Ultra-Performance Liquid Chromatography Coupled with Mass Metabolic Profiling of Ammi majus Roots as Waste Product with Isolation and Assessment of Oral Mucosal Toxicity of Its Psoralen Component Xanthotoxin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals and Solvents
2.3. Extraction of the Plant Material
2.4. UPLC-MS/MS Identification
2.5. Fractionation and Purification
2.6. Studies on Pharmacokinetics and ADME (Absorption, Distribution, Metabolism, and Excretion)
2.7. Biological Study Design
2.8. Statistical Analysis
3. Results
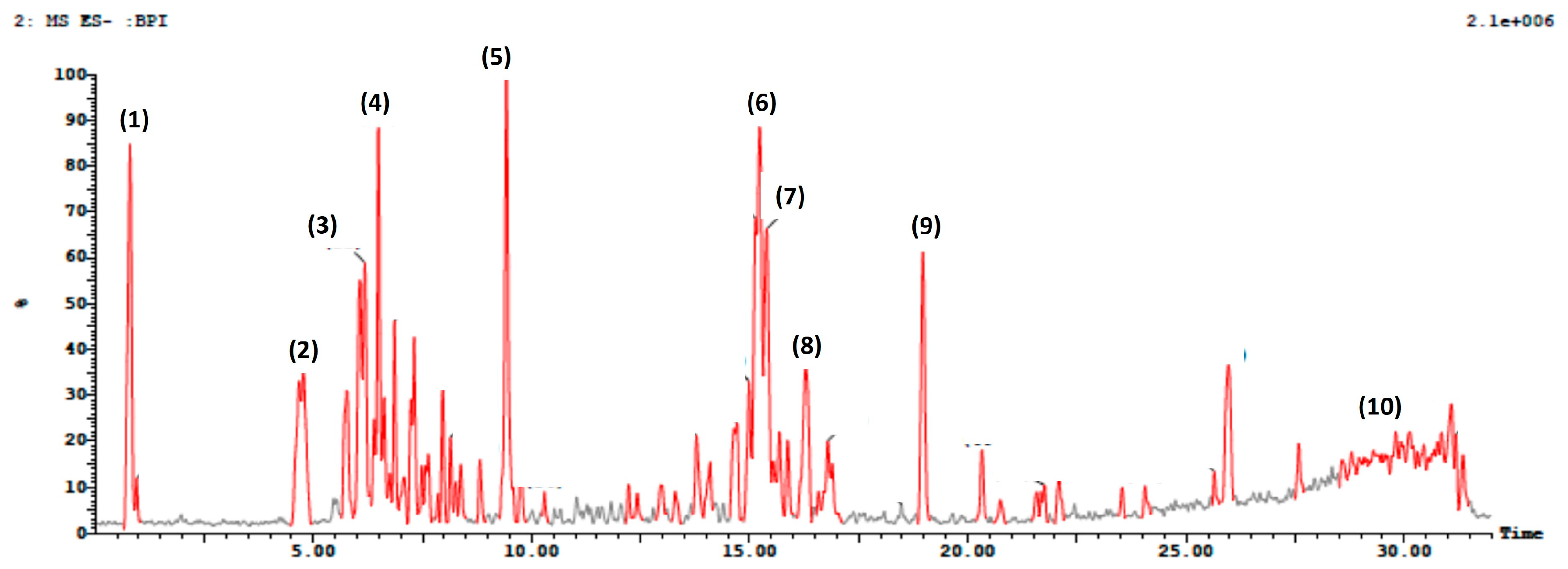
3.1. Ultra-Performance Liquid Chromatography Coupled with Mass (UPLC-MS/MS) Profiling
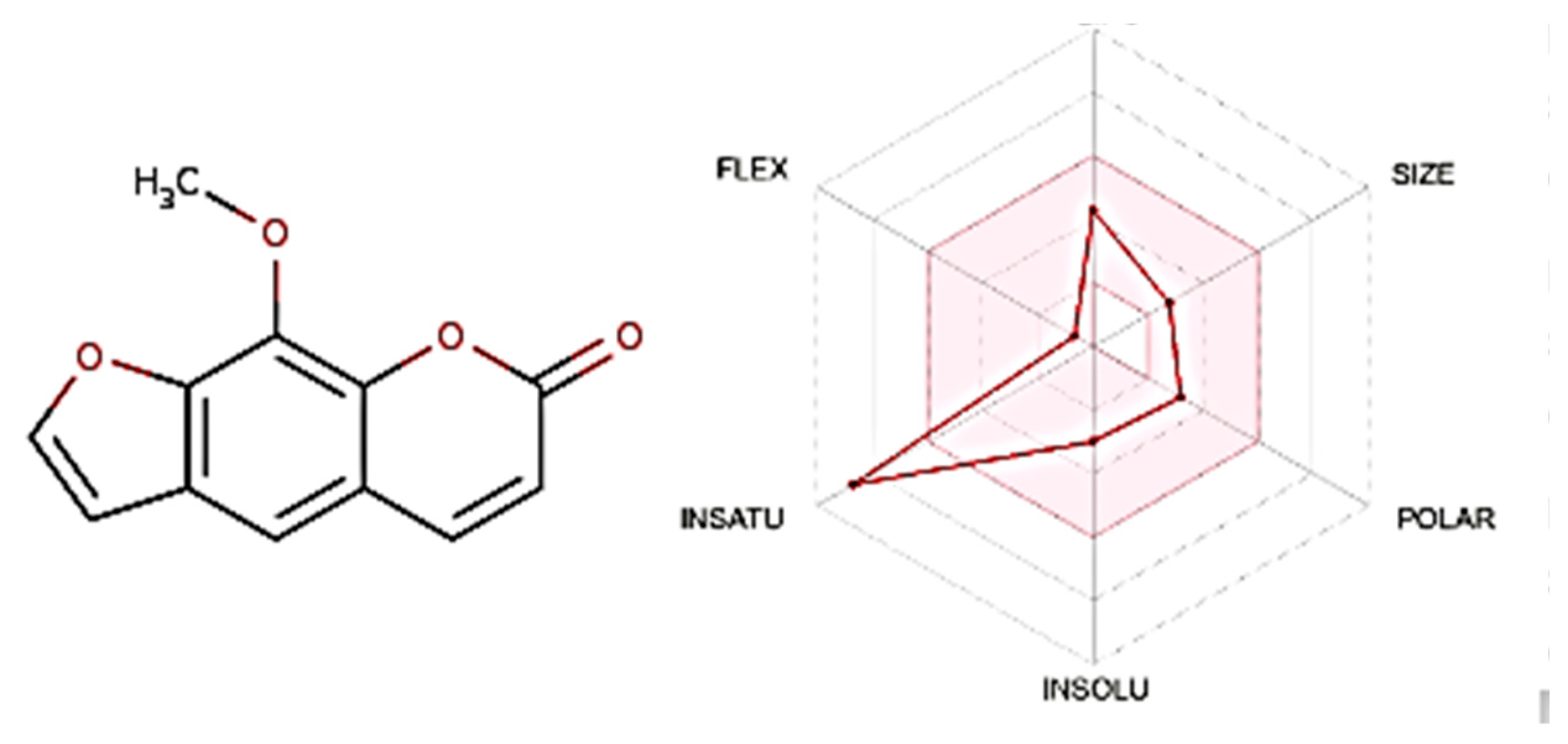
3.2. Structural Isolation and Identification of the Compound
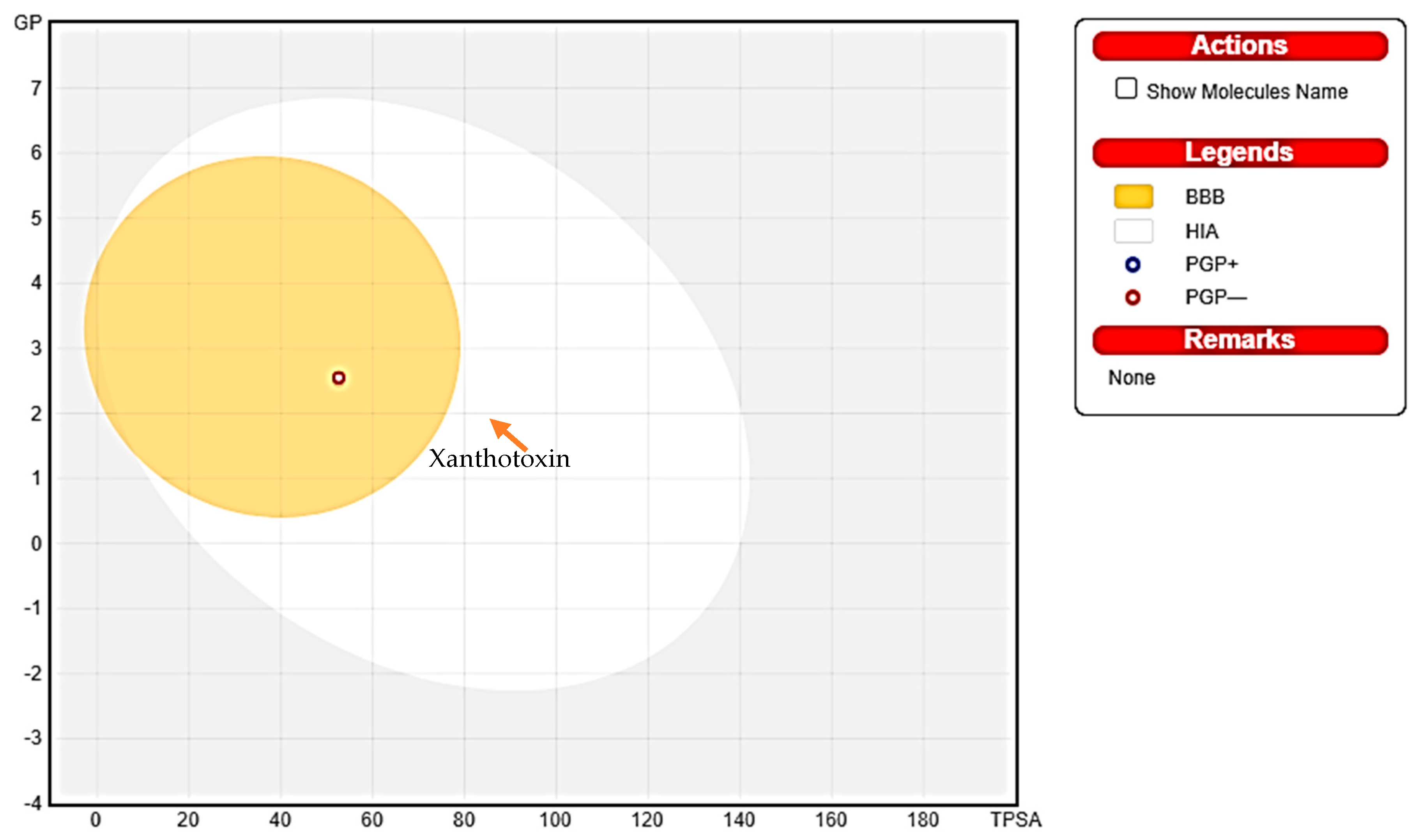
3.3. The Lipinski’s Rule of Five, ADME, and Boiled Egg Techniques
3.4. Histological Results
3.5. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, R.; Shah, T.K. A review on role of plant waste products on fish growth, health and production. J. Entomol. Zool. Stud. 2017, 5, 583–589. [Google Scholar]
- Darley, E.F.; Burleson, F.; Mateer, E.; Middleton, J.T.; Osterli, V. Contribution of burning of agricultural wastes to photochemical air pollution. J. Air. Pollut. Control Assoc. 1966, 16, 685–690. [Google Scholar] [CrossRef]
- El-Mashad, H.M.; Van Loon, W.K.; Zeeman, G.; Bot, G.P.; Lettinga, G. Reuse potential of agricultural wastes in semi-arid regions: Egypt as a case study. Rev. Environ. Sci. Biotechnol. 2003, 2, 53–66. [Google Scholar] [CrossRef]
- Elbasiouny, H.; Elbanna, B.A.; Al-Najoli, E.; Alsherief, A.; Negm, S.; Abou El-Nour, E.; Nofal, A.; Sharabash, S. Agricultural waste management for climate change mitigation: Some implications to Egypt. In Waste Management in MENA Regions; Springer: Berlin/Heidelberg, Germany, 2020; pp. 149–169. [Google Scholar]
- Usmani, Q.I.; Jahan, N.; Aleem, M.; Hasan, S.A. Aatrilal (Ammi majus L.), an important drug of Unani system of medicine: A review. J. Ethnopharmacol. 2021, 276, 114144. [Google Scholar] [CrossRef]
- Duke, J.A. Bishop’s weed (Ammi majus L., Apiaceae). Econ. Bot. 1988, 42, 442–445. [Google Scholar]
- Al-Snafi, A.E. Chemical constituents and pharmacological activities of Ammi majus and Ammi visnaga. A review. Int. J. Pharm. Ind. Res. 2013, 3, 257–265. [Google Scholar]
- Chaachouay, N.; Orch, H.; Zidane, L. Cystitis treatment with phytotherapy within the Rif, Northern Morocco. Future J. Pharm. Sci. 2021, 7, 81. [Google Scholar] [CrossRef]
- Issa, M.Y.; Elshal, M.F.; Fathallah, N.; Abdelkawy, M.A.; Bishr, M.; Salama, O.; Abulfadl, Y.S. Potential Anticancer Activity of the Furanocoumarin Derivative Xanthotoxin Isolated from Ammi majus L. Fruits: In Vitro and In Silico Studies. Molecules 2022, 27, 943. [Google Scholar] [CrossRef]
