Metabolic Changes and Their Associations with Selected Nutrients Intake in the Group of Workers Exposed to Arsenic
Abstract
1. Introduction
2. Materials and Methods
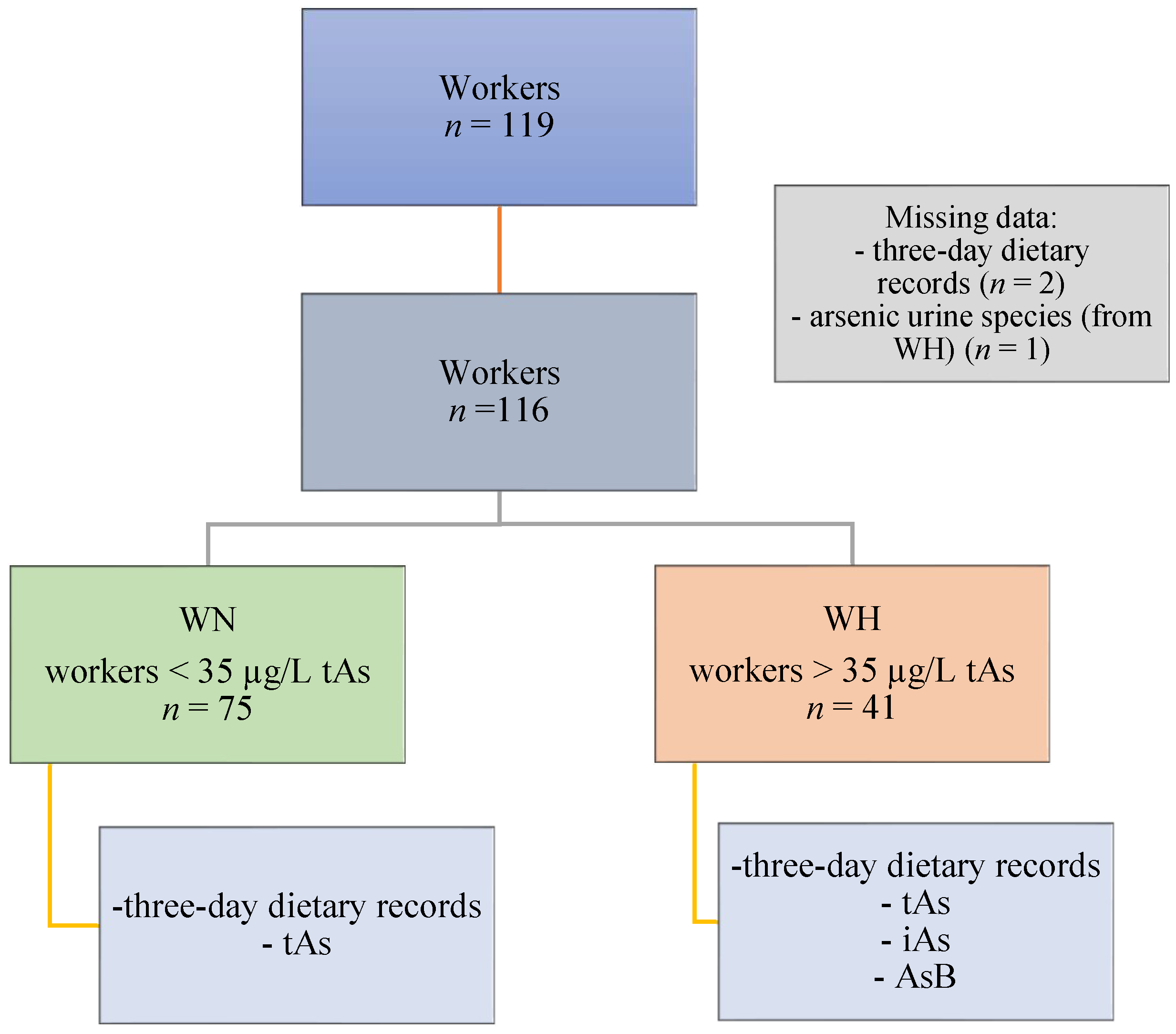
2.1. Study Participants and Design
2.2. Urine Collection and As Analysis
2.3. Diet Assessment
2.4. Sample Preparations for Untargeted Metabolomics
2.5. Metabolomics Analysis
2.6. Bioinformatics and Statistical Analysis
3. Results
3.1. General Characteristic of the Workers
3.2. Differences in Metabolic Profile beetwen WN and WH
3.3. Relationship between Dietary Nutrients Intake Involved in iAs Metabolism and Signal Intensity of Putatively Anotated Metabolites
4. Discussion
4.1. Urinary Metabolomics
4.2. Association between Intake of Nutrients Involved in iAs Metabolism and Putatively Annotated Metabolites
4.3. Strengths and Limitations
- The determination of urinary tAs concentration using well-developed methods;
- The analysis of several nutrients involved in As metabolism;
- Comprehensive analysis of the metabolic profile of workers exposed to iAs (not only urinary As metabolites), which allows for a deeper understanding of the mechanisms that occur during exposure;
- First study to combine the amount of nutrient intake and metabolomics data, which may fill the research gap and provide a direction for further research.
