Differential Effect of Fructose in the Presence or Absence of Fatty Acids on Circadian Metabolism in Hepatocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture Experiments
2.2. Cell Viability Assay
2.3. Western Blot Analysis
2.4. Triglycerides and Cholesterol Measurements
2.5. Lipid Content Measurements
2.6. RNA Extraction and Quantitative Real-Time PCR
2.7. Statistical Analysis
3. Results
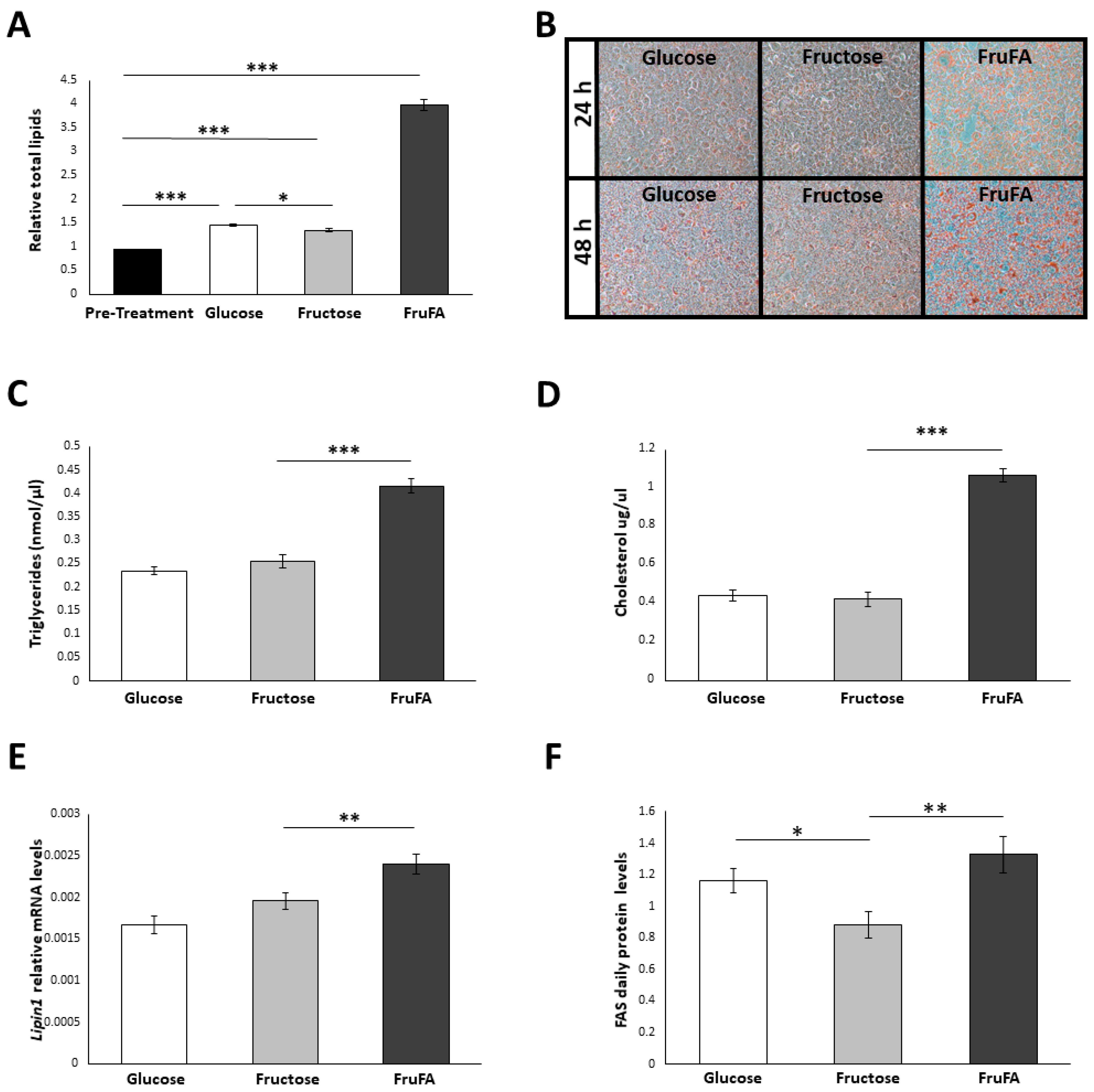
3.1. Steatosis Induction
3.2. Effect of Monosaccharides and/or Steatosis on Lipid Accumulation
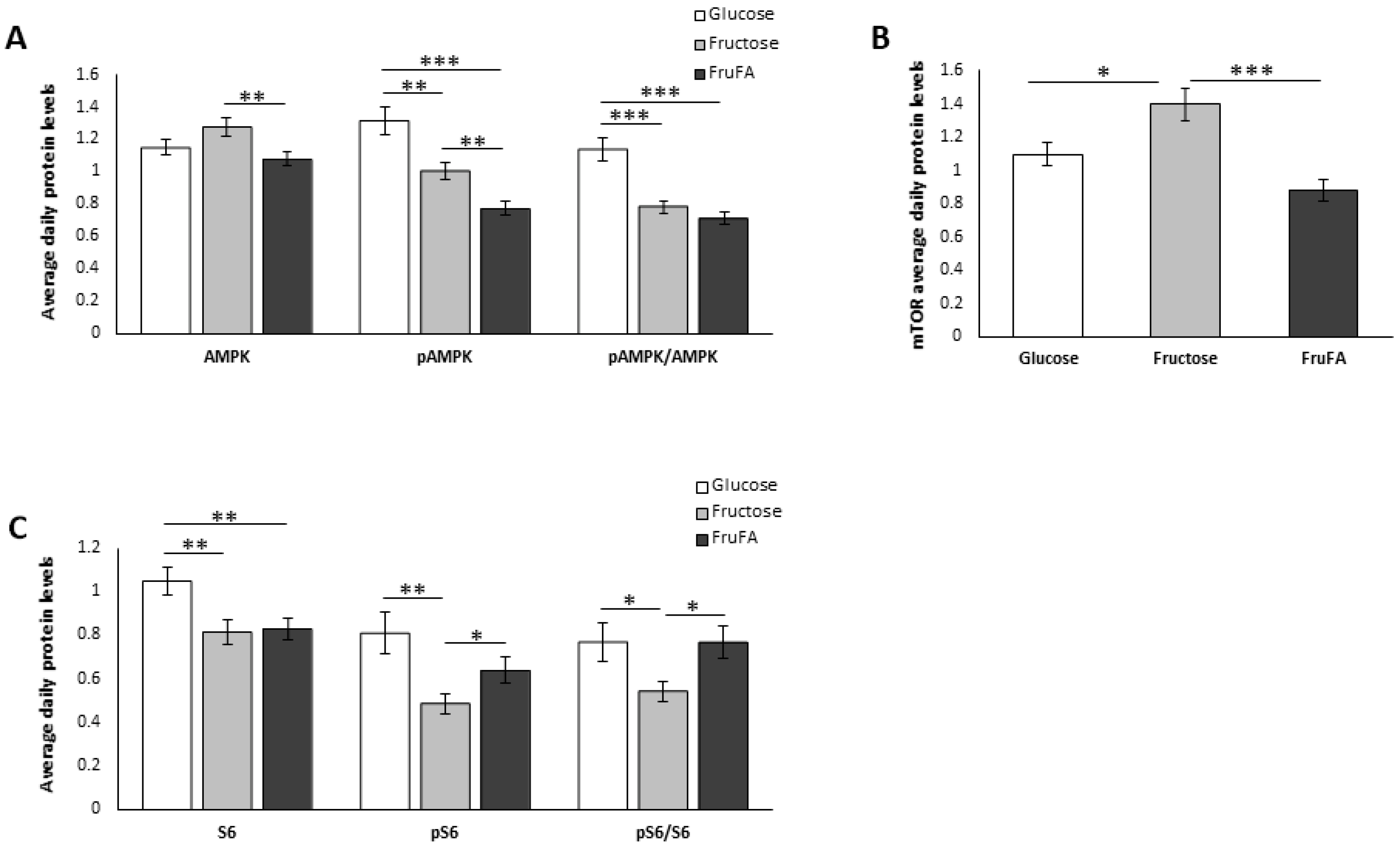
3.3. Effect of Monosaccharides and/or Steatosis on Metabolism
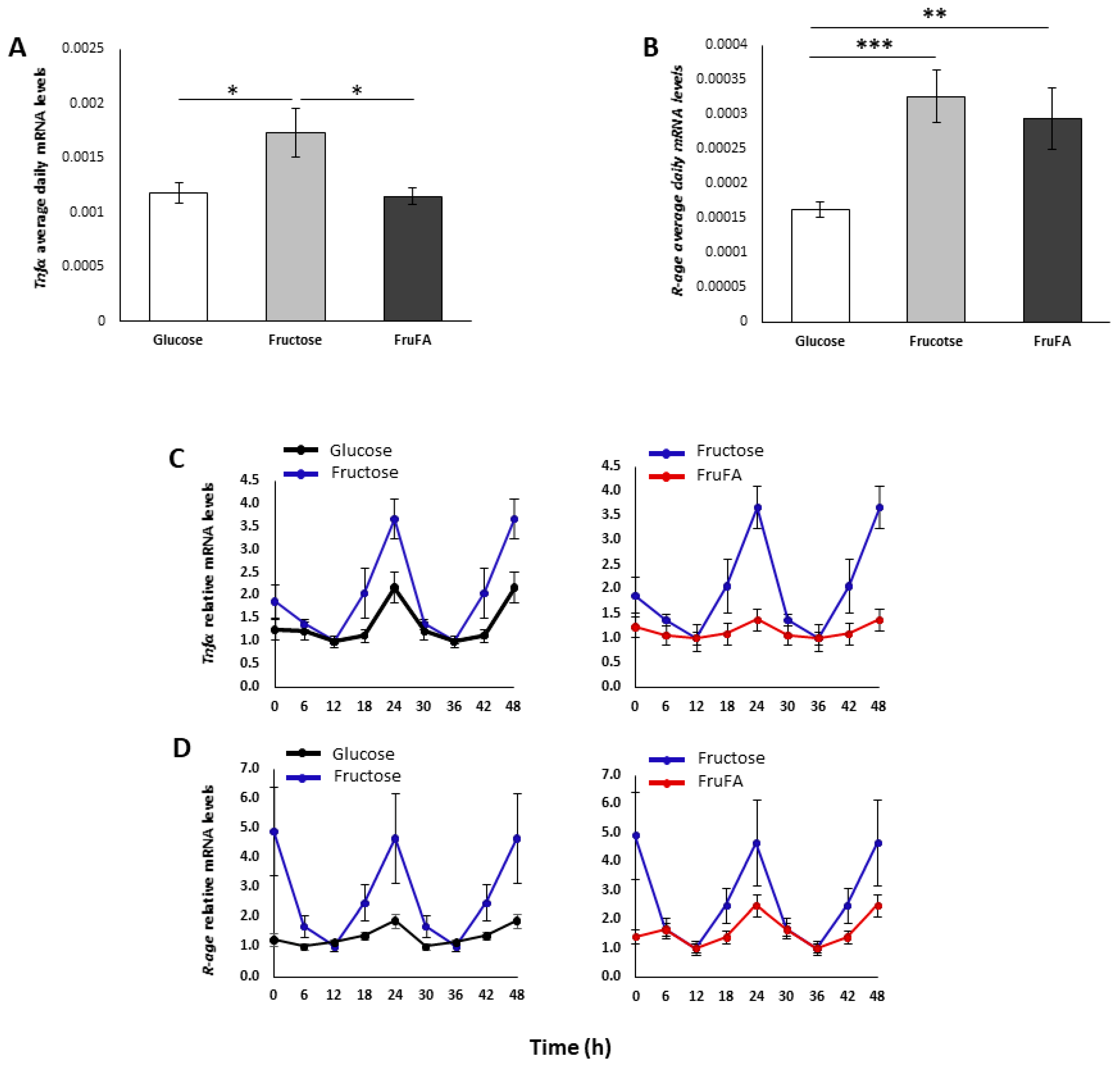
3.4. Effect of Monosaccharides and/or Steatosis on Inflammation
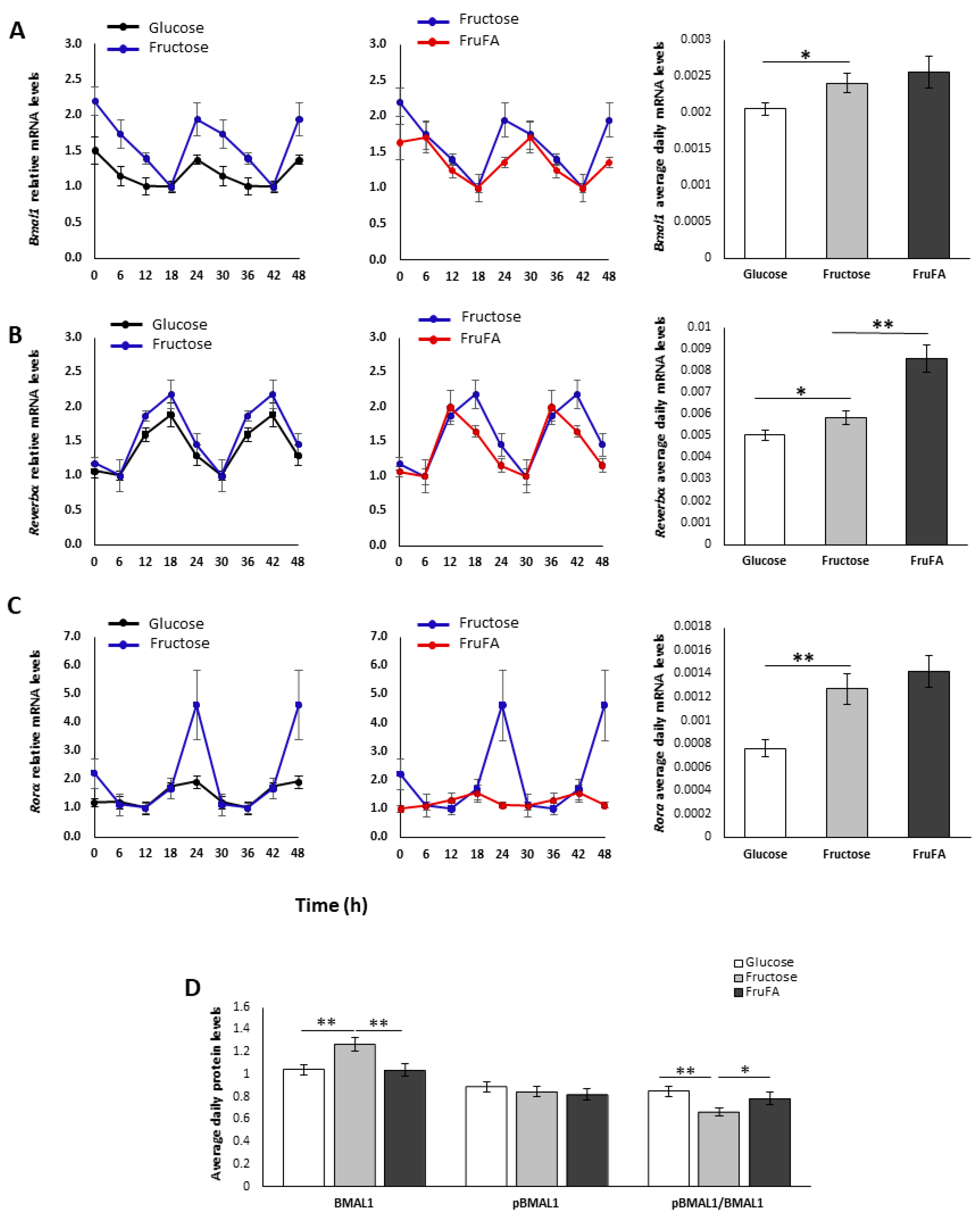
3.5. Effect of Monosaccharides and/or Steatosis on BMAL1-RORα-REV-ERBα Axis
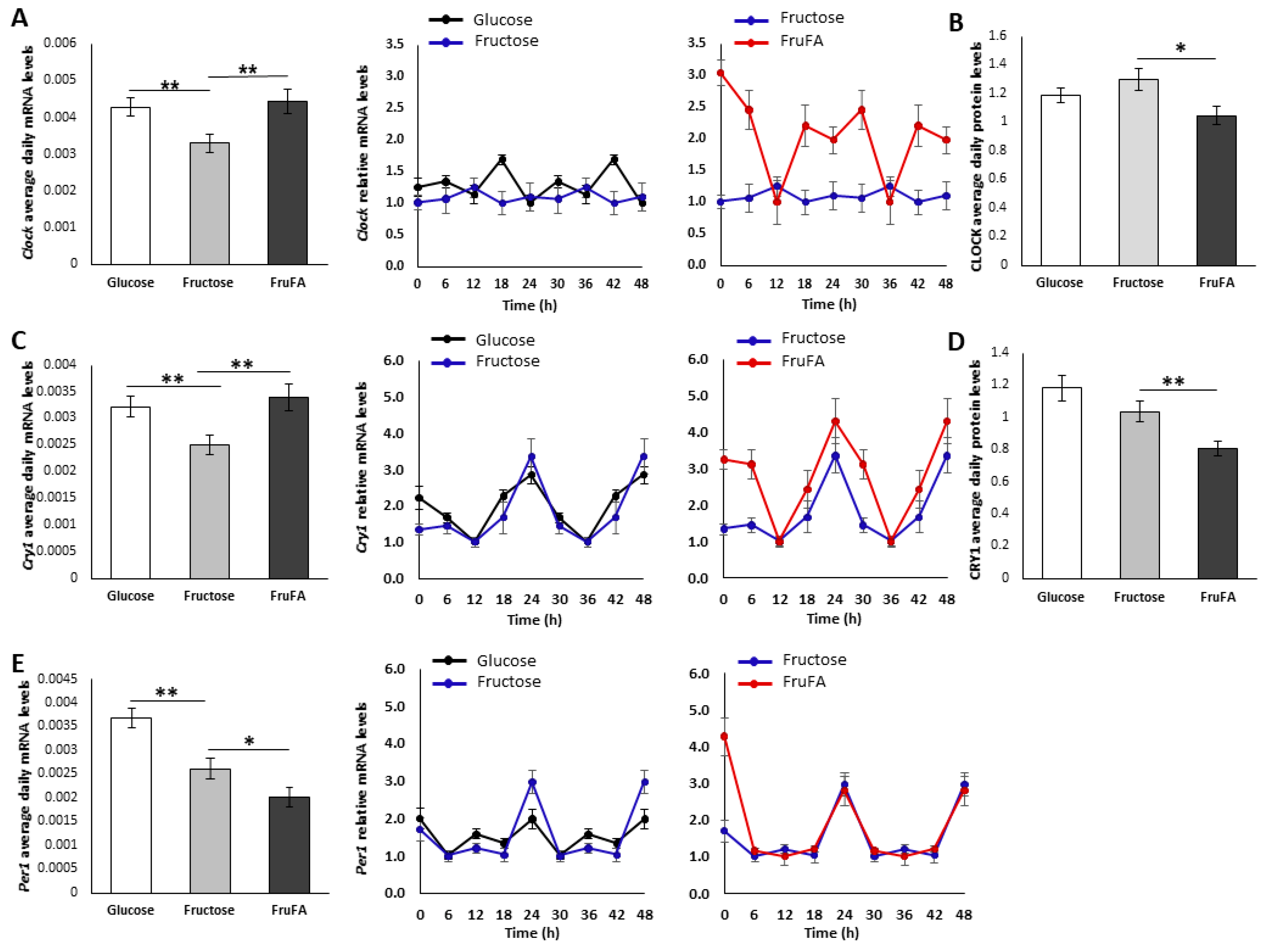
3.6. Effect of Monosaccharides and/or Steatosis on Clock Expression
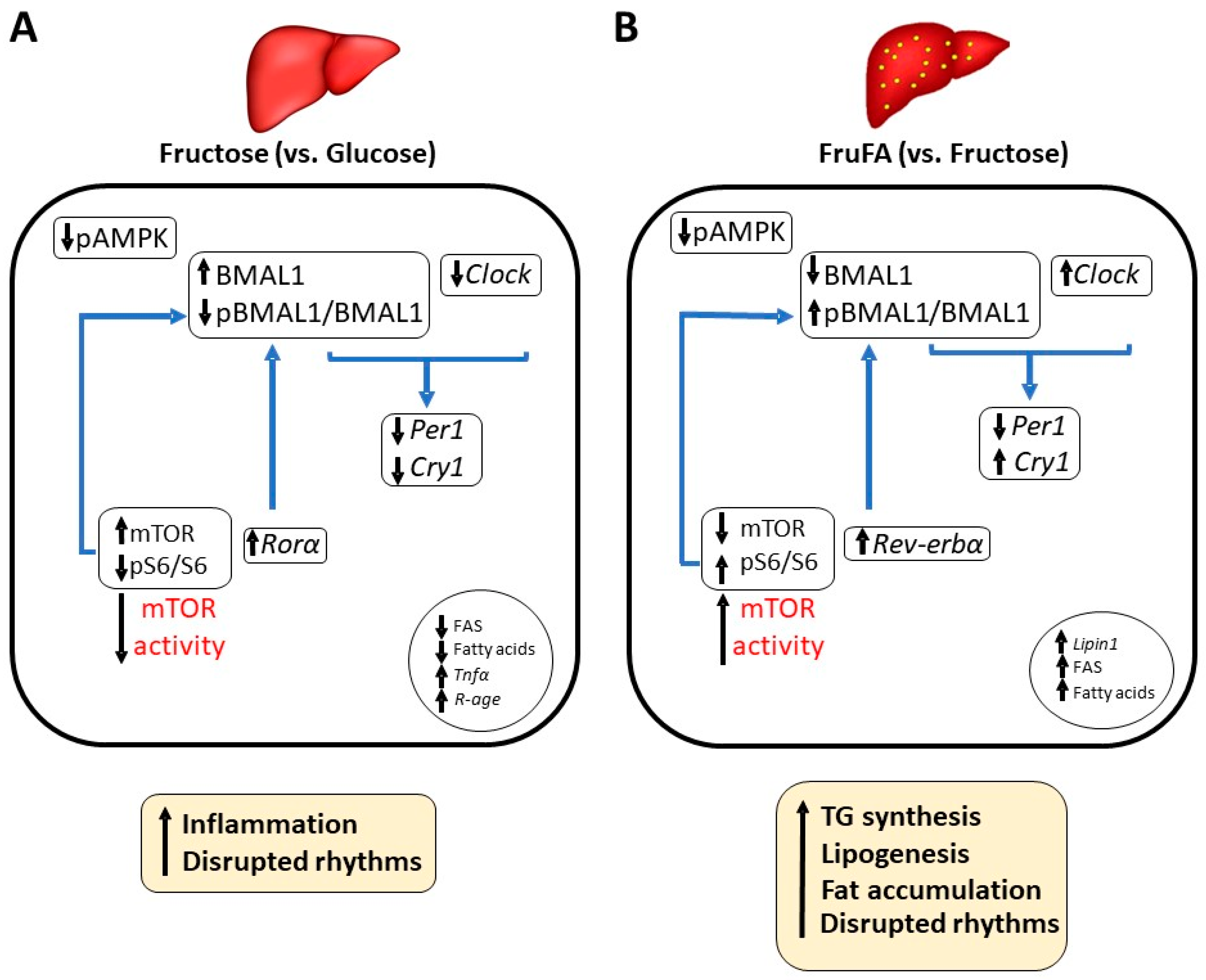
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Froy, O.; Garaulet, M. The Circadian Clock in White and Brown Adipose Tissue: Mechanistic, Endocrine, and Clinical Aspects. Endocr. Rev. 2018, 39, 261–273. [Google Scholar] [CrossRef]
