EPR and Related Magnetic Resonance Imaging Techniques in Cancer Research
Abstract
1. Introduction
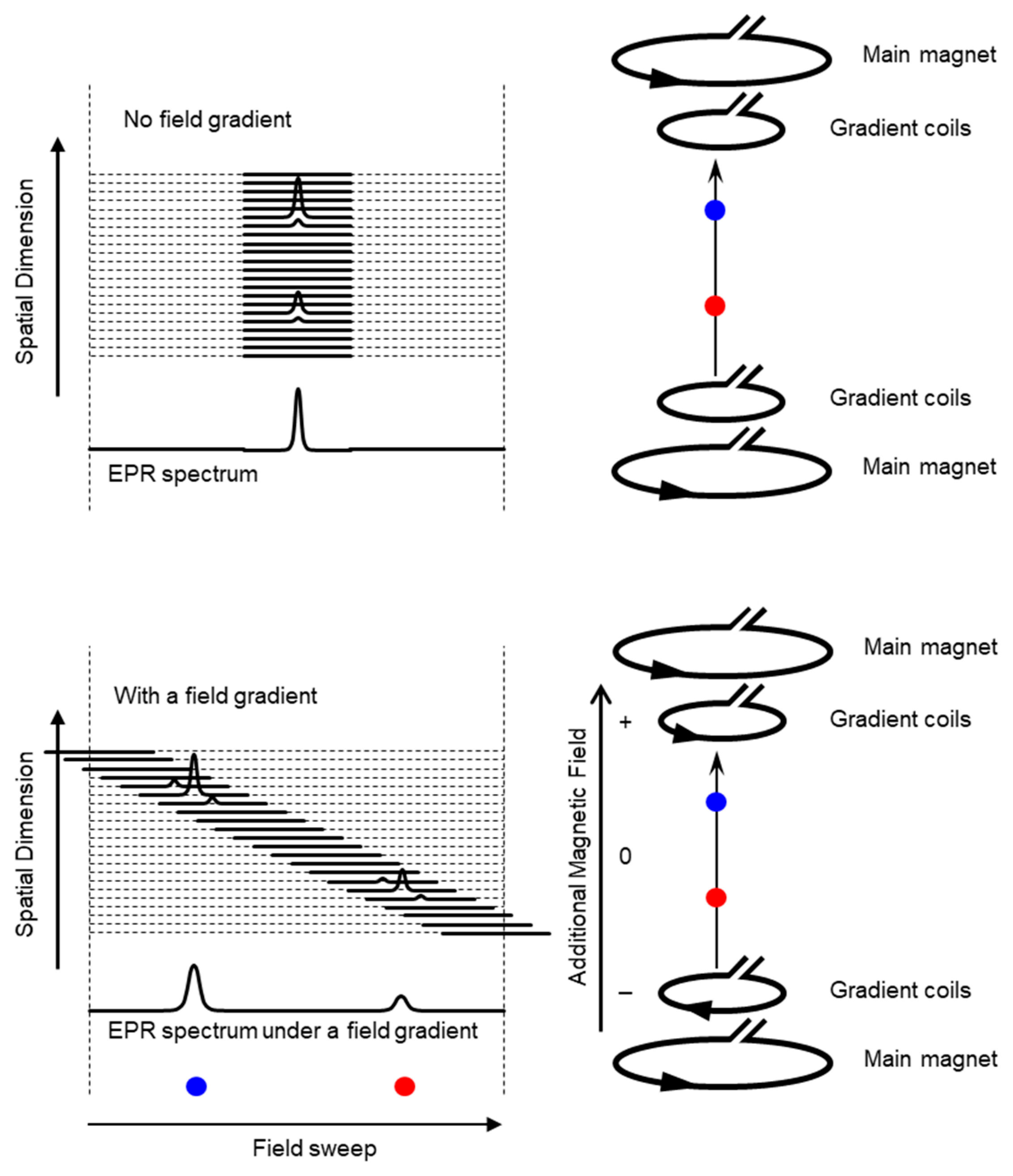
2. Development of Functional EPR Imaging Techniques
2.1. In Vivo EPR Imaging
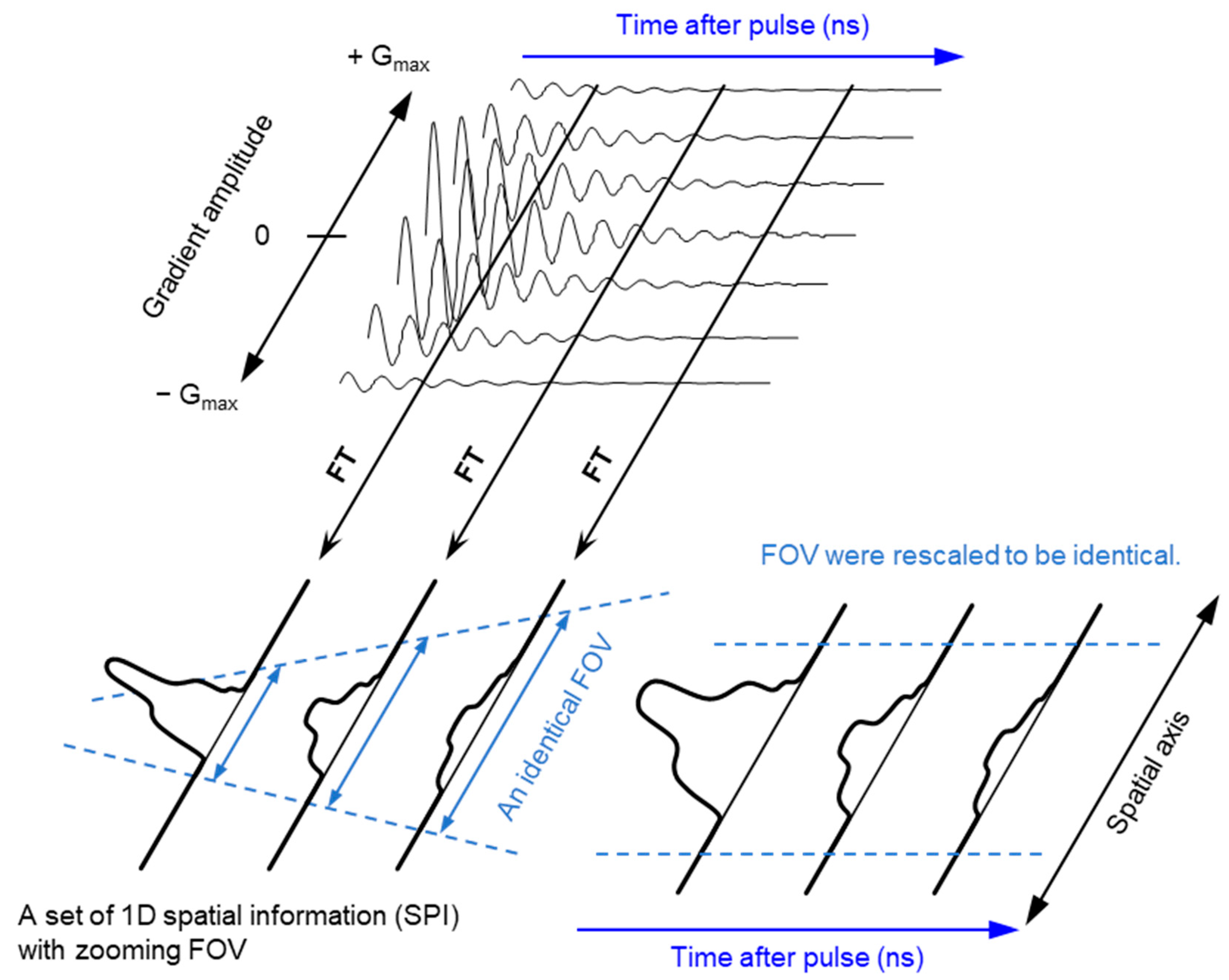
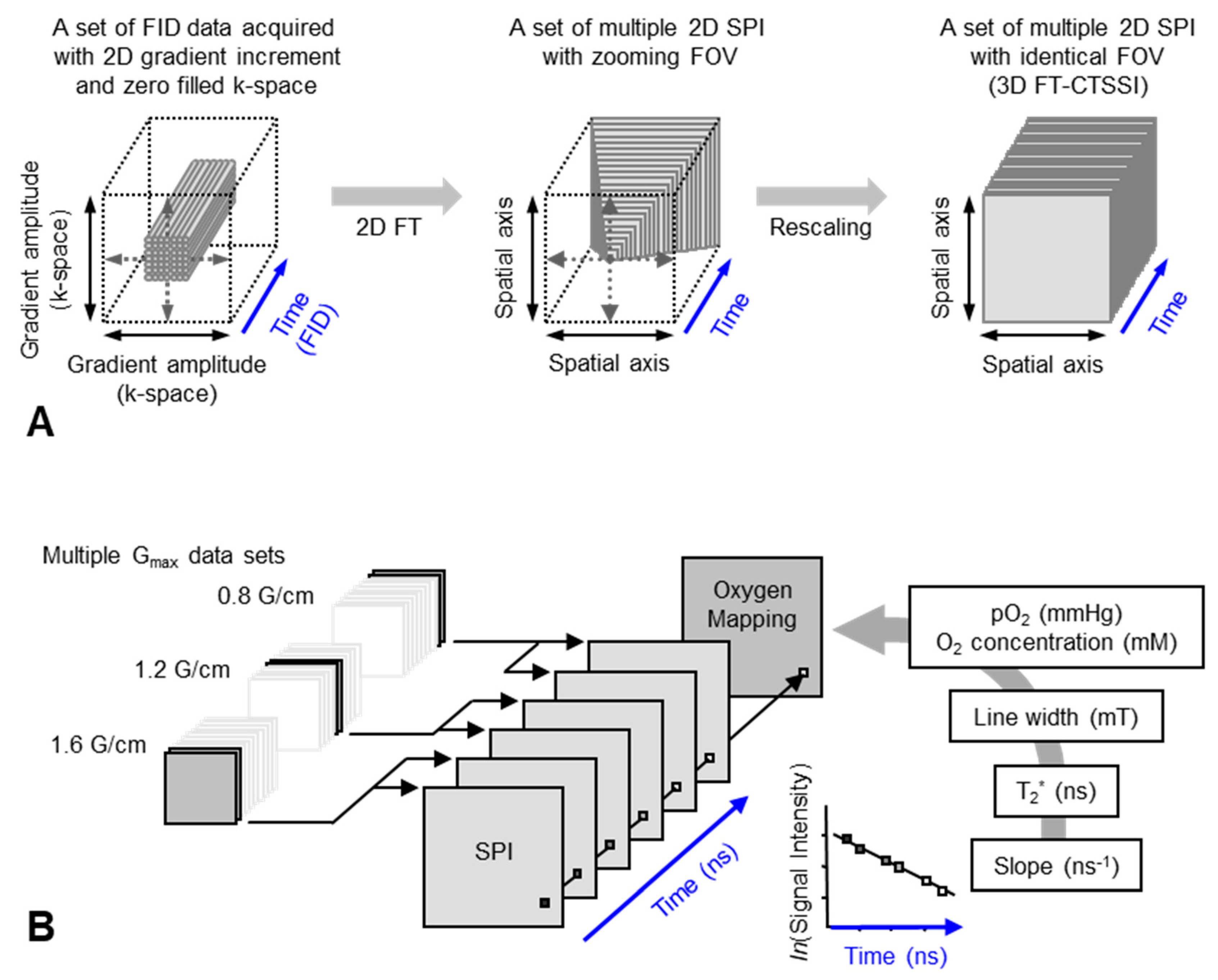
2.2. Pulsed EPR Imaging
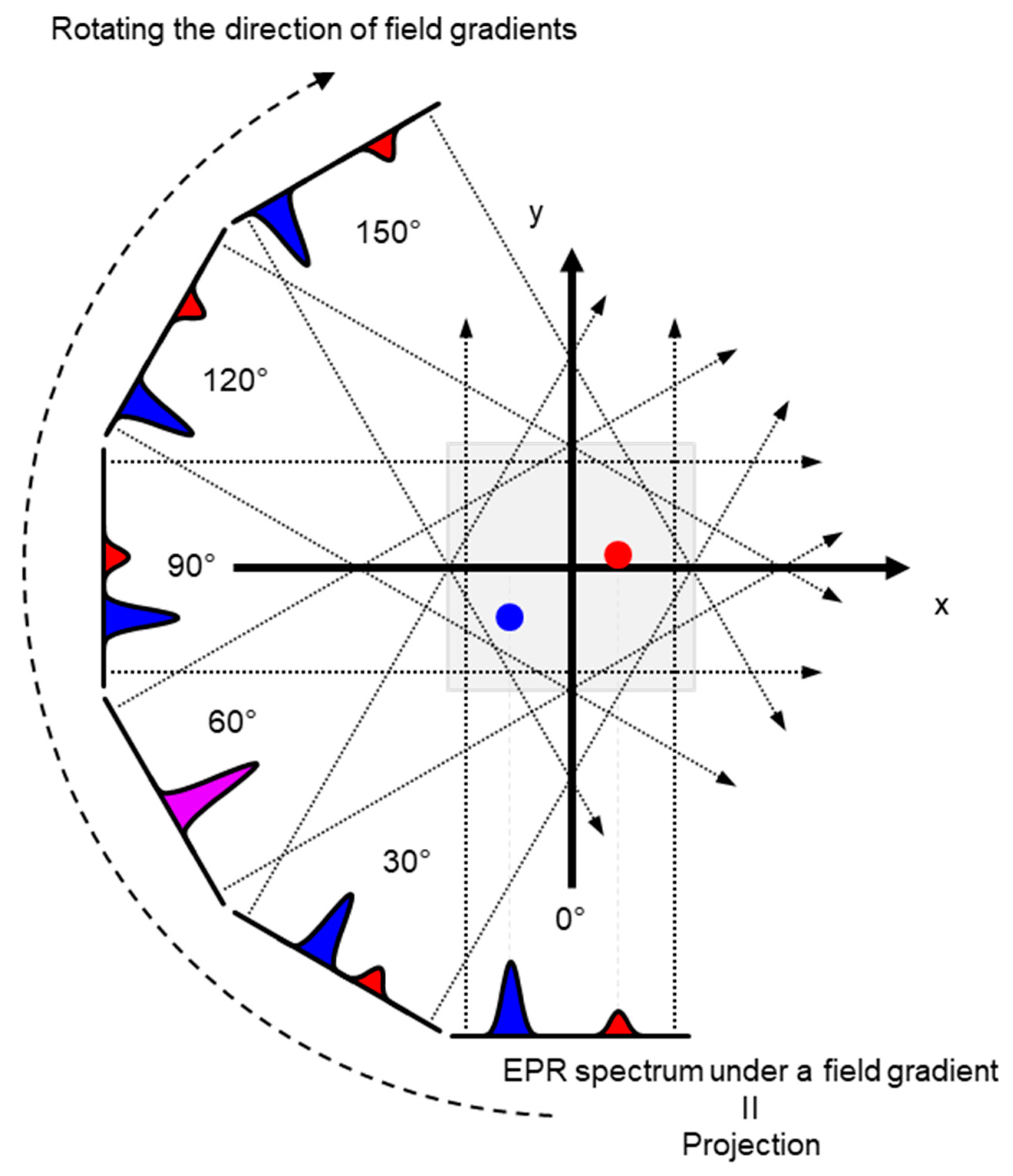
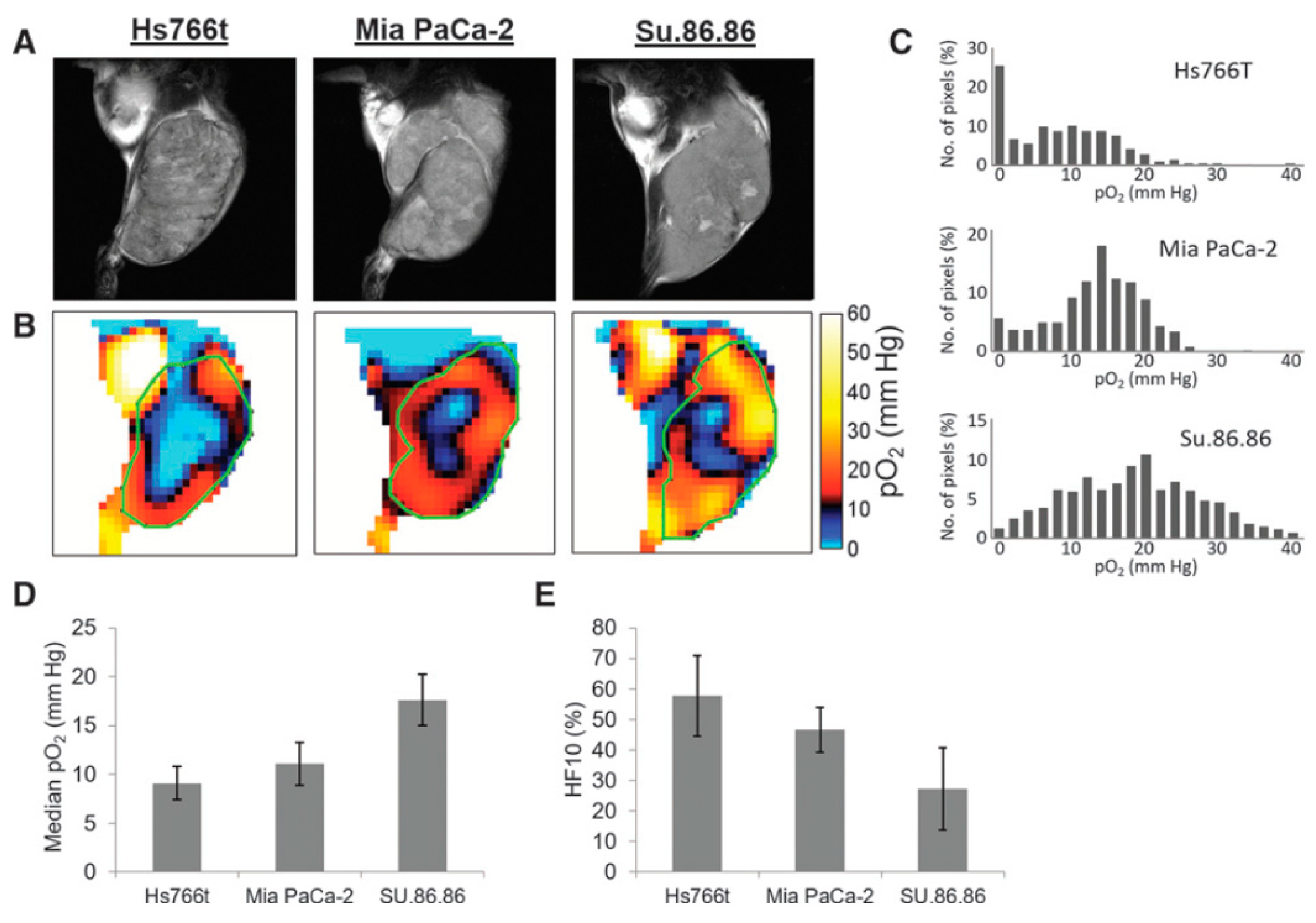
2.3. Spectral–Spatial EPR Imaging (Oxygen Mapping)
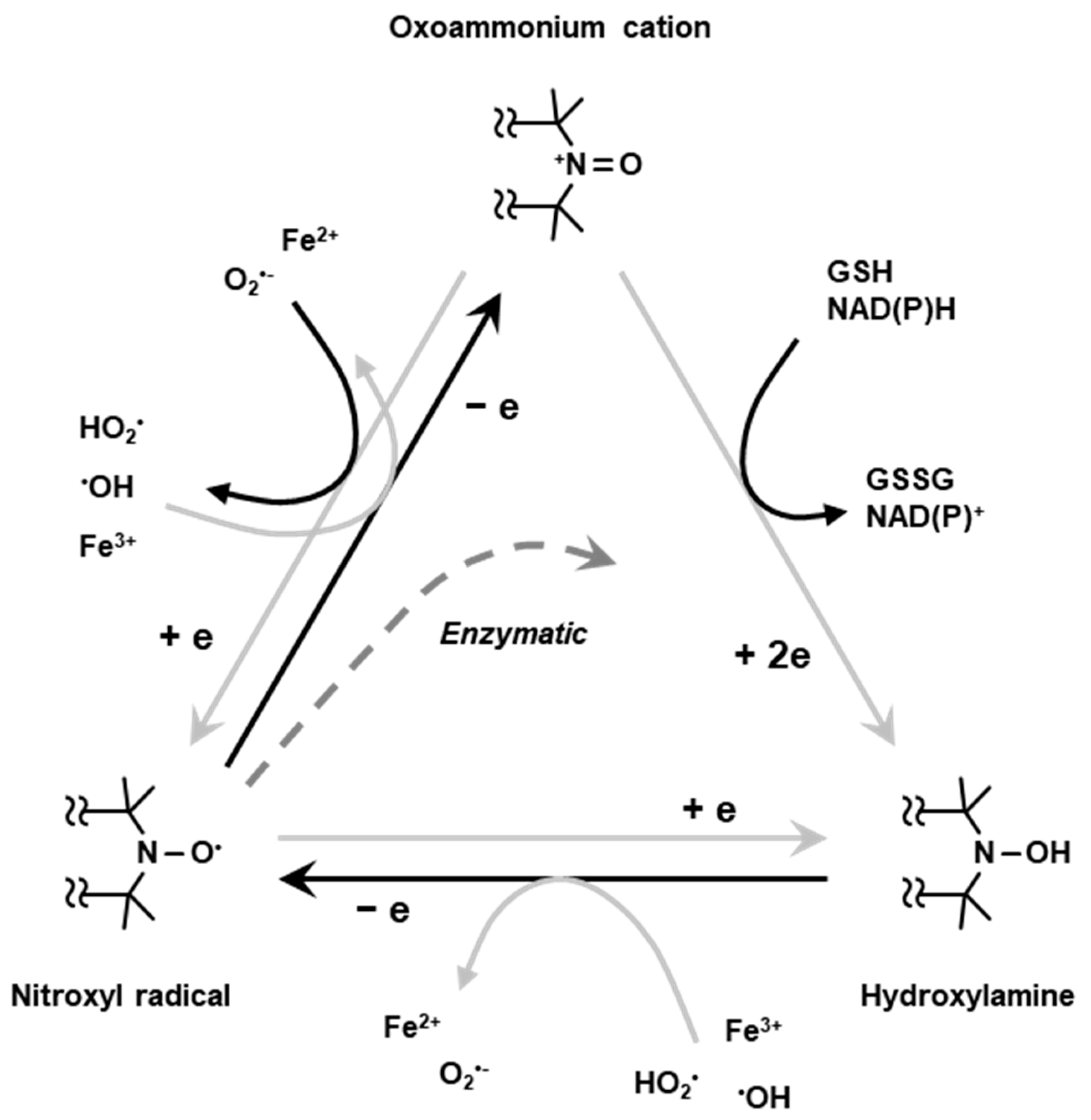
2.4. Dynamic EPR Imaging (Redox Mapping)
2.5. Co-Registration
3. EPR Related Imaging Techniques (OMRI/PEDRI, DNP-Imaging)
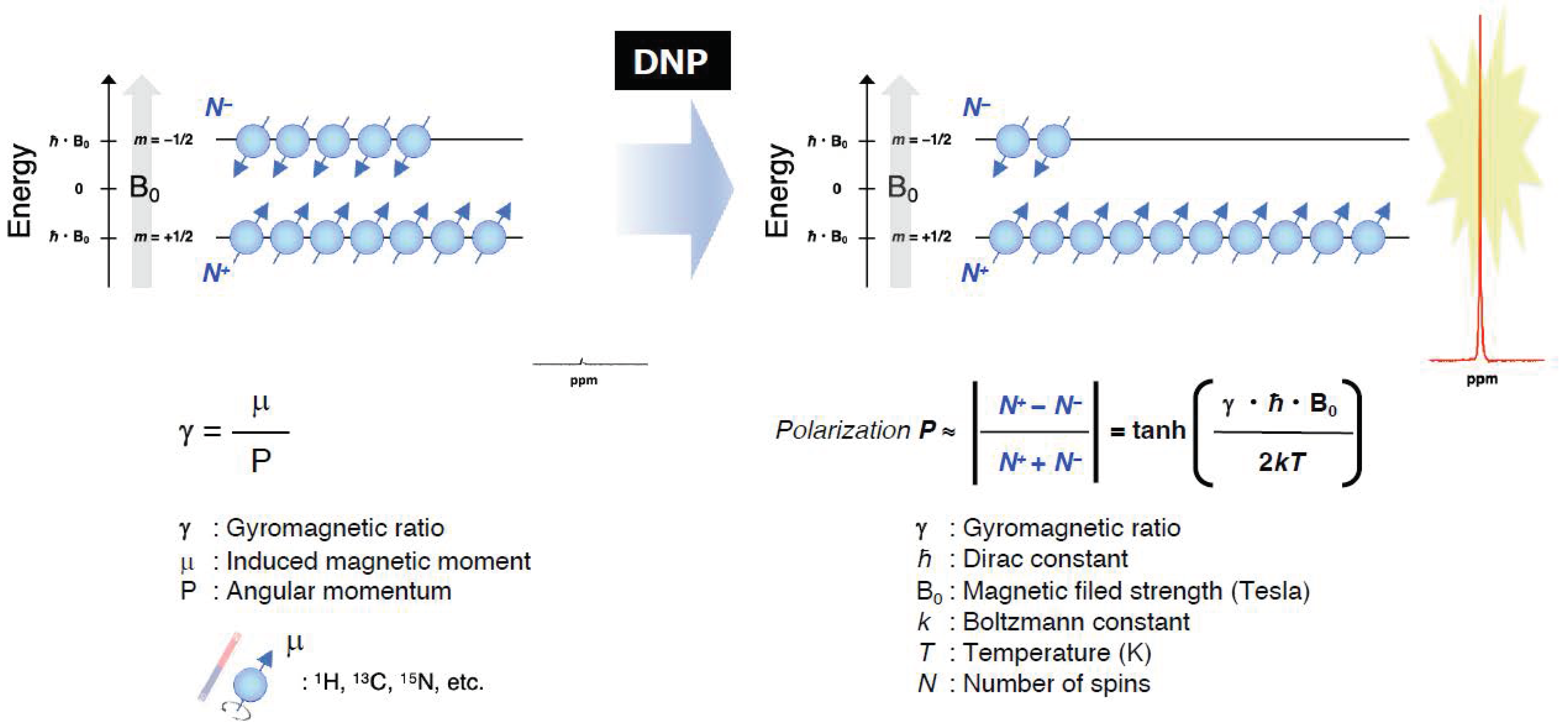
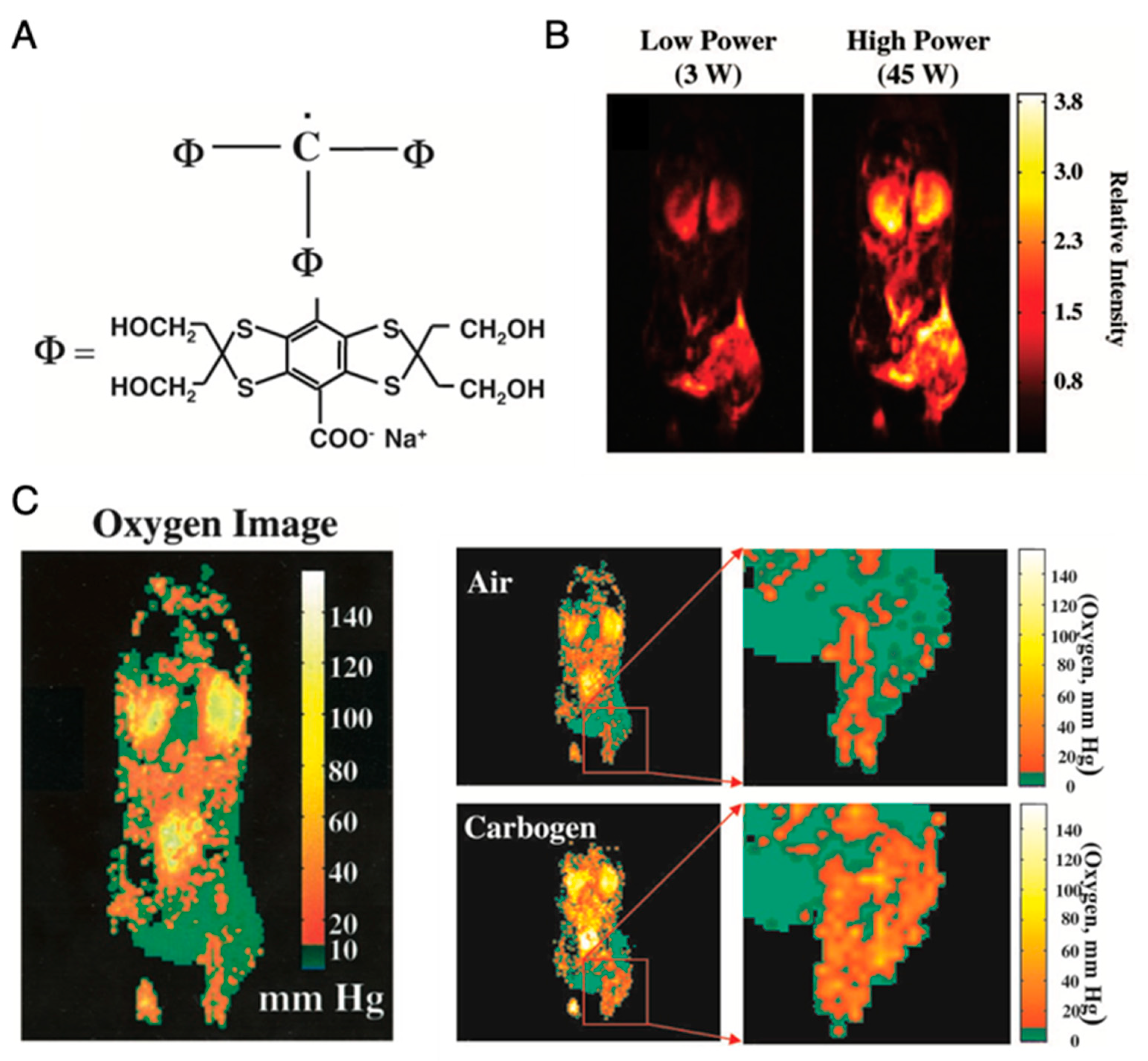
3.1. OMRI/PEDRI
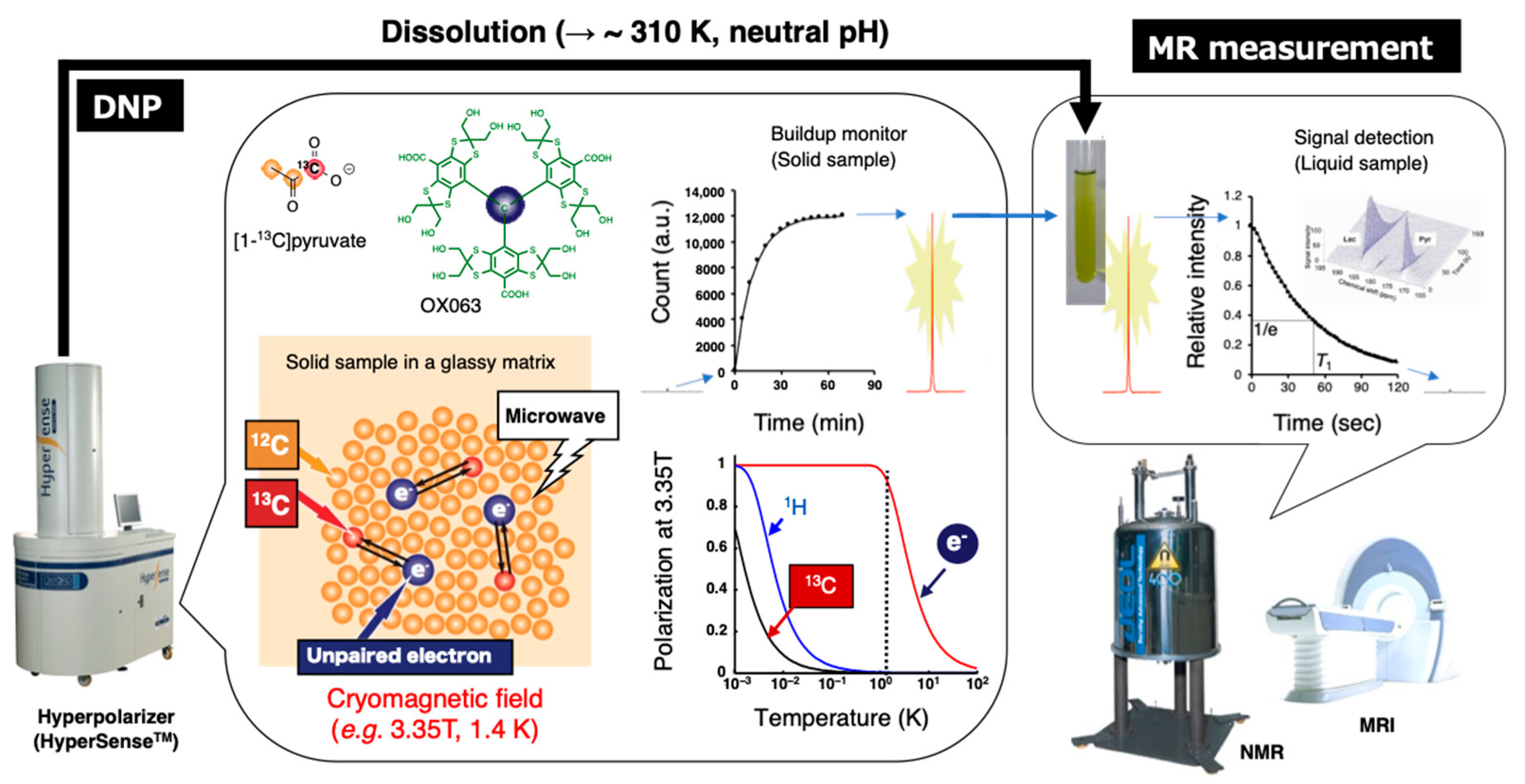
3.2. Hyperpolarized 13C MRI
4. Possibility of Clinical Application of EPRI
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brender, J.R.; Saida, Y.; Devasahayam, N.; Krishna, M.C.; Kishimoto, S. Hypoxia Imaging as a Guide for Hypoxia-Modulated and Hypoxia-Activated Therapy. Antioxid. Redox Signal. 2022, 36, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Torkian, P.; Azadbakht, J.; Andrea Bonaffini, P.; Amini, B.; Chalian, M. Advanced Imaging in Multiple Myeloma: New Frontiers for MRI. Diagnostics 2022, 12, 2182. [Google Scholar] [CrossRef] [PubMed]
- Giovanella, L.; Deandreis, D.; Vrachimis, A.; Campenni, A.; Petranovic Ovcaricek, P. Molecular Imaging and Theragnostics of Thyroid Cancers. Cancers 2022, 14, 1272. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Chow, J.C.L. Recent Advances in Functionalized Nanoparticles in Cancer Theranostics. Nanomaterials 2022, 12, 2826. [Google Scholar] [CrossRef] [PubMed]
- Gallez, B. The Role of Imaging Biomarkers to Guide Pharmacological Interventions Targeting Tumor Hypoxia. Front. Pharmacol. 2022, 13, 853568. [Google Scholar] [CrossRef]
- Matsumoto, K.; Mitchell, J.B.; Krishna, M.C. Multimodal Functional Imaging for Cancer/Tumor Microenvironments Based on MRI, EPRI, and PET. Molecules 2021, 26, 1614. [Google Scholar] [CrossRef]
- Waller, J.; Onderdonk, B.; Flood, A.; Swartz, H.; Shah, J.; Shah, A.; Aydogan, B.; Halpern, H.; Hasan, Y. The clinical utility of imaging methods used to measure hypoxia in cervical cancer. Br. J. Radiol. 2020, 93, 20190640. [Google Scholar] [CrossRef]
- Gallagher, B.M.; Ansari, A.; Atkins, H.; Casella, V.; Christman, D.R.; Fowler, J.S.; Ido, T.; MacGregor, R.R.; Som, P.; Wan, C.N.; et al. Radiopharmaceuticals XXVII. 18F-labeled 2-deoxy-2-fluoro-d-glucose as a radiopharmaceutical for measuring regional myocardial glucose metabolism In Vivo: Tissue distribution and imaging studies in animals. J. Nucl. Med. 1977, 18, 990–996. [Google Scholar]
- Ido, T.; Wan, C.N.; Casella, V.; Fowler, J.S.; Wolf, A.P.; Reivich, M.; Kuhl, D.E. Labeled 2-Deoxy-D-Glucose Analogs—F-18-Labeled 2-Deoxy-2-Fluoro-D-Glucose, 2-Deoxy-2-Fluoro-D-Mannose and C-14-2-Deoxy-2-Fluoro-D-Glucose. J. Label. Compd. Radiopharm. 1978, 14, 175–183. [Google Scholar] [CrossRef]
- Reivich, M.; Kuhl, D.; Wolf, A.; Greenberg, J.; Phelps, M.; Ido, T.; Casella, V.; Fowler, J.; Hoffman, E.; Alavi, A.; et al. The [18F]fluorodeoxyglucose method for the measurement of local cerebral glucose utilization in man. Circ. Res. 1979, 44, 127–137. [Google Scholar] [CrossRef]
- Ogawa, S.; Lee, T.M.; Kay, A.R.; Tank, D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. USA 1990, 87, 9868–9872. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Lee, T.M.; Nayak, A.S.; Glynn, P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn. Reson. Med. 1990, 14, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Subramanian, S.; Murugesan, R.; Mitchell, J.B.; Krishna, M.C. Spatially resolved biologic information from in vivo EPRI, OMRI, and MRI. Antioxid. Redox Signal. 2007, 9, 1125–1141. [Google Scholar] [CrossRef]
