Abstract
The objective of this study was to identify alterations in the vaginal discharge (VD) metabolome and potential biomarkers to predict metritis development and a cure in dairy cows. This prospective cohort study was conducted on two dairies located in CA and TX. Vaginal discharge was evaluated and collected using the Metricheck® device. Cows were examined for metritis at 4, 7, and 9 days in milk (DIM). Cows with a fetid, watery, and reddish-brown uterine discharge were classified as having metritis and randomized to receive ceftiofur (n = 10) or remain untreated (n = 7). A cure was defined as the absence of a fetid, watery, reddish-brown uterine discharge at 14 d after enrollment. Vaginal discharge samples were collected from 86 cows within 6 h after parturition, at 4 and 7 DIM, at metritis diagnosis, and at 4 and 7 days after metritis diagnosis. Cows with metritis (MET; n = 17) were paired with counterparts without metritis (HTH) of a similar DIM and parity (n = 34). The uterine metabolome was evaluated using untargeted gas chromatography time-of-flight mass spectrometry (GC-TOF-MS). Metabolomic data were analyzed using the MetaboAnalyst 5.0. Data were log-transformed and auto-scaled for normalization. Univariate analyses, including the fold-change, were performed to identify the metabolites linked to metritis development and its cure and principal component analysis and partial least squares discriminant analysis were performed to explain metabolite variance between animals developing or not developing metritis and being cured or not being cured of metritis. Comparing HTH with MET cows at calving, 12 metabolites were upregulated, and one was downregulated. At four and seven DIM, 51 and 74 metabolites, respectively, were altered between MET and HTH cows. After metritis development, three and five metabolites were upregulated in cows that were cured and in cows that received treatment and were cured, respectively. In all scenarios, the metabolites lignoceric, malic, and maleic acids, ornithine, and hypotaurine, which are associated with arginine/aminoacyl-tRNA biosynthesis and taurine/purine metabolism, were upregulated in HTH cows. Metritis was associated with changes in the uterine metabolome. Cows not being cured of metritis had changes in the uterus metabolome independent of receiving ceftiofur or remaining untreated. Metabolome analysis may be an important tool to understand the vaginal discharge changes during postpartum and the dynamics of metritis development and cures and help to identify biomarkers to predict metritis being cured.
1. Introduction
Metritis is a painful uterine pathology [1] that involves inflammation of all uterine layers (endometrium, myometrium, and serosa), and it is characterized by an abnormally enlarged uterus and a fetid, watery, red-brown uterine discharge within 21 days after parturition, with the incidence peaking within the first ten days postpartum [2]. Metritis has a high incidence (20–35%) in dairy cows [3] and has a marked negative impact on welfare, health, production, and reproduction [2,3,4]. Economic consequences for the individual animal and the herd range from U$212 to U$884 per case [5,6]. The disease is associated with signs of systemic illness (decreased milk yield, dullness, or other signs of toxemia), and approximately half of the metritic cows can have fever [rectal temperature (RT) ≥ 39.5 °C] [7].
Metritis is a complex multifactorial disease caused by a mixed bacterial infection [8,9]. Previous studies showed that the microbiota is identical between cows that develop metritis and healthy cows up until two days postpartum [10]. But after day two, the uterine microbiota diverges, and dysbiosis of the uterine microbiota is characterized by a loss of heterogeneity, a decrease in bacterial richness, and an increase in Bacteroidetes and Fusobacteria, particularly Bacteroides, Porphyromonas, and Fusobacterium, while the relative abundance of Proteobacteria and Tenericutes decreases [9].
During the transition into lactation, cows enter a negative energy balance, leading to body fat mobilization in the form of non-esterified fatty acids (NEFAs) and the accumulation of products of incomplete oxidation of NEFAs, such as beta-hydroxybutyrate (BHB) [11]. The decrease in glucose and calcium concentrations and the increase in NEFA and BHB concentrations are associated with immunosuppression and an increased risk of metritis [12]. A previous study reported that cows with metritis have alterations in metabolites related to carbohydrate metabolism, acute phase proteins, and proinflammatory cytokines at eight and four weeks before parturition [13]. In addition, products of bacterial metabolism, such as proteins, short-chain fatty acids, and other metabolites, affect immune functions [14]. In cows, the vascular degeneration that occurs shortly after calving allows blood to seep into the uterine lumen, which allows for the exchange of metabolites between blood and the uterine layers (endometrium, myometrium, and serosa) [15]. This exchange suggests that blood metabolites can affect the uterine microbiota, whilst microbial-derived metabolites may affect leukocytes in blood and tissues, and the crosstalk between metabolites of the host and pathogens plays a role in metritis development [16].
Studies have reported the associations of minerals or metabolites with immune function and metritis development [17,18]. Also, studies revealed that predictive models using cows’ sensor data [19] or machine learning algorithms [20] to predict a metritis cure might help improve the judicious use of antibiotics. However, studies characterizing the vaginal-uterine metabolome and potential biomarkers associated with the risk of metritis development and its cure remain scarce. Analytical approaches, such as metabolomics, are advancing and refer to analyzing concentration changes in small-molecule metabolites after organisms experience temporal and external stimuli [21]. The primary analytical approaches used in metabolomics rely on two techniques: nuclear magnetic resonance (NMR) and mass spectrometry (MS) [22]. The former determines the magnetic resonance of nuclei in the molecule and is suitable for detecting all compounds that contain hydrogen atoms, while the latter is the most frequently used platform in metabolomics quantification. Metabolomics improves the precision of identifying and quantifying low-molecular-weight metabolites and their intermediates in different biofluids or tissues, advancing the understanding of disease processes in dairy cows, as reported for milk fever, subclinical mastitis, and urine fingerprinting for metritis risk prediction [23,24,25].