- Schönberg, A.; Sina, A. Xanthotoxin from the fruits of Ammi majus L. Nature 1948, 161, 481–482. [Google Scholar] [CrossRef]
- Abdul-Jalil, T.Z.; Saour, K.; Nasser, A.-M. Phytochemical study of some flavonoids present in the fruits of two Ammi L. species wildly grown in Iraq. Iraqi J. Pharm. Sci. 2010, 19, 48–57. [Google Scholar] [CrossRef]
- Majus, A. Egyptian Herbal Monograph, Pharmacopoeial Wild Medicinal Plants; Egyptian Drug Authority (EDA): Cairo, Egypt, 2022; Volume 2, pp. 128–135.
- Diawara, M.; Trumble, J. Linear furanocoumarins. In Handbook of Plant and Fungal Toxicants; CRC Press: Boca Raton, FL, USA, 2020; pp. 175–189. [Google Scholar]
- Sajjadi, S. Isolation and identification of xanthotoxin (8-methoxypsoralen) from the fruits of Heracleum persicum Desf. ex Fischer. Res. Pharm. Sci. 2008, 2, 13–16. [Google Scholar]
- Beier, R.C.; Ivie, G.W.; Oertli, E.H. Linear furanocoumarins and graveolone from the common herb parsley. Phytochemistry 1994, 36, 869–872. [Google Scholar] [CrossRef]
- Volnukhin, V.; Samsonov, V.; Kravtsova, I.; Tsaregorodtseva, E.; Lukashina, T.; Val, E. Efficiency of PUVA bath therapy in psoriatic patients. Vestn. Dermatol. Venerol. 2006, 5, 56–61. [Google Scholar]
- Kenari, H.M.; Kordafshari, G.; Moghimi, M.; Eghbalian, F.; TaherKhani, D. Review of pharmacological properties and chemical constituents of Pastinaca sativa. J. Pharmacopunct. 2021, 24, 14. [Google Scholar] [CrossRef] [PubMed]
- Dou, C.; Chen, Y.; Ding, N.; Li, N.; Jiang, H.; Zhao, C.; Kang, F.; Cao, Z.; Quan, H.; Luo, F. Xanthotoxin prevents bone loss in ovariectomized mice through the inhibition of RANKL-induced osteoclastogenesis. Osteoporos. Int. 2016, 27, 2335–2344. [Google Scholar] [CrossRef] [PubMed]
- Skalicka-Wozniak, K.; Budzynska, B.; Biala, G.; Boguszewska-Czubara, A. Scopolamine-induced memory impairment is alleviated by xanthotoxin: Role of acetylcholinesterase and oxidative stress processes. ACS Chem. Neurosci. 2018, 9, 1184–1194. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Wei, C.; He, L.-J.; An, J. Xanthotoxin induces apoptosis in SGC-7901 cells through death receptor pathway. Chin. Herb. Med. 2018, 10, 437–444. [Google Scholar] [CrossRef]
- Fu, K.; Zhang, J.; Wang, L.; Zhao, X.; Luo, Y. Xanthotoxin induced photoactivated toxicity, oxidative stress and cellular apoptosis in Caenorhabditis elegans under ultraviolet A. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 251, 109217. [Google Scholar] [CrossRef]
- Zagaja, M.; Bryda, J.; Szewczyk, A.; Szala-Rycaj, J.; Łuszczki, J.J.; Walczak, M.; Kuś, K.; Andres-Mach, M. Xanthotoxin enhances the anticonvulsant potency of levetiracetam and valproate in the 6-Hz corneal stimulation model in mice. Fundam. Clin. Pharmacol. 2022, 36, 133–142. [Google Scholar] [CrossRef]
