Abstract
Drug-induced liver damage is a life-threatening disorder, and one major form of it is the hepatotoxicity induced by the drug cisplatin. In folk medicine, Licorice (Glycyrrhiza glabra (is used for detoxification and is believed to be a potent antioxidant. Currently, the magically self-renewable potential of bone marrow mesenchymal stem cells (BM-MSCs) has prompted us to explore their hepatoregenerative capability. The impact of G. glabra extract (GGE) and BM-MSCs alone and, in combination, on protecting against hepatotoxicity was tested on cisplatin-induced liver injury in rats. Hepatic damage, as revealed by liver histopathology and increased levels of serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and malondialdehyde (MDA), was elevated in rats by received 7 mg/kg of cisplatin intraperitoneally. The combination of GGE and BM-MSCs returned the enzyme levels to near the normal range. It also improved levels of liver superoxide dismutase (SOD) and glutathione (GSH) and reduced MDA levels. Additionally, it was found that when GGE and BM-MSCs were used together, they significantly downregulated caspase9 (Casp9), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), and interleukin-1β (IL-1β), which are involved in severe proinflammatory and apoptotic signaling cascades in the liver. Moreover, combining GGE and BM-MSCs led to the normal result of hepatocytes in several examined liver histological sections. Therefore, our findings suggest that GGE may have protective effects against oxidative liver damage and the promising regenerative potential of BM-MSCs.
1. Introduction
Cis-Diamminedichloroplatinum (II) (cisplatin) is a common platinum-based anticancer medication [1]. Cisplatin has been used in treating 40% to 60% of patients suffering from cancers, for instance, testicular and colorectal tumors, and is considered the most promising drug for these patients [1]. The use of the chemotherapeutic drug cisplatin is associated with the development of acute renal damage (AKI) [2]. Cisplatin-induced kidney injury is predominantly attributable to proximal tubular damage from tubular uptake via the basolateral membrane transporter [3]. Tubulointerstitial damage is histopathological, but AKI only shows acute tubular necrosis [4]. AKI causes acute tubular necrosis, while cisplatin causes interstitial fibrosis [5]. Despite its potent anticancer activity, cisplatin is a double-edged sword because it can lead to serious toxicity in the liver. The liver is the body’s biggest solid organ in the body and is involved in most of the vital metabolic reactions. Cisplatin is characterized by its rapid diffusion into various tissues, especially the liver [6]. Even when cisplatin is administered at low doses, its long-term effect on the liver induces huge hepatotoxicity, including hepatic necrosis, liver cord breakdown, and localized inflammation [6].
Cisplatin-induced hepatotoxicity starts with the excessive generation of reactive oxygen species (ROS) and a concomitant increase in oxidative stress in the liver [7]. The massively generated ROS exhaust the hepatic antioxidant defense system, as is evident by the reduction of glutathione (GSH) levels, the exhaustion of antioxidant enzymes such as superoxide dismutase (SOD), and the increased levels of malondialdehyde (MDA) [8]. This effect is associated with the elevation of alanine transaminase (ALT), aspartate aminotransferase (AST), and alanine phosphatase (ALP) levels in the serum. These specific biomarkers are typically found in the hepatocyte’s cytoplasm and are released in the serum only after hepatocellular damage [9].
Furthermore, the accumulation of the intracellular ROS induces proinflammatory cytokines via the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) and the pro-apoptotic pathways by means of caspase 9 (Casp9) [10]. Additionally, many studies reported that cisplatin itself induced the overexpression of proinflammatory cytokines such as interleukin-1β (IL-1β) [11,12]. Consequently, cisplatin-induced serious histopathological alterations in the liver, including multifocal hepatocellular necrosis and mononuclear inflammatory cell infiltration. In addition, it increased mitotic activity in the liver, leading to dysplastic changes. Many studies have focused on relieving cisplatin-induced liver toxicity because cisplatin has therapeutic value as an anticancer drug [13,14]. Nature provides many herbal plants with beneficial therapeutic effects. One of the widely used herbal medicines is Licorice, that is extracted from the roots of the Glycyrrhiza species (Leguminosae family) [15]. In folk medicine, Glycyrrhiza glabra extract (GGE) has been an agent of choice in treating liver injury for many years [16].
A previous study reported that GGE contains valuable hepatoprotective flavonoids, which displayed a magical effect to reduce inflammation and apoptosis in the liver; this might explain the hepatoprotective effect of GGE [15]. Magnesium isoglycyrrhizinate (MgIG) is a stereoisomer of the licorice root’s glycyrrhizic acid, and it is the main compound that acts as an anti-inflammatory and hepatoprotective medication [15]. Also, glycyrrhetinic acid is hepatoprotective because it inhibits tumor necrosis factor-alpha (TNF-α) and caspase-3 (Casp3) [17]. In 2018, Sohail et al. studied the bioactive components of GGE and revealed that GGE contained various bioactive components with high antioxidant potential [18]. Many researchers studied the hepatoprotective potential of GGE [19]. These studies reported that GGE detoxified cisplatin-induced hepatic damage through the enhancement of cisplatin metabolism and the suppression of the massive release of ROS and their associated hepatic inflammation and apoptosis [7,15].
Recently, regenerative medicine has focused on using bone marrow mesenchymal stem cells (BM-MSCs) to enhance tissue engineering [20]. These self-renewable cells have revolutionized the field of tissue engineering with their ease of isolation, manipulability, and incredible regenerative potential [21]. Their abilities to proliferate, establish daughter cell lines, and repair injured tissues are of great interest to researchers [22]. Another advantage is their ability to dampen the inflammatory responses of various tissues [23]. The therapeutic effects of BM-MSCs are caused by many different stromal cells. This is because BM-MSCs can turn into functional hepatic cells and produce a series of growth factors and cytokines that can stop inflammatory responses, reduce hepatocyte death, reverse liver fibrosis, and improve hepatocyte function [24].
This study’s goal was to investigate the positive ameliorative role of BM-MSCs, GGE, and a combination of BM-MSCs and GGE in improving the hepatotoxic effect of cisplatin in rats. This can be achieved by biochemical, histological, and molecular studies.
2. Materials and Methods
2.1. Sample Collection and Preparation of Methanolic Extract from GGE Roots
The roots of GGE were collected from the online local market (https://www.cosmicelement.com/products/100-pure-cosmic-element-licorice-root-powder-pure-glycyrrhiza-glabra-mulethi-yashtimadhu-natural-halal-and-iso-certified-natural-expectorant-soothes-sore-throat-candy-flavoring-agent-superfood-8oz-227g) (accessed on 21 Novamber 2021). The plant root was ground to a coarse powder, and extractions were performed with methanol (95%) by the Soxhlet extraction procedure. In a rotary evaporator, the methanolic extracts were evaporated until they were dry and then concentrated under a vacuum at 40 to 50 °C. The extract was collected and stored in dark, airtight bottles until use [25].