- Subramanian, S.; Matsumoto, K.; Mitchell, J.B.; Krishna, M.C. Radio frequency continuous-wave and time-domain EPR imaging and Overhauser-enhanced magnetic resonance imaging of small animals: Instrumental developments and comparison of relative merits for functional imaging. NMR Biomed. 2004, 17, 263–294. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Hisahara, S.; Iwahara, N.; Emoto, M.C.; Yokokawa, K.; Suzuki, H.; Manabe, T.; Matsumura, A.; Suzuki, S.; Matsushita, T.; et al. Early administration of galantamine from preplaque phase suppresses oxidative stress and improves cognitive behavior in APPswe/PS1dE9 mouse model of Alzheimer’s disease. Free Radic. Biol. Med. 2019, 145, 20–32. [Google Scholar] [CrossRef]
- Khramtsov, V.V. In Vivo Molecular Electron Paramagnetic Resonance-Based Spectroscopy and Imaging of Tumor Microenvironment and Redox Using Functional Paramagnetic Probes. Antioxid. Redox Signal. 2018, 28, 1365–1377. [Google Scholar] [CrossRef]
- Krishna, M.C.; Devasahayam, N.; Cook, J.A.; Subramanian, S.; Kuppusamy, P.; Mitchell, J.B. Electron paramagnetic resonance for small animal imaging applications. ILAR J. 2001, 42, 209–218. [Google Scholar] [CrossRef]
- Gertsenshteyn, I.; Giurcanu, M.; Vaupel, P.; Halpern, H. Biological validation of electron paramagnetic resonance (EPR) image oxygen thresholds in tissue. J. Physiol. 2021, 599, 1759–1767. [Google Scholar] [CrossRef]
- Kishimoto, S.; Matsumoto, K.; Saito, K.; Enomoto, A.; Matsumoto, S.; Mitchell, J.B.; Devasahayam, N.; Krishna, M.C. Pulsed Electron Paramagnetic Resonance Imaging: Applications in the Studies of Tumor Physiology. Antioxid. Redox Signal. 2018, 28, 1378–1393. [Google Scholar] [CrossRef]
- Bacic, G.; Pavicevic, A.; Peyrot, F. In vivo evaluation of different alterations of redox status by studying pharmacokinetics of nitroxides using magnetic resonance techniques. Redox Biol. 2016, 8, 226–242. [Google Scholar] [CrossRef]
- Krishna, M.C.; Matsumoto, S.; Yasui, H.; Saito, K.; Devasahayam, N.; Subramanian, S.; Mitchell, J.B. Electron paramagnetic resonance imaging of tumor pO2. Radiat. Res. 2012, 177, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Overhauser, A.W. Polarization of Nuclei in Metals. Phys. Rev. 1953, 92, 411. [Google Scholar] [CrossRef]
- Alecci, M.; Lurie, D.J.; Nicholson, I.; Placidi, G.; Sotgiu, A. Young Investigator Award presentation at the 13th Annual Meeting of the ESMRMB, September 1996, Prague. A proton-electron double-resonance imaging apparatus with simultaneous multiple electron paramagnetic resonance irradiation at 10 mT. Magn. Reson. Mater. Phys. Biol. Med. 1996, 4, 187–193. [Google Scholar] [CrossRef]
- Lurie, D.J.; Bussell, D.M.; Bell, L.H.; Mallard, J.R. Proton Electron Double Magnetic-Resonance Imaging of Free-Radical Solutions. J. Magn. Reason. 1988, 76, 366–370. [Google Scholar] [CrossRef]
- Kishimoto, S.; Oshima, N.; Krishna, M.C.; Gillies, R.J. Direct and indirect assessment of cancer metabolism explored by MRI. NMR Biomed. 2019, 32, e3966. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.C.; English, S.; Yamada, K.; Yoo, J.; Murugesan, R.; Devasahayam, N.; Cook, J.A.; Golman, K.; Ardenkjaer-Larsen, J.H.; Subramanian, S.; et al. Overhauser enhanced magnetic resonance imaging for tumor oximetry: Coregistration of tumor anatomy and tissue oxygen concentration. Proc. Natl. Acad. Sci. USA 2002, 99, 2216–2221. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, H.; Yamada, K.; Ichikawa, K.; Sakai, K.; Kinoshita, Y.; Matsumoto, S.; Nagai, M. Simultaneous molecular imaging of redox reactions monitored by Overhauser-enhanced MRI with 14N- and 15N-labeled nitroxyl radicals. Proc. Natl. Acad. Sci. USA 2006, 103, 1463–1468. [Google Scholar] [CrossRef]