- Preitner, N.; Damiola, F.; Lopez-Molina, L.; Zakany, J.; Duboule, D.; Albrecht, U.; Schibler, U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 2002, 110, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.K.; Panda, S.; Miraglia, L.J.; Reyes, T.M.; Rudic, R.D.; McNamara, P.; Naik, K.A.; FitzGerald, G.A.; Kay, S.A.; Hogenesch, J.B. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron 2004, 43, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.R.; Chen, W.; Adachi, A.; Wakamatsu, H.; Hayashi, S.; Takasugi, T.; Nagano, M.; Nakahama, K.; Suzuki, Y.; Sugano, S.; et al. A transcription factor response element for gene expression during circadian night. Nature 2002, 418, 534–539. [Google Scholar] [CrossRef]
- Qian, J.; Scheer, F. Circadian System and Glucose Metabolism: Implications for Physiology and Disease. Trends Endocrinol. Metab. 2016, 27, 282–293. [Google Scholar] [CrossRef]
- Stenvers, D.J.; Jongejan, A.; Atiqi, S.; Vreijling, J.P.; Limonard, E.J.; Endert, E.; Baas, F.; Moerland, P.D.; Fliers, E.; Kalsbeek, A.; et al. Diurnal rhythms in the white adipose tissue transcriptome are disturbed in obese individuals with type 2 diabetes compared with lean control individuals. Diabetologia 2019, 62, 704–716. [Google Scholar] [CrossRef]