Characterizing the vaginal-uterine metabolome during the peripartum can help unravel the crosstalk between host immunological factors and microbes that are key for metritis development and its cure and identify biomarkers that can help predictive models used to select cows that need to be treated for metritis. Therefore, the objectives of this study were to identify changes in the VD metabolome and biomarkers associated with metritis development, its cure, and antimicrobial use in Holstein cows using untargeted gas chromatography time-of-flight mass spectrometry. Our premise is that metritis development and its cure are associated with changes in the uterine metabolome after calving and that antimicrobial treatment is associated with uterine metabolome changes in metritic cows, and biomarkers that can be used for a metritis cure and its development exist.
2. Materials and Methods
2.1. Ethics and Animals
This prospective cohort study was conducted on two dairies in the Central Valley of California (4300 lactating Holstein cows) and one dairy in northwest Texas (2900 lactating Holstein cows) from August to October 2020, and January 2020 to May 2021, respectively. All herds milked only Holstein cows. The rolling herd average milk yield ranged from 10,150 to 12,000 kg. Postpartum pens had sand-bedded stalls and were equipped with sprinklers over the feeding areas activated when the environmental temperature rose above 21 °C. The postpartum diet was formulated to meet or exceed the dietary nutrient requirements for a lactating cow weighing 680 kg and producing 45 kg of 3.5% fat-corrected milk and 3.0% protein (NRC, 2001), and it was delivered as a TMR twice daily.
Considering that the incidence of metritis ranges from 20 to 35% in dairy cows [3] and we were expecting to have at least 15 cases of metritis, vaginal discharge samples were collected from 86 cows within 6 h after parturition, at 4 and 7 days in milk (DIM), at metritis diagnosis, and at 4 and 7 days after metritis diagnosis (Figure 1). All animals enrolled on the parturition day were assessed for metritis diagnosis at 5, 7, and 9 DIM, and vaginal discharge was evaluated using the Metricheck® device (Simcro, Hamilton, Hamilton, New Zealand). Discharge retrieved from the vagina was scored as 1 = not fetid normal lochia, viscous, clear, red, or brown; 2 = cloudy mucoid discharge with flecks of pus; 3 = not fetid, mucopurulent discharge with <50% pus; 4 = not fetid mucopurulent white, yellow or reddish-brownish discharge with ≥50% pus; and, 5 = fetid, thin, serous, or watery, may have been reddish-brownish, with or without pieces of necrotic tissue present (adapted from Chenault et al., 2004 [26]). Cows with a fetid, watery, and reddish-brownish discharge, with or without pieces of necrotic tissue present, were classified as having metritis [27]. From the initial 86 cows from which the vaginal discharge was collected within 6 h after parturition, 17 animals developed metritis. Metritic cows were blocked by parity (primiparous and multiparous) and then randomly assigned to one of two treatments: (1) Ceftiofur [(n = 10) = subcutaneous injections of 6.6 mg/kg of ceftiofur crystalline-free acid (Excede®, Zoetis) in the base of the ear at D0 and D3. Live body weight was estimated using a heart girth measuring tape (Nasco Inc., Atkinson, WI, USA)]; (2) CON [(n = 7) = remained untreated at the time of metritis diagnosis. For vaginal discharge metabolomic analysis, cows with metritis (MET; n = 17) were paired with counterparts without metritis (HTH; n = 34) of a similar DIM and parity that originated from the initial 86 samples for the metabolomic analysis. Our rationale to use a 2:1 versus a 1:1 control-to-case ratio was that by selecting two counterparts without metritis who share similar days in milk and parity with the metritis group, we could enhance statistical efficiency by detecting smaller effect sizes or associations with greater precision, ultimately yielding more informative results. Moreover, the increased control-to-disease ratio enabled us to adjust more effectively for potential confounding variables, enhancing the overall robustness of our analysis. Additionally, the scarcity of research focusing on characterizing uterine metabolites in postpartum cows, especially in healthy individuals, underscores the importance of having a larger sample size for the healthy groups. This not only aids in better representing population dynamics for metritis but also reduces noise in the data. Cows with a vaginal discharge ≤4 were classified as HTH. Cows previously diagnosed with metritis with a vaginal discharge score <5 on d 14 after enrollment were considered cured. The post-enrollment exam on d 14 was performed by a veterinarian from the research team who was unaware of the treatment assignment. Cows within the withdrawal period for any antimicrobial or treated with any nonsteroidal and/or steroidal anti-inflammatory, cows submitted to caesarian section or fetotomy, or cows that aborted (<260 days of gestation) were not eligible for enrollment in the study.
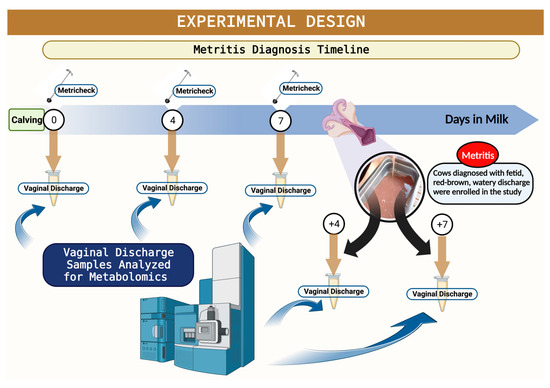
Figure 1.
Schematic representation of the experimental design. Illustration of timeline for metritis diagnosis, metritis enrollment, and sampling for mass spectrophotometry assessment.
2.2. Metabolomic Data Acquisition and Processing
Vaginal discharge was collected using the Metricheck® device, transferred to two polypropylene vials, and stored at −80 °C until it was analyzed in an untargeted gas chromatography time-of-flight mass spectrometer (GC-TOF-MS) at the UC–Davis West Coast Metabolomics Center. The retention index and the complete mass spectrum were encoded as a string. All thresholds reflect the settings for ChromaTOF v. 4.0. Quantification was reported as the peak height using the unique ion as the default, unless a different quantification ion was manually set in the BinBase administration software version 1 BinView. We detected 174 known metabolites from 368 untargeted primary metabolites found in our analysis. A column of a 30 m length by 0.25 mm internal diameter with a 0.25 μm film made of 95% dimethyl/5diphenyl polysiloxanes was used in a Restek corporation Rtx-5Sil MS. The gas helium (99.99% purity) was used as a carrier for the analysis. The column temperature was set between 50 and 330 °C at a flow rate of 1 mL min−1. The oven temperature was set to 50 °C for 1 min, then ramped at 20 °C min−1 to 330 °C, and held constant for 5 min. Finally, the injection temperature was set to 50 °C and ramped to 250 °C by increments of 12 °C The retention of primary metabolites (amino acids, hydroxyl acids, carbohydrates, sugar acids, sterols, aromatics, nucleosides, amines, and various compounds) was evaluated.
