Postmortem Alteration of Purine Metabolism in Coronary Artery Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
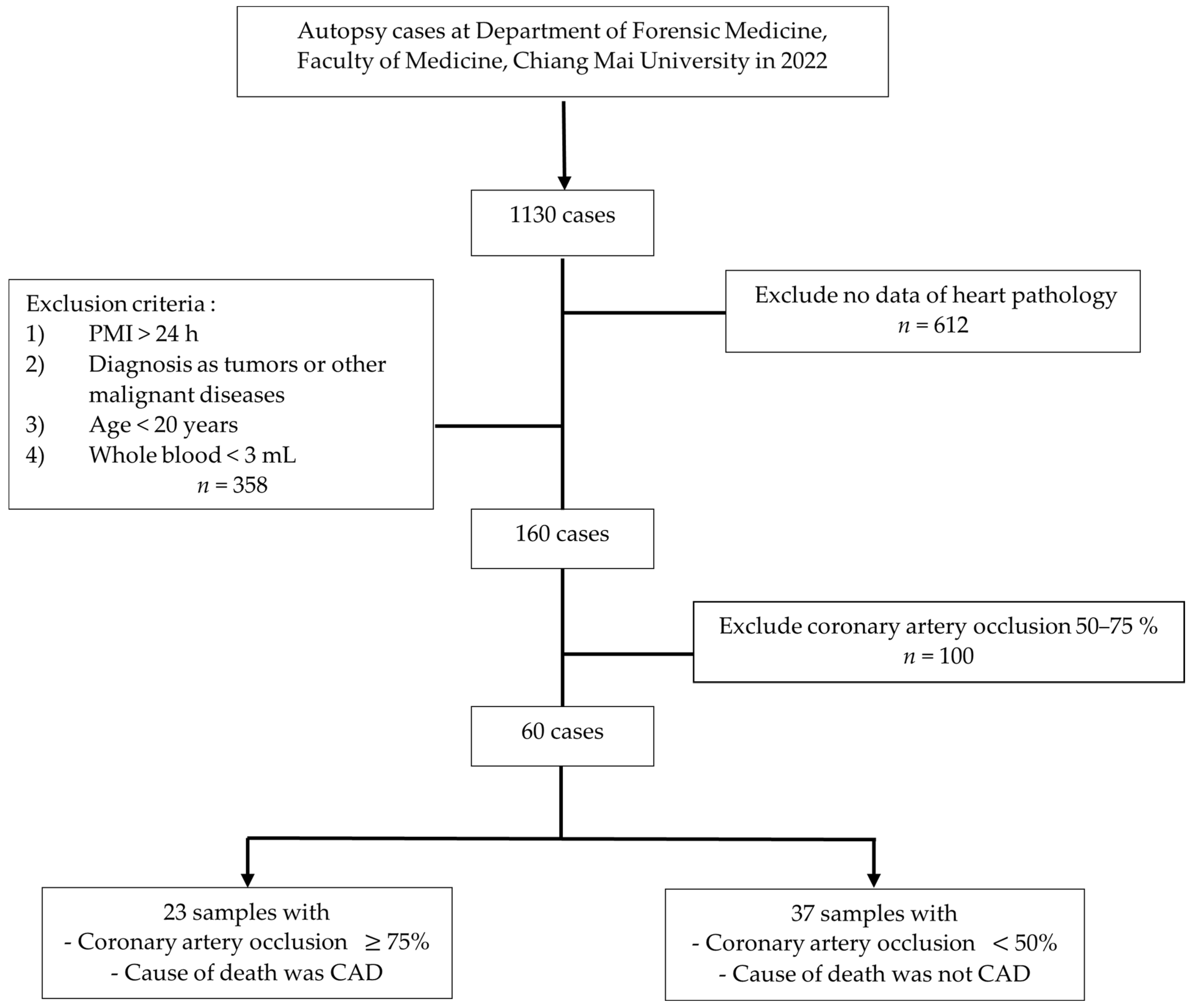
2.2. Subjects and Study Design
2.3. Collection and Preparation of Blood Specimens
2.4. Acquisition Parameters
2.5. Internal Standard (I.S.)
2.6. Quality Control (Q.C.)
2.7. Peak Assignment and Chemical Identification
2.8. 1H-NMR Data Analysis
2.9. Statistical Analysis
3. Results
3.1. Demographic Data of Participants
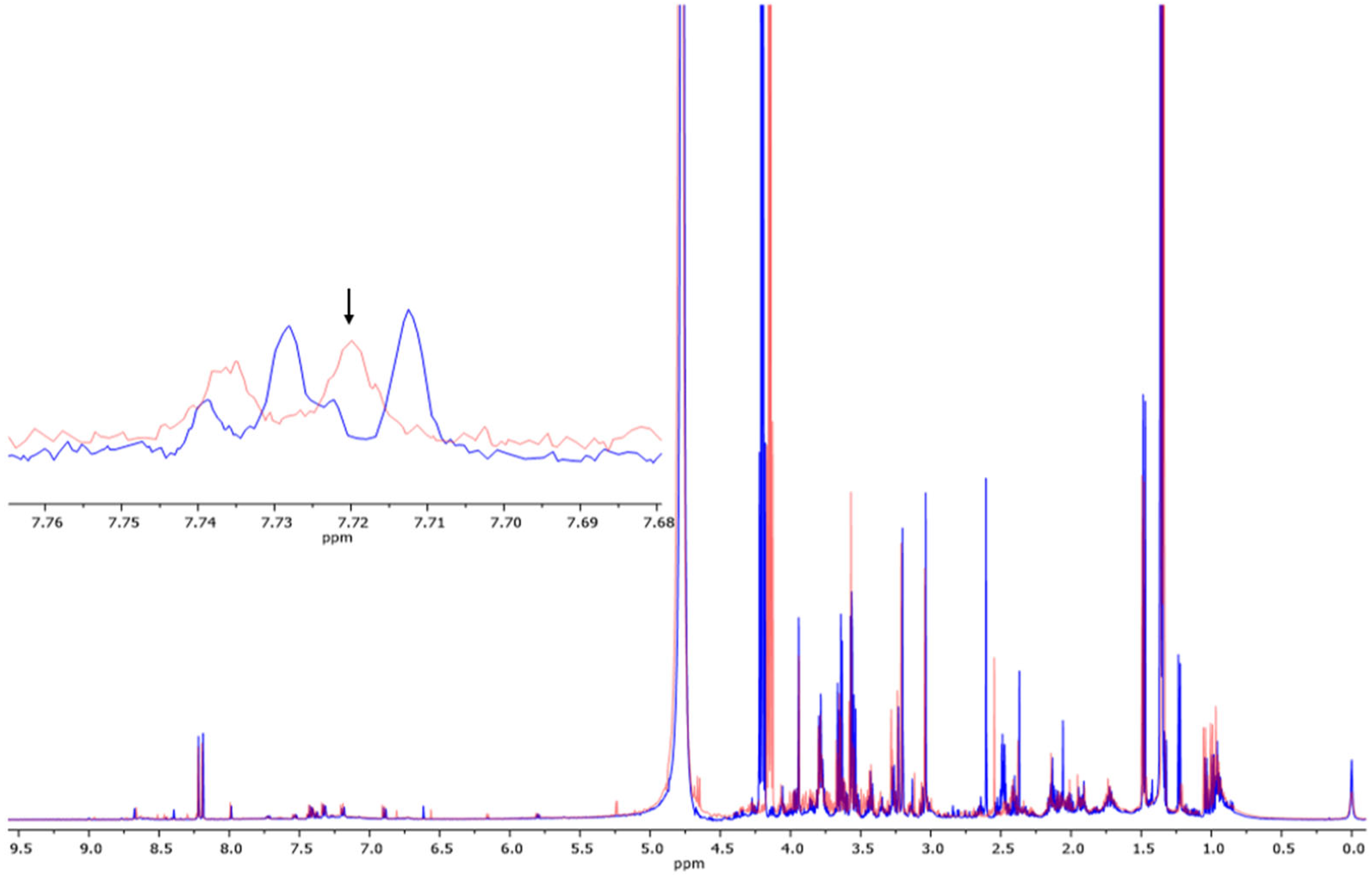
3.2. Determination of Metabolites in Blood Samples via 1H-NMR
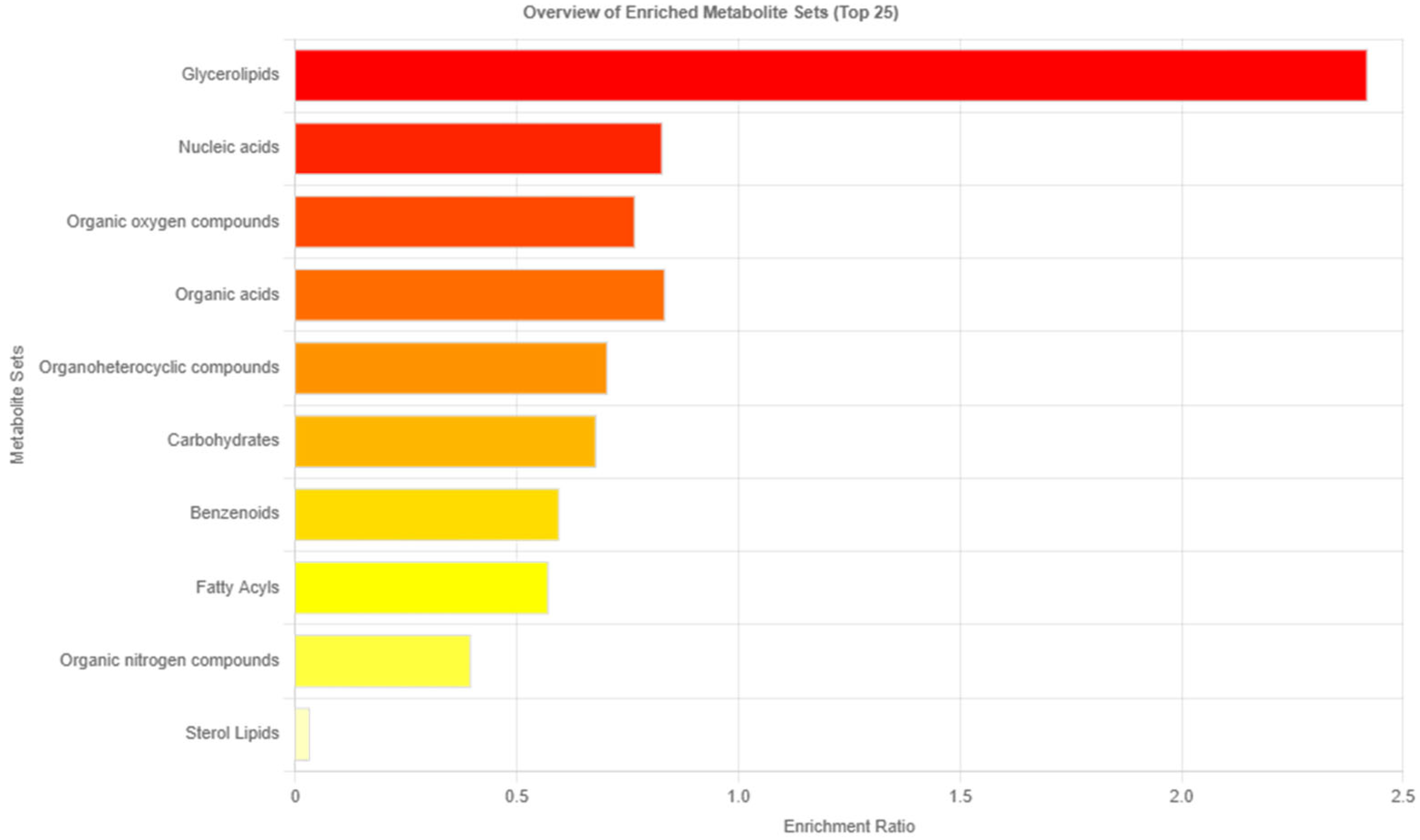
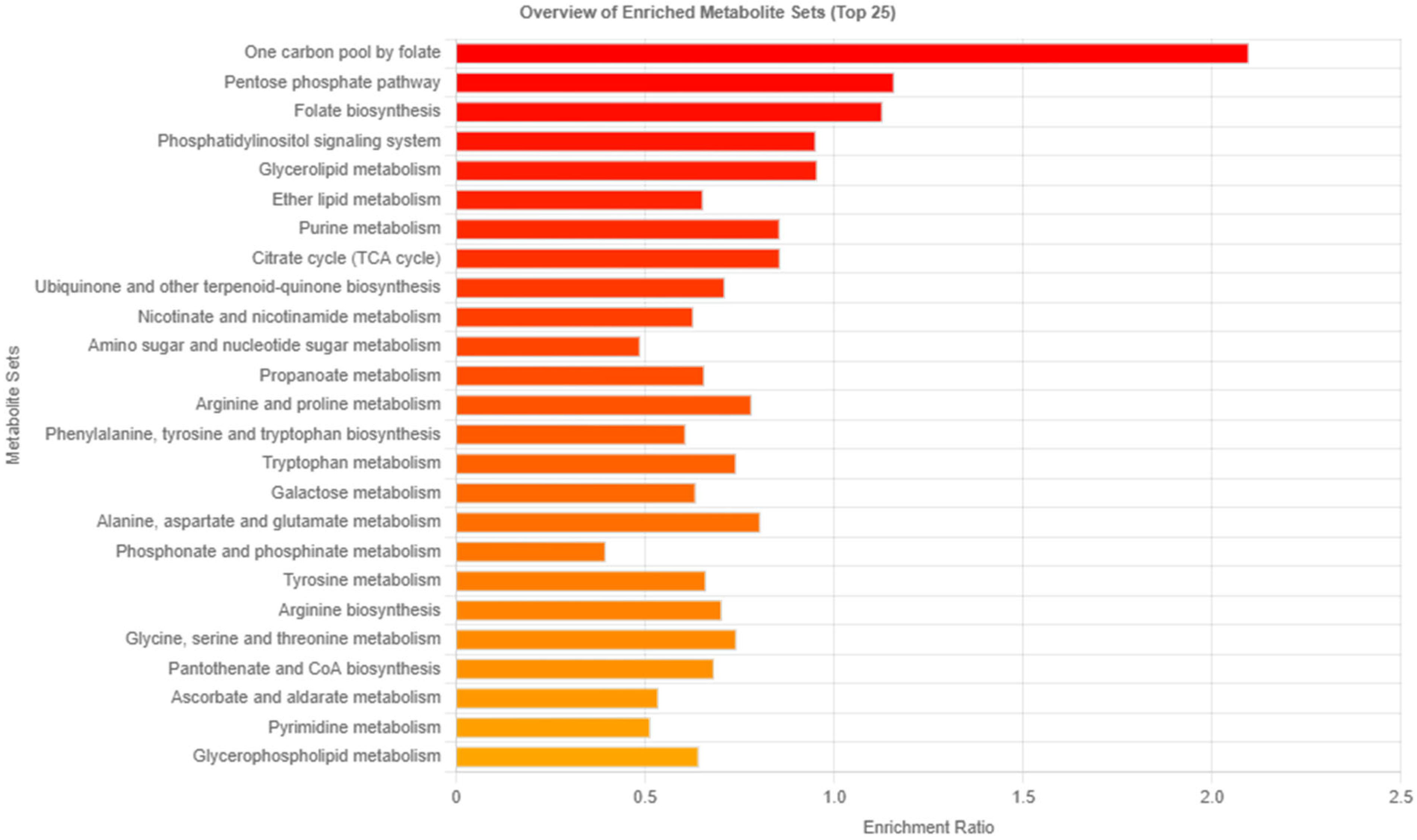
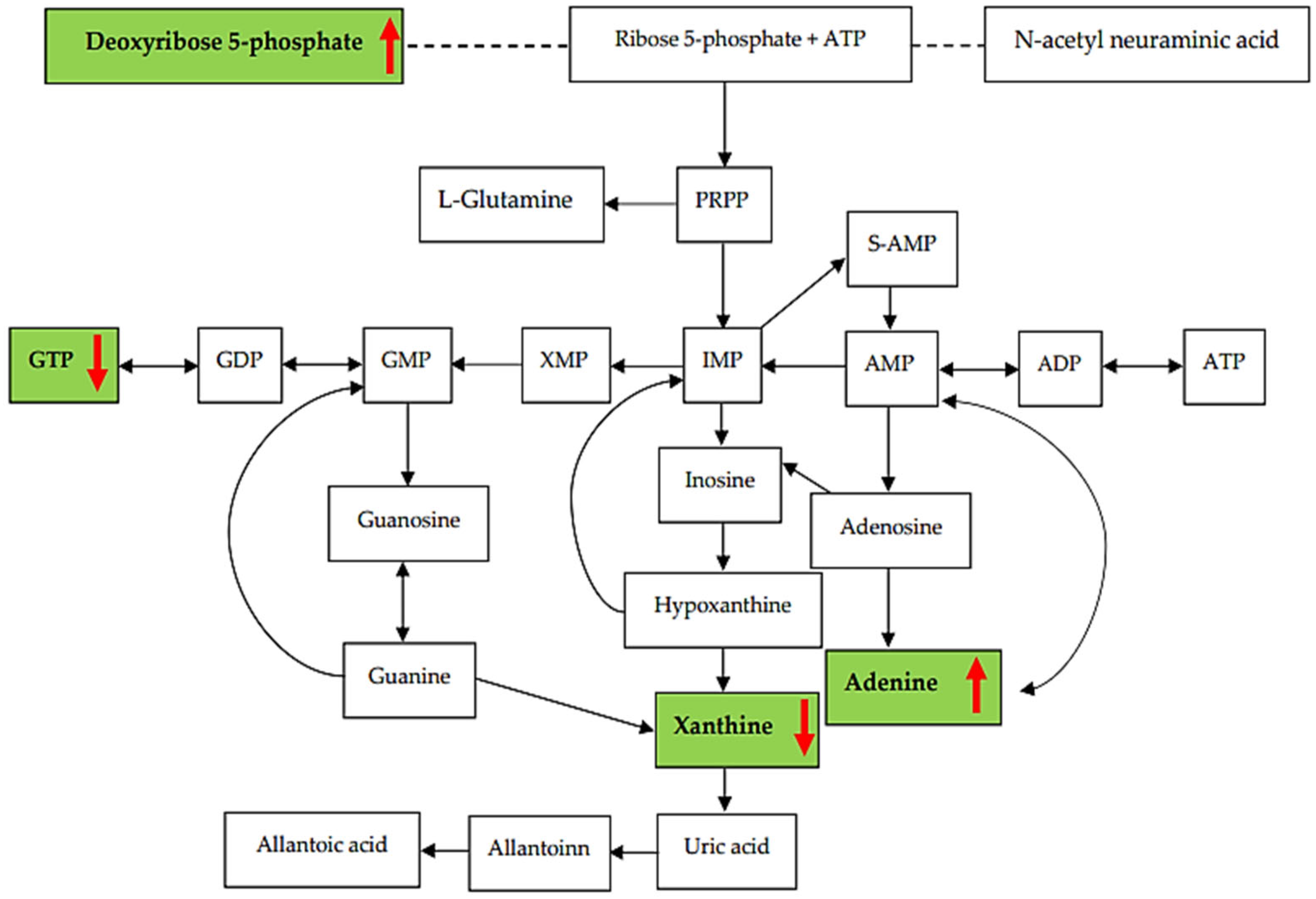
3.3. Analysis of Related Pathways
3.4. Purine Metabolites Analysis in Heart Blood Samples
3.5. Relationship between Metabolites in the Purine Pathway and CAD Group
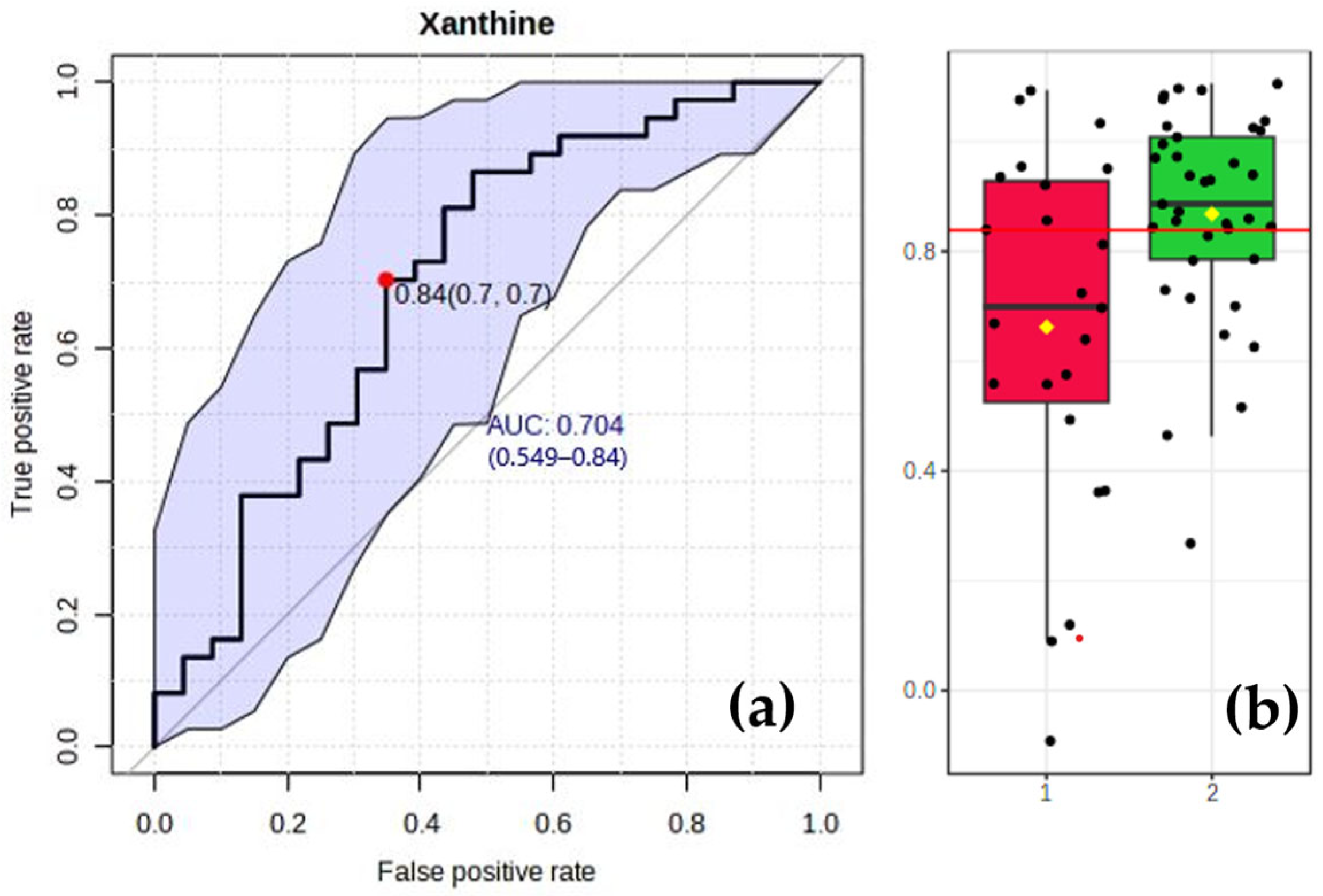
3.6. Analysis of Specific Biomarkers for the CAD Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.; Adams, R.J.; Brown, T.M.; Carnethon, M.; Dai, S.; De Simone, G.; Ferguson, T.B.; Ford, E.; Furie, K.; Gillespie, C.; et al. Executive summary: Heart disease and stroke statistics—2010 update: A report from the American Heart Association. Circulation 2010, 121, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E. Heart disease and stroke statistics—2023 update: A report from the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Theroux, P. Pathophysiology of coronary artery disease. Circulation 2005, 111, 3481–3488. [Google Scholar] [CrossRef]
- Aggarwal, A.; Srivastava, S.; Velmurugan, M. Newer perspectives of coronary artery disease in young. World J. Cardiol. 2016, 8, 728–734. [Google Scholar] [CrossRef]
- Udnoon, J.; Chirachariyavej, T.; Peonim, V. Sudden unexpected deaths in different age groups at Ramathibodi Hospital, Bangkok, Thailand: A retrospective autopsy study during 2003–2007. Southeast Asian J. Trop. Med. Public Health 2009, 40, 162–168. [Google Scholar]