- This study only included men; thus, the results cannot be generalized to the entire population;
- The analysis solely concerned exposure to iAs, without considering the exposure to other compounds that could have influenced the results;
- The disadvantages related to 3-day dietary records hampered the acquirement of certain findings, and include: an underestimation of intake; the failure to account for the seasonality of intake; possible differences between the 3-day records and typical consumption; the fact that the analysis of the consumption of nutrients was based solely on diet, not including dietary supplements (48.3% of the respondents declared their use); and the consumption of rice, seafood, and fish, which may have interfered with the results due to their high As content (however, only 22.4% declared consuming fish in the last 48 h);
- Only one urine sample was taken from each participant (no multiple measurements/serial exposure data);
- Urinary concentrations of DMA and MMA were not determined.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medunić, G.; Fiket, Ž.; Ivanić, M. Arsenic contamination status in Europe, Australia, and other parts of the world. In Arsenic in Drinking Water and Food; Srivastava, S., Ed.; Springer: Singapore, 2020; pp. 183–233. [Google Scholar] [CrossRef]
- Baker, B.A.; Cassano, V.A.; Murray, C. Arsenic Exposure, Assessment, Toxicity, Diagnosis, and Management: Guidance for Occupational and Environmental Physicians. J. Occup. Environ. Med. 2018, 60, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Arsenic; Agency for Toxic Substances and Disease Registry, Division of Toxicology and Human Health Sciences: Atlanta, GA, USA, 2007; pp. 1–559.
- Xi, S.; Zheng, Q.; Zhang, Q.; Sun, G. Metabolic Profile and Assessment of Occupational Arsenic Exposure in Copper- and Steel-Smelting Workers in China. Int. Arch. Occup. Environ. Health 2011, 84, 347–353. [Google Scholar] [CrossRef]
- Halatek, T.; Sinczuk-Walczak, H.; Janasik, B.; Trzcinka-Ochocka, M.; Winnicka, R.; Wasowicz, W. Health Effects and Arsenic Species in Urine of Copper Smelter Workers. J. Environ. Sci. Health Part A 2014, 49, 787–797. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Exposure to arsenic: A major public health concern. In Preventing Disease through Healthy Environments; Department of Public Health, Environmental and Social Determinants of Health, World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Styblo, M.; Del Razo, L.M.; Vega, L.; Germolec, D.R.; LeCluyse, E.L.; Hamilton, G.A.; Reed, W.; Wang, C.; Cullen, W.R.; Thomas, D.J. Comparative Toxicity of Trivalent and Pentavalent Inorganic and Methylated Arsenicals in Rat and Human Cells. Arch. Toxicol. 2000, 74, 289–299. [Google Scholar] [CrossRef]
- Wang, W.; Xie, Z.; Lin, Y.; Zhang, D. Association of Inorganic Arsenic Exposure with Type 2 Diabetes Mellitus: A Meta-Analysis. J. Epidemiol. Community Health 2014, 68, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.A.; Oberoi, S.; Barchowsky, A.; Chen, Y.; Guallar, E.; Nachman, K.E.; Rahman, M.; Sohel, N.; D’Ippoliti, D.; Wade, T.J.; et al. A Dose-Response Meta-Analysis of Chronic Arsenic Exposure and Incident Cardiovascular Disease. Int. J. Epidemiol. 2017, 46, 1924–1939. [Google Scholar] [CrossRef] [PubMed]
- Jalili, C.; Kazemi, M.; Cheng, H.; Mohammadi, H.; Babaei, A.; Taheri, E.; Moradi, S. Associations between Exposure to Heavy Metals and the Risk of Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Crit. Rev. Toxicol. 2021, 51, 165–182. [Google Scholar] [CrossRef]
- Escudero-Lourdes, C. Toxicity Mechanisms of Arsenic That Are Shared with Neurodegenerative Diseases and Cognitive Impairment: Role of Oxidative Stress and Inflammatory Responses. NeuroToxicology 2016, 53, 223–235. [Google Scholar] [CrossRef]
- Rodríguez-Barranco, M.; Lacasaña, M.; Aguilar-Garduño, C.; Alguacil, J.; Gil, F.; González-Alzaga, B.; Rojas-García, A. Association of Arsenic, Cadmium and Manganese Exposure with Neurodevelopment and Behavioural Disorders in Children: A Systematic Review and Meta-Analysis. Sci. Total Environ. 2013, 454–455, 562–577. [Google Scholar] [CrossRef]
- Nunzio, A.D.; Giarra, A.; Toscanesi, M.; Amoresano, A.; Piscopo, M.; Ceretti, E.; Zani, C.; Lorenzetti, S.; Trifuoggi, M.; Montano, L. Comparison between Macro and Trace Element Concentrations in Human Semen and Blood Serum in Highly Polluted Areas in Italy. Int. J. Environ. Res. Public Health 2022, 19, 11635. [Google Scholar] [CrossRef]
