1. Introduction
The treatment of SARS-CoV-2 has differed considerably, and global research has been conducted since January 2020. COVID-19 is the disease caused by this emerging virus and is characterized by interstitial pneumonia and pulmonary thromboembolism. Further, gastrointestinal (GI) and non-GI symptoms are included in COVID-19. The first waves of the pandemic have been found to be harmful to humans [
1]. The use of RNA vaccines has changed the clinical features of COVID-19. For example, anosmia and dysgeusia have been replaced with a higher incidence of conjunctivitis [
2]. Thus, wave by wave, mortality has fallen by 4–5% and is now lower than seasonal flu [
3]. Research performed on the bodies of deceased patients has contributed to the understanding of COVID-19 pathophysiology. It is a hyper-inflammatory syndrome where a non-educated immune system tries to produce antibodies towards a new agent. Subsequently, a cytokine storm is elicited with target organs becoming inflamed, leading to the rapid development of fibrosis [
4]. The lungs become “cement” and patients develop subclinical, but terrifying, respiratory failure [
1,
2].
There is a statistically significant relationship between inflammatory cytokine levels, nutritional status (namely, malnutrition), and COVID-19 mortality [
2]. On the other hand, the stage and gravity of obesity significantly correlate with patients’ morbidity and mortality [
5]. For example, sarcopenia, typical of obesity, is a risk factor for a worse prognosis of COVID-19 and, also, higher SARS-CoV-2 infection susceptibility [
5,
6].
The mainstays of COVID-19 treatment are antibiotics, steroids, and low-molecular-weight heparin administered in the second week of infection [
7]. There is growing literature evidence on the efficacy of the re-establishment of physiologic immune system functioning via nutritional components, both for the prevention and treatment of COVID-19 [
8,
9]. Therefore, immuno-nutrition can be defined as the nutrients or specific components of food, in concentrations significantly higher than those of a normal diet, able to modulate the activation of the immune system or the effects due to its activation [
10]. Specifically, some immuno-nutrients have been used as add-on treatments of COVID-19 vs. evidence-based standard therapy. Initial studies have shown promising results due to the significant down-regulation of the innate and adaptive immune response involved in the “cytokine storm” typical of COVID-19 patients [
11].
Previous data from our study group have already demonstrated the efficacy of a whey-protein-rich enteral feeding formula in ICU-ventilated COVID-19 patients. In detail, we observed an earlier extubation time and improved nutritional status [
12]. Further, data from semi-intensive obese COVID-19 patients have confirmed the preventive effect on sarcopenia and malnutrition of the same formula. This result was joined with a significant drop in inflammatory markers and cytokines [
13]. In fact, the formula used in former studies by our group is rich in whey proteins and, in particular, in bioactive peptides able to modulate the immune response, especially in hyper-inflammatory diseases like COVID-19 [
12,
13].
Thus, this prospective exploratory single-center study aims to evaluate the nutritional and anti-inflammatory effects of an outlined nutritional protocol based on immuno-nutrition (IN) in COVID-19 patients admitted to a mild-intensity clinic.
3. Results
On 1 October and 30 January 2022, we consecutively admitted 52 consecutive patients (mean age 60.9 ± 5.4 years, 17 females, BMI 23.5 Kg/m2) to the mild-intensity COVID-19 Internal Medicine Unit of “Madonna del Soccorso” General Hospital, San Benedetto del Tronto, Italy. Upon informed consent, 14 patients (mean age 67.9 ± 5.4 years, seven females, BMI 26.7 Kg/m2) were accepted to be administered with the immuno-nutrition formula.
The patients included in the study had their infection confirmed via SARS-CoV-2 antigenic nasal swab 3.4 ± 0.5 days prior to admission to the Internal Medicine Unit.
Their main comorbidities were: diabetes (20%, type 2 90%), hyperuricemia (15%), hypertension (38%), chronic ischemic heart disease (12%), COPD (13%), anxiety (10%), and depression (8%).
Considering inflammatory markers at enrollment, the median CRP was 29 [5.5–41] mg/L; IL-6 87 pg/mL; white blood cell count 9020 [5900–14,000].
The HRCT scan results were as follows: mild pneumonitis (35%), moderate pulmonary parenchyma involvement (40%), and severe involvement (20%).
The control group (
n = 18) (COVID-19 patients who did not provide informed consent to receive IN, from the same wave of the pandemic) characteristics are shown in
Table 1. The SARS-CoV-2 infection confirmation prior the admission to the Internal Medicine Unit was conducted within a similar timescale to that of the IN-treated group (Student’s
t-test,
p = NS).