- Rao, D.; Sood, D.; Pathak, P.; Dongre, S.D. A cause of Sudden Cardiac Deaths on Autopsy Findings; a Four-Year Report. Emergency 2014, 2, 12–17. [Google Scholar]
- Basso, C.; Aguilera, B.; Banner, J.; Cohle, S.; d’Amati, G.; de Gouveia, R.H.; di Gioia, C.; Fabre, A.; Gallagher, P.J.; Leone, O.; et al. Guidelines for autopsy investigation of sudden cardiac death: 2017 update from the Association for European Cardiovascular Pathology. Virchows Arch. 2017, 471, 691–705. [Google Scholar] [CrossRef]
- Al-Adnani, M.; Scheimberg, I. How can we improve the rate of autopsies among Muslims? BMJ 2006, 332, 310. [Google Scholar] [CrossRef]
- Netto, J.; Teren, A.; Burkhardt, R.; Willenberg, A.; Beutner, F.; Henger, S.; Schuler, G.; Thiele, H.; Isermann, B.; Thiery, J.; et al. Biomarkers for Non-Invasive Stratification of Coronary Artery Disease and Prognostic Impact on Long-Term Survival in Patients with Stable Coronary Heart Disease. Nutrients 2022, 14, 3433. [Google Scholar] [CrossRef]
- Rahimi, R.; Dahili, N.D.; Zainun, K.A.; Kasim, N.A.M.; Noor, S.M. Post mortem troponin T analysis in sudden death: Is it useful? Malays. J. Pathol. 2018, 40, 143–148. [Google Scholar] [PubMed]
- Lai, P.; Nur Shafina, M.; Mohd Hilmi, S.; Nur Shazuwani, R.; Normaizuwana, M.; Kunasilan, S. Correlation of troponin T levels in the cardiac sudden death cases at hospital kuala lumpur. Int. J. Forensic Sci. Pathol. 2017, 5, 376–383. [Google Scholar]
- Zribi, M.; Ennouri, H.; Turki, M.; Amar, W.B.; Grati, M.; Hammami, Z.; Ayadi, F.; Maatoug, S. Diagnostic value of high-sensitivity troponin T in postmortem diagnosis of sudden cardiac death. J. Forensic Leg. Med. 2021, 78, 102127. [Google Scholar] [CrossRef] [PubMed]
- Santisukwongchote, K.; Amornlertwatana, Y.; Sastraruji, T.; Jaikang, C. Possible Use of Blood Tryptophan Metabolites as Biomarkers for Coronary Heart Disease in Sudden Unexpected Death. Metabolites 2019, 10, 6. [Google Scholar] [CrossRef]
