Food Monitoring: Limitations of Accelerated Storage to Predict Molecular Changes in Hazelnuts (Corylus avellana L.) under Realistic Conditions Using UPLC-ESI-IM-QTOF-MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Samples and Storage Conditions
2.3. Sample Preparation
2.4. UPLC-ESI-IM-QTOF-MS Analysis
2.5. Method for Extracting Antioxidative Compounds
2.6. Analysis of the Antioxidant Capacity Using the Trolox Equivalent Antioxidant Capacity Assay (TEAC)
2.7. Analysis of the Antioxidant Capacity Using the Oxygen Radical Absorbance Capacity Assay (ORAC)
2.8. Determination of the Total Phenolic Content According to Folin-Ciocalteu
2.9. Data Processing and Chemometrics
3. Results and Discussion
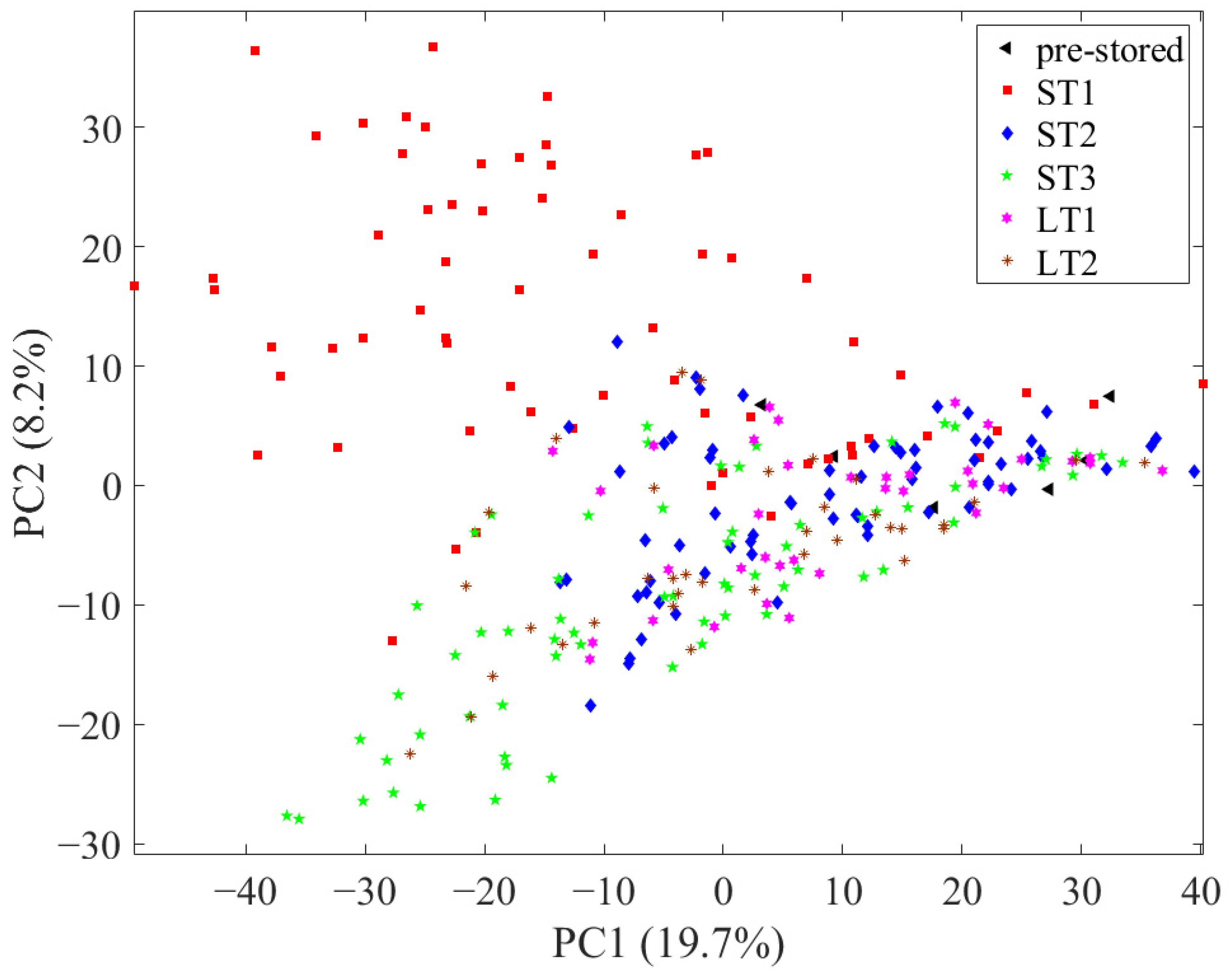
3.1. Principal Component Analysis (PCA) for Evaluation of the Greatest Variances and the Applicability of the Data Processing
3.2. Classification of Pre- and Post-Stored Samples
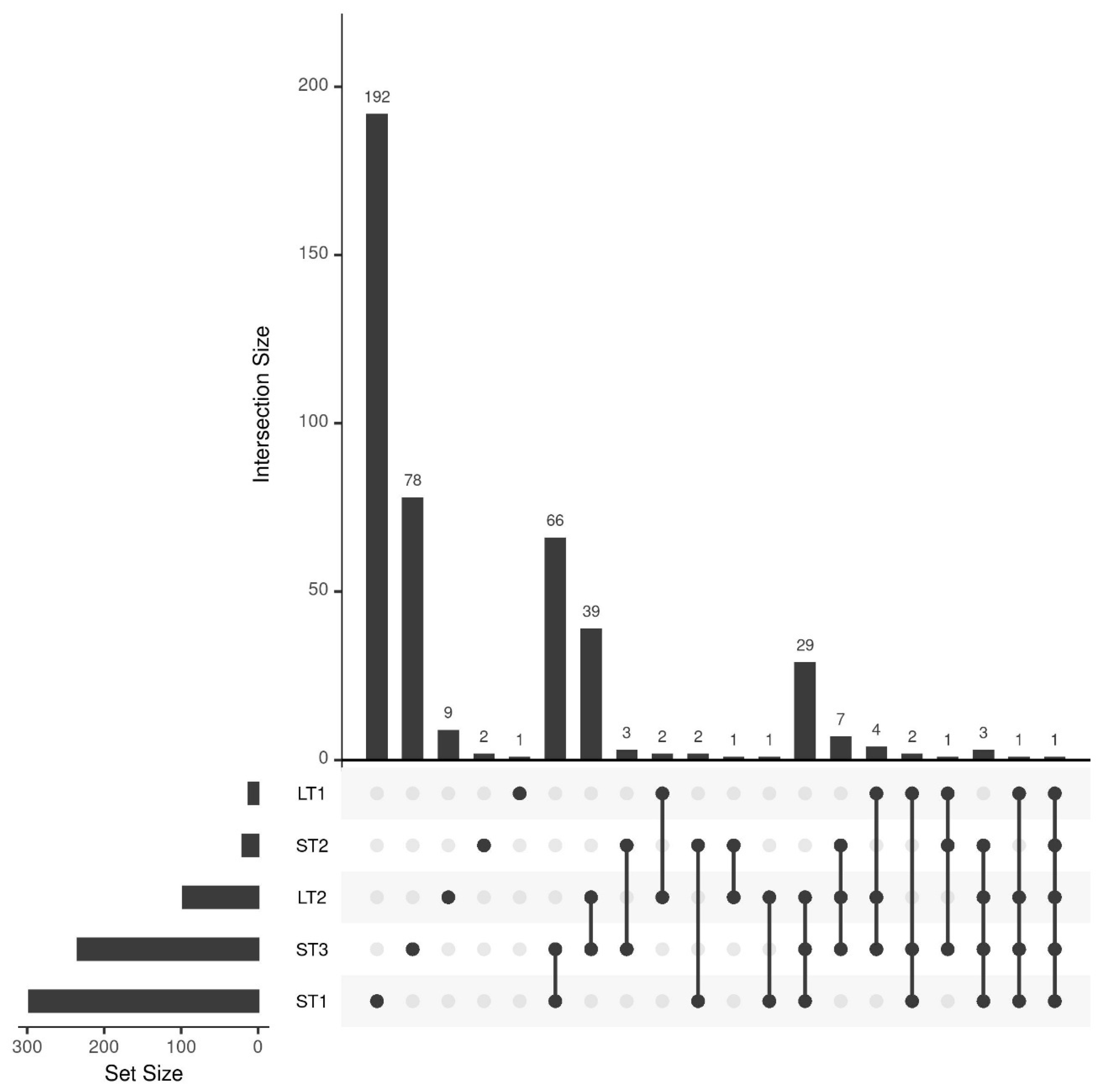
3.3. Comparison of Selected Features for the Different Storage Conditions
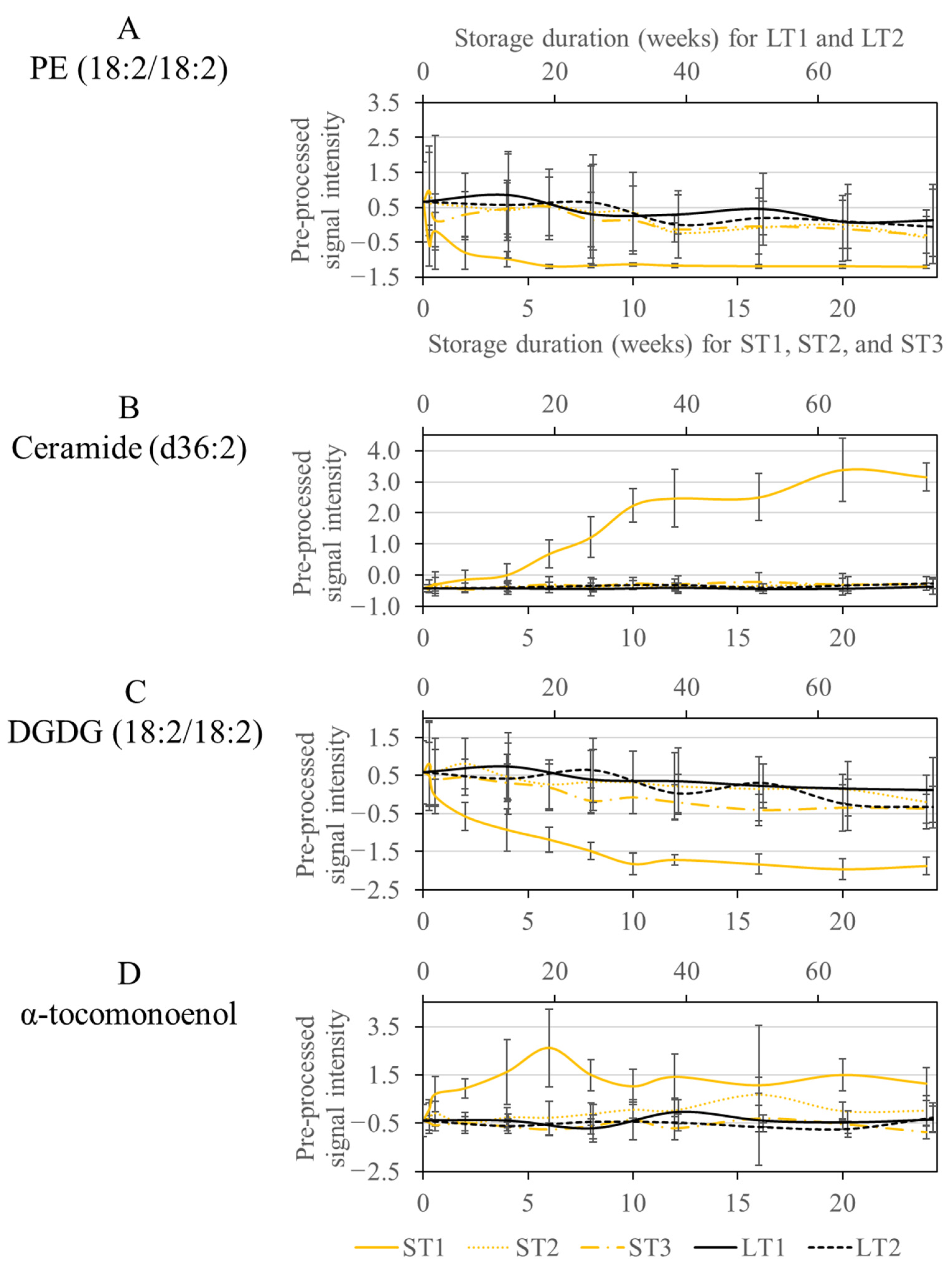
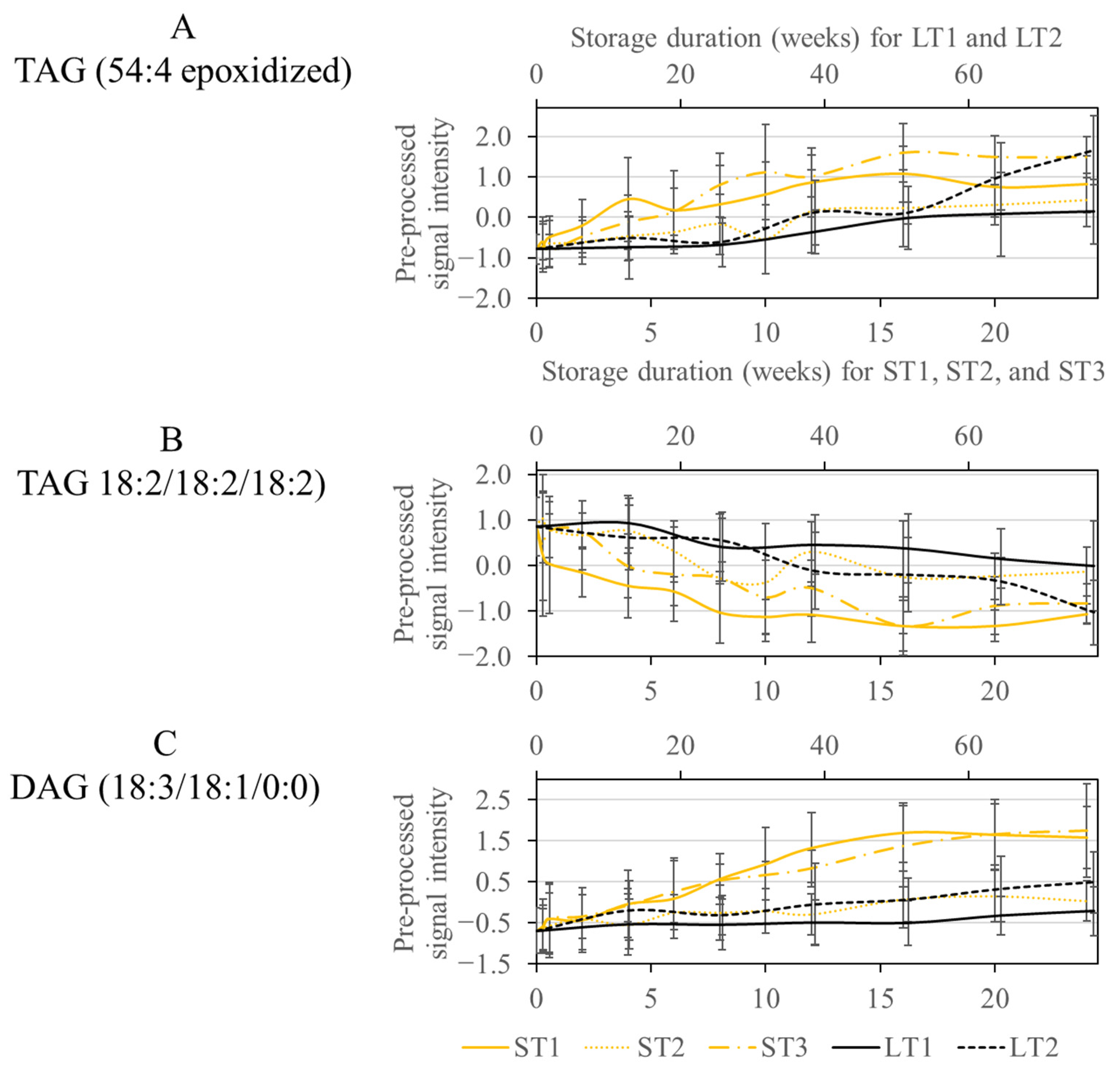
3.4. Analysis and Comparison of Storage-Induced Changes in the Metabolome of Hazelnuts Stored under Accelerated and Realistic Conditions
3.4.1. Analysis of Specific Metabolites for the Accelerated Short-Term Storage Condition with High Temperature (40 °C) and Humidity (75%) (ST1) and Comparison with the other Storage Conditions
3.4.2. Analysis and Comparison of Specific Metabolites Selected for Accelerated Short Term Storage with Increased Temperature (ST1 and ST3) and Realistic Long-Term Storage (LT2)
3.5. Determination of the Antioxidant Capacity Using TEAC, ORAC, and Folin-Ciocalteu Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tous, J. Hazelnut Production in Spain. Acta Hortic. 2005, 686, 659–664. [Google Scholar] [CrossRef]
- Silvestri, C.; Bacchetta, L.; Bellincontro, A.; Cristofori, V. Advances in cultivar choice, hazelnut orchard management, and nut storage to enhance product quality and safety: An overview. J. Sci. Food Agric. 2021, 101, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Islam, A. Hazelnut cultivation in Turkey. Akad. Ziraat Derg. 2018, 7, 251–258. [Google Scholar] [CrossRef]
