Ceramides during Pregnancy and Obstetrical Adverse Outcomes
Abstract
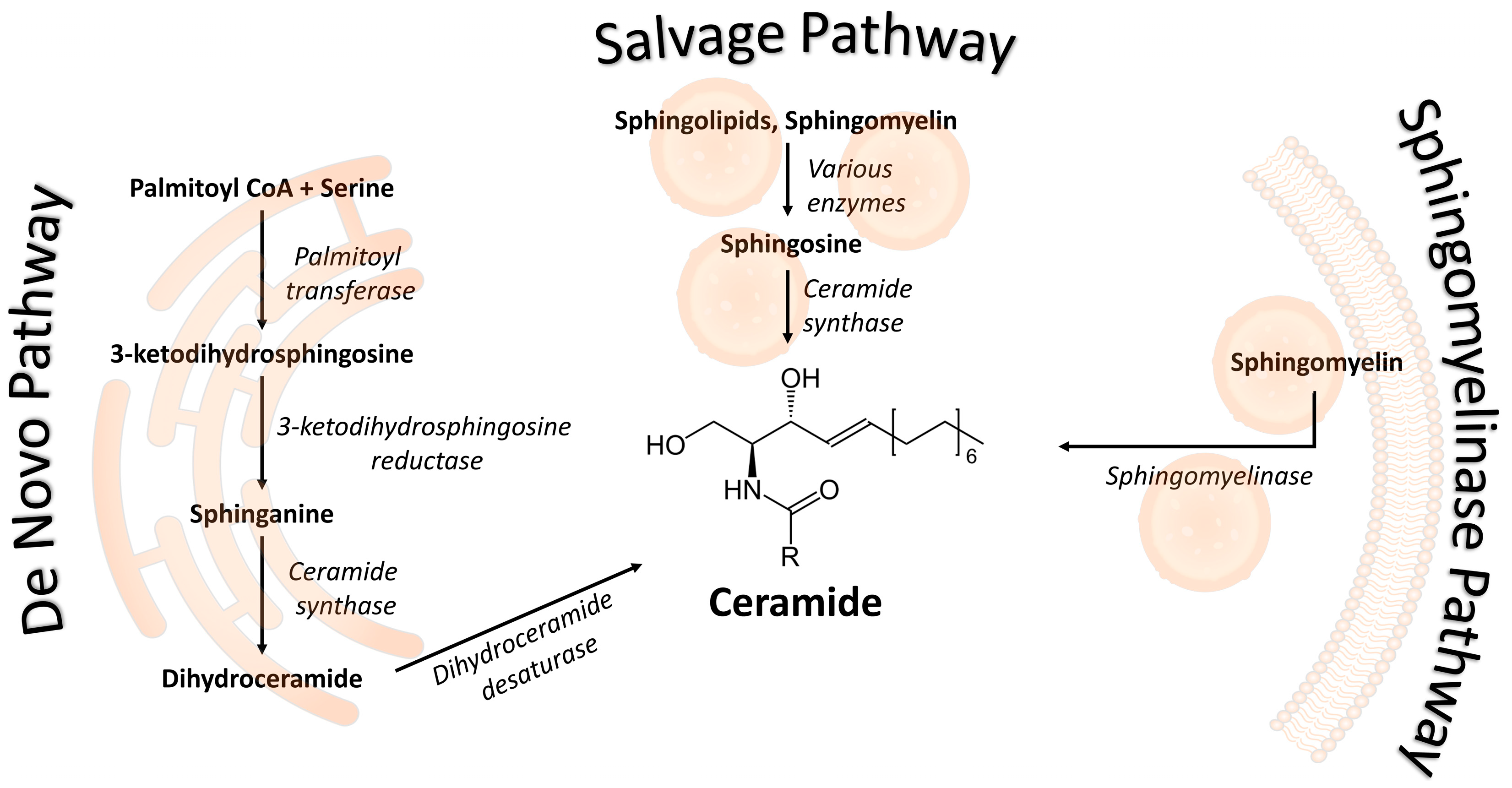
:1. Introduction
2. Relevant Sections
2.1. Ceramides during Pregnancy
2.2. Ceramides in Preeclampsia
2.3. Ceramides in Gestational Diabetes Mellitus
2.4. Ceramides in Other Pregnancy Complications
3. Discussion
4. Conclusions
5. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Kurz, J.; Parnham, M.J.; Geisslinger, G.; Schiffmann, S. Ceramides as Novel Disease Biomarkers. Trends Mol. Med. 2019, 25, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Alexandropoulou, I.; Grammatikopoulou, M.G.; Gkouskou, K.K.; Pritsa, A.A.; Vassilakou, T.; Rigopoulou, E.; Lindqvist, H.M.; Bogdanos, D.P. Ceramides in Autoimmune Rheumatic Diseases: Existing Evidence and Therapeutic Considerations for Diet as an Anticeramide Treatment. Nutrients 2023, 15, 229. [Google Scholar] [CrossRef] [PubMed]
- Al Sazzad, M.A.; Yasuda, T.; Murata, M.; Slotte, J.P. The Long-Chain Sphingoid Base of Ceramides Determines Their Propensity for Lateral Segregation. Biophys. J. 2017, 112, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Ohno, Y.; Kihara, A. Whole Picture of Human Stratum Corneum Ceramides, Including the Chain-Length Diversity of Long-Chain Bases. J. Lipid Res. 2022, 63, 100235. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Goñi, F.M. The Physical Properties of Ceramides in Membranes. Annu. Rev. Biophys. 2018, 47, 633–654. [Google Scholar] [CrossRef]
- Mantovani, A.; Dugo, C. Ceramides and Risk of Major Adverse Cardiovascular Events: A Meta-Analysis of Longitudinal Studies. J. Clin. Lipidol. 2020, 14, 176–185. [Google Scholar] [CrossRef]
- Horbay, R.; Hamraghani, A.; Ermini, L.; Holcik, S.; Beug, S.T.; Yeganeh, B. Role of Ceramides and Lysosomes in Extracellular Vesicle Biogenesis, Cargo Sorting and Release. Int. J. Mol. Sci. 2022, 23, 15317. [Google Scholar] [CrossRef]
- Wigger, D.; Gulbins, E.; Kleuser, B.; Schumacher, F. Monitoring the Sphingolipid de Novo Synthesis by Stable-Isotope Labeling and Liquid Chromatography-Mass Spectrometry. Front. Cell Dev. Biol. 2019, 7, 210. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and Their Metabolism in Physiology and Disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Menaldino, D.S.; Bushnev, A.; Sun, A.; Liotta, D.C.; Symolon, H.; Desai, K.; Dillehay, D.L.; Peng, Q.; Wang, E.; Allegood, J.; et al. Sphingoid Bases and de Novo Ceramide Synthesis: Enzymes Involved, Pharmacology and Mechanisms of Action. Pharmacol. Res. 2003, 47, 373–381. [Google Scholar] [CrossRef]
- Merrill, A.H. De Novo Sphingolipid Biosynthesis: A Necessary, but Dangerous, Pathway. J. Biol. Chem. 2002, 277, 25843–25846. [Google Scholar] [CrossRef] [PubMed]
- Perry, D.K. Serine Palmitoyltransferase: Role in Apoptotic de Novo Ceramide Synthesis and Other Stress Responses. Biochim. Biophys. Acta 2002, 1585, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Kitatani, K.; Idkowiak-Baldys, J.; Hannun, Y.A. The Sphingolipid Salvage Pathway in Ceramide Metabolism and Signaling. Cell. Signal. 