- Bauer, J.A.; Moffatt-Bruce, S.D.; Elton, T.S.; Feldman, D. Purine metabolism in heart failure: Oxidant biology and therapeutic indications. Congest. Heart Fail. 2008, 14, 283–284. [Google Scholar] [CrossRef]
- Bredemeier, M.; Lopes, L.M.; Eisenreich, M.A.; Hickmann, S.; Bongiorno, G.K.; d’Avila, R.; Morsch, A.L.B.; da Silva Stein, F.; Campos, G.G.D. Xanthine oxidase inhibitors for prevention of cardiovascular events: A systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2018, 18, 24. [Google Scholar] [CrossRef]
- Meneshian, A.; Bulkley, G.B. The physiology of endothelial xanthine oxidase: From urate catabolism to reperfusion injury to inflammatory signal transduction. Microcirculation 2002, 9, 161–175. [Google Scholar] [CrossRef]
- Dona, A.C.; Coffey, S.; Figtree, G. Translational and emerging clinical applications of metabolomics in cardiovascular disease diagnosis and treatment. Eur. J. Prev. Cardiol. 2016, 23, 1578–1589. [Google Scholar] [CrossRef]
- Jung, S.; Ahn, E.; Koh, S.B.; Lee, S.H.; Hwang, G.S. Purine metabolite-based machine learning models for risk prediction, prognosis, and diagnosis of coronary artery disease. Biomed. Pharmacother. 2021, 139, 111621. [Google Scholar] [CrossRef]
- Dona, A.C.; Kyriakides, M.; Scott, F.; Shephard, E.A.; Varshavi, D.; Veselkov, K.; Everett, J.R. A guide to the identification of metabolites in NMR-based metabonomics/metabolomics experiments. Comput. Struct. Biotechnol. J. 2016, 14, 135–153. [Google Scholar] [CrossRef]
- Yang, K.M.; Lee, S.Y.; Kim, Y.S.; Seo, J.S.; Lee, Y.S.; Seo, J.W. Guidelines for forensic assessment of natural unexpected cardiovascular death. Basic Appl. Pathol. 2008, 1, 155–163. [Google Scholar] [CrossRef]
- DiMaio, D.; DiMaio, V.J. Forensic Pathology; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Baroldi, G.; Falzi, G.; Mariani, F. Sudden coronary death. A postmortem study in 208 selected cases compared to 97 “control” subjects. Am. Heart J. 1979, 98, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Liu, H.; Zhang, T.; Jiang, Y.; Zhang, A.-H. Untargeted lipidomics study of coronary artery disease by FUPLC-Q-TOF-MS. Anal. Methods 2016, 8, 1229–1234. [Google Scholar] [CrossRef]