- Eide, E.J.; Woolf, M.F.; Kang, H.; Woolf, P.; Hurst, W.; Camacho, F.; Vielhaber, E.L.; Giovanni, A.; Virshup, D.M. Control of mammalian circadian rhythm by CKIepsilon-regulated proteasome-mediated PER2 degradation. Mol. Cell. Biol. 2005, 25, 2795–2807. [Google Scholar] [CrossRef]
- Um, J.H.; Yang, S.; Yamazaki, S.; Kang, H.; Viollet, B.; Foretz, M.; Chung, J.H. Activation of 5′-AMP-activated kinase with diabetes drug metformin induces casein kinase Iepsilon (CKIepsilon)-dependent degradation of clock protein mPER2. J. Biol. Chem. 2007, 282, 20794–20798. [Google Scholar] [CrossRef]
- Lamia, K.A.; Sachdeva, U.M.; DiTacchio, L.; Williams, E.C.; Alvarez, J.G.; Egan, D.F.; Vasquez, D.S.; Juguilon, H.; Panda, S.; Shaw, R.J.; et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 2009, 326, 437–440. [Google Scholar] [CrossRef]
- Lipton, J.O.; Yuan, E.D.; Boyle, L.M.; Ebrahimi-Fakhari, D.; Kwiatkowski, E.; Nathan, A.; Guttler, T.; Davis, F.; Asara, J.M.; Sahin, M. The Circadian Protein BMAL1 Regulates Translation in Response to S6K1-Mediated Phosphorylation. Cell 2015, 161, 1138–1151. [Google Scholar] [CrossRef] [PubMed]