Of note, the CRP and IL-6 values at the time of Emergency Department admission (namely, before Internal Medicine Unit admission) of both groups did not show significant differences (CRP: 37 ± 1.0 vs. 34 ± 0.9 mg/L for IN and control group, respectively, Student’s t-test, p = NS; IL-6: 99 ± 2.3 vs. 98 ± 2.1 pg/mL for IN and control group, respectively, Student’s t-test, p = NS).
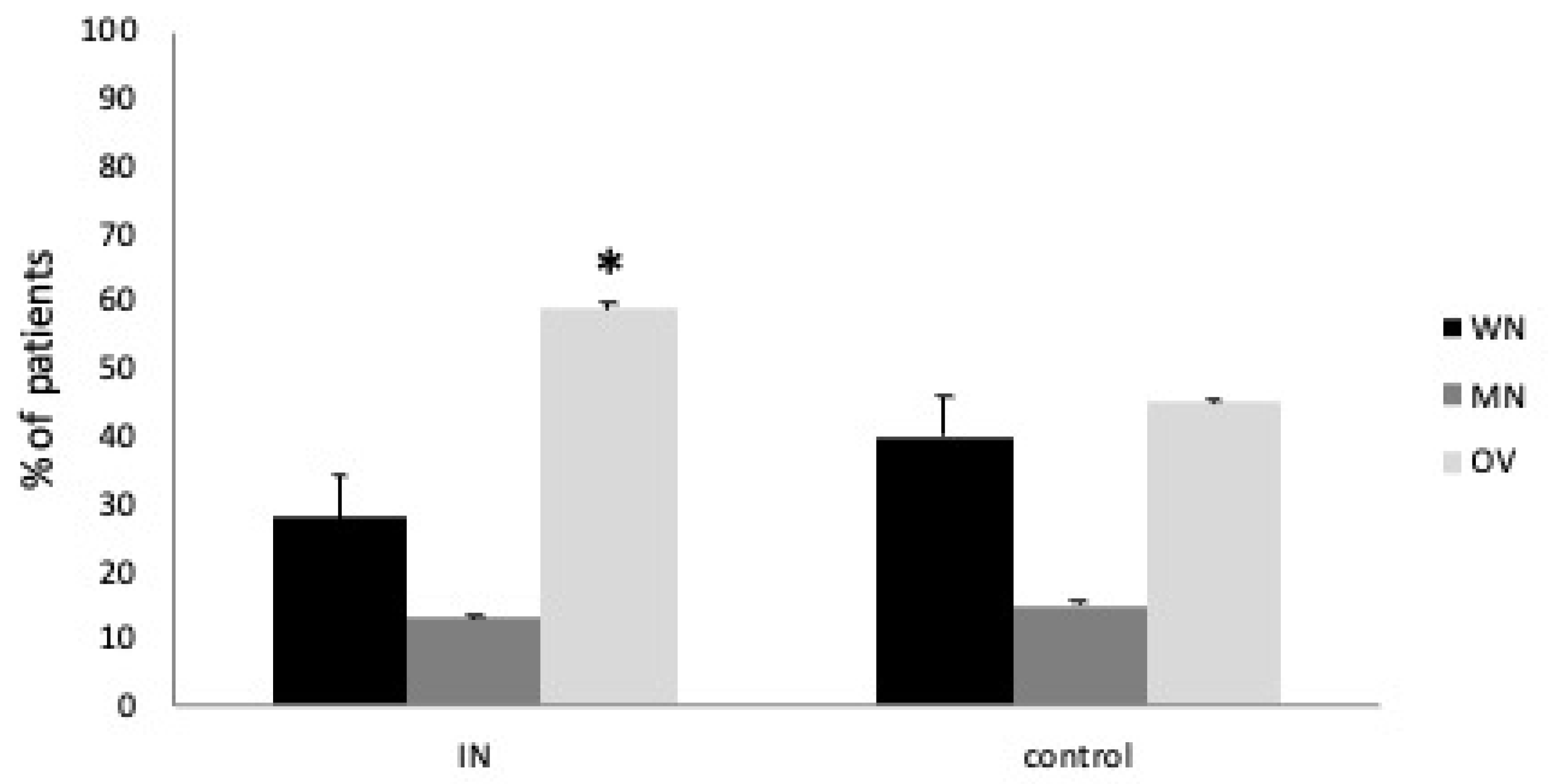
The comorbidity prevalence and other anthropometric, nutritional, and inflammatory characteristics were comparable except for BMI (
p = 0.05). In addition, the MNA test results and BIA confirmed a statistical difference for overweight representation between the study and control group (Mann–Whitney U test, both
p < 0.05) (
Figure 1).