- International Agency for Research Cancer (IARC). Arsenic, Metals, Fibres, and Dusts. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 100 C.; IARC: Lyon, France, 2012; pp. 41–93. ISBN 978-92-832-1320-8. [Google Scholar]
- Thomas, D.J.; Li, J.; Waters, S.B.; Xing, W.; Adair, B.M.; Drobna, Z.; Devesa, V.; Styblo, M. Arsenic (+3 Oxidation State) Methyltransferase and the Methylation of Arsenicals. Exp. Biol. Med. Maywood NJ 2007, 232, 3–13. [Google Scholar]
- Vahter, M. Mechanisms of Arsenic Biotransformation. Toxicology 2002, 181–182, 211–217. [Google Scholar] [CrossRef]
- Wu, M.M.; Chiou, H.Y.; Hsueh, Y.M.; Hong, C.T.; Su, C.L.; Chang, S.-F.; Huang, W.-L.; Wang, H.-T.; Wang, Y.-H.; Hsieh, Y.-C.; et al. Effect of Plasma Homocysteine Level and Urinary Monomethylarsonic Acid on the Risk of Arsenic-Associated Carotid Atherosclerosis. Toxicol. Appl. Pharmacol. 2006, 216, 168–175. [Google Scholar] [CrossRef]
- Pu, Y.S.; Yang, S.M.; Huang, Y.K.; Chung, C.J.; Huang, S.K.; Chiu, A.W.H.; Yang, M.H.; Chen, C.J.; Hsueh, Y.M. Urinary Arsenic Profile Affects the Risk of Urothelial Carcinoma Even at Low Arsenic Exposure. Toxicol. Appl. Pharmacol. 2007, 218, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Chen, Y.; Kibriya, M.G.; Slavkovich, V.; Parvez, F.; Jasmine, F.; Gamble, M.V.; Graziano, J.H. Arsenic Metabolism, Genetic Susceptibility, and Risk of Premalignant Skin Lesions in Bangladesh. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Sijko, M.; Kozłowska, L. Influence of Dietary Compounds on Arsenic Metabolism and Toxicity. Part I—Animal Model Studies. Toxics 2021, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Sijko, M.; Kozłowska, L. Influence of Dietary Compounds on Arsenic Metabolism and Toxicity. Part II—Human Studies. Toxics 2021, 9, 259. [Google Scholar] [CrossRef]
- American Conference of Governmental Industrial Hygienists. TLV® and BEIs® Based on the Documentation of the Threshold Limit Values for Chemical Substances and Physical Agents & Biological Exposure Indices; ACGIH: Cincinnati, OH, USA, 2014. [Google Scholar]
- Janasik, B.; Reszka, E.; Stanislawska, M.; Wieczorek, E.; Fendler, W.; Wasowicz, W. Biological Monitoring and the Influence of Genetic Polymorphism of As3MT and GSTs on Distribution of Urinary Arsenic Species in Occupational Exposure Workers. Int. Arch. Occup. Environ. Health 2015, 88, 807–818. [Google Scholar] [CrossRef]
- Szponar, L.; Wolnicka, K.; Rychlik, E. Album fotografii produktów i potraw; Album of Photographs of Food Products and Dishes; National Food and Nutrition Institute: Warsaw, Poland, 2000; ISBN 83-86060-51-4. [Google Scholar]
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Normy Żywienia dla Populacji Polski i ich Zastosowanie; Nutrition Standards for the Polish Population; National Food and Nutrition Institute: Warsaw, Poland, 2020. [Google Scholar]
- Southam, A.D.; Haglington, L.D.; Najdekr, L.; Jankevics, A.; Weber, R.J.M.; Dunn, W.B. Assessment of Human Plasma and Urine Sample Preparation for Reproducible and High-Throughput UHPLC-MS Clinical Metabolic Phenotyping. Analyst 2020, 145, 6511–6523. [Google Scholar] [CrossRef]
- Kozłowska, L.; Santonen, T.; Duca, R.C.; Godderis, L.; Jagiello, K.; Janasik, B.; Van Nieuwenhuyse, A.; Poels, K.; Puzyn, T.; Scheepers, P.T.J.; et al. HBM4EU Chromates Study: Urinary Metabolomics Study of Workers Exposed to Hexavalent Chromium. Metabolites 2022, 12, 362. [Google Scholar] [CrossRef]
- Wang, S.; Chen, X.; Du, D.; Zheng, W.; Hu, L.; Yang, H.; Cheng, J.; Gong, M. MetaboGroup S: A Group Entropy-Based Web Platform for Evaluating Normalization Methods in Blood Metabolomics Data from Maintenance Hemodialysis Patients. Anal. Chem. 2018, 90, 11124–11130. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC–HRMS Spectra Processing, Multi-Omics Integration and Covariate Adjustment of Global Metabolomics Data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, L.; Janasik, B.; Nowicka, K.; Wąsowicz, W. A Urinary Metabolomics Study of a Polish Subpopulation Environmentally Exposed to Arsenic. J. Trace Elem. Med. Biol. 2019, 54, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shen, H.; Xu, W.; Xia, Y.; Barr, D.B.; Mu, X.; Wang, X.; Liu, L.; Huang, Q.; Tian, M. Urinary Metabolomics Revealed Arsenic Internal Dose-Related Metabolic Alterations: A Proof-of-Concept Study in a Chinese Male Cohort. Environ. Sci. Technol. 2014, 48, 12265–12274. [Google Scholar] [CrossRef]