2.3. Metabolomic Statistical Analysis
Metabolomic analyses were performed using Metaboanalyst 5.0 (www.metaboanalyst.ca (accessed multiple times in February 2022). Before data analysis, a data filtering and integrity check was performed to ensure that all the necessary information was collected (two classes, non-negative numbers for the compound concentration or peak intensity values, and missing value imputations). Data were log-transformed (base 10) and auto-scaled for normalization. For explanatory data analysis, a univariate analysis was performed. A fold-change analysis (FC) was performed with a threshold of 2 to identify candidate metabolites linked to metritis development and its cure. A volcano plot analysis was performed using a fold-change threshold (x) of 2 and t-test threshold (y) of 0.1 to select the essential features based on biological and statistical significance. Principal component analysis (PCA), partial-least square discriminant analysis (PLS-DA), and orthogonal PLS-DA analyses were performed to understand metabolite differences between animals developing or not developing metritis and being cured or not being cured of metritis. Pathways of different metabolites for models using two different organisms [cow (Bos taurus) and E. coli] were further screened using enrichment and topological analyses to identify the key pathway most highly correlated with metabolite differences.
3. Results
3.1. Number of Cows Enrolled Per Farm and Descriptive Data
Overall, 86 animals were enrolled, 44 in California and 42 in Texas. The number of animals developing metritis were nine and eight at the dairy in California and Texas, respectively. The average lactation number was 2.4. The mean DIM at metritis diagnosis was 6 days and the mean BCSs of animals enrolled and developing metritis were 3.2 and 3.5, respectively.
3.2. Changes in Uterine Metabolome in Cows Developing Metritis
A total of 185 known and 236 unknown primary metabolites were identified in the vaginal discharge. Within six hours after calving, a comparison of metabolites revealed an association (adjusted p ≤ 0.05) with metritis development, with HTH vs. MET having 12 metabolites upregulated and one metabolite downregulated (Table 1). The PLS-DA with known metabolites confirmed the differences observed in the uterine metabolome, indicating a dispersion in the metabolite profile between HTH and metritic cows (Figure 2A,B). The top 20 uterine metabolites detected via PLS-DA ranked by variable importance projection (VIP) scores resembled most of the metabolites identified as different by the fold-change analysis (Figure 2C). Pathway analysis based on identified metabolites in cows not developing compared to cows developing metritis allowed for an understanding between the biological pathways for cows (Bos taurus) and E. coli. The subset of important metabolites was associated with glutathione, taurine, hypotaurine, alanine, aspartate, glutamate, D-glutamine, D-glutamate, and phenylalanine metabolism and aminoacyl-tRNA biosynthesis in Bos taurus organisms and associated with glutathione metabolism and aminoacyl-tRNA biosynthesis (Figure 2D).

Table 1.
Important metabolites that were up- and downregulated within 6 h postpartum in healthy cows compared to animals developing metritis.
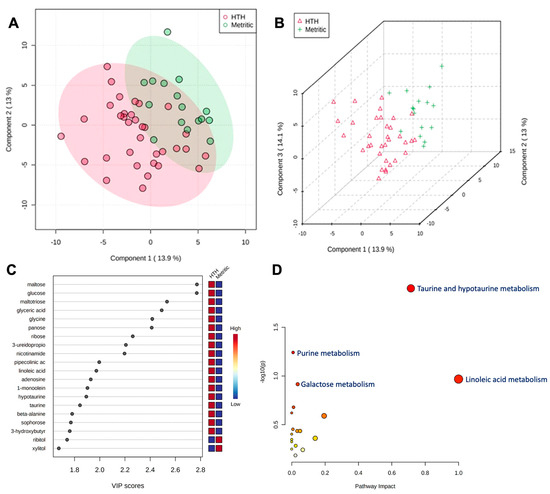
Figure 2.
(A) Partial least square-discriminant analysis (PLS-DA) of vaginal-uterine discharge metabolites within 6 h postpartum in healthy cows compared to animals developing metritis. Score plot between the selected principal components; the explained variances are shown in brackets. (B) A 3-D representation of the known metabolome composition is displayed to demonstrate that there was a slight metabolome difference within 6 h postpartum between cows developing or not developing metritis. (C) Top 20 vaginal discharge metabolites detected via PLS-DA ranked by variable importance projection (VIP) scores. (D) Metabolic pathways associated with metritis at calving (d 0) based on enriched pathway analyses.
At 4 DIM, the fold-change analysis revealed that 51 metabolites were associated with metritis development (adjusted p ≤ 0.05). Comparing HTH to MET groups, 38 metabolites were upregulated, and 13 metabolites were downregulated. The top 50 metabolites are presented in Table 2. The PLS-DA with known metabolites at 4 DIM confirmed the differences observed in the uterine metabolome, indicating a dispersion in the metabolite profile between HTH and metritic cows (Figure 3A,B). The top 20 uterine metabolites detected via PLS-DA ranked by variable importance projection (VIP) scores resembled most of the metabolites identified as different based on the fold-change analysis (Figure 3C).

Table 2.
Metabolites that were up- and downregulated at 4 DIM in healthy cows compared to animals developing metritis.
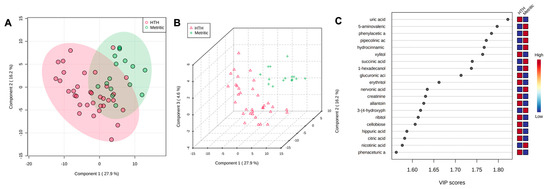
Figure 3.