2.2. Phytochemical Analysis and Antioxidant Activity of GGE
Gas chromatography–mass spectrometry (G.C.–M.S.) analysis, the total phenolic contents (TPC), total flavonoid contents (TFC), and ferric ion-reducing antioxidant power (FRAP) were estimated for GGE [26]. TPC was determined using the Folin–Ciocalteu reagent [27] and expressed in mg/g as the Gallic Acid Equivalent (GAE). The determination of flavonoids was carried out by the Aluminum Chloride Method [28]. TFC was measured in mg/g as the Quercetin Equivalent (Q.E.) [28]. FRAP assay was performed to estimate the antioxidant power of GGE [29], and the positive reference standard was ascorbic acid.
2.3. BM-MSCs Preparation
Rat BM-MSCs were received from the unit of Biochemistry, Faculty of Medicine Cairo University’s. The stem cells were isolated and manipulated as reported [30]. Briefly, the iliac crest was aspirated for bone marrow, and the samples were deposited in heparinized tubes. An ultrasonographic-guided intrasplenic injection was used to apply the bone marrow stromal cells.
2.4. Animal Model’s Design
Male albino rats (Sprague-Dawley) weighing 130 to 160 g each. The animals were housed in a room with a controlled air temperature (25 ± 2 °C) and maintained in a light: dark (12:12) cycle (Figure 1A). All animal experiments techniques were approved by the Local Committee of Bioethics (LCBE), Jouf University (No. 4-05-43). The rats were given an unrestricted supply of pellet food and unrestricted access to water. They were adapted for two days before we started the experiment. Healthy males were chosen at random and split into five groups, each with five rats (Figure 1B):
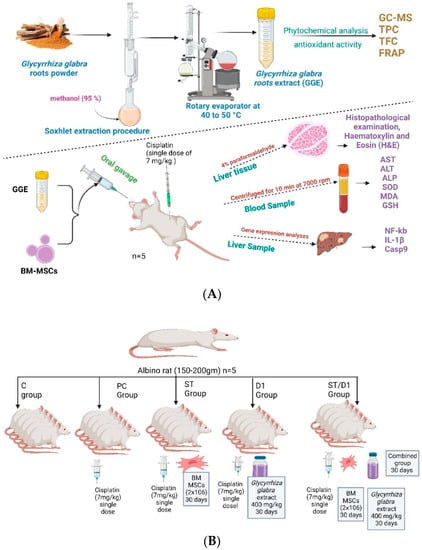
Figure 1.
Experimental design and animal model.: (A) The experimental design. (B) The animal model with doses and duration.
- The control group got saline.
- The cisplatin group (P.C. group): 7 mg/kg of cisplatin was injected intraperitoneally (I.P.) as a single dose to induce liver damage (Positive management).
- The BM-MSCs group (Mesenchymal Stem Cells from Bone Marrow—St group): 7 mg/kg of cisplatin was injected intraperitoneally (I.P.) as a single dose, and on the following day, rats began receiving 2 × 106 BM-MSCs per day in phosphate buffer solution (PBS) by intravenous (IV) injection for 1 month.
- The GGE group (licorice-based drug D1 group): 7 mg/kg of cisplatin was injected intraperitoneally (I.P.) as a single dose, and the following day, rats received 400 mg/kg/day orally of GGE for one month.
- The group consisting of BM-MSCs and GGE (Combinational treatment—St/D1 group) 7 mg/kg of cisplatin was injected intraperitoneally (I.P.) as a single dose, and the following day, rats received 2 × 106 BM-MSCs in PBS by IV injection plus 400 mg/kg/day of GGE one month [31].
2.5. Blood and Liver Tissue Collection
At the end of the experiment, the animals were slaughtered, and blood was collected to evaluate biochemical parameters. After scarification, the liver tissues were rapidly removed, cleaned with physiological saline, and divided into two sections. The first section was placed in a Triazole reagent for the study of gene expression and kept at −20 °C. For histological analysis, the second piece of liver tissue was fixed in a 15% formaldehyde solution.
2.5.1. Biochemical Markers of Serum
Collected blood samples were centrifuged for 10 min at 7000 rpm using a microcentrifuge to isolate the serum. The liver biomarkers (AST, ALT, and ALP) were estimated as reported [8]. The antioxidants and oxidative markers renal malondialdehyde (MDA), superoxidase dismutase (SOD), and glutathione (GSH) levels were measured as reported [32,33,34].
2.5.2. Histopathological Examination, Hematoxylin, and Eosin (H&E)
Specimens that had previously been fixed in formalin were typically dehydrated in an increasing sequence of alcohols, cleaned in xylol, and then ultimately embedded with paraffin. Tissues with a thickness of between 4 and 5 μm were sectioned and prepared for H&E staining. The tissue slides were evaluated, and compared to the controls that corresponded to them, and finally, photographs were taken. Digital image capture equipment was utilized during the gathering of these photos (Olympus CX40; Olympus, Tokyo, Japan) [24].
2.6. Gene Expression Analyses
Changes in the mRNA level of NF-kB, IL-1β, and Casp9 were measured. The total RNA was isolated from the liver tissues using the High Pure RNA Kit according to the manufacturer’s protocol (Roche, Germany). The RNA concentration and quality were determined spectrophotometrically at 260 nm and by the A260/A280 ratio, respectively. Subsequently, 0.1 μg of RNA from each sample was subjected to reverse transcription using the HyperScript™ First-strand synthesis kit (GeneAll, Seoul, Republic of Korea). A real-time polymerase chain reaction (RT-PCR) was performed with a Rotor-Gene Q (Qiagen) using RealQ Plus 2× Master Mix Green (Ampliqon, Odense, Denmark) [35]. The thermal cycling conditions involved an initial activation step for 15 min at 95 °C followed by 40 cycles, including a denaturation step for 20 s at 95 °C and a combined annealing/extension step for 60 s at 60 °C. Melting curves were analyzed to validate a single PCR product of each primer. The fold change in expression of each target mRNA relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was determined using the 2–ΔΔct method. Primer sequences are stated in Table 1.

Table 1.
Genes and primer sequences used for the real-time PCR.