- Koyasu, N.; Hyodo, F.; Iwasaki, R.; Eto, H.; Elhelaly, A.E.; Tomita, H.; Shoda, S.; Takasu, M.; Mori, T.; Murata, M.; et al. Spatiotemporal imaging of redox status using in vivo dynamic nuclear polarization magnetic resonance imaging system for early monitoring of response to radiation treatment of tumor. Free Radic. Biol. Med. 2022, 179, 170–180. [Google Scholar] [CrossRef]
- Yasukawa, K.; Hirago, A.; Yamada, K.; Tun, X.; Ohkuma, K.; Utsumi, H. In vivo redox imaging of dextran sodium sulfate-induced colitis in mice using Overhauser-enhanced magnetic resonance imaging. Free Radic. Biol. Med. 2019, 136, 1–11. [Google Scholar] [CrossRef]
- Ichikawa, K.; Yasukawa, K. Imaging in vivo redox status in high spatial resolution with OMRI. Free Radic. Res. 2012, 46, 1004–1010. [Google Scholar] [CrossRef]
- Chen, A.P.; Albers, M.J.; Cunningham, C.H.; Kohler, S.J.; Yen, Y.F.; Hurd, R.E.; Tropp, J.; Bok, R.; Pauly, J.M.; Nelson, S.J.; et al. Hyperpolarized C-13 spectroscopic imaging of the TRAMP mouse at 3T-initial experience. Magn. Reason. Med. 2007, 58, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Godet, I.; Doctorman, S.; Wu, F.; Gilkes, D.M. Detection of Hypoxia in Cancer Models: Significance, Challenges, and Advances. Cells 2022, 11, 686. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.; Ferreira, S.; Caetano, M. PET/CT in the Evaluation of Hypoxia for Radiotherapy Planning in Head and Neck Tumors: Systematic Literature Review. J. Nucl. Med. Technol. 2021, 49, 107–113. [Google Scholar] [CrossRef]
- O’Connor, J.P.B.; Robinson, S.P.; Waterton, J.C. Imaging tumour hypoxia with oxygen-enhanced MRI and BOLD MRI. Br. J. Radiol. 2019, 92, 20180642. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, F.; Davis, R.M.; Hyodo, E.; Matsumoto, S.; Krishna, M.C.; Mitchell, J.B. The relationship between tissue oxygenation and redox status using magnetic resonance imaging. Int. J. Oncol. 2012, 41, 2103–2108. [Google Scholar] [CrossRef]
- Hirayama, R.; Uzawa, A.; Obara, M.; Takase, N.; Koda, K.; Ozaki, M.; Noguchi, M.; Matsumoto, Y.; Li, H.; Yamashita, K.; et al. Determination of the relative biological effectiveness and oxygen enhancement ratio for micronuclei formation using high-LET radiation in solid tumor cells: An in vitro and in vivo study. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 793, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Epel, B.; Sundramoorthy, S.; Tsai, H.M.; Barth, E.; Gertsenshteyn, I.; Halpern, H.; Hua, Y.; Xie, Q.; Chen, C.T.; et al. Development of a PET/EPRI combined imaging system for assessing tumor hypoxia. J. Instrum. 2021, 16, P03031. [Google Scholar] [CrossRef]
- Crehange, G.; Soussan, M.; Gensanne, D.; Decazes, P.; Thariat, J.; Thureau, S. Interest of positron-emission tomography and magnetic resonance imaging for radiotherapy planning and control. Cancer Radiother. 2020, 24, 398–402. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Meziani, L.; Yakkala, C.; Vozenin, M.C. Expanding the therapeutic index of radiation therapy by normal tissue protection. Br. J. Radiol. 2019, 92, 20180008. [Google Scholar] [CrossRef]
- Matsumoto, S.; Kishimoto, S.; Saito, K.; Takakusagi, Y.; Munasinghe, J.P.; Devasahayam, N.; Hart, C.P.; Gillies, R.J.; Mitchell, J.B.; Krishna, M.C. Metabolic and Physiologic Imaging Biomarkers of the Tumor Microenvironment Predict Treatment Outcome with Radiation or a Hypoxia-Activated Prodrug in Mice. Cancer Res. 2018, 78, 3783–3792. [Google Scholar] [CrossRef]