During their mild-intensity unit stay, all IN and control group patients were treated with guideline-driven treatments (namely, remdesivir, metilprednisolone, piperacillin/tazobactam, and levofloxacin). There was no statistical difference concerning medications used among groups (Student’s t-test, p = NS).
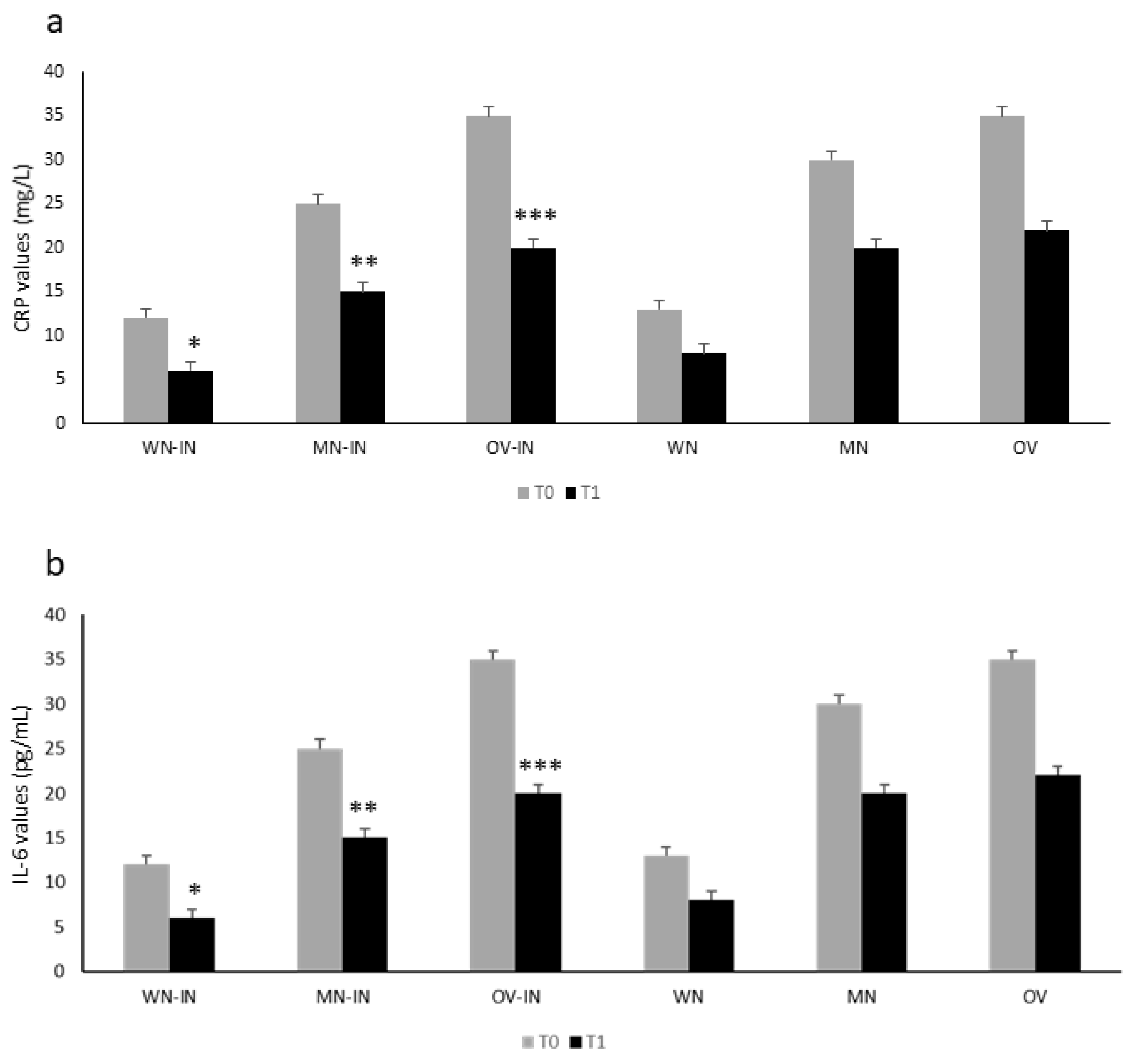
Figure 2 shows the inflammatory marker values in the IN group according to their nutritional status at T0. The control group showed a similar behavior at T0 (
Figure 2). In both groups, malnutrition and being overweight were significantly associated with higher CRP (
Figure 2a) and IL-6 (
Figure 2b) values (Student’s
t-test, both
p < 0.05).
At T1, all patients showed improved HRCT pneumonitis findings (from severe to moderate, from moderate/mild to mild/significant resolution), except for seven patients (six of whom passed from moderate to severe COVID-19 pneumonitis).