- Wu, F.; Chi, L.; Ru, H.; Parvez, F.; Slavkovich, V.; Eunus, M.; Ahmed, A.; Islam, T.; Rakibuz-Zaman, M.; Hasan, R.; et al. Arsenic Exposure from Drinking Water and Urinary Metabolomics: Associations and Long-Term Reproducibility in Bangladesh Adults. Environ. Health Perspect. 2018, 126, 017005. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, Y.; Zhang, Q.; Su, Z.; Yan, T.; Zhou, S.; Wang, T.; Wei, X.; Chen, Z.; Hu, G.; et al. DNA Damage, Serum Metabolomic Alteration and Carcinogenic Risk Associated with Low-Level Air Pollution. Environ. Pollut. 2022, 297, 118763. [Google Scholar] [CrossRef]
- Hsu, Y.S.; Wu, P.J.; Jeng, Y.M.; Hu, C.M.; Lee, W.H. Differential Effects of Glucose and N-Acetylglucosamine on Genome Instability. Am. J. Cancer Res. 2022, 12, 1556–1576. [Google Scholar]
- Ni, G.; Tan, J.; Wang, M.; Ping, N.; Liu, M.; He, Y. Polymorphisms of the AS3MT Gene Are Associated with Arsenic Methylation Capacity and Damage to the P21 Gene in Arsenic Trioxide Plant Workers. Toxicol. Ind. Health 2021, 37, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Liang, Y.; Chen, J.; Cao, G.; Yang, Z.; Zhao, X.; Tian, J.; Xin, X.; Lei, B.; Cai, Z. Urinary Metabolic Characterization with Nephrotoxicity for Residents under Cadmium Exposure. Environ. Int. 2021, 154, 106646. [Google Scholar] [CrossRef]
- Chen, C.H.S.; Kuo, T.C.; Kuo, H.C.; Tseng, Y.J.; Kuo, C.H.; Yuan, T.H.; Chan, C.C. Metabolomics of Children and Adolescents Exposed to Industrial Carcinogenic Pollutants. Environ. Sci. Technol. 2019, 53, 5454–5465. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Lu, Y.; Huang, S.; Gao, L.; Liang, X.; Wu, Y.; Wang, J.; Huang, Q.; Tang, L.; Wang, G.; et al. Identifying Early Urinary Metabolic Changes with Long-Term Environmental Exposure to Cadmium by Mass-Spectrometry-Based Metabolomics. Environ. Sci. Technol. 2014, 48, 6409–6418. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mu, X.; Zhang, J.; Huang, Q.; Alamdar, A.; Tian, M.; Liu, L.; Shen, H. Serum Metabolomics Reveals That Arsenic Exposure Disrupted Lipid and Amino Acid Metabolism in Rats: A Step Forward in Understanding Chronic Arsenic Toxicity. Metallomics 2015, 7, 544–552. [Google Scholar] [CrossRef]
- Pederzolli, C.D.; Sgaravatti, Â.M.; Braum, C.A.; Prestes, C.C.; Zorzi, G.K.; Sgarbi, M.B.; Wyse, A.T.S.; Wannmacher, C.M.D.; Wajner, M.; Dutra-Filho, C.S. 5-Oxoproline Reduces Non-Enzymatic Antioxidant Defenses in Vitro in Rat Brain. Metab. Brain Dis. 2007, 22, 51–65. [Google Scholar] [CrossRef]
- Karakulak, U.N.; Bal, C.; Gunduzoz, M.; Buyuksekerci, M.; Aladag, E.; Sahiner, M.L.; Kaya, E.B.; Ozer, N.; Yilmaz, O.H.; Erel, O. Assessment of Diastolic Function and Thiol-Disulphide Homeostasis in Arsenic-Exposed Workers. Acta Cardiol. Sin. 2021, 37, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bachhawat, A. Pyroglutamic Acid: Throwing Light on a Lightly Studied Metabolite. Curr Sci 2011, 102, 288–297. [Google Scholar]
- García-Sevillano, M.A.; García-Barrera, T.; Navarro, F.; Gómez-Ariza, J.L. Analysis of the Biological Response of Mouse Liver (Mus Musculus) Exposed to As2O3 Based on Integrated -Omics Approaches. Metallomics 2013, 5, 1644. [Google Scholar] [CrossRef]
- García-Sevillano, M.Á.; García-Barrera, T.; Navarro-Roldán, F.; Montero-Lobato, Z.; Gómez-Ariza, J.L. A Combination of Metallomics and Metabolomics Studies to Evaluate the Effects of Metal Interactions in Mammals. Application to Mus Musculus Mice under Arsenic/Cadmium Exposure. J. Proteom. 2014, 104, 66–79. [Google Scholar] [CrossRef]
- Spratlen, M.J.; Grau-Perez, M.; Umans, J.G.; Yracheta, J.; Best, L.G.; Francesconi, K.; Goessler, W.; Bottiglieri, T.; Gamble, M.V.; Cole, S.A.; et al. Targeted Metabolomics to Understand the Association between Arsenic Metabolism and Diabetes-Related Outcomes: Preliminary Evidence from the Strong Heart Family Study. Environ. Res. 2019, 168, 146–157. [Google Scholar] [CrossRef]
- Davalli, A.M.; Perego, C.; Folli, F.B. The Potential Role of Glutamate in the Current Diabetes Epidemic. Acta Diabetol. 2012, 49, 167–183. [Google Scholar] [CrossRef]
