In Vitro Activity of Essential Oils from Piper Species (Piperaceae) against Tachyzoites of Toxoplasma gondii
Abstract
1. Introduction
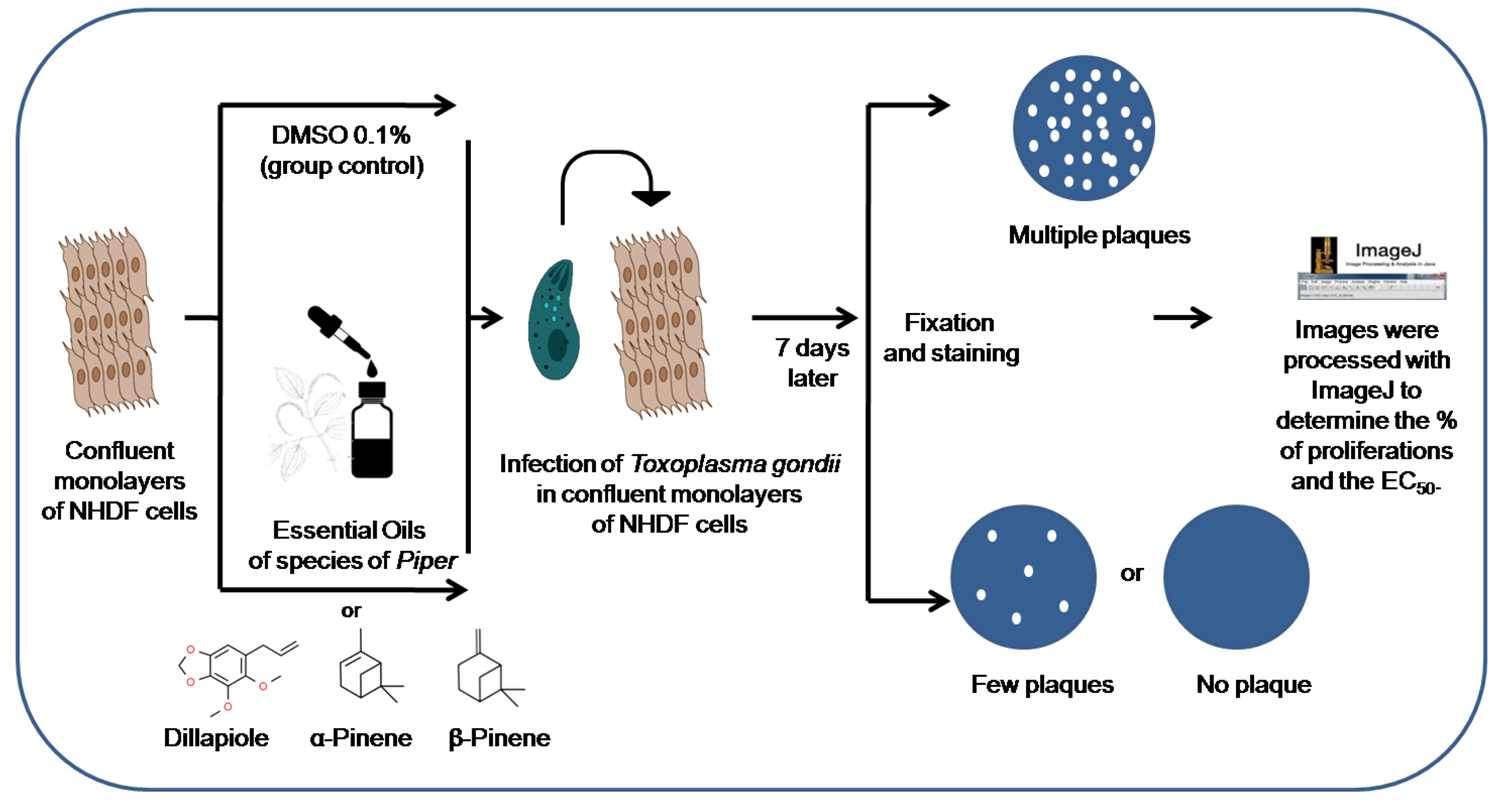
2. Materials and Methods
2.1. Parasites
2.2. Plant Materials and Extraction of Essential Oils
2.3. Antiproliferative RH Strain Tachyzoites Delayed-Death Assays
2.4. Invasion Assay of NHDF Cells by T. gondii Tachyzoites
2.5. Host Cell Viability
2.6. Statistical Analysis
3. Results
3.1. Chemical Composition of Essential Oils (EO)
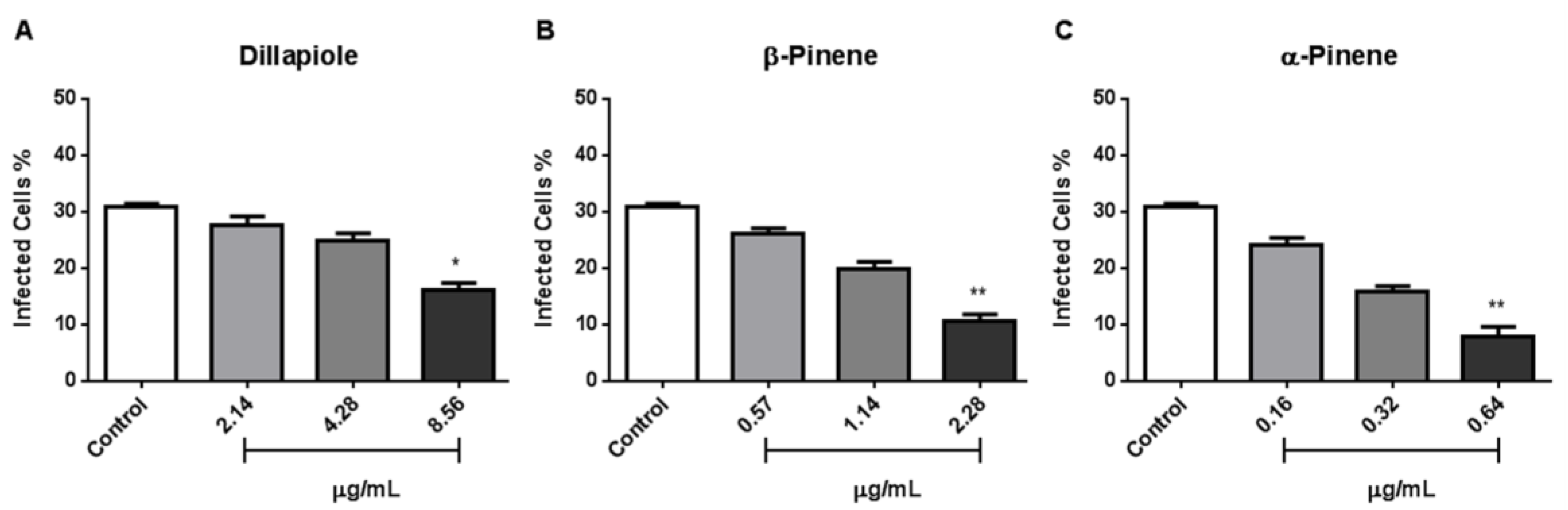
3.2. In Vitro Antiproliferative Effect of Piper EOs and Major Compounds on RH Strain Tachyzoites
3.3. Cytotoxicity Evaluation of Piper EOs and Major Compounds in NHDF Cells
3.4. Evaluation of the Effect of Major Compounds on the Invasion of NHDF Cells by T. gondii Tachyzoites of the RH Strain
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daher, D.; Shaghlil, A.; Sobh, E.; Hamie, M.; Hassan, M.E.; Moumneh, M.B.; Itani, S.; El Hajj, R.; Tawk, L.; El Sabban, M.; et al. Comprehensive Overview of Toxoplasma Gondii-Induced and Associated Diseases. Pathogens 2021, 10, 1351. [Google Scholar] [CrossRef]
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice. Clin. Microbiol. Rev. 2018, 31, e00057-17. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.A.; Longcore, T.; Barbieri, M.; Dabritz, H.; Hill, D.; Klein, P.N.; Lepczyk, C.; Lilly, E.L.; McLeod, R.; Milcarsky, J.; et al. The One Health Approach to Toxoplasmosis: Epidemiology, Control, and Prevention Strategies. EcoHealth 2019, 16, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Robert-Gangneux, F.; Dardé, M.-L. Epidemiology of and Diagnostic Strategies for Toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.-X.; Wei, S.-S.; Lindsay, D.S.; Peng, H.-J. A Systematic Review and Meta-Analysis of the Efficacy of Anti-Toxoplasma Gondii Medicines in Humans. PLoS ONE 2015, 10, e0138204. [Google Scholar] [CrossRef]
- Ben-Harari, R.R.; Goodwin, E.; Casoy, J. Adverse Event Profile of Pyrimethamine-Based Therapy in Toxoplasmosis: A Systematic Review. Drugs RD 2017, 17, 523–544. [Google Scholar] [CrossRef] [PubMed]
- Teil, J.; Dupont, D.; Charpiat, B.; Corvaisier, S.; Vial, T.; Leboucher, G.; Wallon, M.; Peyron, F. Treatment of Congenital Toxoplasmosis: Safety of the Sulfadoxine-Pyrimethamine Combination in Children Based on a Method of Causality Assessment. Pediatr. Infect. Dis. J. 2016, 35, 634–638. [Google Scholar] [CrossRef]