Therefore, after 15 days of admission (T1), three deaths were recorded (mean age 68.9 ± 4.1 years, three females, BMI 27.5 Kg/m2). Overweight significantly correlated with the exitus occurrence (non-parametric Spearman test, r= 0.65).
Specifically, one death was reported for IN-treated patients (mean age 71.1 ± 3.1 years, female sex, BMI 26.6 Kg/m2), and two patients were moved to ICU care because of worsening respiratory performance. The latter was associated with worsened HRCT pneumonitis findings and COPD relapse, respectively.
In the control group, at T1, we observed two deaths (mean age 70.2 ± 2.7 years, one female, BMI 23.1 Kg/m2) and two patients were moved to ICU care because of worsening respiratory performance. The latter was associated with worsened HRCT pneumonitis findings.
After 2 weeks of IN formula administration, we observed a significant reduction in inflammatory markers (PCR, IL-6; ANOVA, *, **, ***
p < 0.05 for both) in the IN group (
Figure 2 a and b). In the control group, a similar trend was observed, without reaching statistical significance (ANOVA,
p = NS) (
Figure 2).
Glycemic assessment was not affected by IN nutrition (p = NS).
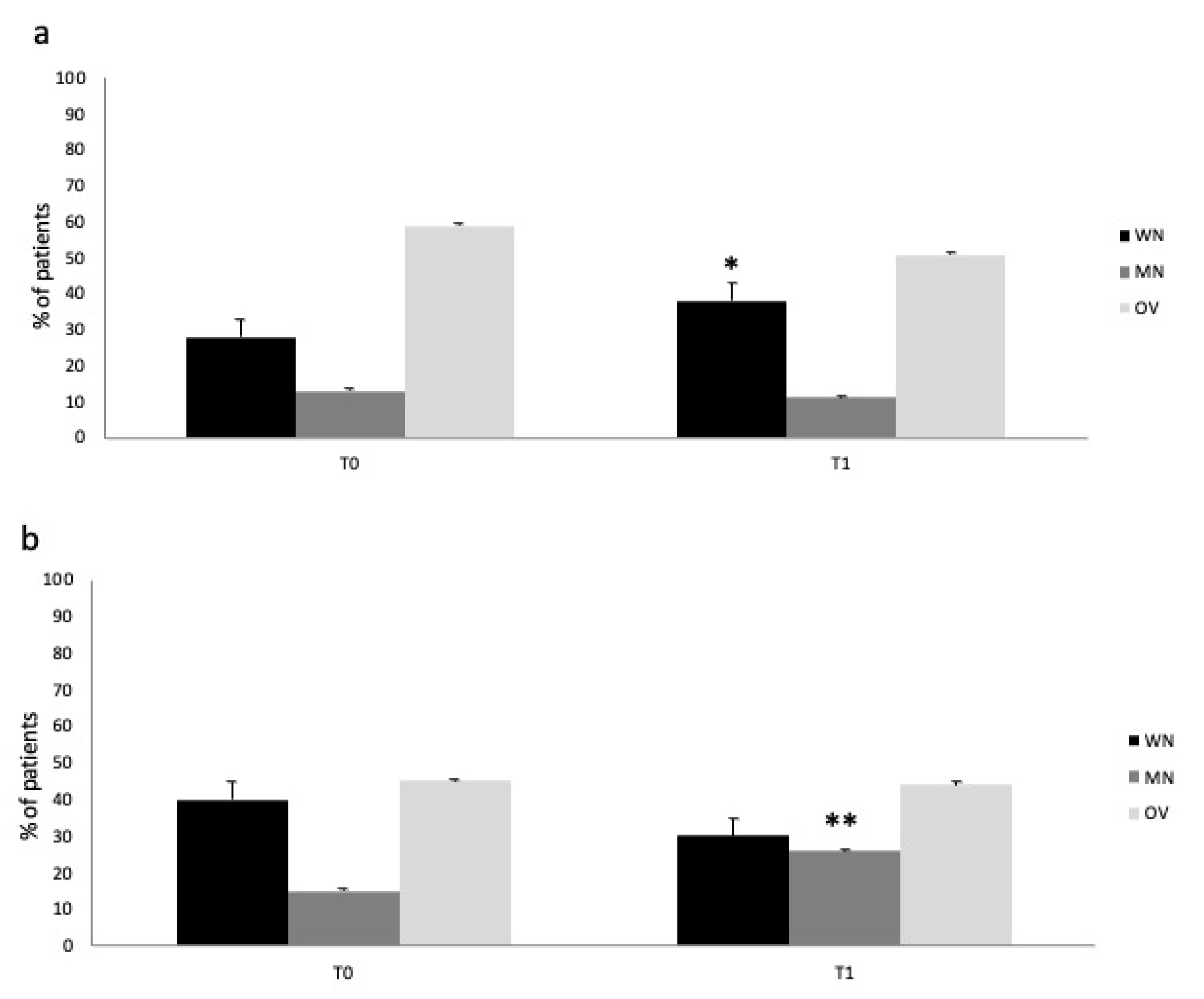
Figure 3 describes the nutritional status change in the IN and control group from T0 to T1. Immuno-nutrition administration was able to prevent the worsening of nutritional status in the treatment vs. control group (ANOVA,
p < 0.05) (IN group:
Figure 3a; control group:
Figure 3b). The finding was due to the significant reduction in malnourished patients (namely, MN and OV) and increase in WN patients in the IN-treated group, respectively (T1: MNA test, percentage of WN, MN, OV patients: 38/11/51%; BIA evaluation PA values for WN, MN, OV patients: 4.6/3.2/8.1°).