- Ghirardello, D.; Contessa, C.; Valentini, N.; Zeppa, G.; Rolle, L.; Gerbi, V.; Botta, R. Effect of storage conditions on chemical and physical characteristics of hazelnut (Corylus avellana L.). Postharvest Biol. Technol. 2013, 81, 37–43. [Google Scholar] [CrossRef]
- Turan, A. Effect of drying on the chemical composition of Çakıldak (cv) hazelnuts during storage. Grasas y Aceites 2019, 70, 296. [Google Scholar] [CrossRef]
- Ozay, G.; Seyhan, F.; Pembeci, C.; Saklar, S.; Yilmaz, A. Factors influencing fungal and aflatoxin levels in Turkish hazelnuts (Corylus avellana L.) during growth, harvest, drying and storage: A 3-year study. Food Addit. Contam. Part. A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 209–218. [Google Scholar] [CrossRef] [PubMed]
- De Santis, D.; Fardelli, A.; Mencarelli, F. Storage Hazelnuts: Effect on Aromatic Profile and Sensory Attributes. Acta Hortic. 2009, 845, 693–700. [Google Scholar] [CrossRef]
- Botondi, R. Hygiene behavior assessment of a hazelnut processing plant. BFJ 2019, 121, 400–410. [Google Scholar] [CrossRef]
- Martin, M.B.S.; Fernandez-Garcia, T.; Romero, A.; Lopez, A. Effect of Modified Atmosphere Storage on Hazelnut Quality. J. Food Process. Preserv. 2001, 25, 309–321. [Google Scholar] [CrossRef]
- Erdogan, V. Hazelnut production in Turkey: Current situation, problems and future prospects. Acta Hortic. 2018, 1226, 13–24. [Google Scholar] [CrossRef]
- Baertschi, S.W.; Dill, A.L.; Kramer, T.T.; Scrivens, G.; Suruzhon, M. Degradation Rate Observations as a Function of Drug Load in Solid-State Drug Products. J. Pharm. Sci. 2019, 108, 1746–1755. [Google Scholar] [CrossRef]
- Waterman, K.C.; Adami, R.C. Accelerated aging: Prediction of chemical stability of pharmaceuticals. Int. J. Pharm. 2005, 293, 101–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lyu, C.; Meng, X.; Dong, W.; Guo, H.; Su, C.; Zhang, X. Effect of Storage Condition on Oil Oxidation of Flat-European Hybrid Hazelnut. J. Oleo Sci. 2019, 68, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Turan, A. Effect of drying methods on fatty acid profile and oil oxidation of hazelnut oil during storage. Eur. Food Res. Technol. 2018, 244, 2181–2190. [Google Scholar] [CrossRef]