2.7. Statistical Analysis
Results are presented as means of 3 independent replicates. Analysis of Variance (one-way ANOVA test) with Tukey post hoc test was used to analyze the data; statistical analysis tests were done with SPSS version 21. Results were shown as mean ± S.D. and were considered significant at a p-value < 0.05 (Tukey post hoc test). Presented distinct lowercase letters denote statistically significant differences in comparison to the group serving as the control. *** p < 0.001, ** p < 0.01, n.s.: p-value > 0.05 is considered non-significant (n = 5).
3. Results
3.1. Phytochemical Analysis and the Antioxidant Power of GGE
The results showed that the TPC of the GGE was 50 mg gallic acid equivalent/g. The TFC was 19 mg quercetin equivalent/g. The FRAP value was 48.4 mg of the ascorbic acid equivalent/g (Figure 2).

Figure 2.
TPC, TFC, and FRAP in Glycyrrhiza glabra methanolic extract.
3.2. Biochemical Parameters
The methanolic extract of G. glabra roots and the BM-MSCs were studied for their hepatoprotective and antioxidant effects on liver damage from cisplatin in rats. Monitoring specific biomarker as ALT, AST, ALP, MDA, SOD, and GSH helped us to determine the extent of liver protection. The levels of liver biomarkers (ALT, AST, ALP), MDA, SOD, and GSH graphically represented the levels in test groups against the negative control group (Figure 3 and Table 2). Cisplatin treatment caused a considerable increase in liver biomarkers and MDA levels (Figure 3), associated with a considerable decline in the levels of SOD and GSH, compared with the control group (Table 2). The groups treated with BM-MSCs, GGE, or BM-MSCs plus GGE had an obvious increase in the levels of SOD and GSH allied with a decline in the levels of liver biomarkers, and MDA compared with the group given cisplatin, but left untreated. In the group receiving the combined BM-MSCs and GGE, levels of SOD and GSH were increased significantly, allied with a decline in the levels of liver biomarkers and MDA compared with the group that received cisplatin but was left untreated, the group that received GGE, and the group that was treated with BM-MSCs (Figure 3). Our findings might support the hepatoprotective potential of GGE combined with the influence exerted by BM-MSCs in the relief of oxidative liver injury.

Figure 3.
The effect of the treatment groups (Control, Cisplatin (P.C.), Cisplatin + GGE (D1), Cisplatin + BM-MSCs (St), Cisplatin + BM-MSCs + GGE (St/D1)) on the renal functions in rats, (A) ALT and (B) AST; (C) ALP and (D) MDA. Different alphabetic letter/s being significantly different (p < 0.05).

Table 2.
The effect of the treatment groups (Control, Cisplatin, Cisplatin + GGE, Cisplatin + BM-MSCs, and Cisplatin + BM-MSCs + GGE) on the SOD and GSH level in rats. Different alphabetic letter/s being significantly different (p < 0.05).
3.3. Liver Histopathology
Hepatocytes in the control group’s livers appeared to be arranged in a normal lobular architecture, and the liver’s central veins and radiating hepatic cords were clearly visible under the microscope. Histological examination of the portal triads revealed typical architecture consisting of branched hepatic arteries, portal veins, and bile ducts. (Figure 4A).

Figure 4.
(A) The control group’s hepatic tissue has normal histological structure with radiating cords of hepatocytes surrounding the central vein. (B) Liver tissue from the Cisplatin group showing necrosis of the hepatic hepatocytes with evidence of dysplastic changes. (C) Liver tissue from the BM-MSCs group showing moderate vacuolated hepatocytes (arrow). (D) Liver tissue from the GGE group showing portal inflammation. (E) Liver tissue from the GGE and BM-MSCs combined group showing an apparently normal portal area with healthy adjacent hepatocytes (n = 5).
The administration of cisplatin resulted in serious histopathological alteration. Multifocal areas of hepatocellular necrosis were frequently detected in the affected individuals. The hepatic lobules showed numerous excessive vacuolated hepatocytes associated with the accumulation of eosinophilic, karyorrhectic debris and a variable number of mononuclear inflammatory cell infiltrations. Dysplastic changes were observed in a few sections associated with increased mitotic activity (Figure 4B).
In the stem cell-treated group, the examined hepatic section showed apparently normal hepatocytes in several hepatic lobules. Fewer sections showed the multifocal aggregation of mononuclear inflammatory cells in the hepatic lobules as well as in the portal areas. The widening of the hepatic sinusoids revealed a marked dilation in some circumstances (Figure 4C). The GGE-treated group showed an increased pathological alteration compared to the BM-MSCs group. Multifocal inflammatory areas were noticed in the affected hepatic parenchyma that was characterized by a moderate number of mononuclear inflammatory cell aggregations. Some examined sections showed apparently normal hepatic tissue (Figure 4D). The GGE and BM-MSCs combined group revealed that hepatic parenchyma has significantly improved. The examination of the hepatic lobules showed apparently normal hepatocytes in several examined sections with intact portal areas and hepatic sinusoids (Figure 4E).
3.4. Gene Expression Analysis
The change in gene expression fold was monitored for NF-kB, IL-1β, and Casp9, which are involved in serious inflammatory and apoptotic signaling cascades in the liver (Figure 5). Normalizing agent, B-actin was utilized to validate the single polymerase chain reaction (PCR) product of each primer, and all data were the means of three replicates and presented as the mean plus or minus the standard deviation (S.D.). It was observed that NF-kB, IL-1β, and Casp9 were upregulated in the group given cisplatin but left untreated, compared with rats that served as the negative control. Rats treated with BM-MSCs, GGE, or BM-MSCs plus GGE, clearly showed that NF-kB, IL-1β, and Casp9 were downregulated compared with the group given cisplatin but left untreated. Interestingly, by monitoring how much each target mRNA gene’s expression changed (NF-kB, IL-1β, and Casp9) in the rats received the BM-MSCs and/or GGE, it was obvious that the NF-kB, IL-1β, and Casp9 were significantly further downregulated compared to the rat’s taken cisplatin, but left untreated, the group taken GGE, and the group given BM-MSCs. This might be attributed to the combined hepatoprotective effect of the GGE plus BM-MSCs.
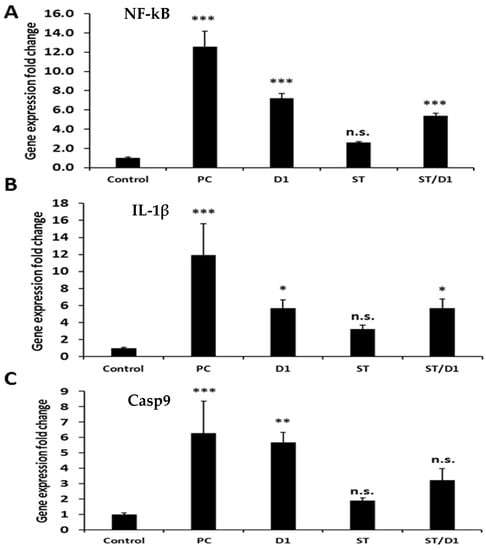
Figure 5.