- Cheng, S.; Rhee, E.P.; Larson, M.G.; Lewis, G.D.; McCabe, E.L.; Shen, D.; Palma, M.J.; Roberts, L.D.; Dejam, A.; Souza, A.L.; et al. Metabolite Profiling Identifies Pathways Associated with Metabolic Risk in Humans. Circulation 2012, 125, 2222–2231. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Zhao, T.; Wang, X.; Qiu, Y.; Su, M.; Jia, W.; Jia, W. Metabonomic Variations in the Drug-Treated Type 2 Diabetes Mellitus Patients and Healthy Volunteers. J. Proteome Res. 2009, 8, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Sung, T.C.; Huang, J.W.; Guo, H.R. Association between Arsenic Exposure and Diabetes: A Meta-Analysis. BioMed Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Toma, I.; Kang, J.J.; Sipos, A.; Vargas, S.; Bansal, E.; Hanner, F.; Meer, E.; Peti-Peterdi, J. Succinate Receptor GPR91 Provides a Direct Link between High Glucose Levels and Renin Release in Murine and Rabbit Kidney. J. Clin. Investig. 2008, 118, 2526–2534. [Google Scholar] [CrossRef]
- Sadagopan, N.; Li, W.; Roberds, S.L.; Major, T.; Preston, G.M.; Yu, Y.; Tones, M.A. Circulating Succinate Is Elevated in Rodent Models of Hypertension and Metabolic Disease. Am. J. Hypertens. 2007, 20, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.; Peitzsch, M.; Rapizzi, E.; Lenders, J.W.; Qin, N.; de Cubas, A.A.; Schiavi, F.; Rao, J.U.; Beuschlein, F.; Quinkler, M.; et al. Krebs Cycle Metabolite Profiling for Identification and Stratification of Pheochromocytomas/Paragangliomas Due to Succinate Dehydrogenase Deficiency. J. Clin. Endocrinol. Metab. 2014, 99, 3903–3911. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Selak, M.A.; Gottlieb, E. Succinate Dehydrogenase and Fumarate Hydratase: Linking Mitochondrial Dysfunction and Cancer. Oncogene 2006, 25, 4675–4682. [Google Scholar] [CrossRef]
- Zhao, T.; Mu, X.; You, Q. Succinate: An Initiator in Tumorigenesis and Progression. Oncotarget 2017, 8, 53819–53828. [Google Scholar] [CrossRef]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate Is an Inflammatory Signal That Induces IL-1β through HIF-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef]
- Mindikoglu, A.L.; Opekun, A.R.; Putluri, N.; Devaraj, S.; Sheikh-Hamad, D.; Vierling, J.M.; Goss, J.A.; Rana, A.; Sood, G.K.; Jalal, P.K.; et al. Unique Metabolomic Signature Associated with Hepatorenal Dysfunction and Mortality in Cirrhosis. Transl. Res. 2018, 195, 25–47. [Google Scholar] [CrossRef]
- Saltzman, A.; Caraway, W.T.; Beck, I.A. Serum Glucuronic Acid Levels in Diabetes Mellitus. Metabolism. 1954, 3, 11–15. [Google Scholar] [PubMed]
- Al-Gayyar, M.M.H.; Ebrahim, M.A.; Shams, M.E.E. Measuring Serum Levels of Glycosaminoglycans for Prediction and Using Viscum Fraxini-2 for Treatment of Patients with Hepatocellular Carcinoma. J. Pharm. Res. 2013, 7, 571–575. [Google Scholar] [CrossRef]
- Ho, A.; Sinick, J.; Esko, T.; Fischer, K.; Menni, C.; Zierer, J.; Matey-Hernandez, M.; Fortney, K.; Morgen, E.K. Circulating Glucuronic Acid Predicts Healthspan and Longevity in Humans and Mice. Aging 2019, 11, 7694–7706. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, T.A.; Leskinen, E. Glucuronic Acid Pathway. In Metabolic Conjugation and Metabolic Hydrolysis; Academic Press, INC.: London, UK, 1970; pp. 157–237. [Google Scholar] [CrossRef]
- Chen, C.H.S.; Yuan, T.H.; Shie, R.H.; Wu, K.Y.; Chan, C.C. Linking Sources to Early Effects by Profiling Urine Metabolome of Residents Living near Oil Refineries and Coal-Fired Power Plants. Environ. Int. 2017, 102, 87–96. [Google Scholar] [CrossRef]
- Shen, H.; Xu, W.; Zhang, J.; Chen, M.; Martin, F.L.; Xia, Y.; Liu, L.; Dong, S.; Zhu, Y.G. Urinary Metabolic Biomarkers Link Oxidative Stress Indicators Associated with General Arsenic Exposure to Male Infertility In a Han Chinese Population. Environ. Sci. Technol. 2013, 47, 8843–8851. [Google Scholar] [CrossRef]
- Cheng, K.C.; Cahill, D.S.; Kasai, H.; Nishimura, S.; Loeb, L.A. 8-Hydroxyguanine, an Abundant Form of Oxidative DNA Damage, Causes G-T and A-C Substitutions. J. Biol. Chem. 1992, 267, 166–172. [Google Scholar] [CrossRef]
- Wang, R.; Kang, H.; Zhang, X.; Nie, Q.; Wang, H.; Wang, C.; Zhou, S. Urinary Metabolomics for Discovering Metabolic Biomarkers of Bladder Cancer by UPLC-MS. BMC Cancer 2022, 22, 214. [Google Scholar] [CrossRef]
- Zhu, C.; Huang, F.; Li, Y.; Zhu, C.; Zhou, K.; Xie, H.; Xia, L.; Xie, G. Distinct Urinary Metabolic Biomarkers of Human Colorectal Cancer. Dis. Markers 2022, 2022, 1758113. [Google Scholar] [CrossRef]