- Rippe, J.M.; Angelopoulos, T.J. Sucrose, high-fructose corn syrup, and fructose, their metabolism and potential health effects: What do we really know? Adv. Nutr. 2013, 4, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Ha, V.; Jayalath, V.H.; Cozma, A.I.; Mirrahimi, A.; de Souza, R.J.; Sievenpiper, J.L. Fructose-containing sugars, blood pressure, and cardiometabolic risk: A critical review. Curr. Hypertens. Rep. 2013, 15, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Chapnik, N.; Rozenblit-Susan, S.; Genzer, Y.; Froy, O. Differential effect of fructose on fat metabolism and clock gene expression in hepatocytes vs. myotubes. Int. J. Biochem. Cell Biol. 2016, 77, 35–40. [Google Scholar] [CrossRef]
- Sherman, H.; Gutman, R.; Chapnik, N.; Meylan, J.; le Coutre, J.; Froy, O. All-trans retinoic acid modifies the expression of clock and disease marker genes. J. Nutr. Biochem. 2012, 23, 209–217. [Google Scholar] [CrossRef]
- Jakubowicz, D.; Landau, Z.; Tsameret, S.; Wainstein, J.; Raz, I.; Ahren, B.; Chapnik, N.; Barnea, M.; Ganz, T.; Menaged, M.; et al. Reduction in Glycated Hemoglobin and Daily Insulin Dose Alongside Circadian Clock Upregulation in Patients with Type 2 Diabetes Consuming a Three-Meal Diet: A Randomized Clinical Trial. Diabetes Care 2019, 42, 2171–2180. [Google Scholar] [CrossRef]
