Lifestyle and Quality of Life of Women Diagnosed with Hypothyroidism in the Context of Non-Alcoholic Fatty Liver
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Methodology
2.3. Data Analysis
2.3.1. Data Interpretation: KomPAN Questionnaire
2.3.2. Data Interpretation: World Health Organization Quality of Life Brief Version
2.3.3. Data Interpretation: Anthropometric and Body Composition
3. Results
3.1. Dietary Behaviors and Lifestyle
3.2. Quality of Life
3.3. Body Composition Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agyapong, G.; Dashti, F.; Banini, B.A. Non-alcoholic liver disease: Epidemiology, risk factors, natural history, and management strategies. Ann. N. Y. Acad. Sci. 2023, 1526, 16–29. [Google Scholar] [CrossRef]
- Vidal-Cevallos, P.; Murúa-Beltrán Gall, S.; Uribe, M.; Chávez-Tapia, N.C. Understanding the Relationship between Non-alcoholic Fatty Liver Disease and Thyroid Disease. Int. J. Mol. Sci. 2023, 24, 14605. [Google Scholar] [CrossRef]
- Janota, B.; Szczepańska, E.; Adamek, B.; Janczewska, E. Hypothyroidism and non-alcoholic fatty liver disease: A coincidence or a causal relationship? World J. Hepatol. 2023, 15, 641–648. [Google Scholar] [CrossRef]
- Elshinshawy, S.; Elhaddad, H.; Abdel, S.; Shaker, O.; Salam, R.; Yosry, A.; Elebrashy, I. The Interrelation Between Hypothyroidism and Non-alcoholic Fatty Liver Disease, a Cross-sectional Study. J. Clin. Exp. Hepatol. 2023, 13, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Ahima, R.S. Endocrine disorders associated with obesity. Best Pract. Res. Clin. Obstet. Gynaecol. 2023, 90, 102394. [Google Scholar] [CrossRef]
- Prashanth, A.; Kothari, R.; Yogesh, S.; Mittal, G.; Gheewala, S.; Bokariya, P.; Palande, A.; Tamrakar, S.; Vemparala, S.S.; Sushmitha, S.; et al. Examining Body Fat Percentage, Galvanic Skin Response, and Muscle Grip Strength in Female Hypothyroid Patients. Cureus 2023, 15, e42023. [Google Scholar] [CrossRef]
- Yang, Q.; Cao, H.; Zeng, Q.; Fu, B. Accumulative prediction values of serum thyroid stimulating hormone and visceral adipose tissue for metabolic syndrome in postmenopausal women: A 10-year follow-up study of Chinese population. J. Diabetes 2023. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Fang, D.; Wang, H.; Wang, J.; Yuan, Y.; Huang, S.; Ma, H.; Gu, T.; Bi, Y. The association between visceral adipocyte hypertrophy and NAFLD in subjects with different degrees of adiposity. Hepatol. Int. 2023, 17, 215–224. [Google Scholar] [CrossRef]
- Okekunle, A.P.; Youn, J.; Song, S.; Chung, G.E.; Yang, S.Y.; Kim, Y.S.; Lee, J.E. Predicted pro-inflammatory hs-CRP score and non-alcoholic fatty liver disease. Gastroenterol. Rep. 2023, 11, goad059. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, K.W.; Lee, J.; Park, T.; Park, H.J.; Song, G.W.; Lee, S.G. Reduction of Visceral Adiposity as a Predictor for Resolution of Non-alcoholic Fatty Liver in Potential Living Liver Donors. Liver Transpl. 2021, 27, 1424–1431. [Google Scholar] [CrossRef]
- Glass, O.; Liu, D.; Bechard, E.; Guy, C.D.; Pendergast, J.; Mae Diehl, A.; Abdelmalek, M.F. Perceptions of Exercise and Its Challenges in Patients With Non-alcoholic Fatty Liver Disease: A Survey-Based Study. Hepatol. Commun. 2022, 6, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Westfall, E.; Jeske, R.; Bader, A.R. Non-alcoholic Fatty Liver Disease: Common Questions and Answers on Diagnosis and Management. Am. Fam. Physician 2020, 102, 603–612. [Google Scholar] [PubMed]
- Stadler, S.; Mohr, A.; Wagner, A.; Bäßler, A.; Fischer, M.; Putz, F.J.; Strack, C.; Li, J.; Arzt, M. Weight loss induced alleviation of sleep-disordered breathing is associated with improvement of non-alcoholic fatty liver disease. Sleep Med. 2023, 112, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, S.; Xu, M.; Zhao, D.; Wang, X.; Zhang, L.; Chen, D.; Du, J.; Yu, R.; Li, H.; et al. The association between sleep duration, quality, and non-alcoholic fatty liver disease: A cross-sectional study. Open Med. 2023, 18, 20230670. [Google Scholar] [CrossRef] [PubMed]