- Ovung, A.; Bhattacharyya, J. Sulfonamide Drugs: Structure, Antibacterial Property, Toxicity, and Biophysical Interactions. Biophys. Rev. 2021, 13, 259–272. [Google Scholar] [CrossRef]
- Silva, L.A.; Fernandes, M.D.; Machado, A.S.; Reis-Cunha, J.L.; Bartholomeu, D.C.; Almeida Vitor, R.W. Efficacy of Sulfadiazine and Pyrimetamine for Treatment of Experimental Toxoplasmosis with Strains Obtained from Human Cases of Congenital Disease in Brazil. Exp. Parasitol. 2019, 202, 7–14. [Google Scholar] [CrossRef]
- Oliveira, C.B.; Meurer, Y.S.; Andrade, J.M.; Costa, M.E.; Andrade, M.M.; Silva, L.A.; Lanza, D.C.; Vítor, R.W.; Andrade-Neto, V.F. Pathogenicity and Phenotypic Sulfadiazine Resistance of Toxoplasma Gondii Isolates Obtained from Livestock in Northeastern Brazil. Mem. Inst. Oswaldo Cruz 2016, 111, 391–398. [Google Scholar] [CrossRef]
- Montazeri, M.; Mehrzadi, S.; Sharif, M.; Sarvi, S.; Tanzifi, A.; Aghayan, S.A.; Daryani, A. Drug Resistance in Toxoplasma Gondii. Front. Microbiol. 2018, 9, 2587. [Google Scholar] [CrossRef] [PubMed]
- Villamizar, L.H.; Cardoso, M.D.G.; Andrade, J.D.; Teixeira, M.L.; Soares, M.J. Linalool, a Piper Aduncum Essential Oil Component, Has Selective Activity against Trypanosoma Cruzi Trypomastigote Forms at 4 °C. Mem. Inst. Oswaldo Cruz 2017, 112, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Esperandim, V.; da Silva Ferreira, D.; Sousa Rezende, K.; Magalhães, L.; Medeiros Souza, J.; Pauletti, P.; Januário, A.; da Silva de Laurentz, R.; Bastos, J.; Símaro, G.; et al. In Vitro Antiparasitic Activity and Chemical Composition of the Essential Oil Obtained from the Fruits of Piper Cubeba. Planta Med. 2013, 79, 1653–1655. [Google Scholar] [CrossRef] [PubMed]
- Ceole, L.F.; Cardoso, M.D.G.; Soares, M.J. Nerolidol, the Main Constituent of Piper Aduncum Essential Oil, Has Anti- Leishmania Braziliensis Activity. Parasitology 2017, 144, 1179–1190. [Google Scholar] [CrossRef]
- Moura do Carmo, D.F.; Amaral, A.C.F.; Machado, G.M.C.; Leon, L.L.; Silva, J.R.D.A. Chemical and Biological Analyses of the Essential Oils and Main Constituents of Piper Species. Molecules 2012, 17, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Houël, E.; Gonzalez, G.; Bessière, J.-M.; Odonne, G.; Eparvier, V.; Deharo, E.; Stien, D. Therapeutic Switching: From Antidermatophytic Essential Oils to New Leishmanicidal Products. Mem. Inst. Oswaldo Cruz 2015, 110, 106–113. [Google Scholar] [CrossRef]