- Anavi, S.; Harmelin, N.B.; Madar, Z.; Tirosh, O. Oxidative stress impairs HIF1alpha activation: A novel mechanism for increased vulnerability of steatotic hepatocytes to hypoxic stress. Free Radic. Biol. Med. 2012, 52, 1531–1542. [Google Scholar] [CrossRef]
- Hodson, L.; Skeaff, C.M.; Fielding, B.A. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog. Lipid Res. 2008, 47, 348–380. [Google Scholar] [CrossRef]
- Guerra, S.; Mocciaro, G.; Gastaldelli, A. Adipose tissue insulin resistance and lipidome alterations as the characterizing factors of non-alcoholic steatohepatitis. Eur. J. Clin. Investig. 2022, 52, e13695. [Google Scholar] [CrossRef]
- Mocciaro, G.; Gastaldelli, A. Obesity-Related Insulin Resistance: The Central Role of Adipose Tissue Dysfunction. Handb. Exp. Pharmacol. 2022, 274, 145–164. [Google Scholar]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef]
- Chen, M.; Huang, N.; Liu, J.; Huang, J.; Shi, J.; Jin, F. AMPK: A bridge between diabetes mellitus and Alzheimer’s disease. Behav. Brain Res. 2021, 400, 113043. [Google Scholar] [CrossRef] [PubMed]