- Janota, B.; Szczepańska, E.; Noras, K.; Janczewska, E. Lifestyle and Quality of Life of Women with Diagnosed Hypothyroidism in the Context of Metabolic Disorders. Metabolites 2023, 13, 1033. [Google Scholar] [CrossRef] [PubMed]
- Taghdir, M.; Salehi, A.; Parastouei, K.; Abbaszadeh, S. Relationship between diet quality and non-alcoholic fatty liver disease predictor indices in Iranian patients with metabolic syndrome: A cross-sectional study. Food Sci. Nutr. 2023, 11, 6133–6139. [Google Scholar] [CrossRef]
- Tryndyak, V.P.; Willett, R.A.; Nagumalli, S.K.; Li, D.; Avigan, M.I.; Beland, F.A.; Rusyn, I.; Pogribny, I.P. Effect of an obesogenic high-fat and high-sucrose diet on hepatic gene expression signatures in male Collaborative Cross mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2023, 324, G232–G243. [Google Scholar] [CrossRef]
- Reddy, A.; Gatta, P.D.; Mason, S.; Nicoll, A.J.; Ryan, M.; Itsiopoulos, C.; Abbott, G.; Johnson, N.A.; Sood, S.; Roberts, S.K.; et al. Adherence to a Mediterranean diet may improve serum adiponectin in adults with non-alcoholic fatty liver disease: The MEDINA randomized controlled trial. Nutr. Res. 2023, 119, 98–108. [Google Scholar] [CrossRef]
- Barrea, L.; Verde, L.; Annunziata, G.; Camajani, E.; Caprio, M.; Sojat, A.S.; Marina, L.V.; Guarnotta, V.; Colao, A.; Muscogiuri, G. Role of Mediterranean diet in endocrine diseases: A joint overview by the endocrinologist and the nutritionist. J. Endocrinol. Investig. 2023. [CrossRef]
- Barrea, L.; Verde, L.; Simancas-Racines, D.; Zambrano, A.K.; Frias-Toral, E.; Colao, A.; Savastano, S.; Muscogiuri, G. Adherence to the Mediterranean diet as a possible additional tool to be used for screening the metabolically unhealthy obesity (MUO) phenotype. J. Transl. Med. 2023, 21, 675. [Google Scholar] [CrossRef]
- Matlock, C.L.; Vanhoof, A.R.; Rangrej, S.B.; Rathore, R. Comparison Between Levothyroxine and Lifestyle Intervention on Subclinical Hypothyroidism in Women: A Review. Cureus 2023, 15, e38309. [Google Scholar] [CrossRef] [PubMed]
- AlAwaji, M.I.; Alhamwy, R.H. The Impact of Hypothyroidism on the Quality of Life of Adults in Riyadh, Saudi Arabia. Cureus 2023, 15, e37636. [Google Scholar] [CrossRef]
- Ghamri, R.; Babaker, R.; Ezzat, S.; Alsaedi, H.; Alkhamisi, M.; Arbaein, R.; Alyahya, R.; Fayraq, S.; Alamri, S. Assessment of Quality of Life Among Patients With Primary Hypothyroidism: A Case-Control Study. Cureus 2022, 14, e29947. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, J.; Liu, Y.; Zhou, H.; Yan, W.; Ren, H. The Relationship between Health-Related Fitness and Quality of Life in Non-alcoholic Fatty Liver Disease. Int. J. Environ. Res. Public. Health 2022, 19, 14215. [Google Scholar] [CrossRef]
- Golubeva, J.A.; Sheptulina, A.F.; Yafarova, A.A.; Mamutova, E.M.; Kiselev, A.R.; Drapkina, O.M. Reduced Quality of Life in Patients with Non-Alcoholic Fatty Liver Disease May Be Associated with Depression and Fatigue. Healthcare 2022, 10, 1699. [Google Scholar] [CrossRef] [PubMed]
- Jeżewska-Zychowicz, M.; Gawęcki, J.; Wądołowska, L.; Czarnocińska, J.; Galiński, G.; Kołłajtis-Dołowy, A.; Roszkowski, W.; Wawrzyniak, A.; Przybyłowicz, K.; Stasiewicz, B.; et al. KomPAN®Kwestionariusz do Badania Pogladów i Zwyczajów Zywieniowychdla Osób w Wieku od 16 do 65 lat, Wersja 1.2—Kwestionariusz do Samodzielnego Wypełnienia przez Respondenta. Rozdz. 2. In KomPAN®Kwestionariusz do Badania Pogladów i Zwyczajów Zywieniowych oraz Procedura Opracowania Danych; Komitet Nauki o Zywieniu Człowieka Polskiej Akademii Nauk: Warszawa, Poland, 2020; pp. 22–34. Available online: https://www.knozc.pan.pl/ (accessed on 11 October 2023).