Internal Medicine Unit length of stay was not affected by IN use (Student’s t-test, p = NS).
4. Discussion
In this single-center prospective pilot study, COVID-19 patients admitted to the mild-intensity Internal Medicine care clinic of our hospital were treated with immuno-nutrition. Its impact on nutritional status and inflammatory state were evaluated. The control group was represented by COVID-19 patients not administered the IN formula.
In agreement with previous results from our research group [
12,
13], we have shown that a specific casein-rich immuno-nutrition formula is able to prevent the worsening of nutritional status in prevalently obese COVID-19 patients (namely, a reduction in the percentage of MN and OV patients). This was followed, in parallel, by an inflammatory response reduction. On the other hand, there was a non-significant reduction in inflammatory marker concentrations in the control group. Indeed, malnutrition was not prevented in this subset of individuals infected with SARS-CoV-2.
This study has been the conclusion of our project exploring the nutritional and immunomodulatory potential of a whey-protein-rich formula that showed promising results in terms of reduced inflammatory state in pediatric IBD patients [
14]. Starting from ICU patients [
12], continuing through semi-intensive ones [
13], and concluding with mild-intensity COVID-19 patients, we can report consistent results.
We recorded a significant impact in terms of a reduction in inflammatory markers such as CRP and interleukin-6 in ICU patients. The inflammatory nutritional marker prealbumin followed this trend, demonstrating its acute phase protein origin. The latter reduction was significantly correlated with shorter extubation time and lower mortality rates. Altogether, this anti-inflammatory effect was accompanied by a reduction in malnutrition risk. The IN formula was administrated enterally through a nasogastric tube [
12]. Among these patients, there was not a significant percentage of obese subjects.
The subsequent exploratory trial in semi-intensive, but obese, COVID-19 patients from our hospital showed that the orally administered immuno-nutrition was able to significantly reduce the inflammatory response of these sub-acute patients without affecting their mortality rate. Interestingly, this formula was able to prevent both malnutrition worsening and occurrence, although these patients were already suffering from sarcopenia [
13].
In the present study, there was no significant correlation between the prevention of malnutrition development and improved mortality or the prevention of worsened clinical course (namely, the need for ICU admission) [
12]. This finding can be explained mainly by the small sample size and also by the short follow-up time of the population in the study, which does not allow further speculation on the impact of IN administration on the prognosis of COVID-19 patients.
Interestingly, the reduction in overweight representation from T0 to T1 in both groups did not reach statistical significance, mainly due to the high percentage of obese patients enrolled in both groups and the small sample size. We must also consider the higher prevalence of obese subjects among IN- vs. non-IN-treated patients. This finding may agree with those from semi-intensive COVID-19 patients previously reported from our group [
13]. More interestingly, this tendency towards a reduction in OV representation in COVID-19 patients by IN has promising implications for the prognosis, morbidity, and, especially, mortality of SARS-CoV-2-infected subjects. Indeed, the significant relationship between higher mortality and obesity in COVID-19 is well known [
6]. In addition, the positive impact of IN on obesity in the general population is also known [
20].
We must also consider the differences between the present study and the study on semi-intensive patients enrolled in the previous investigation conducted by our group [
13]. In fact, mild-intensity clinic patients are the most represented COVID-19 patients. Although their inflammatory status could be lower than semi-intensive ones, they represent the vast majority of admitted and non-admitted patients. Thus, the impact of IN on their inflammatory status and malnutrition risk and status has important economic implications. For example, the early introduction of IN formulas in the diet of these patients could help reduce the hospital admission rate. This is a crucial point in the emergency setting of a pandemic. In fact, the relatively low cost profile and good compliance of IN administration to SARS-CoV-2 patients is one of the pros in favor of its introduction into the daily clinical management of COVID-19.