(A) Partial least square-discriminant analysis (PLS-DA) of vaginal-uterine discharge metabolites at 4 DIM in healthy cows compared to animals developing metritis. Score plot between the selected principal components; the explained variances are shown in brackets. (B) A 3-D representation of the known (B) metabolome composition is displayed to demonstrate that there is a metabolome difference at 4 DIM between cows developing or not developing metritis. (C) Top vaginal discharge metabolites detected via PLS-DA ranked based on variable importance projection (VIP) scores.
At 7 DIM, the fold-change analysis revealed that when comparing HTH to MET groups, 49 metabolites were upregulated, and 22 metabolites were downregulated. The top 50 metabolites are presented in Table 3. The PLS-DA with known metabolites at 7 DIM confirmed the differences observed in the uterine metabolome, indicating a dispersion in metabolite profile between HTH and metritic cows (Figure 4A,B). The top 20 uterine metabolites detected via PLS-DA and ranked by variable importance projection (VIP) scores resembled most of the metabolites identified as different based on the fold-change analysis (Figure 4C).

Table 3.
Important metabolites that were up- and downregulated at 7 DIM in healthy cows compared to animals developing metritis.
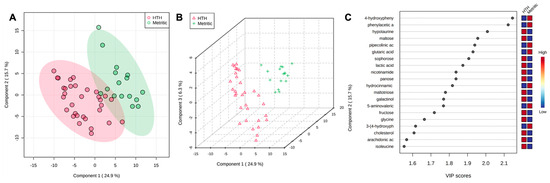
Figure 4.
(A) Partial least square-discriminant analysis (PLS-DA) of vaginal-uterine discharge metabolites at 7 DIM in healthy cows compared to animals developing metritis. Score plot between the selected principal components; the explained variances are shown in brackets. (B) A 3-D representation of the known metabolome composition is displayed to demonstrate that there was a metabolome difference at 7 DIM between cows developing or not developing metritis. (C) Top vaginal discharge metabolites detected via PLS-DA and ranked based on variable importance projection (VIP) scores.
3.3. Changes in Uterine Metabolome in Cows Being Cured versus Cows Not Being Cured of Metritis
After metritis development, three metabolites (lignoceric acid, maleic acid, and ornithine) were upregulated (p < 0.05) in cows that were cured compared to cows that were not cured of metritis and receiving or not receiving treatment. For the group of animals that received treatment and were cured, five metabolites (lignoceric acid, maleic acid, malic acid, N-acetylmannosamine, and ornithine) were upregulated (p < 0.05) compared to cows receiving treatment and not being cured of metritis. The PLS-DA with known metabolites confirmed the differences observed in the uterine metabolome, indicating a dispersion in the metabolite profile between cows being cured and not being cured of metritis (Figure 5A,B). The top 15 uterine metabolites detected via PLS-DA and ranked based on variable importance projection (VIP) scores represented the metabolites identified as being different based on the fold-change analysis (Figure 5C). Pathway analysis based on identified metabolites in cows being cured of metritis allowed for an understanding between the biological pathways for cows (Bos taurus) and E. coli. In both scenarios, the subset of essential metabolites was associated with arginine, proline, glyoxylate, dicarboxylate, and phenylalanine metabolism and aminoacyl-tRNA biosynthesis in Bos taurus organisms and associated with arginine and proline metabolism and arginine biosynthesis in E. coli organisms.
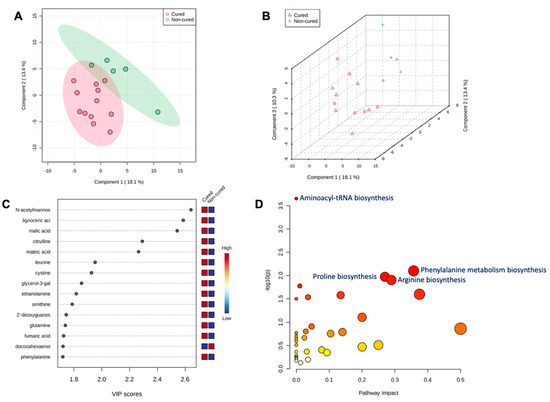
Figure 5.
(A) Partial least square-discriminant analysis (PLS-DA) of vaginal-uterine discharge metabolites of metritic cows 4 days after metritis diagnosis. Score plot between the selected principal components; the explained variances are shown in brackets. (B) The 3-D representation of the known (B) metabolome composition demonstrated that 4 days after metritis diagnosis, there was a metabolome difference in cows being cured and not being cured of metritis. (C) Top vaginal discharge metabolites detected via PLS-DA and ranked based on variable importance projection (VIP) scores. (D) Metabolic pathways associated with metritis at calving (d 0) based on enriched pathway analyses.
4. Discussion
As anticipated by our premise for the study, the characterization of primary metabolites depicted changes in the vaginal discharge metabolome in cows developing metritis that were dynamic over time and underscore differences between cows being cured and failing to be cured of metritis. Changes in metabolic pathways during the transition period, such as amino acid conversion to glucose and increased blood metabolites, such as NEFA and BHB, which are associated with dairy cow health and metritis, have been assessed in the literature throughout the years [11,28,29,30]. On the other hand, studies evaluating metabolomic changes in the uterus and related reproductive tract components during the first weeks postpartum and their association with metritis are only now emerging and need further exploration [16]. Our approach in the current study was supported by a premise that vaginal discharge, a sample that is relatively simpler and less invasive to collect than uterine fluid [16], could still be satisfactory to identify biomarkers for metritis development and its cure that could serve as data entry points to advance current predictive models for a metritis cure [18,20] and shed light on potential mechanisms of disease pathogenesis.