- Sato-Akaba, H.; Emoto, M.C.; Yamada, K.I.; Koshino, H.; Fujii, H.G. Three-dimensional electron paramagnetic resonance imaging of mice using ascorbic acid sensitive nitroxide imaging probes. Free Radic. Res. 2021, 55, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Nakanishi, I.; Zhelev, Z.; Bakalova, R.; Aoki, I. Nitroxyl Radical as a Theranostic Contrast Agent in Magnetic Resonance Redox Imaging. Antioxid. Redox Signal. 2022, 36, 95–121. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, F.; Eto, H.; Naganuma, T.; Koyasu, N.; Elhelaly, A.E.; Noda, Y.; Kato, H.; Murata, M.; Akahoshi, T.; Hashizume, M.; et al. In Vivo Dynamic Nuclear Polarization Magnetic Resonance Imaging for the Evaluation of Redox-Related Diseases and Theranostics. Antioxid. Redox Signal. 2022, 36, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Karthe, W.; Wehrsdofer, E. The measurement of inhomogeneous distributions of paramagnetic centers by means of EPR. J. Magn. Reason. 1979, 33, 107–111. [Google Scholar] [CrossRef]
- Hoch, M.J.R.; Day, A.R. Imaging of paramagnetic centres in diamond. Solid State Commun. 1979, 30, 211–213. [Google Scholar] [CrossRef]
- Lauterbur, P.C. Image formation by induced local interactions: Examples employing nuclear magnetic resonance. Nature 1973, 242, 190–191. [Google Scholar] [CrossRef]
- Ohno, K. A method of EPR imaging: Application to spatial distributions of hydrogen atoms trapped in sulfuric acid ices. Jpn. J. Appl. Phys. 1981, 20, L179–L182. [Google Scholar] [CrossRef]
- Eaton, S.S.; Eaton, G.R. EPR imaging. J. Magn. Reson. 1984, 59, 474–477. [Google Scholar] [CrossRef]
- Ebert, B.; Hanke, T.; Klimes, N. Application of ESR zeugmatography. Stud. Biophys. 1984, 103, 161–168. [Google Scholar]
- Kumar, A.; Welti, D.; Ernst, R.R. NMR Fourier zeugmatography. J. Magn. Reson. 1975, 18, 69–83. [Google Scholar] [CrossRef]
- FONAR History. Available online: https://www.fonar.com/history.html (accessed on 8 September 2022).
- Ray, P.S. Broadband complex refractive indices of ice and water. Appl. Opt. 1972, 11, 1836–1844. [Google Scholar] [CrossRef] [PubMed]
- Berliner, J.L. The evolution of biomedical EPR (ESR). Biomed. Spectrosc. Imaging 2016, 5, 5–26. [Google Scholar] [CrossRef]
- Takeshita, K.; Ozawa, T. Recent progress in in vivo ESR spectroscopy. J. Radiat. Res. 2004, 45, 373–384. [Google Scholar] [CrossRef][Green Version]
- Ono, M.; Ito, K.; Kawamura, N.; Hsieh, K.C.; Hirata, H.; Tsuchihashi, N.; Kamada, H. A surface-coil-type resonator for in vivo ESR measurements. J. Magn. Reson. Ser. B 1994, 104, 180–182. [Google Scholar] [CrossRef]
- Afeworki, M.; van Dam, G.M.; Devasahayam, N.; Murugesan, R.; Cook, J.; Coffin, D.; Larsen, J.H.; Mitchell, J.B.; Subramanian, S.; Krishna, M.C. Three-dimensional whole body imaging of spin probes in mice by time-domain radiofrequency electron paramagnetic resonance. Magn. Reson. Med. 2000, 43, 375–382. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Lukiewicz, S.; Hyde, J.S. Murine in vivo L-band ESR spin-label oximetry with a loop-gap resonator. Magn. Reson. Med. 1986, 3, 747–754. [Google Scholar] [CrossRef]
- He, G.; Dumitrescu, C.; Petryakov, S.; Deng, Y.; Kesselring, E.; Zweier, J.L. Transverse oriented electric field re-entrant resonator (TERR) with automatic tuning and coupling control for EPR spectroscopy and imaging of the beating heart. J. Magn. Reson. 2007, 187, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Berliner, J.L.; Fujii, H. Magnetic resonance imaging of biological specimens by electron paramagnetic resonance of nitroxide spin labels. Science 1985, 227, 517–519. [Google Scholar] [CrossRef]
- Berliner, L.J.; Fujii, H.; Wan, X.M.; Lukiewicz, S.J. Feasibility study of imaging a living murine tumor by electron paramagnetic resonance. Magn. Reson. Med. 1987, 4, 380–384. [Google Scholar] [CrossRef]
- Ishida, S.; Matsumoto, S.; Yokoyama, H.; Mori, N.; Kumashiro, H.; Tsuchihashi, N.; Ogata, T.; Yamada, M.; Ono, M.; Kitajima, T.; et al. An ESR-CT imaging of the head of a living rat receiving an administration of a nitroxide radical. Magn. Reson. Imaging 1992, 10, 109–114. [Google Scholar] [CrossRef]