Further, IN can guarantee a better prognosis perspective because the reduced risk of malnutrition significantly correlates with improved COVID-19 patient survival rates. On the other hand, semi-intensive ventilated patients can show issues in oral IN formula administration vs. non-ventilated patients.
The findings from the present study are in agreement with the solid evidence showing both the positive impact of nutritional assessment and the use of specific food supplements on COVID-19 patients’ morbidity and mortality [
21]. These data are available in both critical and non-critical patients [
22,
23]. In detail, whey-protein-rich formulas and pre-, pro-, and, lately, postbiotics have been used as add-on treatments for steroid, antibiotic, and antiviral therapy in COVID-19 subjects, with promising results [
24,
25].
Thus, our study and the previous data on immuno-nutrition in ICU and semi-intensive patients pave the road and endorse the use of nutritional evaluation with validated questionnaires (namely, MNA, MUST) and/or bioimpedance analysis in current clinical practice. This can have a significant impact on patients’ time of hospital stay, morbidity, and, importantly, mortality.
Specifically, we retrieved one Brazilian study evaluating the impact of a protein-rich normo-caloric diet with or without IN formula as an add-on treatment for the inflammatory response (described also by lymphopenia) on non-ventilated COVID-19 patients [
26]. In this study, there was a significant reduction in the inflammatory cascade via immuno-nutrition formula administration, but no effect on the nutritional status of the patients. This is an important difference with our investigation and can be explained by the type of immuno-nutrition used and, moreover, by its higher content in proteins. Going deeper, bioactive peptides added to the industrial diet may favor mucosal healing in inflammatory bowel disease with a strong anti-inflammatory effect [
27]. These peptides are specific growth factors (namely, transforming growth factor-β (TGF-β)) and control the processes of immune cell differentiation, proliferation, and activation. Thus, these peptides can obtain mucosal immunomodulation with reduced intestinal permeability to inflammatory cytokines, reduced systemic inflammation, and SARS-CoV-2 entrance within the cells [
26].
Although a similar trend was observed in the reduction in inflammatory markers in the control group, only the group of patients treated with IN demonstrated a statistically significant reduction in IL-6 and CRP. Thus, these findings support an anti-inflammatory effect of IN. These findings are in line with those from ICU and semi-intensive COVID-19 patients from our group and with several reports from the literature evaluating other IN examples, such as omega-3 fatty acids successfully used in diabetic and septic patients [
28]. Furthermore, arginine also showed similar promising results in COVID-19 patients [
29].
Our study has several limitations. First, the sample size was small, mainly due to the circumstances of a pandemic and the prospective experimental design. In detail, the small sample size could have affected the lack of significance of the difference in mortality among IN and control groups. Second, our study took into consideration patients enrolled during the third wave of the SARS-CoV-2 pandemic. Their clinical presentation and inflammatory condition differed from those during previous waves (namely, different virus strain, use of vaccine, and antivirals). Third, our cohort had a high representation of obese people with a significant prevalence of sarcopenia that could have benefited from the use of a casein-rich IN formula more than other populations.
Author Contributions
Conceptualization: M.S., E.S. and L.A.; methodology: M.B., L.A. and E.S.; data validation: E.R., M.B., E.S. and L.A.; formal analysis: E.R., M.B., J.T. and E.S.; investigation: M.B., N.G., E.S. and M.S.; data curation: M.S., M.B., N.G. and E.S.; writing—original draft preparation: M.S., E.S., L.A. and E.R.; writing—review and editing: E.S., L.A., J.T. and M.S.; visualization of data: C.R., S.M., L.A. and E.S.; supervision of the study: E.S. and L.A.; project administration: M.S., E.S., L.A. and J.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Regional Ethics Committee of Marche, Ancona, Italy (Ethical Committee Marche, Italy, provisional CERM 2021333IN21012, September 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent was obtained from the patient(s) or relatives to publish this paper.
Data Availability Statement
Data supporting these results can be found in the patient file database of “Madonna del Soccorso General Hospital”, San Benedetto del Tronto, Italy.