- Djekic, D.; Pinto, R.; Repsilber, D.; Hyotylainen, T.; Henein, M. Serum untargeted lipidomic profiling reveals dysfunction of phospholipid metabolism in subclinical coronary artery disease. Vasc. Health Risk Manag. 2019, 15, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Girard, C.; Scarpelli, M.P.; Tettamanti, C.; Palmiere, C. Postmortem evaluation of cholesterol, triglyceride, and apolipoprotein levels. Int. J. Legal Med. 2017, 131, 1777–1782. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-carbon metabolism in health and disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef]
- Rosenberger, F.A.; Moore, D.; Atanassov, I.; Moedas, M.F.; Clemente, P.; Végvári, Á.; El Fissi, N.; Filograna, R.; Bucher, A.-L.; Hinze, Y. The one-carbon pool controls mitochondrial energy metabolism via complex I and iron-sulfur clusters. Sci. Adv. 2021, 7, eabf0717. [Google Scholar]
- Otsu, Y.; Ae, R.; Kuwabara, M. Folate and cardiovascular disease. Hypertens. Res. 2023, 46, 1816–1818. [Google Scholar] [CrossRef]
- Cherepanova, O.A.; Byzova, T.V. Pentose phosphate pathway drives vascular maturation. Nat. Metab. 2022, 4, 15–16. [Google Scholar] [CrossRef]
- Krajnik, A.; Brazzo, J.A., III; Vaidyanathan, K.; Das, T.; Redondo-Muñoz, J.; Bae, Y. Phosphoinositide signaling and mechanotransduction in cardiovascular biology and disease. Front. Cell Dev. Biol. 2020, 8, 595849. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, A.E.; Lamont, I.L. Biochemistry changes that occur after death: Potential markers for determining postmortem interval. PLoS ONE 2013, 8, e82011. [Google Scholar] [CrossRef] [PubMed]