An interesting finding of this study was that the metabolome profile in the vaginal discharge changed from a few hours after calving up to the day of the metritis event, and the number of metabolites up- and downregulated increased over time up to 7 DIM within cows developing and not developing metritis. The leading metabolites up- and downregulated were sugar (e.g., glucose, maltose, panose), amino acid (adenosine, taurine, and hypotaurine)-derived metabolites, and other important organic compounds, such as nicotinamide and linoleic acid. A previous study suggested that preventing uterine disease in dairy cattle depends on avoiding, tolerating, and resisting pathogenic bacteria [31]. In other words, the concept proposed indicates that the prevention of metritis depends on the ability to mitigate tissue damage caused by pathogens through the neutralization of toxins, tissue repair, and immune competence. Important metabolites are known to have an essential role in the immune response and may represent the changes in uterus dysbiosis, toxin neutralization, and tissue repair [31,32,33].
The main pathways related to the metabolite changes in the uterus associated with the Bos taurus at an organism level are taurine, hypotaurine, purine, glutathione, and phenylalanine metabolism, which is positively associated with immunity and health. The nicotinamide concentration was increased in cows not developing metritis, suggesting that this metabolite regulates important physiologic processes in the uterus. Nicotinamide is a critical regulator maintaining important physiologic functions and controlling infection and inflammation [34]. Indeed, the essential redox effect of nicotinamide in promoting cellular oxidative (catabolic) metabolic disorders has been suggested and investigated as an anti-cancer and anti-aging therapeutic target [35]. Other metabolites being upregulated in cows not developing metritis were taurine and hypotaurine; both are antioxidant sulfur-containing amino acids that improve the immune function due to anti-apoptotic activities, antioxidant stress effects, and the regulation of mitochondrial function [36,37] Positive effects associated with these metabolites can be attributed to a potential improved immune response that may translate into the more rapid ability of the uterus tissue to respond to damage and consequently reduced the chances of pathogenic bacterial growth.
Cows have an established uterine microbiota within 20 min of calving [10]. The microbiota structure of a high proportion of proteobacteria is identical between cows that develop metritis and healthy cows up until two days postpartum, after which the bacterial community structure deviates in favor of a greater relative abundance of Bacteroides, Porphyromonas, and Fusobacterium in metritic cows [8,10,38]. Regarding the pathways associated with E. coli in the first week postpartum, it was mainly associated with specific pathways of glutathione, taurine, and hypotaurine metabolism and aminoacyl-tRNA biosynthesis, which are directly associated with protein biosynthesis, which induces translation and E. coli growth [39]. Glutathione, taurine, and hypotaurine metabolism pathways are associated with preventing inflammation and blocking bacterial growth, which could alleviate bacterial dysbiosis [40].
There is a scarcity of studies investigating the uterine postpartum metabolome either for health cows or for cows with the occurrence and progression of metritis. On the other hand, the individual metabolites reported as upregulated in the current study and their action on metabolic pathways have been discussed in other species. An in vitro study investigating the effect of L-arginine on human endometrial cell proliferation reported that supraphysiological concentrations of L-arginine led to increased endometrial RL95-2 cell proliferation and reduced apoptosis, which had a positive impact on endometrial epithelium regeneration, growth, and health [41]. Another relevant aspect of the arginine biosynthesis pathway is the broad role of L-arginine as a precursor of important molecules for cellular physiology, such as nitric oxide and its conversion to ornithine via arginase [42]. In this study, we observed that ornithine was one of the metabolites upregulated in cows not developing metritis. Studies investigating the effect of the abundance of arginine and ornithine in pigs reported a positive effect on the uterus, placental–fetal blood flow, fetoplacental nutrition, and endometrial epithelial cell proliferation [43]. Additionally, some studies highlighted possible connections between L-arginine and Bacteroides spp. and found that an increased arginine concentration correlated with reduced Bacteroides growth. Metritic cows had a greater abundance of total bacteria and a greater abundance of Bacteroides pyogenes (B. pyogenes), Porphyromonas levii (P. levii), and Fusobacterium necrophorum (F. necrophorum) [15]. A possible interpretation to reconcile these studies is that metabolites in the vaginal discharge of cows developing metritis, even after disease establishment, can alter pathogenic growth and reduce dysbiosis. In that case, it could reduce the severity and improve curing independent of treatment, as shown in the present study.
The upregulation of antioxidant amino acids, such as lignoceric, malic, maleic, and hypotaurine in healthy cows was an important finding in this study. Those amino acids are associated with taurine and purine metabolism and have been reported as important compounds in cows’ immune functions [44,45]. In addition to the antioxidant effect, they regulate mitochondrial protein synthesis by enhancing electron transport chain activity and controlling apoptosis due to the regulation of excessive superoxide generation [39]. Cows not being cured of metritis had significant changes in the uterus metabolome independent of receiving ceftiofur or remaining untreated. Cow not being cured had a lower concentration of lignoceric, malic, maleic, and lauric acids and ornithine. The main pathways related to the metabolite changes in the vaginal discharge microbiome associated with the Bos taurus at an organism level were aminoacyl-tRNA biosynthesis, glutathione, taurine, and hypotaurine metabolism, which match with the pathways associated with cows that did not develop metritis and suggest that these pathways are associated with immunity, uterus integrity, and pathogenic bacteria reductions, which may reduce the severity of the disease and improve healing from metritis. The main pathways related to the metabolite changes in the uterus associated with E. coli at the organism level were arginine biosynthesis and arginine and proline metabolism pathways reported as associated with gut microecology and E. coli reductions [40].