2008, 20, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, N.; Hannun, Y.A. Acid and Neutral Sphingomyelinases: Roles and Mechanisms of Regulation. Biochem. Cell Biol. 2004, 82, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, Y.H.; Hannun, Y.A. The Acid Sphingomyelinase/Ceramide Pathway: Biomedical Significance and Mechanisms of Regulation. Curr. Mol. Med. 2010, 10, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, G. The Nutritional Functions of Dietary Sphingomyelin and Its Applications in Food. Front. Nutr. 2022, 9, 1002574. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, Y.; Ye, L.; Gong, S.; Sun, H.; Su, G. Identifying active xenobiotics in humans by use of a suspect screening technique coupled with lipidomic analysis. Environ. Int. 2021, 157, 106844. [Google Scholar] [CrossRef]
- Blachnio-Zabielska, A.U.; Persson, X.-M.T.; Koutsari, C.; Zabielski, P.; Jensen, M.D. A Liquid Chromatography/Tandem Mass Spectrometry Method for Measuring the in Vivo Incorporation of Plasma Free Fatty Acids into Intramyocellular Ceramides in Humans: Muscle Ceramide Enrichment Measured by LC/MS/MS. Rapid Commun. Mass Spectrom. 2012, 26, 1134–1140. [Google Scholar] [CrossRef]
- Carrard, J.; Gallart-Ayala, H.; Weber, N.; Colledge, F.; Streese, L.; Hanssen, H.; Schmied, C.; Ivanisevic, J.; Schmidt-Trucksäss, A. How Ceramides Orchestrate Cardiometabolic Health—An Ode to Physically Active Living. Metabolites 2021, 11, 675. [Google Scholar] [CrossRef]
- Lair, B.; Laurens, C.; Van Den Bosch, B.; Moro, C. Novel Insights and Mechanisms of Lipotoxicity-Driven Insulin Resistance. Int. J. Mol. Sci. 2020, 21, 6358. [Google Scholar] [CrossRef]
- Turpin-Nolan, S.M.; Brüning, J.C. The Role of Ceramides in Metabolic Disorders: When Size and Localization Matters. Nat. Rev. Endocrinol. 2020, 16, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, Z.; Zang, G.; Zhang, L.; Wang, Z. Role of Ceramides in Diabetic Foot Ulcers (Review). Int. J. Mol. Med. 2023, 51, 26. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, Y.M.; Al Aidaros, A.; Valappil, A.; Ali, B.R.; Akawi, N. Role of Ceramides in the Molecular Pathogenesis and Potential Therapeutic Strategies of Cardiometabolic Diseases: What We Know so Far. Front. Cell Dev. Biol. 2022, 9, 816301. [Google Scholar] [CrossRef] [PubMed]
- Burger, B.; Sagiorato, R.N.; Cavenaghi, I.; Rodrigues, H.G. Abnormalities of Sphingolipids Metabolic Pathways in the Pathogenesis of Psoriasis. Metabolites 2023, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Matwiejuk, M.; Mysliwiec, H.; Chabowski, A.; Flisiak, I. The Role of Sphingolipids in the Pathogenesis of Psoriasis. Metabolites 2022, 12, 1171. [Google Scholar] [CrossRef] [PubMed]
- Lantzanaki, M.; Veneti, S.; Mintziori, G.; Begou, O.; Pappas, P.D.; Gika, H.; Goulis, D.G.; Bili, H.; Taousani, E.; Vavilis, D. Plasma Ceramide Concentrations in Full-Term Pregnancies Complicated with Gestational Diabetes Mellitus: A Case-Control Study. Metabolites 2022, 12, 1123. [Google Scholar] [CrossRef] [PubMed]
- Bozzini, N.; Avnet, S.; Baldini, N.; Cortini, M. Epigenetic Regulation Mediated by Sphingolipids in Cancer. Int. J. Mol. Sci. 2023, 24, 5294. [Google Scholar] [CrossRef]
- Bernal-Vega, S.; García-Juárez, M.; Camacho-Morales, A. Contribution of Ceramides Metabolism in Psychiatric Disorders. J. Neurochem. 2023, 164, 708–724. [Google Scholar] [CrossRef]