- Ith, M.; Bigler, P.; Scheurer, E.; Kreis, R.; Hofmann, L.; Dirnhofer, R.; Boesch, C. Observation and identification of metabolites emerging during postmortem decomposition of brain tissue by means of in situ 1H-magnetic resonance spectroscopy. Magn. Reson. Med. 2002, 48, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Jawor, P.; Ząbek, A.; Wojtowicz, W.; Król, D.; Stefaniak, T.; Młynarz, P. Metabolomic studies as a tool for determining the postmortem interval (PMI) in stillborn calves. BMC Vet. Res. 2019, 15, 189. [Google Scholar] [CrossRef]
- Pesko, B.K.; Weidt, S.; McLaughlin, M.; Wescott, D.J.; Torrance, H.; Burgess, K.; Burchmore, R. Postmortomics: The Potential of Untargeted Metabolomics to Highlight Markers for Time Since Death. OMICS 2020, 24, 649–659. [Google Scholar] [CrossRef]
- Rognum, T.O.; Hauge, S.; Øyasaeter, S.; Saugstad, O.D. A new biochemical method for estimation of postmortem time. Forensic Sci. Int. 1991, 51, 139–146. [Google Scholar] [CrossRef]
- Zhu, B.-L.; Ishikawa, T.; Michiue, T.; Tanaka, S.; Zhao, D.; Li, D.-R.; Quan, L.; Oritani, S.; Maeda, H. Differences in postmortem urea nitrogen, creatinine and uric acid levels between blood and pericardial fluid in acute death. Leg. Med. 2007, 9, 115–122. [Google Scholar] [CrossRef]
- Leyva, F.; Wingrove, C.S.; Godsland, I.F.; Stevenson, J.C. The glycolytic pathway to coronary heart disease: A hypothesis. Metabolism 1998, 47, 657–662. [Google Scholar] [CrossRef]
- Nees, S. Coronary flow increases induced by adenosine and adenine nucleotides are mediated by the coronary endothelium: A new principle of the regulation of coronary flow. Eur. Heart J. 1989, 10, 28–35. [Google Scholar] [CrossRef]
- Swain, J.L.; Sabina, R.L.; Peyton, R.B.; Jones, R.N.; Wechsler, A.S.; Holmes, E.W. Derangements in myocardial purine and pyrimidine nucleotide metabolism in patients with coronary artery disease and left ventricular hypertrophy. Proc. Natl. Acad. Sci. USA 1982, 79, 655–659. [Google Scholar] [CrossRef]
- Warangkool, C.; Churdsak, J.; Chaturong, K. Changing of ATP and Its Metabolites in Blood Samples for Post Mortem Interval: In Vitro Study. Indian J. Forensic Med. Toxicol. 2022, 16, 48–52. [Google Scholar] [CrossRef]