- Takeshita, K.; Utsumi, H.; Hamada, A. ESR measurement of radical clearance in lung of whole mouse. Biochem. Biophys. Res. Commun. 1991, 177, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Alecci, M.; Colacicchi, S.; Indovina, P.L.; Momo, F.; Pavone, P.; Sotgiu, A. Three-dimensional in vivo ESR imaging in rats. Magn. Reson. Imaging 1990, 8, 59–63. [Google Scholar] [CrossRef]
- Matsumoto, K.; Hyodo, F.; Anzai, K.; Utsumi, H.; Mitchell, J.B.; Krishna, M.C. Brain redox imaging. Methods Mol. Biol. 2011, 711, 397–419. [Google Scholar] [CrossRef] [PubMed]
- Bacić, G.; Demsar, F.; Zolnai, Z.; Swartz, H.M. Contrast enhancement in ESR imaging: Role of oxygen. Magn. Reson. Med. Biol. 1988, 1, 55–65. [Google Scholar]
- Bacić, G.; Walczak, T.; Demsar, F.; Swartz, H.M. Electron spin resonance imaging of tissues with lipid-rich areas. Magn. Reson. Med. 1988, 8, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Hama, Y.; Matsumoto, K.; Murugesan, R.; Subramanian, S.; Devasahayam, N.; Koscielniak, J.W.; Hyodo, F.; Cook, J.A.; Mitchell, J.B.; Krishna, M.C. Continuous wave EPR oximetric imaging at 300 MHz using radiofrequency power saturation effects. Antioxid. Redox Signal. 2007, 9, 1709–1716. [Google Scholar] [CrossRef]
- Eaton, G.R.; Eaton, S.S. Electron spin-echo-detected EPR imaging. J. Magn. Reson. 1986, 67, 73–77. [Google Scholar] [CrossRef]
- Eaton, G.R.; Eaton, S.S. EPR imaging using T1 selectivity. J. Magn. Reson. 1987, 71, 271–275. [Google Scholar] [CrossRef]
- Maltempo, M.M.; Eaton, S.S.; Eaton, G.R. Spectral-spatial two-dimensional EPR imaging. J. Magn. Reson. 1987, 72, 449–455. [Google Scholar] [CrossRef]
- Maltempo, M.M. Differentiation of spectral and spatial components in EPR imaging using 2-D image reconstruction algorithm. J. Magn. Reson. 1986, 69, 156–161. [Google Scholar] [CrossRef]
- Ewert, U.; Crepeau, R.H.; Dunnam, C.R.; Xu, D.; Lee, S.; Freed, J.H. Fourier Transform electron spin resonance imaging. Chem. Phys. Lett. 1991, 184, 25–33. [Google Scholar] [CrossRef]
- Ewert, U.; Crepeau, R.H.; Dunnam, C.R.; Xu, D.; Lee, S.; Freed, J.H. Spatially resolved two-dimensional Fourier Transform electron spin resonance. Chem. Phys. Lett. 1991, 184, 34–40. [Google Scholar] [CrossRef]
- Murugesan, R.; Cook, J.A.; Devasahayam, N.; Afeworki, M.; Subramanian, S.; Tschudin, R.; Larsen, J.A.; Mitchell, J.B.; Russo, A.; Krishna, M.C. In vivo imaging of a stable paramagnetic probe by pulsed-radiofrequency electron paramagnetic resonance spectroscopy. Magn. Reson. Med. 1997, 38, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Mailer, C.; Sundramoorthy, S.V.; Pelizzari, C.A.; Halpern, H.J. Spin echo spectroscopic electron paramagnetic resonance imaging. Magn. Reson. Med. 2006, 55, 904–912. [Google Scholar] [CrossRef]
- Subramanian, S.; Yamada, K.; Irie, A.; Murugesan, R.; Cook, J.A.; Devasahayam, N.; Van Dam, G.M.; Mitchell, J.B.; Krishna, M.C. Noninvasive in vivo oximetric imaging by radiofrequency FT EPR. Magn. Reson. Med. 2002, 47, 1001–1008. [Google Scholar] [CrossRef]
- Yamada, K.; Murugesan, R.; Devasahayam, N.; Cook, J.A.; Mitchell, J.B.; Subramanian, S.; Krishna, M.C. Evaluation and comparison of pulsed and continuous wave radiofrequency electron paramagnetic resonance techniques for in vivo detection and imaging of free radicals. J. Magn. Reson. 2002, 154, 287–297. [Google Scholar] [CrossRef]
- Di Giuseppe, S.; Placidi, G.; Brivati, J.A.; Alecci, M.; Sotgiu, A. Pulsed EPR imaging: Image reconstruction using selective acquisition sequences. Phys. Med. Biol. 1999, 44, N137–N144. [Google Scholar] [CrossRef]
- Placidi, G.; Brivati, J.A.; Alecci, M.; Testa, L.; Sotgiu, A. Two-dimensional 220 MHz Fourier transform EPR imaging. Phys. Med. Biol. 1998, 43, 1845–1850. [Google Scholar] [CrossRef]
- Subramanian, S.; Devasahayam, N.; Murugesan, R.; Yamada, K.; Cook, J.; Taube, A.; Mitchell, J.B.; Lohman, J.A.; Krishna, M.C. Single-point (constant-time) imaging in radiofrequency Fourier transform electron paramagnetic resonance. Magn. Reson. Med. 2002, 48, 370–379. [Google Scholar] [CrossRef]