One unique aspect of the current study was that we collected vaginal discharge for the metabolome analysis instead of uterine fluid [16], which in itself is a source of variation that can limit the comparison with other studies. However, as acknowledged previously, the vaginal discharge is a less invasive and simpler method to retrieve samples that, unless a cow has severe vaginitis after calving, should mainly represent the contents being released by the enlarged postpartum uterus that remains involuted with an open cervix. The Metricheck tool used has been compared with other standard methods for evaluations of vaginal discharge for the diagnosis of uterine disease, such as cytology, manual evaluations, and vaginoscopy, and it was found to have higher sensitivity than vaginoscopy for the diagnosis of uterine diseases, such endometritis [46,47]. Further studies should focus on increasing the sample size of animals with metritis and assessing the metabolites associated with a spontaneous cure. In this study, we could not analyze metabolite changes in cows being self-cured of metritis due to the limited number of animals left untreated and being cured. Additionally, performing studies using different methods for discharge collection and comparing how the metabolome from plasma and uterine fluid is associated will be essential to determine what metabolites are related to immune functions and pathogenic bacterial proliferation in the uterus. Although the new approaches can be beneficial in identifying key biomarkers associated with an improved immune function, reduced pathogenic bacterial growth in the uterus, and improved uterine health, the current finding already offers a list of potential biomarkers that integrated predictive models for metritis could advance.
5. Conclusions
In summary, untargeted GC-TOF-MS metabolomic analysis of the vaginal discharge of cows in the first week postpartum, at metritis diagnosis, and at 4 and 7 days after metritis diagnosis, highlighted changes in the uterine metabolome in the first week postpartum in cows developing metritis compared to healthy animals. For the metritic group, there were significant changes in the uterine metabolome associated with a cure. In all scenarios, the metabolites lignoceric, malic, and maleic acids, ornithine, and hypotaurine, which are associated with arginine/aminoacyl-tRNA biosynthesis and taurine/purine metabolism, were upregulated in the HTH group and in cows being cured of metritis. Also, cows not being cured of metritis had significant changes in the uterus metabolome independent of receiving ceftiofur or remaining untreated. Metabolome analysis may be an important tool to understand changes in the uterus during the postpartum period and the dynamics of metritis development.
Author Contributions
Conceptualization, E.B.d.O., R.V.P., N.S.D.R., V.S.M. and F.S.L.; Methodology, E.B.d.O., H.F.M., N.S.D.R. and F.S.L.; Formal analysis, E.B.d.O., H.F.M. and F.S.L.; Investigation, E.B.d.O., J.M.V.P. and P.R.M.; Resources, F.S.L.; Data curation, E.B.d.O. and F.S.L.; Writing—original draft, E.B.d.O. and F.S.L.; Writing—review & editing, D.R.W., R.V.P. and F.S.L.; Visualization, F.S.L.; Supervision, F.S.L.; Project administration, F.S.L.; Funding acquisition, R.V.P. and F.S.L. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this research was provided by the U.S. Department of Agriculture Project number CA-V-PHR-4122-H. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA. The authors would like to thank the dairy farmers who agreed to participate in this study.
Institutional Review Board Statement
All experimental procedures carried out in this study were approved by the Institutional Animal Care and Use Committees at the University of California, Davis (protocol: 21,861 from samples collected from California, USA) and Texas Tech University (protocol: 19205-02 for samples collected from Texas, USA).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the. article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stojkov, J.; von Keyserlingk, M.A.; Marchant-Forde, J.N.; Weary, D.M. Assessment of visceral pain associated with metritis in dairy cows. J. Dairy Sci. 2015, 98, 5352–5361. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.S.; Vieira-Neto, A.; Vasconcellos, G.S.; Mingoti, R.D.; Karakaya, E.; Sole, E.; Bisinotto, R.S.; Martinez, N.; Risco, C.A.; Galvao, K.N.; et al. Efficacy of ampicillin trihydrate or ceftiofur hydrochloride for treatment of metritis and subsequent fertility in dairy cows. J. Dairy Sci. 2014, 97, 5401–5414. [Google Scholar] [CrossRef] [PubMed]
- Pinedo, P.; Santos, J.E.P.; Chebel, R.C.; Galvao, K.N.; Schuenemann, G.M.; Bicalho, R.C.; Gilbert, R.O.; Rodriguez Zas, S.; Seabury, C.M.; Rosa, G.; et al. Early-lactation diseases and fertility in 2 seasons of calving across US dairy herds. J. Dairy Sci. 2020, 103, 10560–10576. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, E.B.; Cunha, F.; Daetz, R.; Figueiredo, C.C.; Chebel, R.C.; Santos, J.E.; Risco, C.A.; Jeong, K.C.; Machado, V.S.; Galvão, K.N. Using chitosan microparticles to treat metritis in lactating dairy cows. J. Dairy Sci. 2020, 103, 7377–7391. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.S.; Vieira-Neto, A.; Snodgrass, J.A.; De Vries, A.; Santos, J.E.P. Economic comparison of systemic antimicrobial therapies for metritis in dairy cows. J. Dairy Sci. 2019, 102, 7345–7358. [Google Scholar] [CrossRef]