- Baldus, S.; Mullerleile, K.; Chumley, P.; Steven, D.; Rudolph, V.; Lund, G.K.; Staude, H.J.; Stork, A.; Koster, R.; Kahler, J.; et al. Inhibition of xanthine oxidase improves myocardial contractility in patients with ischemic cardiomyopathy. Free Radic. Biol. Med. 2006, 41, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Grosso, M.A.; Banerjee, A.; St Cyr, J.A.; Rogers, K.B.; Brown, J.M.; Clarke, D.R.; Campbell, D.N.; Harken, A.H. Cardiac 5′-nucleotidase activity increases with age and inversely relates to recovery from ischemia. J. Thorac. Cardiovasc. Surg. 1992, 103, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, L.; Zhang, L. NAD+ metabolism as an emerging therapeutic target for cardiovascular diseases associated with sudden cardiac death. Front. Physiol. 2020, 11, 901. [Google Scholar] [CrossRef] [PubMed]
- Senn, T.; Hazen, S.L.; Tang, W.H. Translating metabolomics to cardiovascular biomarkers. Prog. Cardiovasc. Dis. 2012, 55, 70–76. [Google Scholar] [CrossRef]
- Surendran, A.; Atefi, N.; Zhang, H.; Aliani, M.; Ravandi, A. Defining Acute Coronary Syndrome through Metabolomics. Metabolites 2021, 11, 685. [Google Scholar] [CrossRef]
- Lewis, G.D.; Wei, R.; Liu, E.; Yang, E.; Shi, X.; Martinovic, M.; Farrell, L.; Asnani, A.; Cyrille, M.; Ramanathan, A.; et al. Metabolite profiling of blood from individuals undergoing planned myocardial infarction reveals early markers of myocardial injury. J. Clin. Investig. 2008, 118, 3503–3512. [Google Scholar] [CrossRef]

| Factors | CAD (n = 23) | Control (n = 37) | p-Value |
|---|---|---|---|
| Age (years) | 63 ± 15 | 56 ± 13 | 0.063 |
| Male (%) | 87.0 | 68.4 | 0.094 |
| Hypertension (%) | 52.2 | 26.3 | 0.051 |
| Diabetes mellitus (%) | 21.7 | 15.8 | 0.594 |
| Dyslipidemia (%) | 17.4 | 13.2 | 0.685 |
| Gout (%) | 0 | 7.9 | 0.165 |
| Renal insufficiency (%) | 4.4 | 5.3 | 0.856 |