- The Whoqol Group. Development of the World Health Organization WHOQOL-BREF Quality of Life Assessment. Psychol. Med. 1998, 28, 551–558. [Google Scholar] [CrossRef]
- Karlas, T.; Petroff, D.; Sasso, M.; Fan, J.G.; Mi, Y.Q.; de Lédinghen, V.; Kumar, M.; Lupsor-Platon, M.; Han, K.H.; Cardoso, A.C.; et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J. Hepatol. 2017, 66, 1022–1030. [Google Scholar] [CrossRef]
- Sirli, R.; Sporea, I. Controlled Attenuation Parameter for Quantification of Steatosis: Which Cut-Offs to Use? Can. J. Gastroenterol. Hepatol. 2021, 2021, 6662760. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 15 March 2023).
- Warnes, G.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S.; et al. gplots: Various R Programming Tools for Plotting Data. R Package Version 3.1.3. 2022. Available online: https://CRAN.R-project.org/package=gplots (accessed on 10 October 2023).
- Zejda, J.; Kowalska, M.; Brożek, G. Proste testy statystycznej znamienności zależności. In Biostatystyka. Praktyczne Metody Analizy Danych w Obserwacyjnych Badaniach Epidemiologicznych; Śląski Uniwersytet Medyczny W Katowicach: Katowice, Poland, 2015; ISBN 978-83-7509-297-7. [Google Scholar]
- Bikeyeva, V.; Abdullah, A.; Radivojevic, A.; Jad, A.A.; Ravanavena, A.; Ravindra, C.; Igweonu-Nwakile, E.O.; Ali, S.; Paul, S.; Yakkali, S.; et al. Non-alcoholic Fatty Liver Disease and Hypothyroidism: What You Need to Know. Cureus 2022, 14, e28052. [Google Scholar] [CrossRef]
- R, M.; R, M.; S, R.; Ganga, R. To Assess Non Alcoholic Fatty Liver Disease in Patients with Clinical and Subclinical Hypothyroidism. J. Assoc. Physicians India 2023, 71, 1. [Google Scholar]
- Smith, H.A.; Betts, J.A. Nutrient timing and metabolic regulation. J. Physiol. 2022, 600, 1299–1312. [Google Scholar] [CrossRef]
- Alhussain, M.H.; Macdonald, I.A.; Taylor, M.A. Impact of isoenergetic intake of irregular meal patterns on thermogenesis, glucose metabolism, and appetite: A randomized controlled trial. Am. J. Clin. Nutr. 2022, 115, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Suárez, M.; Boqué, N.; Del Bas, J.M.; Mayneris-Perxachs, J.; Arola, L.; Caimari, A. Mediterranean Diet and Multi-Ingredient-Based Interventions for the Management of Non-Alcoholic Fatty Liver Disease. Nutrients 2017, 9, 1052. [Google Scholar] [CrossRef] [PubMed]
- Curci, R.; Bianco, A.; Franco, I.; Bonfiglio, C.; Campanella, A.; Mirizzi, A.; Giannuzzi, V.; Cozzolongo, R.; Veronese, N.; Osella, A.R. Lifestyle Modification: Evaluation of the Effects of Physical Activity and Low-Glycemic-Index Mediterranean Diet on Fibrosis Score. Nutrients 2023, 15, 3520. [Google Scholar] [CrossRef]