- Ikeda, A.; Nishiumi, S.; Shinohara, M.; Yoshie, T.; Hatano, N.; Okuno, T.; Bamba, T.; Fukusaki, E.; Takenawa, T.; Azuma, T.; et al. Serum Metabolomics as a Novel Diagnostic Approach for Gastrointestinal Cancer: Metabolomics for Gastrointestinal Cancer. Biomed. Chromatogr. 2012, 26, 548–558. [Google Scholar] [CrossRef]
- Bardach, A.E.; Ciapponi, A.; Soto, N.; Chaparro, M.R.; Calderon, M.; Briatore, A.; Cadoppi, N.; Tassara, R.; Litter, M.I. Epidemiology of Chronic Disease Related to Arsenic in Argentina: A Systematic Review. Sci. Total Environ. 2015, 538, 802–816. [Google Scholar] [CrossRef]
- Chen, K.; Liao, Q.L.; Ma, Z.W.; Jin, Y.; Hua, M.; Bi, J.; Huang, L. Association of Soil Arsenic and Nickel Exposure with Cancer Mortality Rates, a Town-Scale Ecological Study in Suzhou, China. Environ. Sci. Pollut. Res. 2015, 22, 5395–5404. [Google Scholar] [CrossRef] [PubMed]
- Heck, J.E.; Nieves, J.W.; Chen, Y.; Parvez, F.; Brandt-Rauf, P.W.; Graziano, J.H.; Slavkovich, V.; Howe, G.R.; Ahsan, H. Dietary Intake of Methionine, Cysteine, and Protein and Urinary Arsenic Excretion in Bangladesh. Environ. Health Perspect. 2009, 117, 99–104. [Google Scholar] [CrossRef]
- Heck, J.E.; Gamble, M.V.; Chen, Y.; Graziano, J.H.; Slavkovich, V.; Parvez, F.; Baron, J.A.; Howe, G.R.; Ahsan, H. Consumption of Folate-Related Nutrients and Metabolism of Arsenic in Bangladesh. Am. J. Clin. Nutr. 2007, 85, 1367–1374. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, T.T.; Li, Y.; Li, J.; Fan, Y.; Huang, F.Q.; Cai, Y.Y.; Ma, G.; Liu, J.F.; Chen, Q.Q.; et al. Functional Metabolomics Characterizes a Key Role for N -Acetylneuraminic Acid in Coronary Artery Diseases. Circulation 2018, 137, 1374–1390. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.B.; Ang, L.; Yau, W.P.; Seow, W.J. Association between Metabolites and the Risk of Lung Cancer: A Systematic Literature Review and Meta-Analysis of Observational Studies. Metabolites 2020, 10, 362. [Google Scholar] [CrossRef] [PubMed]
- Roozbeh, J.; Merat, A.; Bodagkhan, F.; Afshariani, R.; Yarmohammadi, H. Significance of Serum and Urine Neuraminidase Activity and Serum and Urine Level of Sialic Acid in Diabetic Nephropathy. Int. Urol. Nephrol. 2011, 43, 1143–1148. [Google Scholar] [CrossRef]
- Özben, T. Elevated Serum and Urine Sialic Acid Levels in Renal Diseases. Ann. Clin. Biochem. 1991, 28, 44–48. [Google Scholar] [CrossRef]
- Zheng, L.; Kuo, C.C.; Fadrowski, J.; Agnew, J.; Weaver, V.M.; Navas-Acien, A. Arsenic and Chronic Kidney Disease: A Systematic Review. Curr. Environ. Health Rep. 2014, 1, 192–207. [Google Scholar] [CrossRef]
- Dutkiewicz, E.P.; Hsieh, K.T.; Wang, Y.S.; Chiu, H.Y.; Urban, P.L. Hydrogel Micropatch and Mass Spectrometry–Assisted Screening for Psoriasis-Related Skin Metabolites. Clin. Chem. 2016, 62, 1120–1128. [Google Scholar] [CrossRef]
- Mattarucchi, E.; Baraldi, E.; Guillou, C. Metabolomics Applied to Urine Samples in Childhood Asthma; Differentiation between Asthma Phenotypes and Identification of Relevant Metabolites: Metabolomics in Childhood Asthma. Biomed. Chromatogr. 2012, 26, 89–94. [Google Scholar] [CrossRef]
- Shao, X.; Wang, K.; Liu, X.; Gu, C.; Zhang, P.; Xie, J.; Liu, W.; Sun, L.; Chen, T.; Li, Y. Screening and Verifying Endometrial Carcinoma Diagnostic Biomarkers Based on a Urine Metabolomic Profiling Study Using UPLC-Q-TOF/MS. Clin. Chim. Acta 2016, 463, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.H.; Norval, M. The Multiple Roles of Urocanic Acid in Health and Disease. J. Investig. Dermatol. 2021, 141, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Smetana-Just, U.; Matsui, M.; Young, A.R.; John, S.; Norval, M.; Walker, S.L. Cis -Urocanic Acid Initiates Gene Transcription in Primary Human Keratinocytes. J. Immunol. 2008, 181, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Walker, S.L.; Lai-Cheong, J.; Matsui, M.S.; Norval, M.; Young, A.R. Cis-Urocanic Acid Enhances Prostaglandin E2 Release and Apoptotic Cell Death via Reactive Oxygen Species in Human Keratinocytes. J. Investig. Dermatol. 2011, 131, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Argos, M.; Kalra, T.; Pierce, B.L.; Chen, Y.; Parvez, F.; Islam, T.; Ahmed, A.; Hasan, R.; Hasan, K.; Sarwar, G.; et al. A Prospective Study of Arsenic Exposure from Drinking Water and Incidence of Skin Lesions in Bangladesh. Am. J. Epidemiol. 2011, 174, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Yu, J.; Kong, C.; Li, H.; Yang, L.; Xia, Y.; Wu, K. A Follow-up Study of the Development of Skin Lesions Associated with Arsenic Exposure Duration. Environ. Geochem. Health 2018, 40, 2729–2738. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhang, A. Assessing Potential Mechanisms of Arsenic-Induced Skin Lesions and Cancers: Human and in Vitro Evidence. Environ. Pollut. 2020, 260, 113919. [Google Scholar] [CrossRef]