- Kowalczyk, J.; Nakos-Bimpos, M.; Polissidis, A.; Dalla, C.; Kokras, N.; Skalicka-Wozniak, K.; Budzynska, B. Xanthotoxin affects depression-related behavior and neurotransmitters content in a sex-dependent manner in mice. Behav. Brain Res. 2021, 399, 112985. [Google Scholar] [CrossRef]
- Caboni, P.; Saba, M.; Oplos, C.; Aissani, N.; Maxia, A.; Menkissoglu-Spiroudi, U.; Casu, L.; Ntalli, N. Nematicidal activity of furanocoumarins from parsley against Meloidogyne spp. Pest Manag. Sci. 2015, 71, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Diawara, M.M.; Chavez, K.; Hoyer, P.; Williams, D.; Dorsch, J.; Kulkosky, P.; Franklin, M. A novel group of ovarian toxicants: The psoralens. J. Biochem. Mol. Toxicol. 1999, 13, 195–203. [Google Scholar] [CrossRef]
- Diawara, P.; Moussa, M.; Kulkosky, P.; Paul, J. Reproductive toxicity of the psoralens. Pediatr. Pathol. Mol. Med. 2003, 22, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, M.; Fattahi, E.; Kouchesfahani, H.M.; Shockravi, A.; Parivar, K. The adverse effects of methoxsalen on the oogenesis of Balb/C mice. Cell J. 2014, 15, 348. [Google Scholar] [PubMed]
- Farhadi, M.; Kouchesfahani, H.M.; Shockravi, A.; Foroozanfar, M.; Parivar, K. The adverse effects of the methoxsalen and ultraviolent A radiation on spermatogenesis in mice. Iran. J. Reprod. Med. 2015, 13, 489. [Google Scholar]
- Azwanida, N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromat Plants 2015, 4, 196. [Google Scholar]
- Koriem, K.M.M.; Asaad, G.F.; Megahed, H.A.; Zahran, H.; Arbid, M.S. Evaluation of the Antihyperlipidemic, Anti-inflammatory, Analgesic, and Antipyretic Activities of Ethanolic Extract of Ammi majus Seeds in Albino Rats and Mice. Int. J. Toxicol. 2012, 31, 294–300. [Google Scholar] [CrossRef]
- Pohanka, M. Toxicology and the biological role of methanol and ethanol: Current view. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc 2016, 160, 54–63. [Google Scholar] [CrossRef]
- Bartosova, Z.; Ertesvåg, H.; Nyfløt, E.L.; Kämpe, K.; Aasen, I.M.; Bruheim, P. Combined metabolome and lipidome analyses for in-depth characterization of lipid accumulation in the DHA producing Aurantiochytrium sp. T66. Metabolites 2021, 11, 135. [Google Scholar] [CrossRef]
- Abdel Ghani, A.E.; Al-Saleem, M.S.; Abdel-Mageed, W.M.; AbouZeid, E.M.; Mahmoud, M.Y.; Abdallah, R.H. UPLC-ESI-MS/MS Profiling and Cytotoxic, Antioxidant, Anti-Inflammatory, Antidiabetic, and Antiobesity Activities of the Non-Polar Fractions of Salvia hispanica L. Aerial Parts. Plants 2023, 12, 1062. [Google Scholar] [CrossRef]
- Hamming, M. Interpretation of Mass Spectra of Organic Compounds; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Mohammadi, M.; Yousefi, M.; Habibi, Z.; Shafiee, A. Two new coumarins from the chloroform extract of Angelica urumiensis from Iran. Chem. Pharm. Bull. 2010, 58, 546–548. [Google Scholar] [CrossRef] [PubMed]