- Koyuncu, M.A.; Islam, A.; Küçük, M. Fat and fatty acid composition of hazelnut kernels in vacuum packages during storage. Grasas Aceites 2005, 56, 263–266. [Google Scholar] [CrossRef]
- Kinderlerer, J.L.; Johnson, S. Rancidity in hazelnuts due to volatile aliphatic aldehydes. J. Sci. Food Agric. 1992, 58, 89–93. [Google Scholar] [CrossRef]
- Christopoulos, M.V.; Tsantili, E. Effects of temperature and packaging atmosphere on total antioxidants and colour of walnut (Juglans regia L.) kernels during storage. Sci. Hortic. 2011, 131, 49–57. [Google Scholar] [CrossRef]
- Fiehn, O. Combining genomics, metabolome analysis, and biochemical modelling to understand metabolic networks. Comp. Funct. Genom. 2001, 2, 155–168. [Google Scholar] [CrossRef]
- Rosso, M.C.; Liberto, E.; Spigolon, N.; Fontana, M.; Somenzi, M.; Bicchi, C.; Cordero, C. Evolution of potent odorants within the volatile metabolome of high-quality hazelnuts (Corylus avellana L.): Evaluation by comprehensive two-dimensional gas chromatography coupled with mass spectrometry. Anal. Bioanal. Chem. 2018, 410, 3491–3506. [Google Scholar] [CrossRef]
- Stilo, F.; Liberto, E.; Spigolon, N.; Genova, G.; Rosso, G.; Fontana, M.; Reichenbach, S.E.; Bicchi, C.; Cordero, C. An effective chromatographic fingerprinting workflow based on comprehensive two-dimensional gas chromatography—Mass spectrometry to establish volatiles patterns discriminative of spoiled hazelnuts (Corylus avellana L.). Food Chem. 2021, 340, 128135. [Google Scholar] [CrossRef]
- Squara, S.; Caratti, A.; Gavilan, F.O.; Bolzoni, P.; Spigolon, N.; Genova, G.; Castello, G.; González, M.G.B.; Cuadros-Rodriguez, L.; Bicchi, C.; et al. Validation of a high-throughput method for the accurate quantification of secondary products of lipid oxidation in high-quality hazelnuts (Corylus avellana L.): A robust tool for quality assessment. J. Food Compos. Anal. 2022, 114, 104766. [Google Scholar] [CrossRef]