- Di Pietro, P.; Izzo, C.; Abate, A.C.; Iesu, P.; Rusciano, M.R.; Venturini, E.; Visco, V.; Sommella, E.; Ciccarelli, M.; Carrizzo, A.; et al. The Dark Side of Sphingolipids: Searching for Potential Cardiovascular Biomarkers. Biomolecules 2023, 13, 168. [Google Scholar] [CrossRef]
- Signorelli, P.; Avagliano, L.; Reforgiato, M.R.; Toppi, N.; Casas, J.; Fabriàs, G.; Marconi, A.M.; Ghidoni, R.; Caretti, A. De novo ceramide synthesis is involved in acute inflammation during labor. Biol. Chem. 2016, 397, 147–155. [Google Scholar] [CrossRef]
- Birchenall, K.A.; Welsh, G.I.; López Bernal, A. Metabolite changes in maternal and fetal plasma following spontaneous labour at term in humans using untargeted metabolomics analysis: A pilot study. Int. J. Environ. Res. Public Health 2019, 16, 1527. [Google Scholar] [CrossRef] [PubMed]
- Fakhr, Y.; Brindley, D.N.; Hemmings, D.G. Physiological and pathological functions of sphingolipids in pregnancy. Cell. Signal. 2021, 85, 110041. [Google Scholar] [CrossRef] [PubMed]
- Patanapirunhakit, P.; Karlsson, H.; Mulder, M.; Ljunggren, S.; Graham, D.; Freeman, D. Sphingolipids in HDL–Potential markers for adaptation to pregnancy? Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158955. [Google Scholar] [CrossRef] [PubMed]
- Rico, J.E.; Bandaru, V.V.; Dorskind, J.M.; Haughey, N.J.; McFadden, J.W. Plasma ceramides are elevated in overweight Holstein dairy cows experiencing greater lipolysis and insulin resistance during the transition from late pregnancy to early lactation. J. Dairy Sci. 2015, 98, 7757–7770. [Google Scholar] [CrossRef] [PubMed]
- León-Aguilar, L.F.; Croyal, M.; Ferchaud-Roucher, V.; Huang, F.; Marchat, L.A.; Barraza-Villarreal, A.; Romieu, I.; Ramakrishnan, U.; Krempf, M.; Ouguerram, K.; et al. Maternal obesity leads to long-term altered levels of plasma ceramides in the offspring as revealed by a longitudinal lipidomic study in children. Int. J. Obes. 2019, 43, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Hao, S.; Yao, X.; You, J.; Li, X.; Lai, D.; Han, C.; Schilling, J.; Hwa, K.Y.; Thyparambil, S.; et al. High-throughput quantitation of serological ceramides/dihydroceramides by LC/MS/MS: Pregnancy baseline biomarkers and potential metabolic messengers. J. Pharm. Biomed. Anal. 2021, 192, 113639. [Google Scholar] [CrossRef] [PubMed]
- Enthoven, L.F.; Shi, Y.; Fay, E.; Kim, A.; Moreni, S.; Mao, J.; Isoherranen, N.; Totah, R.A.; Hebert, M.F. Effects of Pregnancy on Plasma Sphingolipids Using a Metabolomic and Quantitative Analysis Approach. Metabolites 2023, 13, 1026. [Google Scholar] [CrossRef]
- Kaneko-Tarui, T.; Zhang, L.; Austin, K.J.; Henkes, L.E.; Johnson, J.; Hansen, T.R.; Pru, J.K. Maternal and embryonic control of uterine sphingolipid-metabolizing enzymes during murine embryo implantation. Biol. Reprod. 2007, 77, 658–665. [Google Scholar] [CrossRef]
- Knapp, P.; Dobrzyń, A.; Górski, J. Ceramides, sphinganine, sphingosine and acid sphingomyelinases in the human umbilical cord blood. Horm. Metab. Res. 2005, 37, 433–437. [Google Scholar] [CrossRef]
- Blachnio-Zabielska, A.U.; Koutsari, C.; Tchkonia, T.; Jensen, M.D. Sphingolipid content of human adipose tissue: Relationship to adiponectin and insulin resistance. Obesity 2012, 20, 2341–2347. [Google Scholar] [CrossRef]