- Fathallah, N.; Raafat, M.M.; Issa, M.Y.; Abdel-Aziz, M.M.; Bishr, M.; Abdelkawy, M.A.; Salama, O. Bio-guided fractionation of prenylated benzaldehyde derivatives as potent antimicrobial and antibiofilm from Ammi majus L. fruits-associated Aspergillus amstelodami. Molecules 2019, 24, 4118. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Sumi, D.; Juliet, P.A.; Matsui-Hirai, H.; Asai-Tanaka, Y.; Kano, H.; Fukatsu, A.; Tsunekawa, T.; Miyazaki, A.; Iguchi, A.; et al. Gene transfer of endothelial NO synthase, but not eNOS, plus inducible NOS regressed atherosclerosis in rabbits. Cardiovasc. Res. 2004, 61, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Iyer, D.; Patil, U. Evaluation of antihyperlipidemic and antitumor activities of isolated coumarins from Salvadora indica. Pharm. Biol. 2014, 52, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Niwa, S.; Ueno, S.; Shirasu, R. Alteration of pRb expression in the development of rat tongue carcinoma induced by 4-nitroquinoline 1-oxide. Oral Oncol. 2001, 37, 579–585. [Google Scholar] [CrossRef]
- Rutland, C.S. Histological and histochemical methods 4th edition. J. Anat. 2008, 213, 356. [Google Scholar] [CrossRef]
- Concannon, S.; Ramachandran, V.N.; Smyth, W.F. A study of the electrospray ionisation of selected coumarin derivatives and their subsequent fragmentation using an ion trap mass spectrometer. Rapid Commun. Mass Spectrom. 2000, 14, 1157–1166. [Google Scholar] [CrossRef]
- Cuyckens, F.; Claeys, M. Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef]
- Abu-Mustafa, E.; El-Bay, F.; Fayez, M. Natural coumarins. Part X.: Isoimperatorin, a further constituent of the fruits of Ammi majus L. Recl. Trav. Chim. Pays.-Bas. 1968, 87, 925–928. [Google Scholar] [CrossRef]
- Tian, W.; Cai, J.; Xu, Y.; Luo, X.; Zhang, J.; Zhang, Z.; Zhang, Q.; Wang, X.; Hu, L.; Lin, G. Determination of xanthotoxin using a liquid chromatography-mass spectrometry and its application to pharmacokinetics and tissue distribution model in rat. Int. J. Clin. Exp. Med. 2015, 8, 15164. [Google Scholar] [PubMed]
- Elgamal, M.H.A.; Shalaby, N.M.; Duddeck, H.; Hiegemann, M. Coumarins and coumarin glucosides from the fruits of Ammi majus. Phytochemisty 1993, 34, 819–823. [Google Scholar] [CrossRef]
- Singh, H.; Batish, D.R.; Kohli, R. Autotoxicity: Concept, organisms, and ecological significance. Crit. Rev. Plant Sci. 1999, 18, 757–772. [Google Scholar] [CrossRef]
- Hehmann, M.; Lukačin, R.; Ekiert, H.; Matern, U. Furanocoumarin biosynthesis in Ammi majus L. Cloning of bergaptol O-methyltransferase. Eur. J. Biochem. 2004, 271, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Abad-García, B.; Garmón-Lobato, S.; Berrueta, L.A.; Gallo, B.; Vicente, F. A fragmentation study of dihydroquercetin using triple quadrupole mass spectrometry and its application for identification of dihydroflavonols in Citrus juices. Rapid Commun. Mass Spectrom. 2009, 23, 2785–2792. [Google Scholar] [CrossRef]