Acknowledgments
We thank Mario Gismondi for the strong support during this investigation. Indeed, we must acknowledge the sacrifice and restless work of all of the personnel operating in the mild-intensity Internal Medicine Unit of Madonna del Soccorso General Hospital during the COVID-19 pandemic.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lai, C.; Hsu, C.; Hsueh, S.; Yen, M.; Ko, W.; Hsueh, P. Multisystem inflammatory syndrome in adults: Characteristics, treatment, and outcomes. J. Med. Virol. 2022, 95, 28426. [Google Scholar] [CrossRef] [PubMed]
- Săndulescu, O.; Apostolescu, C.G.; Preoțescu, L.L.; Streinu-Cercel, A.; Săndulescu, M. Therapeutic developments for SARS-CoV-2 infection-Molecular mechanisms of action of antivirals and strategies for mitigating resistance in emerging variants in clinical practice. Front. Microbiol. 2023, 14, 1132501. [Google Scholar] [CrossRef] [PubMed]
- Rabaan, A.A.; Al Mutair, A.; Alhumaid, S.; Al Alawi, Z.; Al Mohaini, M.; Alsalman, A.J.; Fawzy, M.; Al-Tawfiq, J.A.; Almahmoud, S.; Alfouzan, W.; et al. Modulation of host epigenome by coronavirus infections and developing treatment modalities for COVID-19 beyond genetics. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5947–5964. [Google Scholar] [CrossRef]
- Li, M.; Guo, L.; Feng, L. Interplay between swine enteric coronaviruses and host innate immune. Front. Vet. Sci. 2022, 9, 1083605. [Google Scholar] [CrossRef]
- Nindrea, R.D.; Lailani, M.; Masrul Usman, E.; Katar, Y.; Hendriyani, H.; Sari, N.P. Obesity and Mortality of Hospitalized COVID-19 Patients in Asian and Western Countries: A Systematic Review and Meta-Analysis. Int. J. Prev. Med. 2023, 14, 67. [Google Scholar]
- Wang, Y.; Tan, S.; Yan, Q.; Gao, Y. Sarcopenia and COVID-19 Outcomes. Clin. Interv. Aging 2023, 18, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus Disease 2019 (COVID-19) Treatment Guidelines; National Institutes of Health (US): Bethesda, MD, USA, 2021.
- Subedi, L.; Tchen, S.; Gaire, B.P.; Hu, B.; Hu, K. Adjunctive Nutraceutical Therapies for COVID-19. Int. J. Mol. Sci. 2021, 22, 1963. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, H.; Feehan, J.; Al Dhaheri, A.S.; Ali, H.I.; Platat, C.; Ismail, L.C.; Apostolopoulos, V.; Stojanovska, L. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19? Maturitas 2021, 143, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Grimble, R.F. Basics in clinical nutrition: Immuno-nutrition—Nutrients which influence immunity: Effect and mechanism of action. Eur. e-J. Clin. Nutr. Metab. 2009, 4, e10–e13. [Google Scholar] [CrossRef]
- Bae, M.; Kim, H. Mini-Review on the Roles of Vitamin C, Vitamin D, and Selenium in the Immune System against COVID-19. Molecules 2020, 25, 5346. [Google Scholar] [CrossRef]
- Scarcella, M.; Scarpellini, E.; Ascani, A.; Commissari, R.; Scorcella, C.; Zanetti, M.; Parisi, A.; Monti, R.; Milic, N.; Donati, A.; et al. Effect of Whey Proteins on Malnutrition and Extubating Time of Critically Ill COVID-19 Patients. Nutrients 2022, 14, 437. [Google Scholar] [CrossRef] [PubMed]
- Scarcella, M.; Scarpellini, E.; Piergallini, S.; Rinninella, E.; Routhiaux, K.; Rasetti, C.; Abenavoli, L.; De Robertis, E.; Manzi, P.; Commissari, R.; et al. Effect of Immuno-Nutrition on Malnutrition, Inflammatory Response and Clinical Course of Semi-Critically Ill COVID-19 Patients: A Pilot Perspective Study. Nutrients 2023, 15, 1250. [Google Scholar] [CrossRef] [PubMed]
- Matuszczyk, M.; Meglicka, M.; Landowski, P.; Czkwianianc, E.; Sordyl, B.; Szymańska, E.; Kierkuś, J. Oral exclusive enteral nutrition for induction of clinical remission, mucosal healing, and improvement of nutritional status and growth velocity in children with active Crohn’s disease—A prospective multicentre trial. Prz. Gastroenterol. 2021, 16, 346–351. [Google Scholar] [CrossRef]
- Lundin, H.; Sääf, M.; Strender, L.E.; Mollasaraie, H.A.; Salminen, H. Mini nutritional assessment and 10-year mortality in free-living elderly women: A prospective cohort study with 10-year follow-up. Eur. J. Clin. Nutr. 2012, 66, 1050–1053. [Google Scholar] [CrossRef]