- Sanyal, T.; Paul, M.; Bhattacharjee, S.; Bhattacharjee, P. Epigenetic Alteration of Mitochondrial Biogenesis Regulatory Genes in Arsenic Exposed Individuals (with and without Skin Lesions) and in Skin Cancer Tissues: A Case Control Study. Chemosphere 2020, 258, 127305. [Google Scholar] [CrossRef]

| Parameter * | Both Group | WN | WH | p Value ** |
|---|---|---|---|---|
| n = 116 | n = 75 | n = 41 | ||
| Age (years) | 43.5 (21.0–62.0) | 42.1 ± 10.0 | 44.0 (23.0–56.0) | 0.4772 |
| Height (cm) | 177.0 (165.0–198.0) | 176.5 (165.0–198.0) | 178.3 ± 6.1 | 0.9673 |
| Body mass (kg) | 89.4 ± 14.8 | 86.7 ± 14.0 | 94.0 ± 15.1 | 0.0109 |
| BMI (kg/m2) | 28.1 ± 4.3 | 27.2 ± 4.1 | 29.5 ± 4.2 | 0.0047 |
| Period of iAs exposure (years) | 17.5 (1.0–44.0) | 17.0 (1.0–44.0) | 18.5 (2.0–38.0) | 0.7867 |
| Parameter * | Both Group | WN | WH | p Value ** |
|---|---|---|---|---|
| n = 116 | n = 75 | n = 41 | ||
| tAs (µg/L) | 27.3 (1.5–498.1) | 20.6 (1.5–33.9) | 54.7 (35.9–498.1) | 0.0000 |
| iAs (µg/L) | - | - | 39.2 (10.0–87.8) | - |
| AsB (µg/L) | - | - | 20.2 (2.5–433.0) | - |
| tAs (µg/g creat.) | 19.0 (3.3–203.7) | 14.6 (3.3–60.1) | 30.4 (8.0–203.7) | 0.0000 |
| iAs (µg/g creat.) | - | - | 20.1 (6.5–55.8) | - |
| AsB (µg/g creat.) | - | - | 8.5 (0.8–170.8) | - |
| Dietary Intake * | Both Groups | WN | WH | p Value ** |
|---|---|---|---|---|
| n = 116 | n = 75 | n = 41 | ||
| Methionine (mg/kg bm) | 25.05 (8.43–70.48) | 25.23 (8.43–70.48) | 23.75 (14.69–54.47) | 0.5220 |
| Vitamin B2 (mg/kg bm) | 0.02 (0.00–0.04) | 0.02 (0.00–0.04) | 0.02 ± 0.01 | 0.3554 |
| Vitamin B6 (mg/kg bm) | 0.02 (0.01–0.06) | 0.02 (0.01–0.06) | 0.02 ± 0.01 | 0.1506 |
| Vitamin B12 (µg/kg bm) | 0.04 (0.01–0.20) | 0.03 (0.01–0.20) | 0.04 ± 0.01 | 0.8515 |
| Folate (µg/kg bm) | 2.94 (1.03–8.98) | 3.04 (1.13–8.98) | 2.78 ± 0.80 | 0.1819 |
| Zinc (mg/kg bm) | 0.12 (0.03–0.30) | 0.12 (0.03–0.30) | 0.12 ± 0.04 | 0.5418 |
| Annotated Compounds’ Names (ID in HMDB) | p Value (WN vs. WH) | Pathway Name | Sub-Pathway Name |
|---|---|---|---|
| gamma-glutamylcysteine (HMDB0001049) | 0.0000 * | Amino acid metabolism | Aspartate and asparagine metabolism |
| pyroglutamic acid (HMDB0000267) | 0.0000 ** | ||
| D-2-hydroxyglutaric acid (HMDB0000606) | 0.0000 * | ||
| 4-acetamidobutanoic acid (HMDB0003681) | 0.0000 * | ||
| urocanic acid (HMDB0000301) | 0.0000 * | Histidine metabolism | |
| 3-mercaptolactic acid (HMDB0002127) | 0.0034 * | Methionine and cysteine metabolism | |
| L-cystine (HMDB0000192) | 0.0000 * | ||
| succinic acid (HMDB0000254) | 0.0020 * | Carbohydrate metabolism | Butanoate metabolism |
| D-glucose (HMDB0000122) | 0.0286 ** | Glycolysis and Gluconeogenesis | |
| D-xylulose (HMDB0001644) | 0.0000 * | Pentose and Glucuronate Interconversions | |
| 2-ketobutyric acid (HMDB0000005) | 0.0000 * | Propanoate metabolism | |
| hydroxypropionic acid (HMDB0000700) | 0.0000 ** | ||
| iduronic acid (HMDB0002704) | 0.0000 ** | Glycan biosynthesis and metabolism | Heparan sulfate degradation |
| N-acetylneuraminic acid (HMDB0000230) | 0.0000 * | Keratan sulfate degradation | |
| riboflavin (HMDB0000244) | 0.0000 ** | Metabolism of vitamins | Vitamin B2 metabolism |
| pyridoxine (HMDB0000239) | 0.0000 * | Vitamin B6 metabolism | |
| thymine (HMDB0000262) | 0.0000 * | Nucleotide metabolism | Pyrimidine metabolism |
| uridine (HMDB0000296) | 0.0000 ** | ||
| cytosine (HMDB0000630) | 0.0000 * | ||
| cytidine (HMDB0000089) | 0.0000 * | ||
| adenosine monophosphate (HMDB0000045) | 0.0000 * | Many pathways | Aspartate and asparagine metabolism, Histidine metabolism, Methionine and cysteine metabolism, Butanoate metabolism, Glycolysis and Gluconeogenesis, Propanoate metabolism, Vitamin B2 metabolism, Pyrimidine metabolism |