- Donghia, R.; Pesole, P.L.; Castellaneta, A.; Coletta, S.; Squeo, F.; Bonfiglio, C.; De Pergola, G.; Rinaldi, R.; De Nucci, S.; Giannelli, G.; et al. Age-Related Dietary Habits and Blood Biochemical Parameters in Patients with and without Steatosis-MICOL Cohort. Nutrients 2023, 15, 4058. [Google Scholar] [CrossRef]
- Guo, W.; Ge, X.; Lu, J.; Xu, X.; Gao, J.; Wang, Q.; Song, C.; Zhang, Q.; Yu, C. Diet and Risk of Non-Alcoholic Fatty Liver Disease, Cirrhosis, and Liver Cancer: A Large Prospective Cohort Study in UK Biobank. Nutrients 2022, 14, 5335. [Google Scholar] [CrossRef] [PubMed]
- Ivancovsky-Wajcman, D.; Fliss-Isakov, N.; Grinshpan, L.S.; Salomone, F.; Lazarus, J.V.; Webb, M.; Shibolet, O.; Kariv, R.; Zelber-Sagi, S. High Meat Consumption Is Prospectively Associated with the Risk of Non-Alcoholic Fatty Liver Disease and Presumed Significant Fibrosis. Nutrients 2022, 14, 3533. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, Y.; Meng, G.; Zhang, Q.; Liu, L.; Wu, H.; Gu, Y.; Wang, X.; Zhang, J.; Sun, S.; et al. Protein foods from animal sources and risk of non-alcoholic fatty liver disease in representative cohorts from North and South China. J. Intern. Med. 2023, 293, 340–353. [Google Scholar] [CrossRef]
- Das, A.; Tang, Y.L.M.; Althumiri, N.A.; Garcia-Larsen, V.; Schattenberg, J.M.; Alqahtani, S.A. Fatty acid composition but not quantity is an important indicator of non-alcoholic fatty liver disease: A systematic review. Eur. J. Clin. Nutr. 2023. [CrossRef]
- Yabe, Y.; Kim, T.; Oh, S.; Shida, T.; Oshida, N.; Hasegawa, N.; Okada, K.; Someya, N.; Mizokami, Y.; Shoda, J. Relationships of Dietary Habits and Physical Activity Status with Non-Alcoholic Fatty Liver Disease Featuring Advanced Fibrosis. Int. J. Environ. Res. Public Health 2021, 18, 8918. [Google Scholar] [CrossRef]
- Silva, T.J.; Barrera-Arellano, D.; Ribeiro, A.P.B. Margarines: Historical approach, technological aspects, nutritional profile, and global trends. Food Res. Int. 2021, 147, 110486. [Google Scholar] [CrossRef] [PubMed]
- Barroso, L.N.; Salarini, J.; Leite, N.C.; Villela-Nogueira, C.A.; Dávalos, A.; Carmo, M.D.G.T.; Ferreira Peres, W.A. Effect of fish oil supplementation on the concentration of miRNA-122, FGF-21 and liver fibrosis in patients with NAFLD: Study protocol for a randomized, double-blind and placebo-controlled clinical trial. Clin. Nutr. ESPEN 2023, 57, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, X.; Liu, X.; Wu, X.; Xu, Q.; Qu, J.; Li, X.; Zhu, Y.; Wen, L.; Wang, J. High-Carbohydrate Diet Consumption Poses a More Severe Liver Cholesterol Deposition than a High-Fat and High-Calorie Diet in Mice. Int. J. Mol. Sci. 2023, 24, 14700. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.S.; Lin, W.T.; Ting, P.S.; Huang, C.K.; Chen, P.H.; Gonzalez, G.V.; Lin, H.Y. Sugar-Sweetened Beverages and Artificially Sweetened Beverages Consumption and the Risk of Non-alcoholic Fatty Liver (NAFLD) and Non-alcoholic Steatohepatitis (NASH). Nutrients 2023, 15, 3997. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhai, D.; Zhang, T.; Mudoti, N.G.; Chang, Q.; Liu, Y.; Zhao, Y.; Ding, Y.; Xia, Y. Meta-analysis of the association between major foods with added fructose and non-alcoholic fatty liver disease. Food Funct. 2023, 14, 5551–5561. [Google Scholar] [CrossRef] [PubMed]
- Montemayor, S.; García, S.; Monserrat-Mesquida, M.; Tur, J.A.; Bouzas, C. Dietary Patterns, Foods, and Nutrients to Ameliorate Non-Alcoholic Fatty Liver Disease: A Scoping Review. Nutrients 2023, 15, 3987. [Google Scholar] [CrossRef]
- Jafarikhah, R.; Damirchi, A.; Rahmani Nia, F.; Razavi-Toosi, S.M.T.; Shafaghi, A.; Asadian, M. Effect of functional resistance training on the structure and function of the heart and liver in patients with non-alcoholic fatty liver. Sci. Rep. 2023, 13, 15475. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Banini, B.A.; Do, A.; Gunderson, C.; Zaman, S.; Lim, J.K. Exercise Does Not Independently Improve Histological Outcomes in Biopsy-Proven Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Genes 2023, 14, 1811. [Google Scholar] [CrossRef]
- Um, Y.J.; Chang, Y.; Jung, H.S.; Cho, I.Y.; Shin, J.H.; Shin, H.; Wild, S.H.; Byrne, C.D.; Ryu, S. Decrease in Sleep Duration and Poor Sleep Quality over Time Is Associated with an Increased Risk of Incident Non-Alcoholic Fatty Liver Disease. J. Pers. Med. 2022, 12, 92. [Google Scholar] [CrossRef]
- Ezpeleta, M.; Gabel, K.; Cienfuegos, S.; Kalam, F.; Lin, S.; Pavlou, V.; Varady, K.A. Alternate-Day Fasting Combined with Exercise: Effect on Sleep in Adults with Obesity and NAFLD. Nutrients 2023, 15, 1398. [Google Scholar] [CrossRef]
- Roa Dueñas, O.H.; Hofman, A.; Luik, A.I.; Medici, M.; Peeters, R.P.; Chaker, L. The Cross-sectional and Longitudinal Association Between Thyroid Function and Depression: A Population-Based Study. J. Clin. Endocrinol. Metab. 2023, 19, dgad620. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Zhang, R.; Zhao, C.; Wang, Y.; Tu, X.; Duan, W. Associations of depression score with metabolic dysfunction-associated fatty liver disease and liver fibrosis. J. Affect. Disord. 2023, 334, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Asquith, E.; Bould, K.; Catling, J.C.; Day, E.J.; Holt, A. Behaviour regulation and the role of mental health in non-alcoholic fatty liver disease. BMC Gastroenterol. 2023, 23, 306. [Google Scholar] [CrossRef] [PubMed]