- Bauer, J.M.; Kaiser, M.J.; Anthony, P.; Guigoz, Y.; Sieber, C.C. The Mini Nutritional Assessment®—Its history, today’s practice, and future perspectives. Nutr. Clin. Pract. 2008, 23, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Moonen, H.P.F.X.; Van Zanten, A.R.H. Bioelectric impedance analysis for body composition measurement and other potential clinical applications in critical illness. Curr. Opin. Crit. Care 2021, 27, 344–353. [Google Scholar] [CrossRef]
- Mulasi, U.; Kuchnia, A.J.; Cole, A.J.; Earthman, C.P. Bioimpedance at the bedside: Current applications; limitations; and opportunities. Nutr. Clin. Pract. 2015, 30, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Niederer, L.E.; Miller, H.; Haines, K.L.; Molinger, J.; Whittle, J.; MacLeod, D.B.; McClave, S.A.; Wischmeyer, P.E. Prolonged progressive hypermetabolism during COVID-19 hospitalization undetected by common predictive energy equations. Clin. Nutr. ESPEN 2021, 45, 341–350. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, E.; Calcaterra, V.; Verduci, E.; Ghezzi, M.; Lamberti, R.; Vizzuso, S.; Baldassarre, P.; Pendezza, E.; Perico, V.; Bosetti, A.; et al. Immunonutrition and SARS-CoV-2 Infection in Children with Obesity. Nutrients 2022, 14, 1701. [Google Scholar] [CrossRef] [PubMed]
- Alizadehsani, R.; Sani, Z.A.; Behjati, M.; Roshanzamir, Z.; Hussain, S.; Abedini, N.; Hasanzadeh, F.; Khosravi, A.; Shoeibi, A.; Roshanzamir, M.; et al. Risk factors prediction, clinical outcomes, and mortality in COVID-19 patients. J. Med. Virol. 2021, 93, 2307–2320. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Firoozi, D.; Dehghani, M.; Zare, M.; Mehrabi, Z.; Ghaseminasab-Parizi, M.; Masoumi, S.J. Evaluation of Nutritional Status of Intensive Care Unit COVID-19 Patients Based on the Nutritional Risk Screening 2002 Score. Int. J. Clin. Pract. 2022, 2022, 2448161. [Google Scholar] [CrossRef] [PubMed]
- Ting, T.H.Y.; Lo, T.H.M.; Lo, W.W.T.; Ding, Q.; Yuk, D.K.L.; Hui, E.; Tang, M.W.S. Inadequate energy and protein intake; underweight and malnutrition are associated with in-hospital mortality among COVID-19 rehabilitation patients during the omicron outbreak in Hong Kong. Aging Med. 2022, 5, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Khani, N.; Soleimani, R.A.; Noorkhajavi, G.; Soleimani, A.A.; Abbasi, A.; Rad, A.H. Postbiotics as potential promising tools for SARS-CoV-2 disease adjuvant therapy. J. Appl. Microbiol. 2022, 132, 4097–4111. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Sergi, D.; Zauli, E.; Voltan, R.; Lodi, G.; Vaccarezza, M.; Caruso, L.; Previati, M.; Zauli, G. Nutrients, herbal bioactive derivatives and commensal microbiota as tools to lower the risk of SARS-CoV-2 infection. Front. Nutr. 2023, 10, 1152254. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, R.F.W.; Silva, A.P.; Santana, A.I.C.; Silva, D.S.E.; Ramos, M.S.; Souza, M.C.; Suen, V.M.M.; Maduro, I.P.N.N.; Ribas Filho, D.; D’Oliveira Júnior, A.; et al. Effect of immuno-nutrition on serum levels of C-reactive protein and lymphocytes in patients with COVID-19: A ran-domized; controlled; double-blind clinical trial. Nutr. Hosp. 2022, 39, 20–26. [Google Scholar] [PubMed]
- Fell, J.M. Control of systemic and local inflammation with transforming growth factor beta containing formulas. J. Parenter. Enter. Nutr. 2005, 29, S126–S128. [Google Scholar] [CrossRef] [PubMed]
- Naghibi, T.; Shafigh, N.; Mazloomzadeh, S. Role of omega-3 fatty acids in the prevention of delirium in mechanically ventilated patients. J. Res. Med. Sci. 2020, 25, 10. [Google Scholar] [CrossRef] [PubMed]
- Reizine, F.; Lesouhaitier, M.; Gregoire, M.; Pinceaux, K.; Gacouin, A.; Maamar, A.; Painvin, B.; Camus, C.; Le Tulzo, Y.; Tattevin, P.; et al. SARS-CoV-2-Induced ARDS Associates with MDSC Expansion; Lymphocyte Dysfunction; and Arginine Shortage. J. Clin. Immunol. 2021, 41, 515–525. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).