- Matsumoto, K.; Subramanian, S.; Devasahayam, N.; Aravalluvan, T.; Murugesan, R.; Cook, J.A.; Mitchell, J.B.; Krishna, M.C. Electron paramagnetic resonance imaging of tumor hypoxia: Enhanced spatial and temporal resolution for in vivo pO2 determination. Magn. Reson. Med. 2006, 55, 1157–1163. [Google Scholar] [CrossRef]
- Epel, B.; Halpern, H.J. Comparison of pulse sequences for R1-based electron paramagnetic resonance oxygen imaging. J. Magn. Reson. 2015, 254, 56–61. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Epel, B.; Bowman, M.K.; Mailer, C.; Halpern, H.J. Absolute oxygen R1e imaging in vivo with pulse electron paramagnetic resonance. Magn. Reson. Med. 2014, 72, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Ewert, U.; Herrling, T. Spectrally resolved EPR tomography with stationary gradient. Chem. Phys. Lett. 1986, 129, 516–520. [Google Scholar] [CrossRef]
- Stillman, A.E.; Levin, D.N.; Yang, D.B.; Marr, R.B.; Lauterbur, P.C. Back projection reconstruction of spectroscopic NMR images from incomplete sets of projections. J. Magn. Reson. 1986, 69, 168–175. [Google Scholar] [CrossRef]
- Bernardo, M.L.J.; Lauterbur, P.C.; Hedges, L.K. Experimental example of NMR spectroscopic imaging by projection reconstruction involving and intrinsic frequency dimen-sion. J. Magn. Reson. 1985, 61, 168–174. [Google Scholar]
- Lauterbur, P.C.; Levin, D.N.; Marr, R.B. Theory and simulation of NMR spectroscopic imaging and field plotting by projection reconstruction involving and intrinsic frequency dimension. J. Magn. Reson. 1984, 59, 536–541. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Chzhan, M.; Vij, K.; Shteynbuk, M.; Lefer, D.J.; Giannella, E.; Zweier, J.L. Three-dimensional spectral-spatial EPR imaging of free radicals in the heart: A technique for imaging tissue metabolism and oxygenation. Proc. Natl. Acad. Sci. USA 1994, 91, 3388–3392. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Chzhan, M.; Samouilov, A.; Wang, P.; Zweier, J.L. Mapping the spin-density and lineshape distribution of free radicals using 4D spectral-spatial EPR imaging. J. Magn. Reson. Ser. B 1995, 107, 116–125. [Google Scholar] [CrossRef]
- Matsumoto, K.; Utsumi, H. Development of separable electron spin resonance-computed tomography imaging for multiple radical species: An application to ·OH and ·NO. Biophys. J. 2000, 79, 3341–3349. [Google Scholar] [CrossRef][Green Version]
- Matsumoto, K.; Yahiro, T.; Yamada, K.; Utsumi, H. In Vivo EPR spectroscopic imaging for a liposomal drug delivery system. Magn. Reson. Med. 2005, 53, 1158–1165. [Google Scholar] [CrossRef]
- Emid, S.; Creyghton, J.H.N. High-resolution NMR imaging in solids. Physica B+C 1985, 128, 81–83. [Google Scholar] [CrossRef]
- Maresch, G.G.; Mehring, M.; Emid, S. High-resolution electron-spin-resonance imaging. Physica B+C 1986, 138, 261–263. [Google Scholar] [CrossRef]
- Matsumoto, K.; Chandrika, B.; Lohman, J.A.; Mitchell, J.B.; Krishna, M.C.; Subramanian, S. Application of continuous-wave EPR spectral-spatial image reconstruction techniques for in vivo oxymetry: Comparison of projection reconstruction and constant-time modalities. Magn. Reson. Med. 2003, 50, 865–874. [Google Scholar] [CrossRef]
- Matsumoto, K.; Kishimoto, S.; Devasahayam, N.; Chandramouli, G.V.R.; Ogawa, Y.; Matsumoto, S.; Krishna, M.C.; Subramanian, S. EPR-based oximetric imaging: A combination of single point-based spatial encoding and T1 weighting. Magn. Reson. Med. 2018, 80, 2275–2287. [Google Scholar] [CrossRef] [PubMed]
