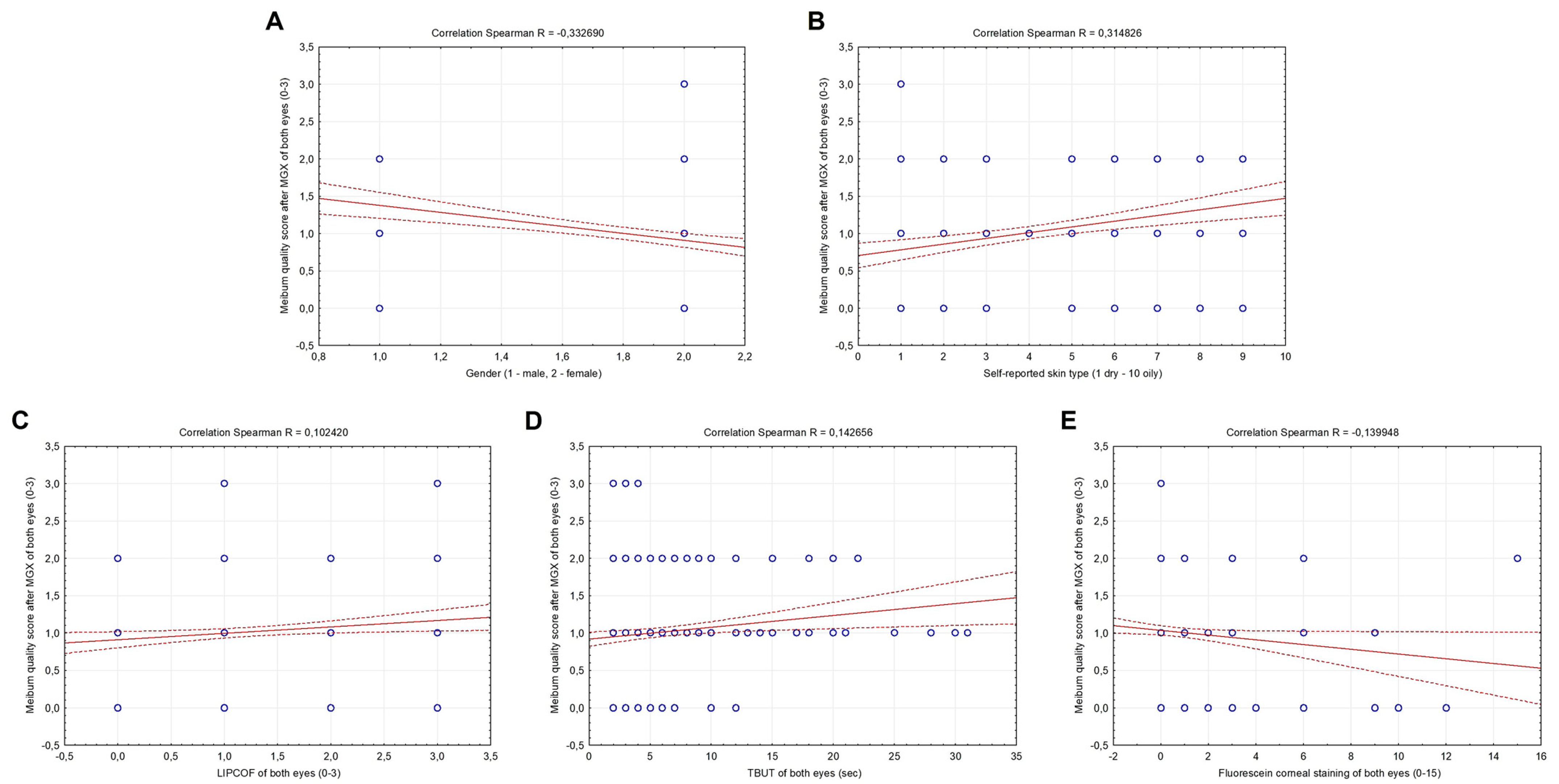
3.3. Correlation between Meibomian Gland Function, Baseline Characteristics, Skin Type, Schein Questionnaire and Objective Dry Eye Signs
3.4. Predictors and Indicators of Dry Eye
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bron, A.J.; de Paiva, G.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Nicholsm, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar]
- Tomlinson, A.; Bron, A.J.; Korb, D.R.; Amano, S.; Paugh, J.R.; Pearce, E.I.; Yee, R.; Yokoi, N.; Arita, R.; Dogru, M. The international workshop on meibomian gland dysfunction: Report of the diagnosis subcommittee. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2006–2049. [Google Scholar] [CrossRef] [PubMed]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef] [PubMed]
- Mathers, W.D.; Shields, W.J.; Sachdev, M.S.; Petroll, W.M.; Jester, J.V. Meibomian gland dysfunction in chronic blepharitis. Cornea 1991, 10, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; Benjamin, L.; Snibson, G.R. Meibomian gland disease. Classification and grading of lid changes. Eye 1991, 5, 395–411. [Google Scholar] [CrossRef]
- Korb, D.R.; Blackie, C.A. Meibomian gland diagnostic expressibility: Correlation with dry eye symptoms and gland location. Cornea 2008, 27, 1142–1147. [Google Scholar] [CrossRef]
- Finis, D.; Pischel, N.; Schrader, S.; Geerling, G. Evaluation of lipid layer thickness measurement of the tear film as a diagnostic tool for Meibomian gland dysfunction. Cornea 2013, 32, 1549–1553. [Google Scholar] [CrossRef] [PubMed]
- Mathers, W.D.; Shields, W.J.; Sachdev, M.S.; Petroll, W.M.; Jester, J.V. Meibomian gland morphology and tear osmolarity: Changes with Accutane therapy. Cornea 1991, 10, 286–290. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Tseng, S.; Sanabria, O.; Kell, H.; Garcia, C.G.; Felix, C.; Reis, B.L. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea 1998, 17, 38–56. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, J.; Goto, E.; Ono, M.; Shimmura, S.; Tsubota, K. Meibomian gland dysfunction in patients with Sjögren syndrome. Ophthalmology 1998, 105, 1485–1488. [Google Scholar] [CrossRef] [PubMed]
- Henriquez, A.S.; Korb, D.R. Meibomian glands and contact lens wear. Br. J. Ophthalmol. 1981, 65, 108–111. [Google Scholar] [CrossRef]
- Azcarate, P.M.; Venincasa, V.D.; Feuer, W.; Stanczyk, F.; Schally, A.V.; Galor, A. Androgen deficiency and dry eye syndrome in the aging male. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5046–5053. [Google Scholar] [CrossRef] [PubMed]