- Varela, M.T.; Lima, M.L.; Galuppo, M.K.; Tempone, A.G.; de Oliveira, A.; Lago, J.H.G.; Fernandes, J.P.S. New Alkenyl Derivative from Piper Malacophyllum and Analogues: Antiparasitic Activity against Trypanosoma Cruzi and Leishmania Infantum. Chem. Biol. Drug Des. 2017, 90, 1007–1011. [Google Scholar] [CrossRef]
- Leesombun, A.; Boonmasawai, S.; Shimoda, N.; Nishikawa, Y. Effects of Extracts from Thai Piperaceae Plants against Infection with Toxoplasma Gondii. PLoS ONE 2016, 11, e0156116. [Google Scholar] [CrossRef]
- Aziz, Z.A.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential Oils: Extraction Techniques, Pharmaceutical And Therapeutic Potential—A Review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef]
- Fanela, T.L.M.; Baldin, E.L.L.; Pannuti, L.E.R.; Cruz, P.L.; Crotti, A.E.M.; Takeara, R.; Kato, M.J. Lethal and Inhibitory Activities of Plant-Derived Essential Oils against Bemisia Tabaci Gennadius (Hemiptera: Aleyrodidae) Biotype B in Tomato. Neotrop. Entomol. 2016, 45, 201–210. [Google Scholar] [CrossRef]
- Pereira Filho, A.A.; Pessoa, G.C.D.; Yamaguchi, L.F.; Stanton, M.A.; Serravite, A.M.; Pereira, R.H.M.; Neves, W.S.; Kato, M.J. Larvicidal Activity of Essential Oils From Piper Species against Strains of Aedes Aegypti (Diptera: Culicidae) Resistant to Pyrethroids. Front. Plant Sci. 2021, 12, 685864. [Google Scholar] [CrossRef] [PubMed]
- Martins-Duarte, E.S.; Portes, J.D.A.; da Silva, R.B.; Pires, H.S.; Garden, S.J.; de Souza, W. In Vitro Activity of N-Phenyl-1,10-Phenanthroline-2-Amines against Tachyzoites and Bradyzoites of Toxoplasma Gondii. Bioorg. Med. Chem. 2021, 50, 116467. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, Y.; Montes, R.; Scull, R.; Sánchez, A.; Cos, P.; Monzote, L.; Setzer, W.N. Chemodiversity Associated with Cytotoxicity and Antimicrobial Activity of Piper Aduncum Var. Ossanum. Chem. Biodivers. 2016, 13, 1715–1719. [Google Scholar] [CrossRef]