- Sun, J.; Hu, P.; Lyu, C.; Tian, J.; Meng, X.; Tan, H.; Dong, W. Comprehensive lipidomics analysis of the lipids in hazelnut oil during storage. Food Chem. 2022, 378, 132050. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Cui, N.; Liu, J.; Ma, Q.; Zhao, T.; Yang, Z.; Zhao, H.; Zhang, B.; Liang, L. Application of metabolomics to explore the automatic oxidation process of hazelnut oil. Food Res. Int. 2022, 162, 111888. [Google Scholar] [CrossRef] [PubMed]
- Chanadang, S.; Chambers, E. Sensory Shelf Life Estimation of Novel Fortified Blended Foods Under Accelerated and Real-Time Storage Conditions. J. Food Sci. 2019, 84, 2638–2645. [Google Scholar] [CrossRef] [PubMed]
- Haouet, M.N.; Tommasino, M.; Mercuri, M.L.; Benedetti, F.; Di Bella, S.; Framboas, M.; Pelli, S.; Altissimi, M.S. Experimental accelerated shelf life determination of a ready-to-eat processed food. Ital. J. Food Saf. 2018, 7, 6919. [Google Scholar] [CrossRef]
- Delvaux, A.; Rathahao-Paris, E.; Alves, S. Different ion mobility-mass spectrometry coupling techniques to promote metabolomics. Mass. Spectrom. Rev. 2021, 41, 695–721. [Google Scholar] [CrossRef]
- Klockmann, S.; Reiner, E.; Bachmann, R.; Hackl, T.; Fischer, M. Food Fingerprinting: Metabolomic Approaches for Geographical Origin Discrimination of Hazelnuts (Corylus avellana) by UPLC-QTOF-MS. J. Agric. Food Chem. 2016, 64, 9253–9262. [Google Scholar] [CrossRef]
- Al-Duais, M.; Müller, L.; Böhm, V.; Jetschke, G. Antioxidant capacity and total phenolics of Cyphostemma digitatum before and after processing: Use of different assays. Eur. Food Res. Technol. 2009, 228, 813–821. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Wenck, S.; Creydt, M.; Hansen, J.; Gärber, F.; Fischer, M.; Seifert, S. Opening the Random Forest Black Box of the Metabolome by the Application of Surrogate Minimal Depth. Metabolites 2021, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, R.A.; Hoefsloot, H.C.J.; Westerhuis, J.A.; Smilde, A.K.; van der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genom. 2006, 7, 142. [Google Scholar] [CrossRef]