| Other heart diseases (%) | 21.7 | 7.9 | 0.134 |
| Metabolites (μM) | HMDB | CAD Group (n = 23) | Control Group (n = 37) | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Q1 | Median | Q3 | Q1 | Median | Q3 | |||
| Adenine | 0000034 | 1.56 | 2.81 | 13.80 | 3.58 | 6.77 | 23.37 | 0.042 * |
| Adenosine | 0000050 | 1.45 | 3.95 | 9.53 | 1.89 | 8.61 | 15.37 | 0.171 |
| Adenyl succinic acid (×) | 0000536 | 0.26 | 0.88 | 2.44 | 0.29 | 0.66 | 2.50 | 0.613 |
| ADP | 0001341 | 0.47 | 1.32 | 6.85 | 1.27 | 6.70 | 15.30 | 0.077 |
| AMP | 0000045 | 0.47 | 10.14 | 38.72 | 1.30 | 5.60 | 15.61 | 0.405 |
| ATP | 0000538 | 0.32 | 8.14 | 31.63 | 2.02 | 5.34 | 12.77 | 0.405 |
| Deoxyribose 5- phosphate (×) | 0001031 | 1.13 | 3.07 | 8.53 | 0.41 | 1.26 | 3.17 | 0.046 * |
| GDP (×) | 0001201 | 0.13 | 0.31 | 1.13 | 0.18 | 0.44 | 1.51 | 0.882 |
| GMP (×) | 0001379 | 0.29 | 0.87 | 2.20 | 0.32 | 0.77 | 2.44 | 0.814 |
| GTP | 0001273 | 0.36 | 0.79 | 3.24 | 0.82 | 3.22 | 8.37 | 0.018 * |
| Guanine | 0000132 | 3.85 | 9.11 | 20.09 | 4.83 | 13.47 | 31.32 | 0.164 |
| Guanosine (×) | 0000133 | 0.65 | 2.02 | 6.95 | 0.47 | 0.89 | 2.29 | 0.084 |
| Hypoxanthine | 0000157 | 1.93 | 8.36 | 40.36 | 4.11 | 18.17 | 38.72 | 0.319 |
| IMP (×) | 0000175 | 0.24 | 0.59 | 1.75 | 0.24 | 0.56 | 1.83 | 0.766 |
| Inosine | 0000195 | 0.62 | 11.87 | 43.62 | 1.15 | 7.49 | 18.40 | 0.258 |
| L-glutamine (×) | 0000641 | 0.60 | 1.03 | 2.08 | 0.50 | 1.40 | 5.01 | 0.847 |
| N-acetylneuraminic acid (×) | 0000230 | 0.76 | 1.98 | 4.11 | 0.51 | 1.13 | 3.09 | 0.157 |
| NAD | 0000902 | 0.61 | 1.54 | 4.29 | 2.06 | 3.81 | 7.03 | 0.036 * |
| NADPH | 0000221 | 0.43 | 1.32 | 6.85 | 0.74 | 4.04 | 11.71 | 0.069 |
| Xanthine (×) | 0000292 | 0.07 | 0.18 | 0.44 | 0.20 | 0.37 | 0.74 | 0.030 * |
| GDP/GTP (×) | - | 0.08 | 0.36 | 1.11 | 0.05 | 0.25 | 0.62 | 0.251 |
| GMP/GDP | - | 1.44 | 1.93 | 2.23 | 1.36 | 1.60 | 2.00 | 0.109 |
| Guanosine/GMP | - | 1.14 | 2.32 | 5.84 | 0.72 | 1.25 | 2.37 | 0.026 * |
| Guanine/guanosine | - | 0.01 | 0.04 | 0.15 | 0.06 | 0.16 | 0.30 | 0.003 * |