- Sharma, K.D.; Karki, S.; Thakur, N.S.; Attri, S. Chemical composition, functional properties and processing of carrot—A review. J. Food Sci. Technol. 2012, 49, 22–32. [Google Scholar] [CrossRef]
- Han, W.-W.; Zhou, Y.-H.; Yao, Y.; Li, Z.-S. Computational studies on bergaptol O-methyltransferase from Ammi majus L.: The substrate specificity. Polymer 2006, 47, 7953–7961. [Google Scholar] [CrossRef]
- Kim, I.; Volkov, D.; Zakharenko, V.; Zakharenko, A.; Golohvast, K.; Klykov, A. Impact of High Temperature on Growth of Embryo and Germination of Heteromorphic Seeds of Anethum graveolens L.(Apiaceae). Agric. Biol. 2020, 55, 995–1003. [Google Scholar]
- Nazik, S.M.; Mona, S.M.; Ramzi, A.M.; Wadah, J.O.; Hassan, S.K. HPTLC fingerprint profiles and UPLC-MS identification of potential antioxidant fractions and compounds from Ambrosia maritima L. and Ammi majus L. Afr. J. Biotechnol. 2020, 19, 249–258. [Google Scholar] [CrossRef]
- Lozhkin, A.; Sakanyan, E. Natural coumarins: Methods of isolation and analysis. Pharm. Chem. J. 2006, 40, 337–346. [Google Scholar] [CrossRef]
- Khalil, N.; Bishr, M.; Desouky, S.; Salama, O. Ammi visnaga L., a potential medicinal plant: A review. Molecules 2020, 25, 301. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C. Antioxidant potential of two Apiaceae plant extracts: A comparative study focused on the phenolic composition. Ind. Crops Prod. 2016, 79, 188–194. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Phenolics and flavonoids contents of medicinal plants, as natural ingredients for many therapeutic purposes-A review. IOSR J. Pharm. 2020, 10, 42–81. [Google Scholar]
- Vitalini, S.; Palmioli, A.; Orlando, F.; Scarì, G.; Airoldi, C.; De Noni, I.; Bocchi, S.; Iriti, M. Phytotoxicity, nematicidal activity and chemical constituents of Peucedanum ostruthium (L.) WDJ Koch (Apiaceae). Ind. Crops Prod. 2021, 166, 113499. [Google Scholar] [CrossRef]
- Radulović, N.S.; Đorđević, N.D. Steroids from poison hemlock (Conium maculatum L.): A GC-MS analysis. J. Serbian Chem. Soc. 2011, 76, 1471–1483. [Google Scholar] [CrossRef]
- Lamnaouer, D.; Fraigui, O.; Martin, M.-T.; Gallard, J.-F.; Bodo, B. Structure of isoferprenin, a 4-hydroxycoumarin derivative from Ferula communis var. genuina. J. Nat. Prod. 1991, 54, 576–578. [Google Scholar] [CrossRef]
- Berenbaum, M.R. Chemical mediation of coevolution: Phylogenetic evidence for Apiaceae and associates. Ann. Mo. Bot. Gard. 2001, 88, 45–59. [Google Scholar] [CrossRef]
- Herrmann, K.; Nagel, C.W. Occurrence and content of hydroxycinnamic and hydroxybenzoic acid compounds in foods. Crit. Rev. Food Sci. Nutr. 1989, 28, 315–347. [Google Scholar] [CrossRef]