- Degenhardt, F.; Seifert, S.; Szymczak, S. Evaluation of variable selection methods for random forests and omics data sets. Brief. Bioinform. 2019, 20, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Tu, J.; Xiong, X.; Shen, X.; Zhu, Z.-J. LipidCCS: Prediction of Collision Cross-Section Values for Lipids with High Precision To Support Ion Mobility-Mass Spectrometry-Based Lipidomics. Anal. Chem. 2017, 89, 9559–9566. [Google Scholar] [CrossRef] [PubMed]
- Keerthirathne, T.P.; Ross, K.; Fallowfield, H.; Whiley, H. A Review of Temperature, pH, and Other Factors that Influence the Survival of Salmonella in Mayonnaise and Other Raw Egg Products. Pathogens 2016, 5, 63. [Google Scholar] [CrossRef]
- Lu, F.S.H.; Bruheim, I.; Haugsgjerd, B.O.; Jacobsen, C. Effect of temperature towards lipid oxidation and non-enzymatic browning reactions in krill oil upon storage. Food Chem. 2014, 157, 398–407. [Google Scholar] [CrossRef]
- Membré, J.-M.; Leporq, B.; Vialette, M.; Mettler, E.; Perrier, L.; Thuault, D.; Zwietering, M. Temperature effect on bacterial growth rate: Quantitative microbiology approach including cardinal values and variability estimates to perform growth simulations on/in food. Int. J. Food Microbiol. 2005, 100, 179–186. [Google Scholar] [CrossRef]
- Kursa, M.B.; Jankowski, A.; Rudnicki, W.R. Boruta—A System for Feature Selection. Fundam. Informaticae 2010, 101, 271–285. [Google Scholar] [CrossRef]
- Byrdwell, W.C.; Neff, W.E. Dual parallel electrospray ionization and atmospheric pressure chemical ionization mass spectrometry (MS), MS/MS and MS/MS/MS for the analysis of triacylglycerols and triacylglycerol oxidation products. Rapid Commun. Mass. Spectrom. 2002, 16, 300–319. [Google Scholar] [CrossRef]
- Dapic, I.; Jakasa, I.; Kobetic, R.; Brkljacic, L. Characterization of Ceramides with Phytosphingosine Backbone by Hydrogen-deuterium Exchange Mass Spectrometry. Croat. Chem. Acta 2019, 92, 411–417. [Google Scholar] [CrossRef]
- Frick, A.A.; Weyermann, C. An untargeted lipidomic approach for qualitative determination of latent fingermark glycerides using UPLC-IMS-QToF-MS E. Analyst 2019, 144, 3590–3600. [Google Scholar] [CrossRef] [PubMed]