The effect of treated groups (Control, Cisplatin (P.C.), Cisplatin + GGE (D1), Cisplatin + BM-MSCs (St), Cisplatin + BM-MSCs + GGE (St/D1)) on gene expression profiling of the liver in rats. (A) NF-kB, (B) IL-1β, and (C) Casp9. *** highly significant p < 0.001, ** significant p < 0.005, * significant p < 0.05 and n.s mean non-significant.
4. Discussion
Hepatotoxicity is one of the many negative effects of cisplatin, an anticancer drug used to treat malignancies of solid organs [36,37]. Clinicians need to be aware of the anticipated consequences of its hepatotoxicity [38]. In the hospital context, recovery following an acute injury is essential to reduce patient morbidity and death [39]. In this investigation, we demonstrated that cisplatin was capable of inducing considerable hepatotoxicity in mice, as shown by increases in their blood-liver enzyme activity, upregulation of proinflammatory markers, and abnormalities in histopathology.
The small size of the cisplatin molecule lets it get through the cell membrane and affects the DNA structure in the nucleus. Although the exact mechanisms by which cisplatin causes liver damage to remain unknown, one possible explanation for such an effect could be that oxidative stress and apoptosis are likely contributors [40]. Other reports have found that cisplatin reduced glutathione as an antioxidant marker and, at the same time, raised lipid peroxidation as an oxidative stress marker [41].
When oxidative stress is present, impairment of the body’s antioxidant system may occur or ROS may be overproduced [42,43]. Therefore, in the present study, the group that received 7 mg/kg of cisplatin I.P., but was left untreated, displayed serious hepatic histopathological alterations, multifocal areas of hepatocellular necrosis, and a variable number of infiltrated mononuclear inflammatory cells [44]. Moreover, this group showed a significant elevation in ALT, AST, ALP, and MDA levels associated with a considerable decline in SOD and GSH levels compared with the negative control group given saline.
Numerous hypotheses about cisplatin-induced hepatotoxicity have been published. These reports have discussed the drug’s pathways in the development of lipid peroxidation, mitochondrial dysfunction, DNA damage [7], and the destruction of structural proteins [40]. Free radicals and ROS are elevated as a result of the DNA damage caused by the binding of cisplatin’s platinum components to DNA. In our study, levels of NF-kB, IL-1β, and Casp9 were upregulated in the group given cisplatin but left untreated, compared with the negative control group. NF-kB and IL-1β are essential inducers of inflammatory cytokines in the liver [10]. Casp9 is a crucial regulator of the initiation of apoptosis and cytokine signaling in the liver [45,46] and belongs to the cysteine-aspartic protease (caspase) family.
GGE and BM-MSCs have been widely utilized in the clinical treatment of cisplatin-induced hepatotoxicity that is directly related to the exhaustion of the hepatic antioxidant defense system [47]. The capacities of GGE or BM-MSCs to mitigate oxidative stress, reduce free radicals, and enhance the overall functioning of the liver have been published in prior research [48,49].
In this study, the examined hepatic sections of rats that had single cisplatin dosage and had been treated with GGE were characterized by a moderate number of mononuclear inflammatory cell aggregations compared with the group given cisplatin but left untreated. There was an obvious increase in the levels of SOD and GSH allied with low levels of ALT, AST, ALP, and MDA, associated with NF-kB, IL-1β, and Casp9 having been downregulated as well, compared with the group given cisplatin, but left untreated. A variety of pharmacologic effects, including anti-inflammatory, antioxidant, immunomodulatory, and hepatoprotection, have been attributed to licorice [50]. Our findings revealed that GGE was rich in considerable amounts of phenolic compounds and antioxidant flavonoids that could relieve hepatic oxidative stress [51]. Furthermore, owing to the molecular similarities between glycyrrhizic acid and adrenal cortex-secreted mineralocorticoid hormones and glycyrrhizic acid’s glucocorticoid action, Licorice has steroid-like anti-inflammatory effects comparable to those of hydrocortisone [52].
The mitigation effect may be due to three mechanisms of action. The first is the inhibition of hepatic apoptosis and necrosis. This inhibition takes place through the blockage of mitochondrial cytochrome C release [17], the suppression of TNF-α and Casp3 [53], and the inhibition of HMGB1 production by Kupffer cells [54]. The second effect occurs through anti-inflammatory and immune regulation [55]. This action is achieved by the suppression of the production of IL-6 and TNF-α produced by the lipid A moiety of lipopolysaccharides [56], as well as the inhibition of the synthesis of mitogen-activated protein kinase (MAPK), NF-kB, and IL-6 induced by paclitaxel [57]. The inhibition of the lytic pathway of the complementary system inhibits tissue damage from the membrane attack complex. The third effect occurs via the induction of liver enzyme activity by the triggering of the CYP3A pathway [58].
Recently, many studies have focused on the excellent regenerative potential of BM-MSCs and their capabilities of repairing injured tissues and dampening the inflammatory responses of various tissues [22,23]. BM-MSCs have paracrine therapeutic activities, mainly through trophic factors [59]. In regenerative medicine, BM-MSCs perform important therapeutic functions as secretors of growth factors, a small number of cytokines, and chemokines, among other soluble factors and trophic factors [60]. By controlling how much cytokines and other substances that cause inflammation are released, BM-MSCs create a hepatoprotective environment: they decrease inflammation, cellular death, and fibrosis while simultaneously promoting angiogenesis and tissue cell regeneration [61].
We used an animal model in our experiment of cisplatin-induced hepatotoxicity that was treated with BM-MSCs, GGE, or BM-MSCs in combination with GGE. Extremely high levels of SOD and GSH was seen, and a decrease in ALT, AST, ALP, and MDA levels associated with the downregulation of NF-kB, IL-1β, and Casp9 in these groups compared with the group given cisplatin but left untreated. Consistent with previous research, our data showed that BM-MSCs were able to recover serum liver function markers dramatically and liver enzyme levels, consequently decreasing liver fibrosis in damaged rat livers [49]. The expression of matrix metalloproteinase (MMP) was upregulated by the BM-MSCs, whereas the expression of the tissue inhibitor of metalloproteinase (TIMP) was downregulated; these changes were mostly associated with fibrosis resolution and dramatically destroyed collagen fibers [62]. Two of the most well-studied trophic factors believed to be released by MSCs are hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF) [63]. HGF is pivotal in the constructive control of hepatocyte proliferation [64]. One study reported that BM-MSCs were an essential component of the connective tissue that provides structural support for various functional cells [65]. Other researchers studied the therapeutic properties of BM-MSCs and their impact on regenerative medicine. It was found that BM-MSCs exerted their therapeutic effects through the secretion of bioactive molecules that regenerated damaged tissues [66] (Figure 6).