- Sibai, B.M. Preeclampsia as a cause of preterm and late preterm (near-term) births. Semin. Perinatol. 2006, 30, 16–19. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Jin, M.; Li, Z.; Zhang, L.; Li, H.; Zhang, Y.; Ye, R.; Li, N. Impact of gestational hypertension and preeclampsia on preterm birth in China: A large prospective cohort study. BMJ Open 2022, 12, e058068. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.S.; Wojdyla, D.; Say, L.; Gulmezoglu, A.M.; Van Look, P.F. WHO analysis of causes of maternal death: A systematic review. Lancet 2006, 367, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Cantwell, R.; Clutton-Brock, T.; Cooper, G.; Dawson, A.; Drife, J.; Garrod, D.; Harper, A.; Hulbert, D.; Lucas, S.; McClure, J.; et al. Saving mothers’ lives: Reviewing maternal deaths to make motherhood safer: 2006–2008. The eighth report of the confidential enquiries into maternal deaths in the United Kingdom. BJOG 2011, 118 (Suppl. S1), 1–203. [Google Scholar] [PubMed]
- Paauw, N.D.; Luijken, K.; Franx, A.; Verhaar, M.C.; Lely, A.T. Long-term renal and cardiovascular risk after preeclampsia: Towards screening and prevention. Clin. Sci. 2016, 130, 239–246. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.D.; Han, Z.; Walsh, M.W.; Gerstein, H.C.; Devereaux, P.J. Kidney disease after preeclampsia: A systematic review and meta-analysis. Am. J. Kidney Dis. 2010, 55, 1026–1039. [Google Scholar] [CrossRef] [PubMed]
- Ristovska, E.C.; Genadieva-Dimitrova, M.; Todorovska, B.; Milivojevic, V.; Rankovic, I.; Samardziski, I.; Bojadzioska, M. The Role of Endothelial Dysfunction in the Pathogenesis of Pregnancy-Related Pathological Conditions: A Review. Prilozi 2023, 44, 113–137. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M. Pathophysiology of ischemic placental disease. Semin. Perinatol. 2014, 38, 139–145. [Google Scholar] [CrossRef]
- Tranquilli, A.L.; Dekker, G.; Magee, L.; Roberts, J.; Sibai, B.M.; Steyn, W.; Zeeman, G.G.; Brown, M.A. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP, Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2014, 4, 97–104. [Google Scholar] [CrossRef]
- Henderson, J.T.; Whitlock, E.P.; O’Connor, E.; Senger, C.A.; Thompson, J.H.; Rowland, M.G. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: A systematic evidence review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2014, 160, 695–703. [Google Scholar] [CrossRef]
- Carty, D.M.; Siwy, J.; Brennand, J.E.; Zürbig, P.; Mullen, W.; Franke, J.; McCulloch, J.W.; Roberts, C.T.; North, R.A.; Chappell, L.C.; et al. Urinary proteomics for prediction of preeclampsia. Hypertension 2011, 57, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Ching, T.; Song, M.A.; Tiirikainen, M.; Molnar, J.; Berry, M.; Towner, D.; Garmire, L.X. Genome-wide hypermethylation coupled with promoter hypomethylation in the chorioamniotic membranes of early onset preeclampsia. Mol. Hum. Reprod. 2014, 20, 885–904. [Google Scholar] [CrossRef] [PubMed]