- Perez-Diaz-Del-Campo, N.; Castelnuovo, G.; Rosso, C.; Nicolosi, A.; Guariglia, M.; Dileo, E.; Armandi, A.; Caviglia, G.P.; Bugianesi, E. Impact of Health Related QoL and Mediterranean Diet on Liver Fibrosis in Patients with NAFLD. Nutrients 2023, 15, 3018. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Aggarwal, P.; Shrestha, I.; Fernandes, J.; Johansen, P.; Augusto, M.; Nair, S. The burden of non-alcoholic steatohepatitis: A systematic review of health-related quality of life and patient-reported outcomes. JHEP Rep. 2022, 4, 100525. [Google Scholar] [CrossRef]
- Musio, A.; Perazza, F.; Leoni, L.; Stefanini, B.; Dajti, E.; Menozzi, R.; Petroni, M.L.; Colecchia, A.; Ravaioli, F. Osteosarcopenia in NAFLD/MAFLD: An Underappreciated Clinical Problem in Chronic Liver Disease. Int. J. Mol. Sci. 2023, 24, 7517. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Vachliotis, I.D.; Mantzoros, C.S. Sarcopenia, sarcopenic obesity and non-alcoholic fatty liver disease. Metabolism 2023, 147, 155676. [Google Scholar] [CrossRef]
- Szlejf, C.; Suemoto, C.K.; Janovsky, C.C.P.S.; Barreto, S.M.; Diniz, M.F.H.S.; Lotufo, P.A.; Bensenor, I.M. Thyroid Function and Sarcopenia: Results from the ELSA-Brasil Study. J. Am. Geriatr. Soc. 2020, 68, 1545–1553. [Google Scholar] [CrossRef]
- Pekgor, S.; Duran, C.; Kutlu, R.; Solak, I.; Pekgor, A.; Eryilmaz, M.A. Visceral Adiposity Index Levels in Patients with Hypothyroidism. J. Natl. Med. Assoc. 2018, 110, 606–613. [Google Scholar] [CrossRef]
- Properzi, C.; Adams, L.A.; Lo, J.; Sherriff, J.L.; Jeffrey, G.P.; O’Sullivan, T.A. Higher Overall Intakes Are the Defining Feature of Dietary Intakes in NAFLD and Compared to the General Population. Nutrients 2023, 15, 2669. [Google Scholar] [CrossRef]
- Tian, H.; Qu, H.; Zheng, Y.; Sun, Y.; Wang, W.; Wu, Y. Association of dietary inflammatory potential and non-alcoholic fatty liver disease in US adults. Eur. J. Gastroenterol. Hepatol. 2023, 35, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
| Group H NAFL Hypothyroidism and NAFL | Group H Hypothyroidism without NAFL | Group NAFL NAFL without Hypothyroidism | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age | 59.5 | 13 | 56.6 | 16 | 56.8 | 12 |
| BMI a | 28.4 | 6.6 | 24.4 | 2.9 | 30.9 | 7.1 |
| WHR b | 0.87 | 0.08 | 0.83 | 0.08 | 0.95 | 0.09 |
| WHtR c | 0.54 | 0.16 | 0.5 | 0.11 | 0.6 | 0.16 |