- Perez-Baez, J.; Silva, T.V.; Risco, C.A.; Chebel, R.C.; Cunha, F.; De Vries, A.; Santos, J.E.P.; Lima, F.S.; Pinedo, P.; Schuenemann, G.M.; et al. The economic cost of metritis in dairy herds. J. Dairy Sci. 2021, 104, 3158–3168. [Google Scholar] [CrossRef]
- Benzaquen, M.E.; Risco, C.A.; Archbald, L.F.; Melendez, P.; Thatcher, M.J.; Thatcher, W.W. Rectal temperature, calving-related factors, and the incidence of puerperal metritis in postpartum dairy cows. J. Dairy Sci. 2007, 90, 2804–2814. [Google Scholar] [CrossRef]
- Jeon, S.J.; Lima, F.S.; Vieira-Neto, A.; Machado, V.S.; Lima, S.F.; Bicalho, R.C.; Santos, J.E.P.; Galvao, K.N. Shift of uterine microbiota associated with antibiotic treatment and cure of metritis in dairy cows. Vet. Microbiol. 2018, 214, 132–139. [Google Scholar] [CrossRef]
- Galvao, K.N.; Bicalho, R.C.; Jeon, S.J. Symposium review: The uterine microbiome associated with the development of uterine disease in dairy cows. J. Dairy Sci. 2019, 102, 11786–11797. [Google Scholar] [CrossRef]
- Jeon, S.J.; Vieira-Neto, A.; Gobikrushanth, M.; Daetz, R.; Mingoti, R.D.; Parize, A.C.; de Freitas, S.L.; da Costa, A.N.; Bicalho, R.C.; Lima, S.; et al. Uterine Microbiota Progression from Calving until Establishment of Metritis in Dairy Cows. Appl. Environ. Microbiol. 2015, 81, 6324–6332. [Google Scholar] [CrossRef]
- Hubner, A.; Canisso, I.F.; Peixoto, P.M.; Coelho, W.M., Jr.; Ribeiro, L.; Aldridge, B.M.; Menta, P.; Machado, V.S.; Lima, F.S. Characterization of metabolic profile, health, milk production, and reproductive outcomes of dairy cows diagnosed with concurrent hyperketonemia and hypoglycemia. J. Dairy Sci. 2022, 105, 9054–9069. [Google Scholar] [CrossRef] [PubMed]
- Martinez, N.; Risco, C.A.; Lima, F.S.; Bisinotto, R.S.; Greco, L.F.; Ribeiro, E.S.; Maunsell, F.; Galvao, K.; Santos, J.E. Evaluation of peripartal calcium status, energetic profile, and neutrophil function in dairy cows at low or high risk of developing uterine disease. J. Dairy Sci. 2012, 95, 7158–7172. [Google Scholar] [CrossRef] [PubMed]
- Dervishi, E.; Zhang, G.; Hailemariam, D.; Goldansaz, S.A.; Deng, Q.; Dunn, S.M.; Ametaj, B.N. Alterations in innate immunity reactants and carbohydrate and lipid metabolism precede occurrence of metritis in transition dairy cows. Res. Vet. Sci. 2016, 104, 30–39. [Google Scholar] [CrossRef]
- Contreras, G.A.; Sordillo, L.M. Lipid mobilization and inflammatory responses during the transition period of dairy cows. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.J.; Cunha, F.; Vieira-Neto, A.; Bicalho, R.C.; Lima, S.; Bicalho, M.L.; Galvao, K.N. Blood as a route of transmission of uterine pathogens from the gut to the uterus in cows. Microbiome 2017, 5, 109. [Google Scholar] [CrossRef]
- Figueiredo, C.C.; Balzano-Nogueira, L.; Bisinotto, D.Z.; Ruiz, A.R.; Duarte, G.A.; Conesa, A.; Galvao, K.N.; Bisinotto, R.S. Differences in uterine and serum metabolome associated with metritis in dairy cows. J. Dairy Sci. 2023, 106, 3525–3536. [Google Scholar] [CrossRef]
- Chapinal, N.; Carson, M.; Duffield, T.F.; Capel, M.; Godden, S.; Overton, M.; Santos, J.E.; LeBlanc, S.J. The association of serum metabolites with clinical disease during the transition period. J. Dairy Sci. 2011, 94, 4897–4903. [Google Scholar] [CrossRef]
- Machado, V.S.; Celestino, M.L.; Oliveira, E.B.; Lima, F.S.; Ballou, M.A.; Galvao, K.N. The association of cow related factors assessed at metritis diagnosis with metritis cure risk, fertility, milk yield, and culling for untreated and ceftiofur-treated dairy cows. J. Dairy Sci. 2020, 103, 9261–9276. [Google Scholar] [CrossRef]
- Merenda, V.R.; Ruiz-Munoz, J.; Zare, A.; Chebel, R.C. Predictive models to identify Holstein cows at risk of metritis and clinical cure and reproductive/productive failure following antimicrobial treatment. Prev. Vet. Med. 2021, 194, 105431. [Google Scholar] [CrossRef]
- de Oliveira, E.B.; Ferreira, F.C.; Galvao, K.N.; Youn, J.; Tagkopoulos, I.; Silva-Del-Rio, N.; Pereira, R.V.V.; Machado, V.S.; Lima, F.S. Integration of statistical inferences and machine learning algorithms for prediction of metritis cure in dairy cows. J. Dairy Sci. 2021, 104, 12887–12899. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, T.; Gan, Z.; Li, H.; Li, Y.; Zhang, Y.; Zhao, X. Metabolomic analysis of untargeted bovine uterine secretions in dairy cows with endometritis using ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. Res. Vet. Sci. 2021, 139, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Djukovic, D.; Gowda, G.A.N.; Raftery, D. Mass Spectrometry and NMR Spectroscopy-Based Quantitative Metabolomics. In Proteomic and Metabolomic Approaches to Biomarker Discovery; Academic Press: Cambridge, MA, USA, 2013; pp. 279–297. [Google Scholar] [CrossRef]
- Hailemariam, D.; Mandal, R.; Saleem, F.; Dunn, S.M.; Wishart, D.S.; Ametaj, B.N. Metabolomics Approach Reveals Altered Plasma Amino Acid and Sphingolipid Profiles Associated with Patholological State in Transition Dairy Cows. Curr. Metabol. 2014, 2, 184–195. [Google Scholar] [CrossRef]
- Dervishi, E.; Zhang, G.; Dunn, S.M.; Mandal, R.; Wishart, D.S.; Ametaj, B.N. GC-MS Metabolomics Identifies Metabolite Alterations That Precede Subclinical Mastitis in the Blood of Transition Dairy Cows. J. Proteome Res. 2017, 16, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Dervishi, E.; Zhang, G.; Hailemariam, D.; Mandal, R.; Wishart, D.S.; Ametaj, B.N. Urine metabolic fingerprinting can be used to predict the risk of metritis and highlight the pathobiology of the disease in dairy cows. Metabolomics 2018, 14, 83. [Google Scholar] [CrossRef] [PubMed]