- Hankin, J.A.; Murphy, R.C.; Barkley, R.M.; Gijón, M.A. Ion Mobility and Tandem Mass Spectrometry of Phosphatidylglycerol and Bis(monoacylglycerol)phosphate (BMP). Int. J. Mass. Spectrom. 2015, 378, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Le Thanh, T.; van Anh, N.T.; Hue, P.T. Molecular species of glycolipid and anti-inflammation activity of lipid fractions in the green algae Halimeda incrassata Lamx. collected from Truong Sa, Viet Nam. Vietnam. J. Chem. 2021, 59, 639–647. [Google Scholar] [CrossRef]
- Pi, J.; Wu, X.; Feng, Y. Fragmentation patterns of five types of phospholipids by ultra-high-performance liquid chromatography electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Anal. Methods 2016, 8, 1319–1332. [Google Scholar] [CrossRef]
- Zeb, A. Chemistry and liquid chromatography methods for the analyses of primary oxidation products of triacylglycerols. Free Radic. Res. 2015, 49, 549–564. [Google Scholar] [CrossRef]
- Zeb, A. Triacylglycerols composition, oxidation and oxidation compounds in camellia oil using liquid chromatography-mass spectrometry. Chem. Phys. Lipids 2012, 165, 608–614. [Google Scholar] [CrossRef]
- Zeb, A.; Murkovic, M. Characterization of the effects of β-carotene on the thermal oxidation of triacylglycerols using HPLC-ESI-MS. Eur. J. Lipid Sci. Technol. 2010, 112, 1218–1228. [Google Scholar] [CrossRef]
- Pournik, S.; Abbasi-Rostami, M.; Sadeghipour, H.R.; Ghaderi-Far, F. True lipases beside phospholipases contribute to walnut kernel viability loss during controlled deterioration and natural aging. Environ. Exp. Bot. 2019, 164, 71–83. [Google Scholar] [CrossRef]
- Olsen, I.; Jantzen, E. Sphingolipids in Bacteria and Fungi. Anaerobe 2001, 7, 103–112. [Google Scholar] [CrossRef]
- Barnholz, Y.; Roitman, A.; Gatt, S. Enzymatic Hydrolysis of Sphingolipids. J. Biol. Chem. 1966, 241, 3731–3737. [Google Scholar] [CrossRef]
- Djordjevic, J.T. Role of phospholipases in fungal fitness, pathogenicity, and drug development—Lessons from cryptococcus neoformans. Front. Microbiol. 2010, 1, 125. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-M.; Yu, C.-W. Chloroplast Galactolipids: The Link Between Photosynthesis, Chloroplast Shape, Jasmonates, Phosphate Starvation and Freezing Tolerance. Plant Cell Physiol. 2018, 59, 1128–1134. [Google Scholar] [CrossRef]