| Mean | N | Mean | N | Mean | N | |
| Glucose (g/dL) | 106 | 42 | 95.2 | 15 | 95.9 | 10 |
| Total cholesterol (g/dL) | 213.2 | 15 | 188.8 | 14 | 207.1 | 7 |
| N | % | N | % | N | % | |
| Smoking (Yes) | 9 | 11.5 | 5 | 17.9 | 3 | 10.7 |
| Eating Behavior | H NAFL | H | NAFL | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Number of consumed meals | 3.6 | 0.7 | 3.9 | 0.9 | 3.8 | 0.9 |
| N | % | N | % | N | % | |
| Declaration of regular meal consumption | 47 | 60.3 | 20 | 71.4 | 64.3 | 18 |
| Declaration of snack consumption | 67 | 86.9 | 26 | 92.9 | 22 | 78.6 |
| Declaration of sweetening drinks (with sugar or honey) | 27 | 34.6 | 7 | 25 | 6 | 21.4 |
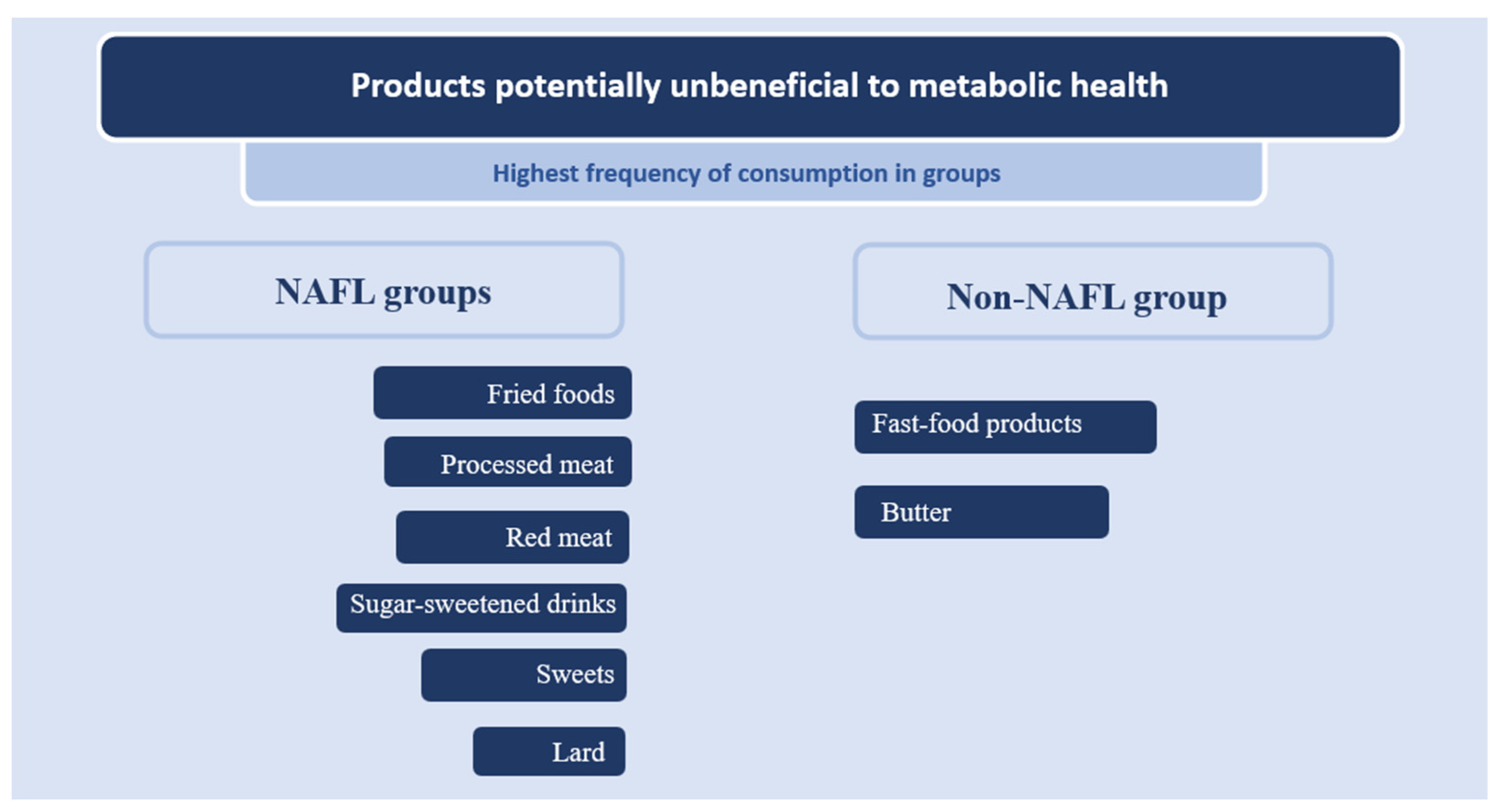
| Product | Frequency of Consumption | |||||
|---|---|---|---|---|---|---|
| H NAFL | H | NAFL | ||||
| Mean | SD | Mean | SD | Mean | SD | |
| Vegetables | 4.77 | 0.95 | 4.75 | 1.27 | 4.46 | 0.74 |
| Fruits | 4.55 | 1.11 | 4.71 | 1.33 | 4.39 | 1.10 |
| Whole-grain bread | 3.31 | 1.78 | 3.48 | 1.78 | 3.43 | 1.77 |
| Legume seeds | 2.32 | 0.89 | 2.46 | 0.96 | 2.18 | 0.72 |
| Whole grains | 3.10 | 1.25 | 3.04 | 1.02 | 3.11 | 1.47 |
| Fish | 2.76 | 0.81 | 2.86 | 0.85 | 2.64 | 0.83 |
| Fermented dairy products | 3.53 | 1.26 | 3.44 | 1.37 | 3.46 | 1.07 |
| White meat | 3.54 | 0.77 | 3.29 | 1.15 | 3.43 | 0.84 |
| Eggs | 3.50 | 0.83 | 3.85 | 0.86 | 3.39 | 0.92 |
| Freshly squeezed juices | 2.17 | 1.29 | 2.18 | 1.09 | 2.21 | 1.10 |
| Lard | 2.13 | 1.53 | 1.52 | 0.85 | 1.71 | 1.01 |
| Butter | 4.12 | 1.65 | 4.41 | 1.76 | 4.32 | 1.36 |
| Cottage cheese | 3.29 | 1.23 | 2.89 | 0.93 | 3.04 | 1.23 |
| Fried foods | 3.08 | 1.21 | 2.89 | 1.31 | 3.29 | 1.18 |
| Processed meat | 3.63 | 1.23 | 3.74 | 1.51 | 3.96 | 0.79 |
| Red meat | 2.94 | 1.01 | 2.54 | 1.10 | 2.96 | 1.07 |
| Fast food | 1.63 | 0.79 | 1.71 | 0.60 | 1.57 | 0.74 |
| Sweets | 3.31 | 1.46 | 2.96 | 1.37 | 3.54 | 1.53 |
| Sugar-sweetened drinks | 1.49 | 1.04 | 1.29 | 0.53 | 1.29 | 0.46 |
| Packaged juices | 1.55 | 1.03 | 1.64 | 0.87 | 1.32 | 0.67 |
| Lifestyle Factor | H NAFL | H | NAFL | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Number of hours of sleep on weekdays | 1.67 | 0.53 | 1.70 | 0.61 | 1.71 | 0.53 |
| Number of hours of sleep on weekends | 1.87 | 0.53 | 1.89 | 0.58 | 1.79 | 0.57 |
| Assessment of activity during work or school days | 1.62 | 0.69 | 1.74 | 0.66 | 1.57 | 0.50 |
| Assessment of activity on weekends | 1.67 | 0.66 | 1.78 | 0.70 | 1.57 | 0.57 |
| Number of hours spent using electronic devices | 2.63 | 1.42 | 2.56 | 1.25 | 2.75 | 1.55 |
| Selected Element | H NAFL | H | NAFL |