| IMP/GMP | - | 0.74 | 0.80 | 0.91 | 0.70 | 0.78 | 0.90 | 0.155 |
| IMP/AMP | - | 5.94 | 6.99 | 77.23 | 6.05 | 9.70 | 48.68 | 0.538 |
| Inosine/IMP | - | 0.05 | 0.17 | 0.19 | 0.02 | 0.13 | 0.19 | 0.425 |
| Hypoxanthine/inosine | - | 0.27 | 2.52 | 4.63 | 1.09 | 4.04 | 6.54 | 0.099 |
| Xanthine/hypoxanthine | - | 1.12 | 1.60 | 5.63 | 1.27 | 3.70 | 7.54 | 0.277 |
| Xanthine/guanine | - | 0.99 | 2.14 | 5.78 | 1.50 | 2.42 | 4.62 | 0.569 |
| AMP/adenyl succinic acid | - | 0.01 | 0.10 | 0.12 | 0.02 | 0.08 | 0.13 | 0.744 |
| Adenyl succinic acid/IMP | - | 1.16 | 1.30 | 1.47 | 0.96 | 1.25 | 1.42 | 0.233 |
| ADP/ATP | - | 0.09 | 0.38 | 1.55 | 0.45 | 1.35 | 2.53 | 0.031 * |
| Adenine/adenosine | - | 0.29 | 1.24 | 2.97 | 0.54 | 1.62 | 3.83 | 0.342 |
| Adenosine/AMP | - | 0.20 | 0.85 | 1.96 | 0.53 | 1.66 | 3.37 | 0.127 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somtua, P.; Jaikang, C.; Konguthaithip, G.; Intui, K.; Watcharakhom, S.; O’Brien, T.E.; Amornlertwatana, Y. Postmortem Alteration of Purine Metabolism in Coronary Artery Disease. Metabolites 2023, 13, 1135. https://doi.org/10.3390/metabo13111135
Somtua P, Jaikang C, Konguthaithip G, Intui K, Watcharakhom S, O’Brien TE, Amornlertwatana Y. Postmortem Alteration of Purine Metabolism in Coronary Artery Disease. Metabolites. 2023; 13(11):1135. https://doi.org/10.3390/metabo13111135
Chicago/Turabian StyleSomtua, Phakchira, Churdsak Jaikang, Giatgong Konguthaithip, Kanicnan Intui, Somlada Watcharakhom, Timothy E. O’Brien, and Yutti Amornlertwatana. 2023. "Postmortem Alteration of Purine Metabolism in Coronary Artery Disease" Metabolites 13, no. 11: 1135. https://doi.org/10.3390/metabo13111135
APA StyleSomtua, P., Jaikang, C., Konguthaithip, G., Intui, K., Watcharakhom, S., O’Brien, T. E., & Amornlertwatana, Y. (2023). Postmortem Alteration of Purine Metabolism in Coronary Artery Disease. Metabolites, 13(11), 1135. https://doi.org/10.3390/metabo13111135