- Chenault, J.R.; McAllister, J.F.; Chester, S.T., Jr.; Dame, K.J.; Kausche, F.M.; Robb, E.J. Efficacy of ceftiofur hydrochloride sterile suspension administered parenterally for the treatment of acute postpartum metritis in dairy cows. J. Am. Vet. Med. Assoc. 2004, 224, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Lewis, G.S.; LeBlanc, S.; Gilbert, R.O. Defining postpartum uterine disease in cattle. Theriogenology 2006, 65, 1516–1530. [Google Scholar] [CrossRef]
- Ospina, P.; Nydam, D.; Stokol, T.; Overton, T. Comparison between Individual and Pooled Samples of Non-esterified Fatty Acids (NEFA) and B-hydroxybutyrate (BHBA) in Transition Dairy Cows to Determine Herd Alarm Level Status. In Proceedings of the Forty-Third Annual Conference of the American Association of Bovine Practitioners, Knoxville, TN, USA, 10–11 February 2010; p. 185. [Google Scholar]
- Reynolds, C.K.; Aikman, P.C.; Lupoli, B.; Humphries, D.J.; Beever, D.E. Splanchnic metabolism of dairy cows during the transition from late gestation through early lactation. J. Dairy Sci. 2003, 86, 1201–1217. [Google Scholar] [CrossRef]
- Luo, Z.Z.; Shen, L.H.; Jiang, J.; Huang, Y.X.; Bai, L.P.; Yu, S.M.; Yao, X.P.; Ren, Z.H.; Yang, Y.X.; Cao, S.Z. Plasma metabolite changes in dairy cows during parturition identified using untargeted metabolomics. J. Dairy Sci. 2019, 102, 4639–4650. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.G.; Bromfield, J.J. Tolerance and Innate Immunity Shape the Development of Postpartum Uterine Disease and the Impact of Endometritis in Dairy Cattle. Annu. Rev. Anim. Biosci. 2019, 7, 361–384. [Google Scholar] [CrossRef]
- Bromfield, J.J.; Santos, J.E.; Block, J.; Williams, R.S.; Sheldon, I.M. Physiology and endocrinology symposium: Uterine infection: Linking infection and innate immunity with infertility in the high-producing dairy cow. J. Anim. Sci. 2015, 93, 2021–2033. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.G.; Pospiech, M.; Turner, M.L. Symposium review: Mechanisms linking metabolic stress with innate immunity in the endometrium. J. Dairy Sci. 2018, 101, 3655–3664. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD(+) metabolism: Pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2020, 5, 227. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, H.B.S.; Williams, C.; King, S.J.; Allison, S.J. Nicotinamide adenine dinucleotide (NAD+): Essential redox metabolite, co-substrate and an anti-cancer and anti-ageing therapeutic target. Biochem. Soc. Trans. 2020, 48, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Jong, C.J.; Azuma, J.; Schaffer, S. Mechanism underlying the antioxidant activity of taurine: Prevention of mitochondrial oxidant production. Amino Acids 2012, 42, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, J.; Strus, M.; Walczewska, M.; Machul, A.; Mikolajczyk, D. Influence of taurine haloamines (TauCl and TauBr) on the development of Pseudomonas aeruginosa biofilm: A preliminary study. Adv. Exp. Med. Biol. 2013, 775, 269–283. [Google Scholar] [CrossRef]
- Jeon, S.J.; Galvao, K.N. An Advanced Understanding of Uterine Microbial Ecology Associated with Metritis in Dairy Cows. Genom. Inform. 2018, 16, e21. [Google Scholar] [CrossRef]
- Putzer, H.; Laalami, S.; Brakhage, A.A.; Condon, C.; Grunberg-Manago, M. Aminoacyl-tRNA synthetase gene regulation in Bacillus subtilis: Induction, repression and growth-rate regulation. Mol. Microbiol. 1995, 16, 709–718. [Google Scholar] [CrossRef]
- Yu, H.; Guo, Z.; Shen, S.; Shan, W. Effects of taurine on gut microbiota and metabolism in mice. Amino Acids 2016, 48, 1601–1617. [Google Scholar] [CrossRef]
- Greene, J.M.; Feugang, J.M.; Pfeiffer, K.E.; Stokes, J.V.; Bowers, S.D.; Ryan, P.L. L-Arginine enhances cell proliferation and reduces apoptosis in human endometrial RL95-2 cells. Reprod. Biol. Endocrinol. 2013, 11, 15. [Google Scholar] [CrossRef]
- Yang, Z.; Luo, F.; Liu, G.; Luo, Z.; Ma, S.; Gao, H.; He, H.; Tao, J. Plasma Metabolomic Analysis Reveals the Relationship between Immune Function and Metabolic Changes in Holstein Peripartum Dairy Cows. Metabolites 2022, 12, 953. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Tuo, W.; Flynn, S.P. Unusual abundance of arginine and ornithine in porcine allantoic fluid. Biol. Reprod. 1996, 54, 1261–1265. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Osorio, J.S.; Trevisi, E.; Ji, P.; Drackley, J.K.; Luchini, D.; Bertoni, G.; Loor, J.J. Biomarkers of inflammation, metabolism, and oxidative stress in blood, liver, and milk reveal a better immunometabolic status in peripartal cows supplemented with Smartamine M or MetaSmart. J. Dairy Sci. 2014, 97, 7437–7450. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wu, T.; Zhang, H.; Loor, J.J.; Wang, M.; Peng, A.; Wang, H. Jugular infusion of arginine has a positive effect on antioxidant mechanisms in lactating dairy cows challenged intravenously with lipopolysaccharide1. J. Anim. Sci. 2018, 96, 3850–3855. [Google Scholar] [CrossRef]
- McDougall, S.; Macaulay, R.; Compton, C. Association between endometritis diagnosis using a novel intravaginal device and reproductive performance in dairy cattle. Anim. Reprod. Sci. 2007, 99, 9–23. [Google Scholar] [CrossRef]
- Pleticha, S.; Drillich, M.; Heuwieser, W. Evaluation of the Metricheck device and the gloved hand for the diagnosis of clinical endometritis in dairy cows. J. Dairy Sci. 2009, 92, 5429–5435. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).