Figure 6.
A summary of the suggested mechanism of Glycyrrhiza glabra and/or BM-MSCs on cisplatin-induced hepatocellular damage in rats.
In the group treated with BM-MSCs plus GGE, our results revealed a considerable downregulation of Casp9, NF-kB, and IL-1β that might be attributed to the potent antioxidant potential of GGE and the complementary regenerative potential of the BM-MSCs and their ability to dampen various inflammatory responses. These results were strongly supported by the remarkable improvement of the hepatic parenchyma and the obvious amelioration of hepatocytes in several examined sections after treatment with the combined BM-MSCs and GGE. Our previous research showed that compared with either BM-MSCs alone or the drug silymarin alone, their combination was more effective [67]. The levels of liver enzymes in the serum were restored to those of the control group, suggesting that the combined therapies strongly augment the hepatic environment. As a consequence of this, the BM-MSCs were able to survive longer in the host tissue and were able to differentiate into hepatocytes more effectively, and the hepatic function was greatly improved. [49]. Interestingly, it was observed that the specific biomarkers, which are strongly related to oxidative liver injury, were normalized by the antioxidant potential of GGE combined with the regenerative potential of the BM-MSCs. Many studies stated that the combined synergistic effect of GGE and BM-MSCs could be because of the acceleration of BM-MSC differentiation and migration when BM-MSCs are combined with GGE [68,69]. Others have suggested that the combined effect reveals an enhancement of angiogenesis and neurogenesis [70,71]. Finally, they effectively regulate astrocytes and improve the flow of blood to the brain and the metabolism of glucose [72,73,74]. The actions of GGE, BM-MSCs, and the combined BM-MSCs and GGE are summarized in Figure 6.
5. Conclusions
In traditional medicine, GGE is used to treat liver dysfunction. G. glabra has a well-known hepatoprotective capability. Furthermore, BM-MSCs have extraordinary regenerative capabilities. In our research, we explored the hepatoprotective impact of GGE, BM-MSCs, and combined GGE and BM-MSCs on cisplatin-induced liver damage in rats. GGE combined with BM-MSCS significantly inhibited the elevated hepatic enzymes caused by cisplatin intoxication. This combination led to a remarkable improvement in the liver parenchyma and showed that hepatic function was improved and that blood liver enzyme levels were returned to those of healthy controls, demonstrating that the combined therapy worked synergistically to alter the hepatic environment.
Author Contributions
Conceptualization, M.A.M. and N.S.A.; methodology, M.A.M., D.M.K., N.Y.H., M.O.G. and N.S.A.; software, M.A.M., D.M.K. and N.S.A.; validation, N.Y.H., M.O.G. and N.S.A.; formal analysis, M.A.M., D.M.K., N.Y.H., M.O.G., E.I.A. and N.S.A.; investigation, M.A.M., D.M.K., N.Y.H., M.O.G. and N.S.A.; resources, N.Y.H., M.O.G., E.I.A. and N.S.A.; data curation, M.A.M., D.M.K. and N.S.A.; writing—original draft preparation, M.A.M., D.M.K., E.I.A. and N.S.A.; writing—review and editing, M.A.M., D.M.K., N.Y.H., M.O.G. and N.S.A.; visualization, D.M.K. and N.Y.H.; supervision, N.Y.H., M.O.G., E.I.A. and N.S.A.; project administration, M.A.M. and N.S.A.; funding acquisition, M.A.M., N.Y.H., M.O.G., E.I.A. and N.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at Jouf University for funding this work through research grant No (DSR-2021-01-0110).
Institutional Review Board Statement
All experimental protocols followed the criteria for animal experimentation, which was authorized by the Local Committee of Bioethics (LCBE), Jouf University (No. 4-05-43).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aminuddin, A.; Ng, P.Y.; Leong, C.-O.; Chua, E.W. Mitochondrial DNA Alterations May Influence the Cisplatin Responsiveness of Oral Squamous Cell Carcinoma. Sci. Rep. 2020, 10, 7885. [Google Scholar] [CrossRef] [PubMed]
- Arany, I.; Safirstein, R.L. Cisplatin Nephrotoxicity. Semin. Nephrol. 2003, 23, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Pabla, N.; Dong, Z. Cisplatin Nephrotoxicity: Mechanisms and Renoprotective Strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Caetano-Pinto, P.; Justian, N.; Dib, M.; Fischer, J.; Somova, M.; Burchardt, M.; Wolff, I. In Vitro Characterization of Renal Drug Transporter Activity in Kidney Cancer. Int. J. Mol. Sci. 2022, 23, 10177. [Google Scholar] [CrossRef] [PubMed]
- Hosohata, K. Role of Oxidative Stress in Drug-Induced Kidney Injury. Int. J. Mol. Sci. 2016, 17, 1826. [Google Scholar] [CrossRef]
- Quintanilha, J.C.F.; de Sousa, V.M.; Visacri, M.B.; Amaral, L.S.; Santos, R.M.M.; Zambrano, T.; Salazar, L.A.; Moriel, P. Involvement of Cytochrome P450 in Cisplatin Treatment: Implications for Toxicity. Cancer Chemother. Pharmacol. 2017, 80, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Abd Rashid, N.; Abd Halim, S.A.S.; Teoh, S.L.; Budin, S.B.; Hussan, F.; Ridzuan, N.R.A.; Jalil, N.A.A. The Role of Natural Antioxidants in Cisplatin-Induced Hepatotoxicity. Biomed. Pharmacother. 2021, 144, 112328. [Google Scholar] [CrossRef]