- Navaratnam, K.; Alfirevic, Z.; Baker, P.N.; Gluud, C.; Grüttner, B.; Kublickiene, K.; Zeeman, G.; Kenny, L.C. A multi-centre phase IIa clinical study of predictive testing for preeclampsia: Improved pregnancy outcomes via early detection (IMPROvED). BMC Pregnancy Childbirth 2013, 13, 226. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.C.; Nicolaides, K.H. Early prediction of preeclampsia. Obstet. Gynecol. Int. 2014, 2014, 297397. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; You, J.; Chen, L.; Zhao, H.; Huang, Y.; Zheng, L.; Tian, L.; Maric, I.; Liu, X.; Li, T.; et al. Changes in pregnancy-related serum biomarkers early in gestation are associated with later development of preeclampsia. PLoS ONE 2020, 15, e0230000. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Nien, J.K.; Espinoza, J.; Todem, D.; Fu, W.; Chung, H.; Kusanovic, J.P.; Gotsch, F.; Erez, O.; Mazaki-Tovi, S.; et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J. Matern. Fetal Neonatal Med. 2008, 21, 9–23. [Google Scholar]
- Baig, S.; Lim, J.Y.; Fernandis, A.Z.; Wenk, M.R.; Kale, A.; Su, L.L.; Biswas, A.; Vasoo, S.; Shui, G.; Choolani, M. Lipidomic analysis of human placental syncytiotrophoblast microvesicles in adverse pregnancy outcomes. Placenta 2013, 34, 436–442. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Liu, Y.; Maurya, M.R.; Benny, P.; Lassiter, C.; Li, H.; Subramaniam, S.; Garmire, L.X. The maternal blood lipidome is indicative of the pathogenesis of severe preeclampsia. J. Lipid Res. 2021, 62, 100118. [Google Scholar] [CrossRef]
- Dobierzewska, A.; Soman, S.; Illanes, S.E.; Morris, A.J. Plasma cross-gestational sphingolipidomic analyses reveal potential first trimester biomarkers of preeclampsia. PLoS ONE 2017, 12, e0175118. [Google Scholar] [CrossRef]
- Huang, Q.; Hao, S.; You, J.; Yao, X.; Li, Z.; Schilling, J.; Thyparambil, S.; Liao, W.-L.; Zhou, X.; Mo, L.; et al. Early-pregnancy prediction of risk for preeclampsia using maternal blood leptin/ceramide ratio: Discovery and confirmation. BMJ Open 2021, 11, e050963. [Google Scholar] [CrossRef]
- Amraoui, F.; Hassani Lahsinoui, H.; Spijkers, L.J.A.; Vogt, L.; Peters, S.L.M.; Wijesinghe, D.S.; Warncke, U.O.; Chalfant, C.E.; Ris-Stalpers, C.; van den Born, B.H.; et al. Plasma ceramide is increased and associated with proteinuria in women with preeclampsia and HELLP syndrome. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2020, 19, 100–105. [Google Scholar] [CrossRef]
- Charkiewicz, K.; Goscik, J.; Blachnio-Zabielska, A.; Raba, G.; Sakowicz, A.; Kalinka, J.; Chabowski, A.; Laudanski, P. Sphingolipids as a new factor in the pathomechanism of preeclampsia—Mass spectrometry analysis. PLoS ONE 2017, 12, e0177601. [Google Scholar] [CrossRef] [PubMed]
- Melland-Smith, M.; Ermini, L.; Chauvin, S.; Craig-Barnes, H.; Tagliaferro, A.; Todros, T.; Post, M.; Caniggia, I. Disruption of sphingolipid metabolism augments ceramide-induced autophagy in preeclampsia. Autophagy 2015, 11, 653–669. [Google Scholar] [CrossRef] [PubMed]
- Ausman, J.; Abbade, J.; Ermini, L.; Farrell, A.; Tagliaferro, A.; Post, M.; Caniggia, I. Ceramide-induced BOK promotes mitochondrial fission in preeclampsia. Cell Death Dis. 2018, 9, 298. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.W.; Reep, D.T.; Alam, S.M.K.; Washington, S.L.; Al Dulaimi, M.; Lee, S.M.; Springel, E.H.; Strauss, J.F., 3rd; Stephenson, D.J.; Chalfant, C.E. Placental production of eicosanoids and sphingolipids in women who developed preeclampsia on low-dose aspirin. Reprod. Sci. 2020, 27, 2158–2169. [Google Scholar] [CrossRef] [PubMed]