- Truong, S.; Cole, N.; Stapleton, F.; Golebiowski, B. Sex hormones and the dry eye. Clin. Exp. Optom. 2014, 97, 324–336. [Google Scholar] [CrossRef]
- Gagliano, C.; Caruso, S.; Napolitano, G.; Malaguarnera, G.; Cicinelli, M.V.; Amato, R.; Reibaldi, M.; Incarbone, G.; Bucolo, C.; Drago, F.; et al. Low levels of 17-β-oestradiol, oestrone and testosterone correlate with severe evaporative dysfunctional tear syndrome in postmenopausal women: A case–control study. Br. J. Ophthalmol. 2014, 98, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Takayasu, S.; Wakimoto, H.; Itami, S.; Sano, S. Activity of testosterone 5 alpha-reductase in various tissues of human skin. J. Investig. Dermatol. 1980, 74, 187–191. [Google Scholar] [CrossRef]
- Sullivan, D.A.; Jensen, R.V.; Suzuki, T.; Richards, S.M. Do sex steroids exert sex-specific and/or opposite effects on gene expression in lacrimal and meibomian glands? Mol. Vis. 2009, 15, 1553–1572. [Google Scholar]
- Steagall, R.J.; Yamagami, H.; Wickham, L.A.; Sullivan, D.A. Androgen control of gene expression in the rabbit meibomian gland. Adv. Exp. Med. Biol. 2002, 506, 465–476. [Google Scholar]
- Yamagami, H.; Schirra, F.; Liu, M.; Richards, S.M.; Sullivan, B.D.; Sullivan, D.A. Androgen influence on gene expression in the meibomian gland. Adv. Exp. Med. Biol. 2002, 506 Pt A, 477–481. [Google Scholar]
- Schirra, F.; Richards, S.M.; Liu, M.; Suzuki, T.; Yamagami, H.; Sullivan, D.A. Androgen regulation of lipogenic pathways in the mouse meibomian gland. Exp. Eye Res. 2006, 83, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Schirra, F.; Richards, S.M.; Sullivan, D.A. Androgen influence on cholesterogenic enzyme mRNA levels in the mouse meibomian gland. Curr. Eye Res. 2007, 32, 393–398. [Google Scholar] [CrossRef]
- Sullivan, B.D.; Evans, J.E.; Cermak, J.M.; Krenzer, K.L.; Dana, M.R.; Sullivan, D.A. Complete androgen insensitivity syndrome: Effect on human meibomian gland secretions. Arch. Ophthalmol. 2002, 120, 1689–1699. [Google Scholar] [CrossRef]
- Yamagami, H.; Richards, S.M.; Sullivan, B.D.; Liu, M.; Steagall, R.J.; Sullivan, D.A. Gender-associated differences in gene expression of the meibomian gland. Adv. Exp. Med. Biol. 2002, 506, 459–463. [Google Scholar]
- Labrie, F.; Bélanger, A.; Cusan, L.; Gomez, J.L.; Candas, B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J. Clin. Endocrinol. Metab. 1997, 82, 2396–2402. [Google Scholar] [CrossRef] [PubMed]