- Nunes, T.A.L.; Costa, L.H.; De Sousa, J.M.S.; De Souza, V.M.R.; Rodrigues, R.R.L.; Val, M.D.C.A.; da Cunha Pereira, A.C.T.; Ferreira, G.P.; Da Silva, M.V.; Da Costa, J.M.A.R.; et al. Eugenia Piauhiensis Vellaff. Essential Oil and γ-Elemene Its Major Constituent Exhibit Antileishmanial Activity, Promoting Cell Membrane Damage and in Vitro Immunomodulation. Chem. Biol. Interact. 2021, 339, 109429. [Google Scholar] [CrossRef]
- Mammari, N.; Halabi, M.A.; Yaacoub, S.; Chlala, H.; Dardé, M.-L.; Courtioux, B. Toxoplasma Gondii Modulates the Host Cell Responses: An Overview of Apoptosis Pathways. BioMed Res. Int. 2019, 2019, 6152489. [Google Scholar] [CrossRef]
- Sanchez, S.G.; Besteiro, S. The Pathogenicity and Virulence of Toxoplasma Gondii. Virulence 2021, 12, 3095–3114. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.C.; de Souza, G.; Borges, B.C.; de Araújo, T.E.; Rosini, A.M.; Aguila, F.A.; Ambrósio, S.R.; Veneziani, R.C.S.; Bastos, J.K.; Silva, M.J.B.; et al. Copaifera Spp. Oleoresins Impair Toxoplasma Gondii Infection in Both Human Trophoblastic Cells and Human Placental Explants. Sci. Rep. 2020, 10, 15158. [Google Scholar] [CrossRef]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of In-Vitro Bioassay Methods: Application in Herbal Drug Research. In Profiles of Drug Substances, Excipients and Related Methodology; Elsevier: Cambridge, MA, USA; Academic Press: Cambridge, MA, USA, 2021; Volume 46, pp. 273–307. ISBN 978-0-12-824127-1. [Google Scholar]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Krivogorsky, B.; Grundt, P.; Yolken, R.; Jones-Brando, L. Inhibition of Toxoplasma Gondii by Indirubin and Tryptanthrin Analogs. Antimicrob. Agents Chemother. 2008, 52, 4466–4469. [Google Scholar] [CrossRef]
- Evans, S.M.; Casartelli, A.; Herreros, E.; Minnick, D.T.; Day, C.; George, E.; Westmoreland, C. Development of a High Throughput in Vitro Toxicity Screen Predictive of High Acute in Vivo Toxic Potential. Toxicol. In Vitro 2001, 15, 579–584. [Google Scholar] [CrossRef]
- Nwaka, S.; Hudson, A. Innovative Lead Discovery Strategies for Tropical Diseases. Nat. Rev. Drug Discov. 2006, 5, 941–955. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; García, M.; Montalvo, A.M.; Scull, R.; Miranda, M. Chemistry, Cytotoxicity and Antileishmanial Activity of the Essential Oil from Piper Auritum. Mem. Inst. Oswaldo Cruz 2010, 105, 168–173. [Google Scholar] [CrossRef] [PubMed]


| Compounds | RIa | RIb | PAD | PLD | PCE |
|---|---|---|---|---|---|
| α-Pinene S | 932 | 932 | 0.2 | 61.7 | 16.6 |