- Amara, S.; Lafont, D.; Parsiegla, G.; Point, V.; Chabannes, A.; Rousset, A.; Carrière, F. The galactolipase activity of some microbial lipases and pancreatic enzymes. Eur. J. Lipid Sci. Technol. 2013, 115, 442–451. [Google Scholar] [CrossRef]
- Matsumoto, A.; Takahashi, S.; Nakano, K.; KIJIMA, S. Identification of new vitamin E in plant oil. J. Jpn. Oil Chem. Soc. 1995, 44, 593–597. [Google Scholar] [CrossRef]
- Fiorentino, A.; Mastellone, C.; D’Abrosca, B.; Pacifico, S.; Scognamiglio, M.; Cefarelli, G.; Caputo, R.; Monaco, P. δ-Tocomonoenol: A new vitamin E from kiwi (Actinidia chinensis) fruits. Food Chem. 2009, 115, 187–192. [Google Scholar] [CrossRef]
- Azzi, A. Molecular mechanism of alpha-tocopherol action. Free Radic. Biol. Med. 2007, 43, 16–21. [Google Scholar] [CrossRef]
- Montoya-Arroyo, A.; Wagner, T.; Sus, N.; Müller, M.; Kröpfl, A.; Vetter, W.; Frank, J. Cytotoxicity, cellular uptake, and metabolism to short-chain metabolites of 11′-α-tocomonoenol is similar to RRR-α-tocopherol in HepG2 cells. Free Radic. Biol. Med. 2021, 177, 24–30. [Google Scholar] [CrossRef]
- Jaeger, K.-E.; Eggert, T. Lipases for biotechnology. Curr. Opin. Biotechnol. 2002, 13, 390–397. [Google Scholar] [CrossRef]
- Gray, J.I. Measurement of lipid oxidation: A review. J. Am. Oil Chem. Soc. 1978, 55, 539–546. [Google Scholar] [CrossRef]
- Baysal, T.; Demirdöven, A. Lipoxygenase in fruits and vegetables: A review. Enzym. Microb. Technol. 2007, 40, 491–496. [Google Scholar] [CrossRef]
- Bolling, B.W.; Chen, C.-Y.O.; McKay, D.L.; Blumberg, J.B. Tree nut phytochemicals: Composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr. Res. Rev. 2011, 24, 244–275. [Google Scholar] [CrossRef] [PubMed]
- Köksal, A.İ.; Artik, N.; Şimşek, A.; Güneş, N. Nutrient composition of hazelnut (Corylus avellana L.) varieties cultivated in Turkey. Food Chem. 2006, 99, 509–515. [Google Scholar] [CrossRef]
| Abbreviation | Temperature [°C] | Rel. Humidity [%] | Maximum Storage Time |