- Versura, P.; Campos, E.C. Menopause and dry eye. A possible relationship. Gynecol. Endocrinol. 2005, 20, 289–298. [Google Scholar] [CrossRef]
- Suzuki, T.; Schirra, F.; Richards, S.M.; Jensen, R.V.; Sullivan, D.A. Estrogen and Progesterone Control of Gene Expression in the Mouse Meibomian Gland. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1797–1808. [Google Scholar] [CrossRef]
- Chen, F.; Hu, X.; He, Y.; Huang, D. Lipidomics demonstrates the association of sex hormones with sebum. J. Cosmet. Dermatol. 2021, 20, 2015–2019. [Google Scholar] [CrossRef]
- Petriček, I. The Influence of Tear Film on Visual Function. Ph.D. Thesis, University of Zagreb School of Medicine, Zagreb, Croatia, 2011. [Google Scholar]
- Clayton, R.W.; Langan, E.A.; Ansell, D.M.; de Vos, I.J.H.M.; Göbel, K.; Schneider, M.R.; Picardo, M.; Lim, X.; van Steensel, M.A.M.; Paus, R. Neuroendocrinology and Neurobiology of Sebaceous Glands. Biol. Rev. Camb. Philos. Soc. 2020, 95, 592–624. [Google Scholar] [CrossRef]
- Vidas Pauk, S. Non-Invasive Tear Break-Up Time Measurement Using Handheld Lipid Layer Thickness Assessment Tool. Ph.D. Thesis, University of Zagreb School of Medicine, Zagreb, Croatia, 2019. [Google Scholar]
- Sunwoo, Y.; Chou, C.; Takeshita, J.; Murakami, M.; Tochihara, Y. Physiological and subjective responses to low relative humidity. J. Physiol. Anthropol. 2006, 25, 7–14. [Google Scholar] [CrossRef]
- Petriček, I.; Pauk, S.V.; Tomić, M.; Bulum, T. Dry eye and dry skin—Is there a connection? Ophthalmic Epidemiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Hahnel, E.; Lichterfeld, A.; Blume-Peytavi, U.; Kottner, J. The epidemiology of skin conditions in the aged: A systematic review. J. Tissue Viability 2017, 26, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Moniaga, C.S.; Tominaga, M.; Takamori, K. Mechanisms and Management of Itch in Dry Skin. Acta Derm. Venereol. 2020, 100, adv00024. [Google Scholar] [CrossRef]
- Guliani, B.P.; Bhalla, A.; Naik, M.P. Association of the severity of meibomian gland dysfunction with dyslipidemia in Indian population. Indian J. Ophthalmol. 2018, 66, 1411–1416. [Google Scholar] [PubMed]
- Schein, O.D.; Tielsch, J.M.; Munõz, B.; Bandeen-Roche, K.; West, S. Relation between signs and symptoms of dry eye in the elderly. A population-based perspective. Ophthalmology 1997, 104, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Lemp, M.A.; Foulks, G.N. The definition and classification of dry eye disease: Report of definition and classification subcommittee of international dry eye workshop. Ocul. Surf. 2007, 5, 75–92. [Google Scholar]