| Camphene S | 947 | 946 | - | 1.7 | 0.1 |
| β-Pinene S | 975 | 974 | 0.3 | 1.4 | 11.5 |
| β-Myrcene S | 992 | 988 | - | 0.3 | 1.0 |
| α-Phellandrene S | 1004 | 1002 | 0.1 | - | 0.2 |
| 2-Carene S | 1010 | 1008 | 0.1 | - | 0.2 |
| α-Terpinene | 1016 | 1014 | - | - | 4.5 |
| p-Cymene S | 1024 | 1020 | 0.1 | 1.0 | 9.2 |
| Limonene S | 1039 | 1024 | 0.1 | 5.3 | 0.8 |
| (Z)-β-Ocimene S | 1039 | 1032 | 1.6 | - | 0.1 |
| (E)-β-Ocimene S | 1049 | 1044 | 3.4 | - | 0.3 |
| γ-Terpinene | 1059 | 1054 | 0.2 | - | 9.9 |
| α-Terpinolene S | 1088 | 1086 | 0.4 | - | 2.7 |
| Linalool S | 1100 | 1095 | - | 1.6 | - |
| (E)-4,8-Dimethyl-1,3,7-nonatriene (DMNT) S | 1117 | 1114 | - | - | 0.3 |
| Camphor S | 1144 | 1141 | - | 1.1 | - |
| Terpinen-4-ol | 1178 | 1174 | - | 0.1 | 0.3 |
| α-Terpineole | 1191 | 1186 | - | 2.3 | 0.3 |
| Oxygenated monoterpene * | 1209 | - | 0.1 | 0.1 | - |
| (+)-Piperitone | 1255 | 1249 | 0.7 | - | - |
| δ-Elemene | 1339 | 1335 | 0.1 | 1.1 | 0.4 |
| α-Cubebene | 1352 | 1345 | - | 0.1 | 0.3 |
| α-Ylangene | 1374 | 1373 | 0.1 | - | - |
| α-Copaene S | 1378 | 1374 | 0.2 | 6.4 | 2.1 |
| β-Bourbonene | 1387 | 1387 | - | 0.2 | 0.6 |
| β-Elemene | 1394 | 1389 | 0.2 | 0.3 | 4.4 |
| α-Gurjunene S | 1412 | 1409 | 0.1 | - | 0.1 |
| (E)-β-Caryophyllene S | 1422 | 1417 | 0.8 | 0.5 | 7.0 |
| β-Gurjunene | 1432 | 1431 | 0.2 | 0.6 | 0.5 |
| (+)-Aromadendrene S | 1442 | 1439 | - | 0.2 | 0.4 |
| α-Humulene S | 1457 | 1452 | 0.9 | 0.2 | 2.1 |
| (-)-Alloaromadendrene S | 1464 | 1458 | - | 0.9 | 0.1 |
| Dehydro-aromadendrane | 1466 | 1460 | - | 0.9 | - |
| γ-Muurolene | 1480 | 1479 | - | 1.3 | 0.5 |
| Germacrene D | 1484 | 1481 | 2.7 | - | 5.2 |
| β-Selinene | 1490 | 1490 | - | 0.3 | 0.5 |
| α-Selinene | 1500 | 1498 | 1.4 | 0.1 | - |
| Bicyclogermacrene | 1500 | 1500 | 2.3 | - | 10.7 |
| α-Muurolene | 1503 | 1500 | 0.1 | 1.0 | - |
| α-Bulnesene | 1510 | 1509 | 0.2 | - | 0.7 |
| γ-Cadinene | 1517 | 1513 | 0.1 | 1.2 | 0.4 |
| δ-Cadinene | 1522 | 1522 | 1.2 | - | - |
| (E)-Cadina-1.4-diene | 1527 | 1533 | - | 1.3 | 0.3 |
| Germacrene B | 1561 | 1559 | 0.2 | - | 0.1 |
| (E)-Nerolidol S | 1566 | 1561 | 0.1 | - | 0.9 |
| Palustrol | 1572 | 1567 | - | - | 0.1 |
| Spathulenol | 1581 | 1577 | 0.1 | 0.3 | 0.7 |
| (-)-Caryophyllene oxide S | 1587 | 1582 | - | 3.4 | 0.8 |
| Veridiflorol | 1596 | 1592 | 0.3 | 0.5 | 0.6 |