| L-glutamic acid (HMDB0000148) | 0.0000 * | Aspartate and asparagine metabolism, Histidine metabolism, Butanoate metabolism, Vitamin B9 metabolism | |
| 2-hydroxybutyric acid (HMDB0000008) | 0.0000 * | Butanoate metabolism, Propanoate metabolism | |
| D-glucuronic acid (HMDB0000127) | 0.0000 * | Pentose and Glucuronate Interconversions, Heparan sulfate degradation, Hyaluronan metabolism | |
| N-acetyl-D-glucosamine (HMDB0000215) | 0.0000 ** | Heparan sulfate degradation, Keratan sulfate degradation, Hyaluronan metabolism |
| Correlation between Nutrient Intake *** and Metabolite | Both Group | WN | WH | |||
|---|---|---|---|---|---|---|
| R | p | R | p | R | p | |
| vitamin B2 with cytosine | −0.1886 * | 0.0464 | −0.2554 | 0.0316 | NS | |
| vitamin B6 with cytosine | −0.2175 * | 0.0213 | −0.2346 | 0.0489 | NS | |
| vitamin B2 with D-glucuronic acid | NS | NS | −0.3956 * | 0.0100 | ||
| vitamin B6 with D-glucuronic acid | NS | NS | −0.3646 * | 0.0190 | ||
| vitamin B12 with D-glucuronic acid | NS | NS | −0.3479 * | 0.0260 | ||
| folate with D-glucuronic acid | −0.1996 * | 0.0349 | −0.2603* | 0.0283 | NS | |
| methionine with hydroxypropionic acid | −0.2163 * | 0.0220 | NS | NS | ||
| vitamin B12 with hydroxypropionic acid | −0.2017 * | 0.0330 | NS | NS | ||
| vitamin B2 with L-glutamic acid | NS | NS | −0.4211 ** | 0.0061 | ||
| vitamin B6 with L-glutamic acid | NS | NS | −0.3653 ** | 0.0188 | ||
| folate with L-glutamic acid | NS | NS | −0.3794 ** | 0.0144 | ||
| methionine with N-acetyl-D-glucosamine | −0.1954 * | 0.0390 | −0.2728 * | 0.0214 | NS | |
| vitamin B2 with N-acetyl-D-glucosamine | −0.2504 * | 0.0077 | −0.3150 * | 0.0075 | −0.3253 * | 0.0380 |
| vitamin B6 with N-acetyl-D-glucosamine | −0.2374 * | 0.0117 | NS | NS | ||
| vitamin B12 with N-acetyl-D-glucosamine | −0.1871 * | 0.0482 | −0.3261 * | 0.0055 | NS | |
| zinc with N-acetyl-D-glucosamine | −0.1917 * | 0.0429 | −0.2710 * | 0.0223 | NS | |
| vitamin B2 with N-acetylneuraminic acid | −0.1915 * | 0.0431 | NS | NS | ||
| vitamin B6 with N-acetylneuraminic acid | −0.2246 * | 0.0173 | NS | NS | ||
| vitamin B12 with N-acetylneuraminic acid | NS | NS | −0.3171 * | 0.0430 | ||
| vitamin B2 with pyroglutamic acid | NS | −0.2832 * | 0.0167 | NS | ||
| vitamin B6 with pyroglutamic acid | NS | −0.2430 * | 0.0412 | NS | ||
| vitamin B12 with pyroglutamic acid | NS | −0.2667 * | 0.0246 | NS | ||
| folate with pyroglutamic acid | −0.1894 * | 0.0454 | NS | NS | ||
| zinc with pyroglutamic acid | NS | −0.2548 * | 0.0320 | NS | ||
| vitamin B2 with uridine | −0.1944 * | 0.0400 | NS | NS | ||
| vitamin B6 with uridine | −0.1955 * | 0.0388 | NS | NS | ||
| vitamin B12 with uridine | NS | −0.2559 * | 0.0313 | −0.3308 * | 0.0350 | |
| methionine with urocanic acid | NS | −0.2569 * | 0.0305 | NS | ||
| vitamin B6 with urocanic acid | NS | −0.2457 * | 0.0389 | NS | ||
| folate with urocanic acid | −0.2067 * | 0.0288 | −0.2665 * | 0.0247 | NS | |
| zinc with urocanic acid | NS | −0.2722 * | 0.0217 | NS | ||
| folate with 3-mercaptolactic acid | NS | NS | 0.3337 * | 0.0330 | ||
| zinc with 3-mercaptolactic acid | NS | NS | 0.3658 * | 0.0190 | ||
| methionine with succinic acid | NS | NS | 0.3284 ** | 0.0361 | ||
| folate with succinic acid | NS | NS | 0.3359 * | 0.0320 | ||
| zinc with succinic acid | NS | NS | 0.3498 * | 0.0250 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sijko, M.; Janasik, B.; Wąsowicz, W.; Kozłowska, L. Metabolic Changes and Their Associations with Selected Nutrients Intake in the Group of Workers Exposed to Arsenic. Metabolites 2023, 13, 70. https://doi.org/10.3390/metabo13010070
Sijko M, Janasik B, Wąsowicz W, Kozłowska L. Metabolic Changes and Their Associations with Selected Nutrients Intake in the Group of Workers Exposed to Arsenic. Metabolites. 2023; 13(1):70. https://doi.org/10.3390/metabo13010070
Chicago/Turabian StyleSijko, Monika, Beata Janasik, Wojciech Wąsowicz, and Lucyna Kozłowska. 2023. "Metabolic Changes and Their Associations with Selected Nutrients Intake in the Group of Workers Exposed to Arsenic" Metabolites 13, no. 1: 70. https://doi.org/10.3390/metabo13010070
APA StyleSijko, M., Janasik, B., Wąsowicz, W., & Kozłowska, L. (2023). Metabolic Changes and Their Associations with Selected Nutrients Intake in the Group of Workers Exposed to Arsenic. Metabolites, 13(1), 70. https://doi.org/10.3390/metabo13010070