- Bakija, I.; Filipčić, I.; Bogadi, M.; Šimunović Filipčić, I.; Gotovac, M.; Kaštelan, S. Comparison of the Schein and Osdi Questionnaire as Indicator of Tear Film Stability in Patients with Schizophrenia. Psychiatr. Danub. 2021, 33, 596–603. [Google Scholar]
- Bandeen-Roche, K.; Muñoz, B.; Tielsch, J.M.; West, S.K.; Schein, O.D. Self-reported assessment of dry eye in a population-based setting. Investig. Ophthalmol. Vis. Sci. 1997, 38, 2469–2475. [Google Scholar]
- Terry, R.L.; Schnider, C.M.; Holden, B.A.; Cornish, R.; Grant, T.; Sweeney, D.; La Hood, D.; Back, A. CCLRU standards for success of daily and extended wear contact lenses. Optom. Vis. Sci. 1993, 70, 234–243. [Google Scholar] [CrossRef]
- Höh, H.; Schirra, F.; Kienecker, C.; Ruprecht, K.W. Lid-parallel conjunctival folds are a sure diagnostic sign of dry eye. Ophthalmologe 1995, 92, 802–808. [Google Scholar]
- Korb, D.R. The tear film—Its role today and in the future. In The Tear Film, Structure, Function and Clinical Examination; Butterworth-Heinemann: Oxford, UK, 2002; pp. 174–177. [Google Scholar]
- Thulasi, P.; Djalilian, A.R. Update in current diagnostics and therapeutics of dry eye disease. Ophthalmology 2017, 124, S27–S33. [Google Scholar] [CrossRef] [PubMed]
- Korb, D.R.; Henriquez, A.S. Meibomian gland dysfunction and contact lens intolerance. J. Am. Optom. Assoc. 1980, 51, 243–251. [Google Scholar] [PubMed]
- Ito, K.; Takamatsu, K.; Nohno, K.; Sugano, A.; Funayama, S.; Katsura, K.; Kaneko, N.; Ogawa, M.; Meurman, J.H.; Inoue, M. Factors associated with mucosal dryness in multiple regions and skin: A web-based study in women. J. Obstet. Gynaecol. Res. 2017, 43, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Werschler, W.P.; Trookman, N.S.; Rizer, R.L.; Ho, E.T.; Mehta, R. Enhanced efficacy of a facial hydrating serum in subjects with normal or self-perceived dry skin. J. Clin. Aesthetic Dermatol. 2011, 4, 51–55. [Google Scholar]
| Dry Eye Group (n = 100) | Control Group (n = 100) | t a Chi b | p | |
|---|---|---|---|---|
| Age (years) * | 51.17 ± 15.16 | 41.39 ± 16.08 | 4.426 a | <0.001 a |
| Gender (m/f) ** | 10/90 | 35/65 | 17.921 b | <0.001 b |
| Schein questionnaire score * | 7 (1–20) | 0 (0–0) | 12.216 c | <0.001 c |
| Self-reported skin type (1 dry–10 oily) † | 2 (1–9) | 5 (1–9) | −5.049 c | <0.001 c |
| Dry Eye Group (n = 100) | Control Group (n = 100) | Z | p | |
|---|---|---|---|---|
| CCRLU of both eyes (0–4) | 0 (0–2) | 0 (0–0) | 0.487 | 0.626 |