- Andersen, O.M.; Markham, K.R. Flavonoids: Chemistry, Biochemistry and Applications; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Kaouadji, M.; Pouget, C. Additional phthalide derivatives from Meum athamanticum. J. Nat. Prod. 1986, 49, 184–185. [Google Scholar] [CrossRef]
- Hussain, F.; Jahan, N.; Rahman, K.-u.; Sultana, B.; Jamil, S. Identification of hypotensive biofunctional compounds of Coriandrum sativum and evaluation of their angiotensin-converting enzyme (ACE) inhibition potential. Oxidative Med. Cell. Longev. 2018, 2018, 4643736. [Google Scholar] [CrossRef]
- Cornara, L.; Borghesi, B.; Mariotti, M.G. Micromorphological investigation on the leaves of the rock samphire (Crithmum maritimum L.): Occurrence of hesperidin. Plant Biosyst. 2009, 143, 282–293. [Google Scholar] [CrossRef]
- Gebhardt, Y.; Witte, S.; Forkmann, G.; Lukačin, R.; Matern, U.; Martens, S. Molecular evolution of flavonoid dioxygenases in the family Apiaceae. Phytochemistry 2005, 66, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Bourgaud, F.; Hehn, A.; Larbat, R.; Doerper, S.; Gontier, E.; Kellner, S.; Matern, U. Biosynthesis of coumarins in plants: A major pathway still to be unravelled for cytochrome P450 enzymes. Phytochem. Rev. 2006, 5, 293–308. [Google Scholar] [CrossRef]
- Attique, S.A.; Hassan, M.; Usman, M.; Atif, R.M.; Mahboob, S.; Al-Ghanim, K.A.; Bilal, M.; Nawaz, M.Z. A molecular docking approach to evaluate the pharmacological properties of natural and synthetic treatment candidates for use against hypertension. Int. J. Environ. Res. Public Health 2019, 16, 923. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Zoete, V. A boiled-egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Obi, F.; Ugwuishiwu, B.; Nwakaire, J. Agricultural waste concept, generation, utilization and management. Niger. J. Technol. 2016, 35, 957–964. [Google Scholar] [CrossRef]
- Okonko, I.O.; Adeola, O.; Aloysius, F.; Damilola, A.; Adewale, O. Utilization of food wastes for sustainable development. Electr. J. Environ. Agric. Food Chem. 2009, 8, 263–286. [Google Scholar]
- Mowaka, S.; Ayoub, B.M. Comparative study between UHPLC-UV and UPLC-MS/MS methods for determination of alogliptin and metformin in their pharmaceutical combination. Die Pharm.-Int. J. Pharm. Sci. 2017, 72, 67–72. [Google Scholar]
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yordi, E.G. Coumarins—An important class of phytochemicals. Phytochem.-Isol. Characterisation Role Hum. Health 2015, 25, 533–538. [Google Scholar]
- Skalicka-Woźniak, K.; Zagaja, M.; Głowniak, K.; Łuszczki, J.J. Purification and anticonvulsant activity of xanthotoxin (8-methoxypsoralen). Cent. Eur. J. Biol. 2014, 9, 431–436. [Google Scholar] [CrossRef]