- Alecci, M.; Ferrari, M.; Quaresima, V.; Sotgiu, A.; Ursini, C.L. Simultaneous 280 MHz EPR imaging of rat organs during nitroxide free radical clearance. Biophys. J. 1994, 67, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Hyodo, F.; Matsumoto, A.; Koretsky, A.P.; Sowers, A.L.; Mitchell, J.B.; Krishna, M.C. High-resolution mapping of tumor redox status by magnetic resonance imaging using nitroxides as redox-sensitive contrast agents. Clin. Cancer Res. 2006, 12, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Sato-Akaba, H.; Kuwahara, Y.; Fujii, H.; Hirata, H. Half-life mapping of nitroxyl radicals with three-dimensional electron paramagnetic resonance imaging at an interval of 3.6 seconds. Anal. Chem. 2009, 81, 7501–7506. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, F.; Matsumoto, K.; Matsumoto, A.; Mitchell, J.B.; Krishna, M.C. Probing the intracellular redox status of tumors with magnetic resonance imaging and redox-sensitive contrast agents. Cancer Res. 2006, 66, 9921–9928. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Hyodo, F.; Subramanian, S.; Devasahayam, N.; Munasinghe, J.; Hyodo, E.; Gadisetti, C.; Cook, J.A.; Mitchell, J.B.; Krishna, M.C. Low-field paramagnetic resonance imaging of tumor oxygenation and glycolytic activity in mice. J. Clin. Investig. 2008, 118, 1965–1973. [Google Scholar] [CrossRef]
- Takakusagi, Y.; Naz, S.; Takakusagi, K.; Ishima, M.; Murata, H.; Ohta, K.; Miura, M.; Sugawara, F.; Sakaguchi, K.; Kishimoto, S.; et al. A Multimodal Molecular Imaging Study Evaluates Pharmacological Alteration of the Tumor Microenvironment to Improve Radiation Response. Cancer Res. 2018, 78, 6828–6837. [Google Scholar] [CrossRef]
- Yasui, H.; Matsumoto, S.; Devasahayam, N.; Munasinghe, J.P.; Choudhuri, R.; Saito, K.; Subramanian, S.; Mitchell, J.B.; Krishna, M.C. Low-field magnetic resonance imaging to visualize chronic and cycling hypoxia in tumor-bearing mice. Cancer Res. 2010, 70, 6427–6436. [Google Scholar] [CrossRef] [PubMed]
- Grucker, D. In Vivo detection of injected free radicals by Overhauser effect imaging. Magn. Reson. Med. 1990, 14, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Grucker, D.; Chambron, J. Oxygen imaging in perfused hearts by dynamic nuclear polarization. Magn. Reson. Imaging 1993, 11, 691–696. [Google Scholar] [CrossRef]
- Golman, K.; Petersson, J.S.; Ardenkjaer-Larsen, J.H.; Leunbach, I.; Wistrand, L.G.; Ehnholm, G.; Liu, K. Dynamic In Vivo oxymetry using overhauser enhanced MR imaging. J. Magn. Reson. Imaging 2000, 12, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Golman, K.; Leunbach, I.; Ardenkjaer-Larsen, J.H.; Ehnholm, G.J.; Wistrand, L.G.; Petersson, J.S.; Jarvi, A.; Vahasalo, S. Overhauser-enhanced MR imaging (OMRI). Acta Radiol. 1998, 39, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Ardenkjaer-Larsen, J.H.; Laursen, I.; Leunbach, I.; Ehnholm, G.; Wistrand, L.G.; Petersson, J.S.; Golman, K. EPR and DNP properties of certain novel single electron contrast agents intended for oximetric imaging. J. Magn. Reson. 1998, 133, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, S.; Krishna, M.C.; Khramtsov, V.V.; Utsumi, H.; Lurie, D.J. In Vivo Application of Proton-Electron Double-Resonance Imaging. Antioxid. Redox Signal. 2018, 28, 1345–1364. [Google Scholar] [CrossRef]
- Lurie, D.J.; Davies, G.R.; Foster, M.A.; Hutchison, J.M. Field-cycled PEDRI imaging of free radicals with detection at 450 mT. Magn. Reson. Imaging 2005, 23, 175–181. [Google Scholar] [CrossRef]
- Lurie, D.J.; Li, H.; Petryakov, S.; Zweier, J.L. Development of a PEDRI free-radical imager using a 0.38 T clinical MRI system. Magn. Reson. Med. 2002, 47, 181–186. [Google Scholar] [CrossRef]
- Lurie, D.J.; Foster, M.A.; Yeung, D.; Hutchison, J.M. Design, construction and use of a large-sample field-cycled PEDRI imager. Phys. Med. Biol. 1998, 43, 1877–1886. [Google Scholar] [CrossRef]
- Matsumoto, S.; Yasui, H.; Batra, S.; Kinoshita, Y.; Bernardo, M.; Munasinghe, J.P.; Utsumi, H.; Choudhuri, R.; Devasahayam, N.; Subramanian, S.; et al. Simultaneous imaging of tumor oxygenation and microvascular permeability using Overhauser enhanced MRI. Proc. Natl. Acad. Sci. USA 2009, 106, 17898–17903. [Google Scholar] [CrossRef] [PubMed]