- Softic, S.; Meyer, J.G.; Wang, G.X.; Gupta, M.K.; Batista, T.M.; Lauritzen, H.; Fujisaka, S.; Serra, D.; Herrero, L.; Willoughby, J.; et al. Dietary Sugars Alter Hepatic Fatty Acid Oxidation via Transcriptional and Post-translational Modifications of Mitochondrial Proteins. Cell Metab. 2019, 30, 735–753.e734. [Google Scholar] [CrossRef]
- Geidl-Flueck, B.; Hochuli, M.; Nemeth, A.; Eberl, A.; Derron, N.; Kofeler, H.C.; Tappy, L.; Berneis, K.; Spinas, G.A.; Gerber, P.A. Fructose- and sucrose- but not glucose-sweetened beverages promote hepatic de novo lipogenesis: A randomized controlled trial. J. Hepatol. 2021, 75, 46–54. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; Mehta, V.; Onkaramurthy, N.; O’Keefe, J.H. Fructose-induced inflammation and increased cortisol: A new mechanism for how sugar induces visceral adiposity. Prog. Cardiovasc. Dis. 2018, 61, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Rizkalla, S.W. Health implications of fructose consumption: A review of recent data. Nutr. Metab. (Lond.) 2010, 7, 82. [Google Scholar] [CrossRef]
- Dekker, M.J.; Su, Q.; Baker, C.; Rutledge, A.C.; Adeli, K. Fructose: A highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E685–E694. [Google Scholar] [CrossRef] [PubMed]