|---|---|---|---|
| Mean | Mean | Mean | |
| Quality of life | 3.75 | 3.70 | 3.50 |
| Satisfaction with health * | 3.30 | 3.36 | 2.89 |
| Amount of joy in life | 3.72 | 3.75 | 3.54 |
| Concentration of attention | 3.89 | 3.64 | 3.54 |
| Level of energy | 3.57 | 3.54 | 3.29 |
| Physical appearance | 3.76 | 3.61 | 3.50 |
| Adaptability to circumstances * | 3.87 | 3.54 | 3.50 |
| Money compared to the needs | 3.45 | 3.86 | 3.64 |
| Quality of sleep | 3.10 | 3.25 | 2.93 |
| Self-satisfaction | 3.67 | 3.89 | 3.57 |
| Support of friends | 4.14 | 3.93 | 4.04 |
| Negative emotions (sadness, melancholy, depression) | 3.5 | 3.4 | 3.6 |
| Average: World Health Organization Quality of Life Brief Version | 3.70 | 3.69 | 3.57 |
| Body Composition Parameters | H NAFL | H | NAFL | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| SMM (skeletal muscle mass) (kg) | 26.1 | 3.6 | 23.7 | 37 | 26.3 | 4.7 |
| PBF (percentage of body fat) (%) * | 38.7 | 7.9 | 32.8 | 5.6 | 40.4 | 8.5 |
| Extracellular water index | 0.4 | 0 | 0.4 | 0 | 0.4 | 0 |
| VFA (visceral fat area) (cm2) * | 154.7 | 57.0 | 106.4 | 37.8 | 165.3 | 67.3 |
| Phase angle (°) | 5 | 0.6 | 5 | 0.7 | 5.2 | 0.5 |
| FFMI (free fat mass index) (kg/m2) * | 17.7 | 1.7 | 16.5 | 1.4 | 18.1 | 2.1 |
| FMI (fat mass index) (kg/m2) * | 11.9 | 4.6 | 8.2 | 2.4 | 13.2 | 5.4 |
| Waist circumference (cm) * | 94.4 | 12.99 | 84.3 | 9.4 | 101 | 176 |
| Hip circumference (cm) * | 108.2 | 12.52 | 102 | 7.8 | 106 | 10.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janota, B.; Adamek, B.; Szczepańska, E.; Biernacki, K.; Janczewska, E. Lifestyle and Quality of Life of Women Diagnosed with Hypothyroidism in the Context of Non-Alcoholic Fatty Liver. Metabolites 2023, 13, 1174. https://doi.org/10.3390/metabo13121174
Janota B, Adamek B, Szczepańska E, Biernacki K, Janczewska E. Lifestyle and Quality of Life of Women Diagnosed with Hypothyroidism in the Context of Non-Alcoholic Fatty Liver. Metabolites. 2023; 13(12):1174. https://doi.org/10.3390/metabo13121174
Chicago/Turabian StyleJanota, Barbara, Brygida Adamek, Elżbieta Szczepańska, Krzysztof Biernacki, and Ewa Janczewska. 2023. "Lifestyle and Quality of Life of Women Diagnosed with Hypothyroidism in the Context of Non-Alcoholic Fatty Liver" Metabolites 13, no. 12: 1174. https://doi.org/10.3390/metabo13121174
APA StyleJanota, B., Adamek, B., Szczepańska, E., Biernacki, K., & Janczewska, E. (2023). Lifestyle and Quality of Life of Women Diagnosed with Hypothyroidism in the Context of Non-Alcoholic Fatty Liver. Metabolites, 13(12), 1174. https://doi.org/10.3390/metabo13121174