- Huo, H.Z.; Wang, B.; Liang, Y.K.; Bao, Y.Y.; Gu, Y. Hepatoprotective and Antioxidant Effects of Licorice Extract against CCl4-Induced Oxidative Damage in Rats. Int. J. Mol. Sci. 2011, 12, 6529–6543. [Google Scholar] [CrossRef]
- Contreras-Zentella, M.L.; Hernández-Muñoz, R. Is Liver Enzyme Release Really Associated with Cell Necrosis Induced by Oxidant Stress? Oxidative Med. Cell. Longev. 2016, 2016, 3529149. [Google Scholar] [CrossRef]
- Sadhukhan, P.; Saha, S.; Dutta, S.; Sil, P.C. Mangiferin Ameliorates Cisplatin Induced Acute Kidney Injury by Upregulating Nrf-2 via the Activation of PI3K and Exhibits Synergistic Anticancer Activity with Cisplatin. Front. Pharmacol. 2018, 9, 638. [Google Scholar] [CrossRef]
- Cüre, M.C.; Erkan, C.; Kalkan, Y.; Kirbaş, A.; Tümkaya, L.; Yilmaz, A.; Türkyilmaz, A.K.; Şehitoğlu, İ.; Süleyman, Y. Infliximab Modulates Cisplatin-Induced Hepatotoxicity in Rats. Balk. Med. J. 2016, 33, 504–511. [Google Scholar] [CrossRef]
- El-Naggar, S.A.; El-Said, K.S.; Mobasher, M.; Elbakry, M. Enhancing antitumor efficacy of cisplatin low dose by EDTA in Ehrlich ascetic carcinoma bearing mice. Braz. Arch. Biol. Technol. 2019, 62, e19180716. [Google Scholar] [CrossRef]
- Florea, A.-M.; Büsselberg, D. Cisplatin as an Anti-Tumor Drug: Cellular Mechanisms of Activity, Drug Resistance and Induced Side Effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef]
- Liao, Y.; Lu, X.; Lu, C.; Li, G.; Jin, Y.; Tang, H. Selection of Agents for Prevention of Cisplatin-Induced Hepatotoxicity. Pharmacol. Res. 2008, 57, 125–131. [Google Scholar] [CrossRef]
- Wahab, S.; Annadurai, S.; Abullais, S.S.; Das, G.; Ahmad, W.; Ahmad, M.F.; Kandasamy, G.; Vasudevan, R.; Ali, M.S.; Amir, M. Glycyrrhiza Glabra (Licorice): A Comprehensive Review on Its Phytochemistry, Biological Activities, Clinical Evidence and Toxicology. Plants 2021, 10, 2751. [Google Scholar] [CrossRef]
- Zhang, Q.; Ye, M. Chemical Analysis of the Chinese Herbal Medicine Gan-Cao (Licorice). J. Chromatogr. A 2009, 1216, 1954–1969. [Google Scholar] [CrossRef]
- Tang, B.; Qiao, H.; Meng, F.; Sun, X. Glycyrrhizin Attenuates Endotoxin- Induced Acute Liver Injury after Partial Hepatectomy in Rats. Braz. J. Med. Biol. Res. 2007, 40, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.; Rakha, A.; Butt, M.S.; Asghar, M. Investigating the Antioxidant Potential of Licorice Extracts Obtained through Different Extraction Modes. J. Food Biochem. 2018, 42, e12466. [Google Scholar] [CrossRef]
- Yin, G.; Cao, L.; Xu, P.; Jeney, G.; Nakao, M.; Lu, C. Hepatoprotective and Antioxidant Effects of Glycyrrhiza Glabra Extract against Carbon Tetrachloride (CCl4)-Induced Hepatocyte Damage in Common Carp (Cyprinus Carpio). Fish Physiol. Biochem. 2011, 37, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Zahran, F.; Nabil, A.; El Karef, A.; Lotfy, A.; Mahmoud, K.; Hozayen, W.G.; Sobh, M. Effect of Antioxidants and Mesenchymal Stem Cells on Cisplatin Induced Renal Fibrosis in Rats. J. Stem Cell Res. Ther. 2016, 1, 1. [Google Scholar]
- Rosenbaum, A.J.; Grande, D.A.; Dines, J.S. The Use of Mesenchymal Stem Cells in Tissue Engineering: A Global Assessment. Organogenesis 2008, 4, 23–27. [Google Scholar] [CrossRef]
- Solter, D.; Gearhart, J. Putting Stem Cells to Work. Science 1999, 283, 1468–1470. [Google Scholar] [CrossRef] [PubMed]
- Zahran, F.; El-Ghareb, M.; Nabil, A. Bone Marrow Derived Mesenchymal Stem Cells As A Therapy for Renal Injury. IJAR 2014, 4, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.R.; El-Demerdash, R.S.; Elminshawy, H.H.; Mehanna, E.T.; Mesbah, N.M.; Abo-Elmatty, D.M. Therapeutic Effect of Bone Marrow Mesenchymal Stem Cells in a Rat Model of Carbon Tetrachloride Induced Liver Fibrosis. Biomed. J. 2021, 44, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Akram, R.; Saqib, M.; Khalid, B.; Arshad, A. Effects of Extraction Media and Techniques on the Antioxidant Properties and Recovery of Phenolics from Roots of Glycyrrhiza Glabra. J. Mol. Pathophysiol. 2015, 4, 138–143. [Google Scholar] [CrossRef]
- Koldaş, S.; Demirtas, I.; Ozen, T.; Demirci, M.A.; Behçet, L. Phytochemical Screening, Anticancer and Antioxidant Activities of Origanum Vulgare L. Ssp. Viride (Boiss.) Hayek, a Plant of Traditional Usage. J. Sci. Food Agric. 2015, 95, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin–Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Sahu, R.; Saxena, J. Screening of Total Phenolic and Flavonoid Content in Conventional and Non-Conventional Species of Curcuma. J. Pharmacogn. Phytochem. 2013, 21, 24–26. [Google Scholar]
- Jang, H.-J.; Lee, H.-J.; Yoon, D.-K.; Ji, D.-S.; Kim, J.-H.; Lee, C.-H. Antioxidant and Antimicrobial Activities of Fresh Garlic and Aged Garlic By-Products Extracted with Different Solvents. Food Sci. Biotechnol. 2018, 27, 219–225. [Google Scholar] [CrossRef]
- Amin, M.A.; Sabry, D.; Rashed, L.A.; Aref, W.M.; el-Ghobary, M.A.; Farhan, M.S.; Fouad, H.A.; Youssef, Y.A.-A. Short-Term Evaluation of Autologous Transplantation of Bone Marrow–Derived Mesenchymal Stem Cells in Patients with Cirrhosis: Egyptian Study. Clin. Transplant. 2013, 27, 607–612. [Google Scholar] [CrossRef]