- Le, K.A.; Ith, M.; Kreis, R.; Faeh, D.; Bortolotti, M.; Tran, C.; Boesch, C.; Tappy, L. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am. J. Clin. Nutr. 2009, 89, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Maersk, M.; Belza, A.; Stodkilde-Jorgensen, H.; Ringgaard, S.; Chabanova, E.; Thomsen, H.; Pedersen, S.B.; Astrup, A.; Richelsen, B. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: A 6-mo randomized intervention study. Am. J. Clin. Nutr. 2012, 95, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Bravo, S.; Lowndes, J.; Sinnett, S.; Yu, Z.; Rippe, J. Consumption of sucrose and high-fructose corn syrup does not increase liver fat or ectopic fat deposition in muscles. Appl. Physiol. Nutr. Metab. 2013, 38, 681–688. [Google Scholar] [CrossRef]
- Sun, S.; Hanzawa, F.; Kim, D.; Umeki, M.; Nakajima, S.; Sakai, K.; Ikeda, S.; Mochizuki, S.; Oda, H. Circadian rhythm-dependent induction of hepatic lipogenic gene expression in rats fed a high-sucrose diet. J. Biol. Chem. 2019, 294, 15206–15217. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Jiao, X.; Sun, X.; Huang, Y.; Xu, P.; Xue, Y.; Fu, T.; Liu, J.; Li, Z. Short-Term High Fructose Intake Impairs Diurnal Oscillations in the Murine Cornea. Investig. Ophthalmol. Vis. Sci. 2021, 62, 22. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, D.; Liu, S.; Burkewitz, K.; Kory, N.; Knudsen, N.H.; Alexander, R.K.; Unluturk, U.; Li, X.; Kong, X.; Hyde, A.L.; et al. Hepatic Bmal1 Regulates Rhythmic Mitochondrial Dynamics and Promotes Metabolic Fitness. Cell Metab. 2015, 22, 709–720. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsameret, S.; Chapnik, N.; Froy, O. Differential Effect of Fructose in the Presence or Absence of Fatty Acids on Circadian Metabolism in Hepatocytes. Metabolites 2023, 13, 138. https://doi.org/10.3390/metabo13020138
Tsameret S, Chapnik N, Froy O. Differential Effect of Fructose in the Presence or Absence of Fatty Acids on Circadian Metabolism in Hepatocytes. Metabolites. 2023; 13(2):138. https://doi.org/10.3390/metabo13020138
Chicago/Turabian StyleTsameret, Shani, Nava Chapnik, and Oren Froy. 2023. "Differential Effect of Fructose in the Presence or Absence of Fatty Acids on Circadian Metabolism in Hepatocytes" Metabolites 13, no. 2: 138. https://doi.org/10.3390/metabo13020138
APA StyleTsameret, S., Chapnik, N., & Froy, O. (2023). Differential Effect of Fructose in the Presence or Absence of Fatty Acids on Circadian Metabolism in Hepatocytes. Metabolites, 13(2), 138. https://doi.org/10.3390/metabo13020138