- Del Gaudio, I.; Sasset, L.; Lorenzo, A.D.; Wadsack, C. Sphingolipid signature of human feto-placental vasculature in preeclampsia. Int. J. Mol. Sci. 2020, 21, 1019. [Google Scholar] [CrossRef] [PubMed]
- Romanowicz, L.; Bańkowski, E. Sphingolipids of human umbilical cord vein and their alteration in preeclampsia. Mol. Cell. Biochem. 2010, 340, 81–89. [Google Scholar] [CrossRef]
- Romanowicz, L.; Bańkowski, E. Altered sphingolipid composition in Wharton’s jelly of pre-eclamptic newborns. Pathobiology 2010, 77, 78–87. [Google Scholar] [CrossRef]
- Charkiewicz, K.; Blachnio-Zabielska, A.; Zbucka-Kretowska, M.; Wolczynski, S.; Laudanski, P. Maternal plasma and amniotic fluid sphingolipids profiling in fetal Down syndrome. PLoS ONE 2015, 10, e0127732. [Google Scholar] [CrossRef]
- Sokolowska, E.; Blachnio-Zabielska, A. The role of ceramides in insulin resistance. Front. Endocrinol. 2019, 10, 577. [Google Scholar] [CrossRef]
- Bailey, L.J.; Alahari, S.; Tagliaferro, A.; Post, M.; Caniggia, I. Augmented trophoblast cell death in preeclampsia can proceed via ceramide-mediated necroptosis. Cell Death Dis. 2017, 8, e2590. [Google Scholar] [CrossRef] [PubMed]
- Pantham, P.; Heazell, A.E.; Mullard, G.; Begley, P.; Chen, Q.; Brown, M.; Dunn, W.B.; Chamley, L.W. Antiphospholipid antibodies alter cell-death-regulating lipid metabolites in first and third trimester human placentae. Am. J. Reprod. Immunol. 2015, 74, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.D.; Louis, J.M. Does race or ethnicity play a role in the origin, pathophysiology, and outcomes of preeclampsia? An expert review of the literature. Am. J. Obstet. Gynecol. 2022, 226, S876–S885. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Clausen, C.F.; Dolinsky, V.W.; Morton, J.S.; Proctor, S.D.; Dyck, J.R.; Davidge, S.T. Hypoxia-induced intrauterine growth restriction increases the susceptibility of rats to high-fat diet-induced metabolic syndrome. Diabetes 2011, 60, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Rico, J.E.; Specker, B.; Perry, C.A.; McFadden, J.W. Plasma ceramides and triglycerides are elevated during pregnancy in association with markers of insulin resistance in Hutterite women. Lipids 2020, 55, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Y.; Wu, P.; Ye, Y.; Sun, F.; Yang, X.; Lu, Q.; Yuan, J.; Liu, Y.; Zeng, H.; et al. Plasma lipidomics in early pregnancy and risk of gestational diabetes mellitus: A prospective nested case-control study in Chinese women. Am. J. Clin. Nutr. 2021, 114, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, J.; Yang, K.; Leng, J.; Li, W.; Yang, W.; Huo, X.; Yu, Z.; Ma, R.C.; Hu, G.; et al. Ceramides and their interactive effects with trimethylamine-N-oxide metabolites on risk of gestational diabetes: A nested case-control study. Diabetes Res. Clin. Pract. 2021, 171, 108606. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Francis, E.; Hu, G.; Chen, L. Metabolomic profiling of women with gestational diabetes mellitus and their offspring: Review of metabolomics studies. J. Diabetes Complicat. 2018, 32, 512–523. [Google Scholar] [CrossRef]