- Hassen, M.T.; Mohamed, H.K.; Montaser, M.M.; El-Sharnouby, M.E.; Awad, N.; Ebiya, R.A. Molecular, Immunomodulatory, and Histopathological Role of Mesenchymal Stem Cells and Beetroot Extract on Cisplatin Induced Testicular Damage in Albino Rats. Animals 2021, 11, 1142. [Google Scholar] [CrossRef] [PubMed]
- Mathai, C.K.; Beutler, E. Blood Preservatives for the Glutathione Stability Test. Nature 1965, 207, 204–205. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Kawada, K.; Shimada, T.; Mori, M. Lipid Peroxidation in Maternal and Cord Blood and Protective Mechanism against Activated-Oxygen Toxicity in the Blood. Am. J. Obstet. Gynecol. 1979, 135, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Haddadi, P.; Mahdavi, M.; Rahbarghazi, R. Down-Regulation of Bcl2 and Survivin, and up-Regulation of Bax Involved in Copper (II) Phenylthiosemicarbazone Complex-Induced Apoptosis in Leukemia Stem-like KG1a Cells. Process Biochem. 2020, 92, 190–196. [Google Scholar] [CrossRef]
- Taghizadeh, F.; Hosseinimehr, S.J.; Zargari, M.; Karimpour Malekshah, A.; Mirzaei, M.; Talebpour Amiri, F. Alleviation of Cisplatin-induced Hepatotoxicity by Gliclazide: Involvement of Oxidative Stress and Caspase-3 Activity. Pharm. Res. Perspect 2021, 9, e00788. [Google Scholar] [CrossRef]
- Francis, P.; Navarro, V.J. Drug Induced Hepatotoxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Mobasher, M.A.; Valverde, Á.M. Signaling pathways involved in paracetamol-induced hepatotoxicity: New insights on the role of protein tyrosine phosphatase 1B. Arch. Physiol. Biochem. 2014, 120, 51–63. [Google Scholar] [CrossRef]
- Kuna, L.; Božić, I.; Kizivat, T.; Bojanić, K.; Mršo, M.; Kralj, E.; Smolić, R.; Wu, G.Y.; Smolić, M. Models of Drug Induced Liver Injury (DILI)—Current Issues and Future Perspectives. Curr. Drug Metab. 2018, 19, 830–838. [Google Scholar] [CrossRef]
- Bilgic, Y.; Akbulut, S.; Aksungur, Z.; Erdemli, M.E.; Ozhan, O.; Parlakpinar, H.; Vardi, N.; Turkoz, Y. Protective Effect of Dexpanthenol against Cisplatin-Induced Hepatotoxicity. Exp. Ther. Med. 2018, 16, 4049–4057. [Google Scholar] [CrossRef]
- Ellabban, M.A.; Fattah, I.O.A.; Kader, G.A.; Eldeen, O.S.; Mehana, A.E.; Khodeer, D.M.; Hosny, H.; Elbasiouny, M.S.; Masadeh, S. The Effects of Sildenafil and/or Nitroglycerin on Random-Pattern Skin Flaps After Nicotine Application in Rats. Sci. Rep. 2020, 10, 3212. [Google Scholar] [CrossRef]
- Almaeen, A.H.; Alduraywish, A.A.; Mobasher, M.A.; Almadhi, O.I.M.; Nafeh, H.M.; El-Metwally, T.H. Oxidative stress, immunological and cellular hypoxia biomarkers in hepatitis C treatment-naïve and cirrhotic patients. Arch Med Sci. 2020, 17, 368–375. [Google Scholar] [CrossRef]
- El-Said, K.S.; Atta, A.; Mobasher, M.A.; Germoush, M.O.; Mohamed, T.M.; Salem, M.M. Quercetin mitigates rheumatoid arthritis by inhibiting adenosine deaminase in rats. Mol. Med. 2022, 28, 24. [Google Scholar] [CrossRef] [PubMed]
- Real, M.; Barnhill, M.S.; Higley, C.; Rosenberg, J.; Lewis, J.H. Drug-Induced Liver Injury: Highlights of the Recent Literature. Drug Saf. 2019, 42, 365–387. [Google Scholar] [CrossRef] [PubMed]
- Avrutsky, M.I.; Troy, C.M. Caspase-9: A Multimodal Therapeutic Target with Diverse Cellular Expression in Human Disease. Front. Pharmacol. 2021, 12, 1728. [Google Scholar] [CrossRef]
- Kuida, K. Caspase-9. Int. J. Biochem. Cell Biol. 2000, 32, 121–124. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Beshbishy, A.M.; El-Mleeh, A.; Abdel-Daim, M.M.; Devkota, H.P. Traditional Uses, Bioactive Chemical Constituents, and Pharmacological and Toxicological Activities of Glycyrrhiza Glabra L.(Fabaceae). Biomolecules 2020, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-C.; Lee, Y.-H.; Kim, S.H.; Kim, K.-J.; Kim, K.-M.; Oh, S.; Jung, Y.-S. Hepatoprotective Effect of Licorice, the Root of Glycyrrhiza Uralensis Fischer, in Alcohol-Induced Fatty Liver Disease. BMC Complement Altern. Med. 2016, 16, 19. [Google Scholar] [CrossRef]
- Aithal, A.P.; Bairy, L.K.; Seetharam, R.N.; Kumar, N. Hepatoprotective Effect of Bone Marrow-Derived Mesenchymal Stromal Cells in CCl4-Induced Liver Cirrhosis. 3 Biotech. 2021, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Kaur, H.; Dhindsa, A.S. Glycyrrhiza glabra: A phytopharmacological review. Int. J. Pharm. Sci. Res. 2013, 4, 2470. [Google Scholar]
- Vijayalakshmi, U.; Shourie, A. Evaluation of Different Methods for Extraction of Antioxidant Phenolic Compounds from Glycyrrhiza Glabra Roots. World J. Pharm. Res. 2015, 4, 1524–1537. [Google Scholar]
- Damle, M. Glycyrrhiza Glabra (Liquorice)—A Potent Medicinal Herb. Int. J. Herb. Med. 2014, 2, 132–136. [Google Scholar]
- El-Tahawy, N.F.; Ali, A.H.; Saied, S.R.; Abdel-Wahab, Z. Effect of Glycyrrhizin on Lipopolysaccharide/D-Galactosamine-Induced Acute Hepatitis in Albino Rats: A Histological and Immunohistochemical Study. Egypt. J. Histol. 2011, 34, 518–527. [Google Scholar] [CrossRef]