- Zagaja, M.; Andres-Mach, M.; Patrzylas, P.; Pyrka, D.; Szpringer, M.; Florek-Łuszczki, M.; Żółkowska, D.; Skalicka-Woźniak, K.; Łuszczki, J.J. Influence of xanthotoxin (8-methoxypsoralen) on the anticonvulsant activity of various novel antiepileptic drugs against maximal electroshock-induced seizures in mice. Fitoterapia 2016, 115, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Couperus, M. Ammoidin (xanthotoxin) in the treatment of vitiligo. Calif. Med. 1954, 81, 402. [Google Scholar] [PubMed]
- Del Río, J.A.; Díaz, L.; García-Bernal, D.; Blanquer, M.; Ortuno, A.; Correal, E.; Moraleda, J.M. Furanocoumarins: Biomolecules of therapeutic interest. Stud. Nat. Prod. Chem. 2014, 43, 145–195. [Google Scholar]
- Alireza, H. Investigation of the processes of absorption, distribution, metabolism and elimination (ADME) as vital and important factors for modulating drug action and toxicity. Open Access J. Oncol. 2019, 2, 180010. [Google Scholar]
- McKerrow, J.H.; Lipinski, C.A. The rule of five should not impede anti-parasitic drug development. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Kullaa-Mikkonen, A.; Hynynen, M.; Hyvönen, P. Filiform Papillae of Human, Rat and Swine Tongue. Acta Anat. 2008, 130, 280–284. [Google Scholar] [CrossRef]
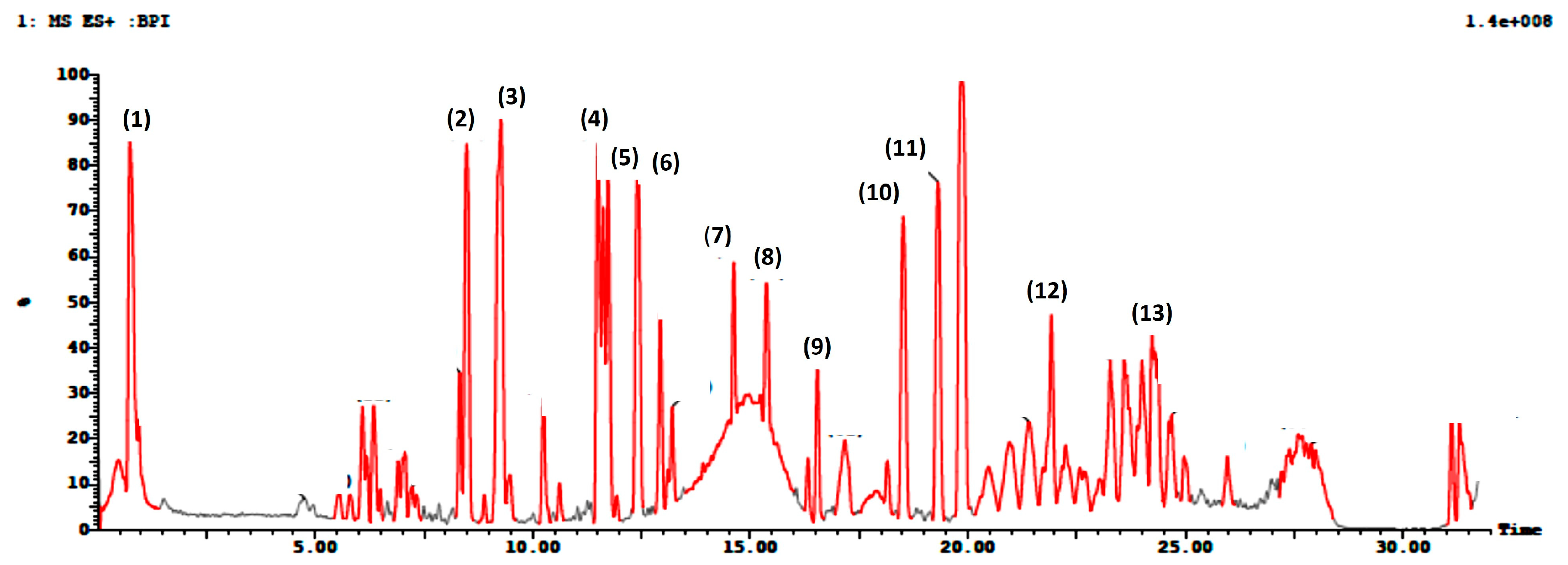


| Peak ID | Name | Rt (min) | Molecular Formula | Area % | [M+H]+ | Fragmentation (MS2) | References |
|---|---|---|---|---|---|---|---|
| 1 | Bergaptol-O-hexoside | 0.7 | C17H16O9 | 5% | 365 | 364, 202, 184, 104 | [44] |
| 2 | Xanthotoxin | 8.4 | C12H8O4 | 5.5% | 217 | 216, 202, 189, 185, 174, 161 | [45] |
| 3 | (Iso)arnottinin | 9.2 | C14H14O4 | 6% | 247 | 246, 228, 213, 175, 174, 145, 131, 70 | [46] |
| 4 | Di hydro chalcone (Phloretin) | 11.5 | C15H14O5 | 4% | 275 | 274, 269,209 | [47] |
| 5 | Bergaptol | 12.4 | C11H6O4 | 4% | 203 | 203, 175, 157, 147, 131, 129, 103, 91 | [48,49] |
| 6 | p-Coumaroyl glycolic acid | 12.9 | C11H10O5 | 2% | 223 | 208, 165 | [50] |
| 7 | Taxifolin | 14.6 | C15H12O7 | 4.5% | 305 | 305, 123, 95 | [51] |
| 8 | Pelargonidin | 15.3 | C26H29O14 | 4% | 566 | 566, 334 | [52] |
| 9 | Gallic acid 4-O-glucoside | 16.5 | C13H16O10 | 1% | 333 | 333, 332,122 | [53] |
| 10 | Coumestrol | 18.5 | C15H8O5 | 3.5% | 269 | 269, 268, 221 | [54,55] |
| 11 | p-Coumaric acid (p-Coumaroyl tartaric acid) | 19.3 | C13H12O8 | 3.5% | 297 | 297, 256, | [56] |
| 12 | p-Coumaroyl malic acid | 21.9 | C13H12O7 | 2% | 281 | 281, 247 | [56] |
| 13 | 3,5-Diferuloylquinicc acid | 23.2 | C27H28O12 | 2% | 544 | 383, 355, 337, 323 | [57,58] |
| Peak ID | Name | Rt (min) | Molecular Formula | Area % | [M-H]− | Fragmentation (MS2) | References |
|---|---|---|---|---|---|---|---|
| 1 | Albafuran | 0.8 | C24H26O4 | 4% | 377 | 377, 361, 345, 329 | [59] |
| 2 | 7-Hydroxycoumarin | 4.7 | C9H6O3 | 2% | 162 | 161, 146, 133 | [60,61] |
| 3 | Sinapoylquinic acid isome | 6.1 | C18 H22 O10 | 2.5% | 397 | 397, 232, 166 | [62] |
| 4 | Quercetin 5,3’,4’-trimethyl ether 3-galactosyl-(1->2)-rhamnoside-7-rhamnoside | 6.4 | C36H46O20 | 4.5% | 797 | 797, 284 | [63] |