- Wang, H.; Li, N.; Chivese, T.; Werfalli, M.; Sun, H.; Yuen, L.; Hoegfeldt, C.A.; Elise Powe, C.; Immanuel, J.; Karuranga, S.; et al. IDF Diabetes Atlas: Estimation of global and regional gestational diabetes mellitus prevalence for 2021 by International Association of Diabetes in Pregnancy study group’s criteria. Diabetes Res. Clin. Pract. 2022, 183, 109050. [Google Scholar] [CrossRef]
- Juchnicka, I.; Kuźmicki, M.; Zabielski, P.; Krętowski, A.; Błachnio-Zabielska, A.; Szamatowicz, J. Serum C18:1-Cer as a potential biomarker for early detection of gestational diabetes. J. Clin. Med. 2022, 11, 384. [Google Scholar] [CrossRef]
- Mustaniemi, S.; Keikkala, E.; Kajantie, E.; Nurhonen, M.; Jylhä, A.; Morin-Papunen, L.; Öhman, H.; Männistö, T.; Laivuori, H.; Eriksson, J.G.; et al. Serum ceramides in early pregnancy as predictors of gestational diabetes. Sci. Rep. 2023, 13, 13274. [Google Scholar] [CrossRef] [PubMed]
- Mejia, J.F.; Hirschi, K.M.; Tsai, K.Y.F.; Long, M.G.; Tullis, B.C.; Bitter, E.E.K.; Bikman, B.T.; Reynolds, P.R.; Arroyo, J.A. Differential placental ceramide levels during gestational diabetes mellitus (GDM). Reprod. Biol. Endocrinol. 2019, 17, 81. [Google Scholar] [CrossRef] [PubMed]
- Hallman, M.; Bry, K.; Pitkänen, O. Ceramide lactoside in amniotic fluid: High concentration in chorioamnionitis and in preterm labor. Am. J. Obstet. Gynecol. 1989, 161, 313–318. [Google Scholar] [CrossRef]
- Edwin, S.S.; Mitchell, M.D.; Silver, R.M.; Branch, D.W.; Dudley, D.J. Ceramide stimulates prostaglandin production by human amnion and decidual cells. J. Soc. Gynecol. Investig. 1997, 4, 274–278. [Google Scholar] [CrossRef]
- Laudanski, P.; Charkiewicz, K.; Kisielewski, R.; Kuc, P.; Koc-Zorawska, E.; Raba, G.; Kraczkowski, J.; Dymicka-Piekarska, V.; Chabowski, A.; Kacerovsky, M.; et al. Plasma C16-Cer levels are increased in patients with preterm labor. Prostaglandins Other Lipid Mediat. 2016, 123, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Morillon, A.C.; Yakkundi, S.; Thomas, G.; Gethings, L.A.; Langridge, J.I.; Baker, P.N.; Kenny, L.C.; English, J.A.; McCarthy, F.P. Association between phospholipid metabolism in plasma and spontaneous preterm birth: A discovery lipidomic analysis in the cork pregnancy cohort. Metabolomics 2020, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Liu, H.; Zhao, S.J.; Shen, L.; Xie, T.; Luo, J.; Mor, G.; Liao, A.H. The metabolic landscape of decidua in recurrent pregnancy loss using a global metabolomics approach. Placenta 2021, 112, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Borkowski, K.; Newman, J.W.; Aghaeepour, N.; Mayo, J.A.; Blazenović, I.; Fiehn, O.; Stevenson, D.K.; Shaw, G.M.; Carmichael, S.L. Mid-gestation serum lipidomic profile associations with spontaneous preterm birth are influenced by body mass index. PLoS ONE 2020, 15, e0239115. [Google Scholar] [CrossRef]
- Sun, X.; Qu, T.; Wang, W.; Li, C.; Yang, X.; He, X.; Wang, Y.; Xing, G.; Xu, X.; Yang, L.; et al. Untargeted lipidomics analysis in women with intrahepatic cholestasis of pregnancy: A cross-sectional study. BJOG 2022, 129, 880–888. [Google Scholar] [CrossRef]