|---|---|---|---|
| ST1 | 40 | 75 | 24 weeks |
| ST2 | 25 | 60 | 24 weeks |
| ST3 | 40 | 25 | 24 weeks |
| LT1 | 10 | 75 | 18 months |
| LT2 | 19 | 50 | 18 months |
| Predicted | |||||
|---|---|---|---|---|---|
| Pre-Stored | Post-Stored | Cohens Kappa | |||
| True | ST1 | Pre-stored | 6 | 0 | 1.00 |
| Post-stored | 0 | 24 | |||
| ST2 | Pre-stored | 1 | 5 | –0.01 | |
| Post-stored | 0 | 24 | |||
| ST3 | Pre-stored | 5 | 1 | 0.89 | |
| Post-stored | 0 | 24 | |||
| LT1 | Pre-stored | 1 | 5 | 0.14 | |
| Post-stored | 1 | 17 | |||
| LT2 | Pre-stored | 3 | 3 | 0.60 | |
| Post-stored | 0 | 18 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loesel, H.; Shakiba, N.; Wenck, S.; Le Tan, P.; Karstens, T.-O.; Creydt, M.; Seifert, S.; Hackl, T.; Fischer, M. Food Monitoring: Limitations of Accelerated Storage to Predict Molecular Changes in Hazelnuts (Corylus avellana L.) under Realistic Conditions Using UPLC-ESI-IM-QTOF-MS. Metabolites 2023, 13, 1031. https://doi.org/10.3390/metabo13101031
Loesel H, Shakiba N, Wenck S, Le Tan P, Karstens T-O, Creydt M, Seifert S, Hackl T, Fischer M. Food Monitoring: Limitations of Accelerated Storage to Predict Molecular Changes in Hazelnuts (Corylus avellana L.) under Realistic Conditions Using UPLC-ESI-IM-QTOF-MS. Metabolites. 2023; 13(10):1031. https://doi.org/10.3390/metabo13101031
Chicago/Turabian StyleLoesel, Henri, Navid Shakiba, Soeren Wenck, Phat Le Tan, Tim-Oliver Karstens, Marina Creydt, Stephan Seifert, Thomas Hackl, and Markus Fischer. 2023. "Food Monitoring: Limitations of Accelerated Storage to Predict Molecular Changes in Hazelnuts (Corylus avellana L.) under Realistic Conditions Using UPLC-ESI-IM-QTOF-MS" Metabolites 13, no. 10: 1031. https://doi.org/10.3390/metabo13101031
APA StyleLoesel, H., Shakiba, N., Wenck, S., Le Tan, P., Karstens, T.-O., Creydt, M., Seifert, S., Hackl, T., & Fischer, M. (2023). Food Monitoring: Limitations of Accelerated Storage to Predict Molecular Changes in Hazelnuts (Corylus avellana L.) under Realistic Conditions Using UPLC-ESI-IM-QTOF-MS. Metabolites, 13(10), 1031. https://doi.org/10.3390/metabo13101031