| β-Asarone | 1623 | 1616 | - | 0.3 | - |
| Dillapiole S | 1632 | 1620 | 81.0 | - | 0.1 |
| epi-α-Muurolol | 1646 | 1640 | 0.3 | 0.6 | 0.6 |
| Torreyol | 1650 | 1644 | - | 0.3 | 0.4 |
| α-Cadinol | 1659 | 1652 | - | 0.9 | 1.2 |
| Apiole S | 1686 | 1677 | 0.2 | - | - |
| EO/Compound | EC50 (95% Confidence Intervals) µg/mL | R |
|---|---|---|
| Piper aduncum | 1.749 (1.498 to 2.043) | 0.9807 |
| P. cernuum | 3.687 (2.190 to 6.208) | 0.7144 |
| P. lindbergii | 0.839 (0.6492 to 1.084) | 0.9358 |
| Dillapiole | 4.287 (3.511 to 5.234) | 0.9331 |
| β-Pinene | 1.145 (1.008 to 1.300) | 0.9836 |
| α-Pinene | 0.326 (0.295 to 0.360) | 0.9805 |
| EO/Compound | CC50 (95% Confidence Intervals) µg/mL | R | SI |
|---|---|---|---|
| Piper aduncum | 169.8 (135.6 to 212.8) | 0.8074 | 97 |
| Piper cernuum | 172.1 (125.6 to 235.7) | 0.6826 | 46 |
| Piper lindbergii | 83.80 (75.42 to 91.34) | 0.9998 | 99 |
| Dillapiole | 210.8 (194.6 to 228.3) | 0.9906 | 49 |
| β-Pinene | 70.78 (53.22 to 94.12) | 0.9682 | 61 |
| α-Pinene | 41.37 (37.64 to 45.09) | 0.9991 | 126 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira Filho, A.A.; Cunha, M.M.; Alves Stanton, M.; Fumiko Yamaguchi, L.; Jorge Kato, M.; Martins-Duarte, É.S. In Vitro Activity of Essential Oils from Piper Species (Piperaceae) against Tachyzoites of Toxoplasma gondii. Metabolites 2023, 13, 95. https://doi.org/10.3390/metabo13010095
Pereira Filho AA, Cunha MM, Alves Stanton M, Fumiko Yamaguchi L, Jorge Kato M, Martins-Duarte ÉS. In Vitro Activity of Essential Oils from Piper Species (Piperaceae) against Tachyzoites of Toxoplasma gondii. Metabolites. 2023; 13(1):95. https://doi.org/10.3390/metabo13010095
Chicago/Turabian StylePereira Filho, Adalberto Alves, Mariana Maciel Cunha, Mariana Alves Stanton, Lydia Fumiko Yamaguchi, Massuo Jorge Kato, and Érica S. Martins-Duarte. 2023. "In Vitro Activity of Essential Oils from Piper Species (Piperaceae) against Tachyzoites of Toxoplasma gondii" Metabolites 13, no. 1: 95. https://doi.org/10.3390/metabo13010095
APA StylePereira Filho, A. A., Cunha, M. M., Alves Stanton, M., Fumiko Yamaguchi, L., Jorge Kato, M., & Martins-Duarte, É. S. (2023). In Vitro Activity of Essential Oils from Piper Species (Piperaceae) against Tachyzoites of Toxoplasma gondii. Metabolites, 13(1), 95. https://doi.org/10.3390/metabo13010095