- Mikucka-Niczyporuk, A.; Pierzynski, P.; Lemancewicz, A.; Kosinski, P.; Charkiewicz, K.; Knas, M.; Kacerovsky, M.; Blachnio-Zabielska, A.; Laudanski, P. Role of sphingolipids in the pathogenesis of intrahepatic cholestasis. Prostaglandins Other Lipid Mediat. 2020, 147, 106399. [Google Scholar] [CrossRef]
- Diboun, I.; Ramanjaneya, M.; Ahmed, L.; Bashir, M.; Butler, A.E.; Albagha, O.; Abou-Samra, A.B.; Atkin, S.L.; Mazloum, N.A.; Elrayess, M.A. Metabolomic profiling of pregnancies with polycystic ovary syndrome identifies a unique metabolic signature and potential predictive biomarkers of low birth weight. Front. Endocrinol. 2021, 12, 638727. [Google Scholar] [CrossRef]
| Tissue | Study | Pathology | Ceramide Concentration Changes | Gestational Stage | Group Size (PE vs. Control) | Method |
|---|---|---|---|---|---|---|
| Embryo | ||||||
| Umbilical cord: veins | Romanowicz, 2010 | PE | Decrease (total SAFA, MUFA, PUFA) | late ** | 10 vs. 10 | TLC-HPLC |
| Umbilical cord: Wharton jelly | Romanowicz, 2010 | PE | Increase (total, SAFA, MUFA) | late | 10 vs. 11 | TLC-HPLC |
| Placenta | ||||||
| Placenta | Amraoui, 2020; Walsh, 2020; Melland-Smith, 2015 | PE + HELLP; PE + aspirin; PE | No difference; Increase: D-e-C18:0 (severe risk + aspirin); Increase (16–18–20–24) | late; late; late | 11 vs. 24/13; 13 vs. 14; 45 vs. 40 | LC-MS; UPLC ESI-MS/MS; LC-MS/MS |
| Mitochondria | Ausman, 2018 | PE | Increase: 16, 18 | late | 4 vs. 4 | LC-MS/MS |
| Arteries and endothelial cells | Delgaudio, 2020 | PE | Decrease: 20 | late | 8 vs. 10 | LC-MS/MS |
| Syncytiotrophoblast microvesicles | Nbaig, 2013; Pantham, 2015 | PE and RM; PE model * | No difference; Increase 18:1/24:1) in first trimester and ceramide-1-phosphate (d18:1/12:0) in third trimester | late; early and late | 6/9 vs. 6/9; 90 early and 54 late | LC-MS; UHPLC-MS |
| Mother | ||||||
| Plasma | Dobierzewska, 2017; Amraoui, 2020; Charkiewicz, 2017 | PE; PE + HELLP; PE mild | Decrease: 24 in third, 14 in first trimester; Increase (total); increase: 16, 18, 18:1, 20, 22, 24 | all; late; late | 7 vs. 7; 11 vs. 24/13; 21 vs. 36; | HPLC-ESI-MS/MS; LC-MS; UHPLC/MS/MS |
| Serum | Huang, 2021; He, 2021; Melland-Smith, 2015 | PE; PE severe; PE | Decrease: d18:1/25:0 in second trimester Decrease: Cer-NS d30:1; Increase: 16–18–20–24 | all; late; late | 20 vs. 20; 44 vs. 20; 45 vs. 40 | LC-MS/MS; LC-MS/MS; LC-MS/MS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lantzanaki, M.; Vavilis, T.; Harizopoulou, V.C.; Bili, H.; Goulis, D.G.; Vavilis, D. Ceramides during Pregnancy and Obstetrical Adverse Outcomes. Metabolites 2023, 13, 1136. https://doi.org/10.3390/metabo13111136
Lantzanaki M, Vavilis T, Harizopoulou VC, Bili H, Goulis DG, Vavilis D. Ceramides during Pregnancy and Obstetrical Adverse Outcomes. Metabolites. 2023; 13(11):1136. https://doi.org/10.3390/metabo13111136
Chicago/Turabian StyleLantzanaki, Maria, Theofanis Vavilis, Vikentia C. Harizopoulou, Helen Bili, Dimitrios G. Goulis, and Dimitrios Vavilis. 2023. "Ceramides during Pregnancy and Obstetrical Adverse Outcomes" Metabolites 13, no. 11: 1136. https://doi.org/10.3390/metabo13111136
APA StyleLantzanaki, M., Vavilis, T., Harizopoulou, V. C., Bili, H., Goulis, D. G., & Vavilis, D. (2023). Ceramides during Pregnancy and Obstetrical Adverse Outcomes. Metabolites, 13(11), 1136. https://doi.org/10.3390/metabo13111136