| 5 | 3,7-Dimethylquercetin | 9.4 | C17H14O7 | 6% | 329 | 328, 302, 287 | [64,65] |
| 6 | p-Coumaroyl tartaric acid | 15.2 | C13H12O8 | 7% | 295 | 294, 263, 251 | [56] |
| 7 | Hesperidin | 15.3 | C28H34O15 | 5% | 610 | 610, 600, 163 | [66] |
| 8 | p-Coumaroyl tartaric acid | 16.7 | C13H12O8 | 1% | 295 | 294, 263, 251 | [56] |
| 9 | Naringenin | 18.9 | C15H14O5 | 4% | 271 | 271, 243, 230 | [67] |
| 10 | 1,2-Benzopyrone(coumarin) | 29.8 | C9H6O2 | 1% | 145 | 145, 116 | [68] |
| No. | Name | M.wt | Lipophilicity | Hydrogen Bond Donors | Hydrogen Bond Acceptors | No. of Rule Violations | Drug-Likeness |
|---|---|---|---|---|---|---|---|
| Less than 500 g/mol | Less than 5 | Less than 5 | Less than 10 | Less than 2 Violations | Lipinski’s Rule Follows Rule | ||
| 1 | Xanthotoxin | 216.19 | 1.18 | 0 | 4 | 0 | Yes |
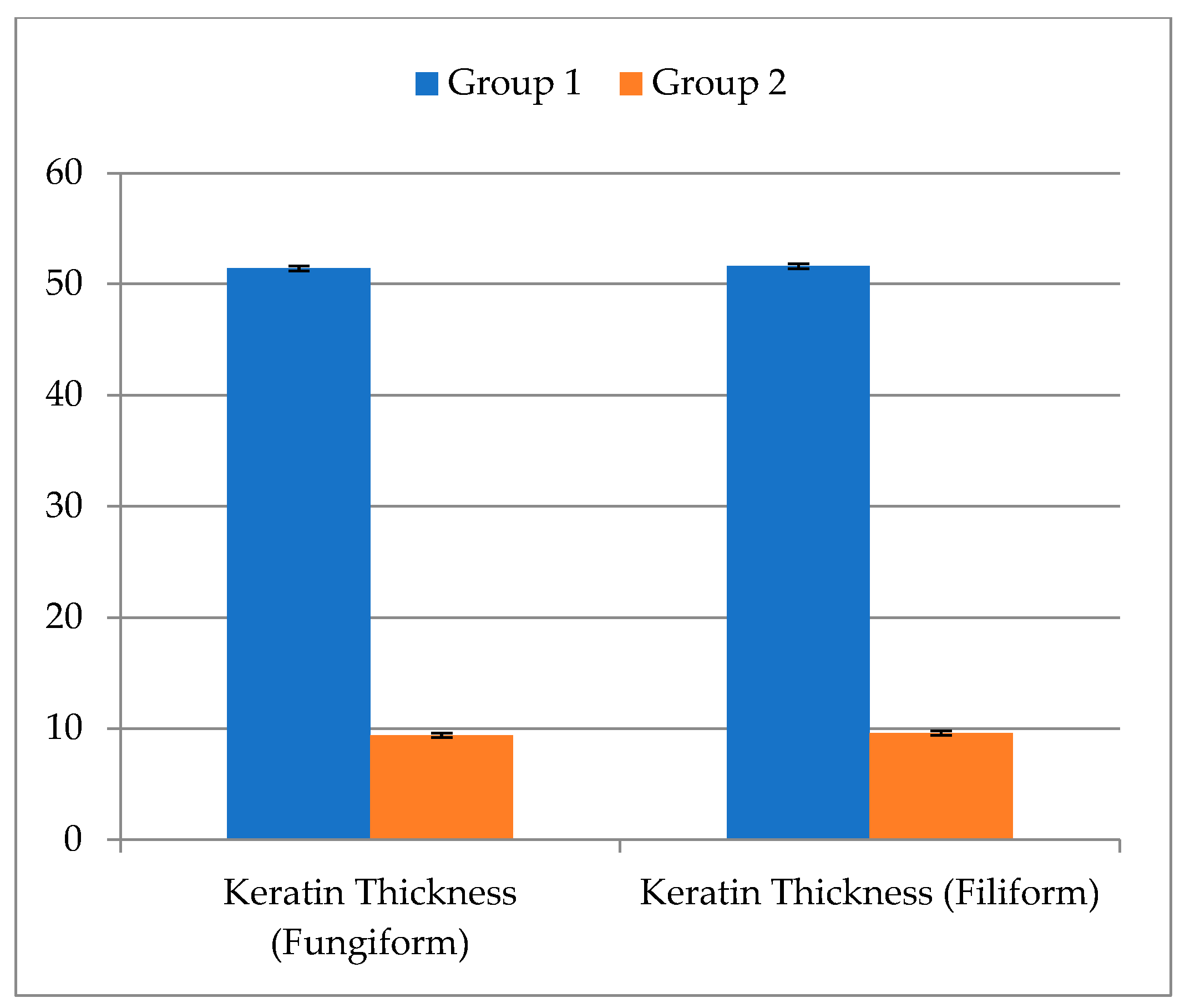
| Group 1, µm | Group 2, µm | t-Test | p-Value | |
|---|---|---|---|---|
| Keratin Thickness (Fungiform) | ||||
| Mean ± SD | 51.40 ± 0.22 | 9.40 ± 0.22 | 363.731 | <0.001 ** |
| Range | 51.1–51.7 | 9.1–9.7 | ||
| Keratin Thickness (Filliform) | ||||
| Mean ± SD | 51.60 ± 0.22 | 9.60 ± 0.22 | 341.696 | <0.001 ** |
| Range | 51.3–51.9 | 9.3–9.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fathallah, N.; El Deeb, M.; Rabea, A.A.; Almehmady, A.M.; Alkharobi, H.; Elhady, S.S.; Khalil, N. Ultra-Performance Liquid Chromatography Coupled with Mass Metabolic Profiling of Ammi majus Roots as Waste Product with Isolation and Assessment of Oral Mucosal Toxicity of Its Psoralen Component Xanthotoxin. Metabolites 2023, 13, 1044. https://doi.org/10.3390/metabo13101044
Fathallah N, El Deeb M, Rabea AA, Almehmady AM, Alkharobi H, Elhady SS, Khalil N. Ultra-Performance Liquid Chromatography Coupled with Mass Metabolic Profiling of Ammi majus Roots as Waste Product with Isolation and Assessment of Oral Mucosal Toxicity of Its Psoralen Component Xanthotoxin. Metabolites. 2023; 13(10):1044. https://doi.org/10.3390/metabo13101044
Chicago/Turabian StyleFathallah, Noha, Mona El Deeb, Amany A. Rabea, Alshaimaa M. Almehmady, Hanaa Alkharobi, Sameh S. Elhady, and Noha Khalil. 2023. "Ultra-Performance Liquid Chromatography Coupled with Mass Metabolic Profiling of Ammi majus Roots as Waste Product with Isolation and Assessment of Oral Mucosal Toxicity of Its Psoralen Component Xanthotoxin" Metabolites 13, no. 10: 1044. https://doi.org/10.3390/metabo13101044
APA StyleFathallah, N., El Deeb, M., Rabea, A. A., Almehmady, A. M., Alkharobi, H., Elhady, S. S., & Khalil, N. (2023). Ultra-Performance Liquid Chromatography Coupled with Mass Metabolic Profiling of Ammi majus Roots as Waste Product with Isolation and Assessment of Oral Mucosal Toxicity of Its Psoralen Component Xanthotoxin. Metabolites, 13(10), 1044. https://doi.org/10.3390/metabo13101044