| LIPCOF of both eyes (0–3) | 1.5 (0–3) | 1 (0–3) | 5.142 | <0.001 |
| TBUT of both eyes (s) | 4 (2–12) | 5 (2–28) | −5.035 | <0.001 |
| Fluorescein corneal staining of both eyes (0–15) | 0 (0–15) | 0 (0–3) | 1.980 | 0.047 |
| Meibum quantity score of both eyes (0–3) | 1 (0–3) | 2 (0–3) | −3.532 | <0.001 |
| Meibum quality score of both eyes (0–3) | 1 (0–3) | 1 (0–2) | −1.259 | 0.208 |
| Parameter Estimate | Wald’s Chi-Square | p | OR (95%CI) | Parameter Estimate | Wald’s Chi-Square | p | AOR (95% CI) * | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 0.03908 | 16.956 | <0.001 | 1.64 (1.09–2.48) | / | |||
| Gender (female) | 1.57818 | 16.056 | <0.001 | 4.85 (2.23–9.94) | / | |||
| Self-reported drier or oilier facial skin (1–10) | −0.31468 | 20.799 | <0.001 | 0.73 (0.64–0.84) | −0.1732 | 4.886 | 0.027 | 0.84 (0.72–0.98) |
| LIPCOF of both eyes | 1.13481 | 26.879 | <0.001 | 1.04 (1.02–1.06) | 0.77804 | 10.022 | 0.002 | 1.18 (1.04–1.54) |
| TBUT of the right eye | −0.26232 | 20.889 | <0.001 | 0.77 (0.69–0.86) | −0.23057 | 15.263 | <0.001 | 0.79 (0.71–0.89) |
| Fluorescein corneal staining of both eyes | 0.43914 | 5.537 | 0.019 | 1.05 (1.01–1.24) | 0.49599 | 5.617 | 0.028 | 1.14 (1.09–1.48) |
| Meibum quantity score of both eyes | −0.53517 | 12.957 | <0.001 | 0.59 (0.44–0.79) | −0.42891 | 7.044 | 0.008 | 0.65 (0.47–0.89) |
| Omnibus tests χ2 = 84.840, df = 7, p < 0.001 Cox & Snell R2 = 0.346, Nagelkerke R2 = 0.461 Hosmer and Lemeshow test χ2 = 5.354, df = 8, p = 0.719 | Omnibus tests χ2 = 77.145, df = 5, p < 0.001 Cox & Snell R2 = 0.320, Nagelkerke R2 = 0.427 Hosmer and Lemeshow test χ2 = 7.069, df = 8, p = 0.529 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petriček, I.; Tomić, M.; Bulum, T.; Lešin Gaćina, D.; Vidas Pauk, S. Meibomian Gland Assessment in Routine Ophthalmology Practice. Metabolites 2023, 13, 157. https://doi.org/10.3390/metabo13020157
Petriček I, Tomić M, Bulum T, Lešin Gaćina D, Vidas Pauk S. Meibomian Gland Assessment in Routine Ophthalmology Practice. Metabolites. 2023; 13(2):157. https://doi.org/10.3390/metabo13020157
Chicago/Turabian StylePetriček, Igor, Martina Tomić, Tomislav Bulum, Dina Lešin Gaćina, and Sania Vidas Pauk. 2023. "Meibomian Gland Assessment in Routine Ophthalmology Practice" Metabolites 13, no. 2: 157. https://doi.org/10.3390/metabo13020157
APA StylePetriček, I., Tomić, M., Bulum, T., Lešin Gaćina, D., & Vidas Pauk, S. (2023). Meibomian Gland Assessment in Routine Ophthalmology Practice. Metabolites, 13(2), 157. https://doi.org/10.3390/metabo13020157