- Mohamed, H.K.; Mobasher, M.A.; Ebiya, R.A.; Hassen, M.T.; Hagag, H.M.; El-Sayed, R.; Abdel-Ghany, S.; Said, M.M.; Awad, N.S. Anti-Inflammatory, Anti-Apoptotic, and Antioxidant Roles of Honey, Royal Jelly, and Propolis in Suppressing Nephrotoxicity Induced by Doxorubicin in Male Albino Rats. Antioxidants 2022, 11, 1029. [Google Scholar] [CrossRef]
- Li, J.; Cao, H.; Liu, P.; Cheng, G.; Sun, M. Glycyrrhizic Acid in the Treatment of Liver Diseases: Literature Review. Biomed. Res. Int. 2014, 2014, 872139. [Google Scholar] [CrossRef]
- Honda, H.; Nagai, Y.; Matsunaga, T.; Saitoh, S.-I.; Akashi-Takamura, S.; Hayashi, H.; Fujii, I.; Miyake, K.; Muraguchi, A.; Takatsu, K. Glycyrrhizin and Isoliquiritigenin Suppress the LPS Sensor Toll-like Receptor 4/MD-2 Complex Signaling in a Different Manner. J. Leukoc. Biol. 2012, 91, 967–976. [Google Scholar] [CrossRef]
- Schröfelbauer, B.; Raffetseder, J.; Hauner, M.; Wolkerstorfer, A.; Ernst, W.; Szolar, O.H.J. Glycyrrhizin, the Main Active Compound in Liquorice, Attenuates pro-Inflammatory Responses by Interfering with Membrane-Dependent Receptor Signalling. Biochem. J. 2009, 421, 473–482. [Google Scholar] [CrossRef]
- Tu, J.-H.; He, Y.-J.; Chen, Y.; Fan, L.; Zhang, W.; Tan, Z.-R.; Huang, Y.-F.; Guo, D.; Hu, D.-L.; Wang, D.; et al. Effect of Glycyrrhizin on the Activity of CYP3A Enzyme in Humans. Eur. J. Clin. Pharmacol. 2010, 66, 805–810. [Google Scholar] [CrossRef]
- Feng, J.; Yao, W.; Zhang, Y.; Xiang, A.P.; Yuan, D.; Hei, Z. Intravenous Anesthetics Enhance the Ability of Human Bone Marrow-Derived Mesenchymal Stem Cells to Alleviate Hepatic Ischemia-Reperfusion Injury in a Receptor-Dependent Manner. Cell Physiol. Biochem. 2018, 47, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Eom, Y.W.; Shim, K.Y.; Baik, S.K. Mesenchymal Stem Cell Therapy for Liver Fibrosis. Korean J. Intern. Med. 2015, 30, 580–589. [Google Scholar] [CrossRef]
- Gazdic, M.; Arsenijevic, A.; Markovic, B.S.; Volarevic, A.; Dimova, I.; Djonov, V.; Arsenijevic, N.; Stojkovic, M.; Volarevic, V. Mesenchymal Stem Cell-Dependent Modulation of Liver Diseases. Int. J. Biol. Sci. 2017, 13, 1109–1117. [Google Scholar] [CrossRef]
- Sakaida, I.; Terai, S.; Yamamoto, N.; Aoyama, K.; Ishikawa, T.; Nishina, H.; Okita, K. Transplantation of Bone Marrow Cells Reduces CCl4-Induced Liver Fibrosis in Mice. Hepatology 2004, 40, 1304–1311. [Google Scholar] [CrossRef]
- Efimenko, A.; Starostina, E.; Kalinina, N.; Stolzing, A. Angiogenic Properties of Aged Adipose Derived Mesenchymal Stem Cells after Hypoxic Conditioning. J. Transl. Med. 2011, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.; Yeganeh, M.; Ramanathan, S.; Leblanc, C.; Pomerleau, V.; Ferbeyre, G.; Saucier, C.; Ilangumaran, S. SOCS1 Controls Liver Regeneration by Regulating HGF Signaling in Hepatocytes. J. Hepatol. 2011, 55, 1300–1308. [Google Scholar] [CrossRef]
- Galland, S.; Stamenkovic, I. Mesenchymal Stromal Cells in Cancer: A Review of Their Immunomodulatory Functions and Dual Effects on Tumor Progression. J. Pathol. 2020, 250, 555–572. [Google Scholar] [CrossRef]
- Miceli, V.; Bulati, M.; Iannolo, G.; Zito, G.; Gallo, A.; Conaldi, P.G. Therapeutic Properties of Mesenchymal Stromal/Stem Cells: The Need of Cell Priming for Cell-Free Therapies in Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 763. [Google Scholar] [CrossRef] [PubMed]
- Aithal, A.P.; Bairy, L.K.; Seetharam, R.N. Safety Assessment of Human Bone Marrow-Derived Mesenchymal Stromal Cells Transplantation in Wistar Rats. J. Clin. Diagn. Res. 2017, 11, FF01–FF03. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Ho, T.-Y.; Lee, E.-J.; Su, S.-Y.; Tang, N.-Y.; Hsieh, C.-L. Ferulic Acid Reduces Cerebral Infarct through Its Antioxidative and Anti-Inflammatory Effects Following Transient Focal Cerebral Ischemia in Rats. Am. J. Chin. Med. 2008, 36, 1105–1119. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Deng, Z.; Lai, X.; Tu, W. Differentiation of Human Bone Marrow Stromal Cells into Neural-like Cells Induced by Sodium Ferulate in Vitro. Cell Mol. Immunol. 2005, 2, 225–229. [Google Scholar] [PubMed]
- Chen, J.; Zhang, Z.G.; Li, Y.; Wang, L.; Xu, Y.X.; Gautam, S.C.; Lu, M.; Zhu, Z.; Chopp, M. Intravenous Administration of Human Bone Marrow Stromal Cells Induces Angiogenesis in the Ischemic Boundary Zone after Stroke in Rats. Circ. Res. 2003, 92, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Zhang, R.; Katakowski, M.; Gautam, S.C.; Xu, Y.; Lu, M.; Zhang, Z.; Chopp, M. Combination Therapy of Stroke in Rats with a Nitric Oxide Donor and Human Bone Marrow Stromal Cells Enhances Angiogenesis and Neurogenesis. Brain Res. 2004, 1005, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Gabryel, B.; Trzeciak, H.I. Role of Astrocytes in Pathogenesis of Ischemic Brain Injury. Neurotox. Res. 2001, 3, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Kowiański, P.; Lietzau, G.; Steliga, A.; Waśkow, M.; Moryś, J. The Astrocytic Contribution to Neurovascular Coupling--Still More Questions than Answers? Neurosci. Res. 2013, 75, 171–183. [Google Scholar] [CrossRef]
- Girouard, H.; Iadecola, C. Neurovascular Coupling in the Normal Brain and in Hypertension, Stroke, and Alzheimer Disease. J. Appl. Physiol. 2006, 100, 328–335. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).