- Ardenkjaer-Larsen, J.H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M.H.; Servin, R.; Thaning, M.; Golman, K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. USA 2003, 100, 10158–10163. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Nonaka, H.; Takakusagi, Y.; Sando, S. Design of Nuclear Magnetic Resonance Molecular Probes for Hyperpolarized Bioimaging. Angew. Chem. Int. Ed. 2021, 60, 14779–14799. [Google Scholar] [CrossRef] [PubMed]
- Keshari, K.R.; Wilson, D.M. Chemistry and biochemistry of 13C hyperpolarized magnetic resonance using dynamic nuclear polarization. Chem. Soc. Rev. 2014, 43, 1627–1659. [Google Scholar] [CrossRef]
- Golman, K.; Zandt, R.i.’t.; Thaning, M. Real-time metabolic imaging. Proc. Natl. Acad. Sci. USA 2006, 103, 11270–11275. [Google Scholar] [CrossRef]
- De Kouchkovsky, I.; Chen, H.Y.; Ohliger, M.A.; Wang, Z.J.; Bok, R.A.; Gordon, J.W.; Larson, P.E.Z.; Frost, M.; Okamoto, K.; Cooperberg, M.R.; et al. Hyperpolarized 1-[(13)C]-Pyruvate Magnetic Resonance Imaging Detects an Early Metabolic Response to Immune Checkpoint Inhibitor Therapy in Prostate Cancer. Eur. Urol. 2022, 81, 219–221. [Google Scholar] [CrossRef]
- Aggarwal, R.; Vigneron, D.B.; Kurhanewicz, J. Hyperpolarized 1-[(13)C]-Pyruvate Magnetic Resonance Imaging Detects an Early Metabolic Response to Androgen Ablation Therapy in Prostate Cancer. Eur. Urol. 2017, 72, 1028–1029. [Google Scholar] [CrossRef]
- Nelson, S.J.; Kurhanewicz, J.; Vigneron, D.B.; Larson, P.E.; Harzstark, A.L.; Ferrone, M.; van Criekinge, M.; Chang, J.W.; Bok, R.; Park, I.; et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-(1)(3)C]pyruvate. Sci. Transl. Med. 2013, 5, 198ra108. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Oshima, N.; Ishida, R.; Kishimoto, S.; Beebe, K.; Brender, J.R.; Yamamoto, K.; Urban, D.; Rai, G.; Johnson, M.S.; Benavides, G.; et al. Dynamic Imaging of LDH Inhibition in Tumors Reveals Rapid In Vivo Metabolic Rewiring and Vulnerability to Combination Therapy. Cell Rep. 2020, 30, 1798–1810.e4. [Google Scholar] [CrossRef]
- Saito, K.; Matsumoto, S.; Takakusagi, Y.; Matsuo, M.; Morris, H.D.; Lizak, M.J.; Munasinghe, J.P.; Devasahayam, N.; Subramanian, S.; Mitchell, J.B.; et al. 13C-MR Spectroscopic Imaging with Hyperpolarized [1-13C]pyruvate Detects Early Response to Radiotherapy in SCC Tumors and HT-29 Tumors. Clin. Cancer Res. 2015, 21, 5073–5081. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Ohliger, M.A.; Larson, P.E.Z.; Gordon, J.W.; Bok, R.A.; Slater, J.; Villanueva-Meyer, J.E.; Hess, C.P.; Kurhanewicz, J.; Vigneron, D.B. Hyperpolarized 13C MRI: State of the Art and Future Directions. Radiology 2019, 291, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Kurhanewicz, J.; Vigneron, D.B.; Ardenkjaer-Larsen, J.H.; Bankson, J.A.; Brindle, K.; Cunningham, C.H.; Gallagher, F.A.; Keshari, K.R.; Kjaer, A.; Laustsen, C.; et al. Hyperpolarized 13C MRI: Path to Clinical Translation in Oncology. Neoplasia 2019, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, F.A.; Kettunen, M.I.; Hu, D.E.; Jensen, P.R.; Zandt, R.I.; Karlsson, M.; Gisselsson, A.; Nelson, S.K.; Witney, T.H.; Bohndiek, S.E.; et al. Production of hyperpolarized [1,4-13C2]malate from [1,4-13C2]fumarate is a marker of cell necrosis and treatment response in tumors. Proc. Natl. Acad. Sci. USA 2009, 106, 19801–19806. [Google Scholar] [CrossRef] [PubMed]
- Clatworthy, M.R.; Kettunen, M.I.; Hu, D.E.; Mathews, R.J.; Witney, T.H.; Kennedy, B.W.; Bohndiek, S.E.; Gallagher, F.A.; Jarvis, L.B.; Smith, K.G.; et al. Magnetic resonance imaging with hyperpolarized [1,4-13C2]fumarate allows detection of early renal acute tubular necrosis. Proc. Natl. Acad. Sci. USA 2012, 109, 13374–13379. [Google Scholar] [CrossRef]
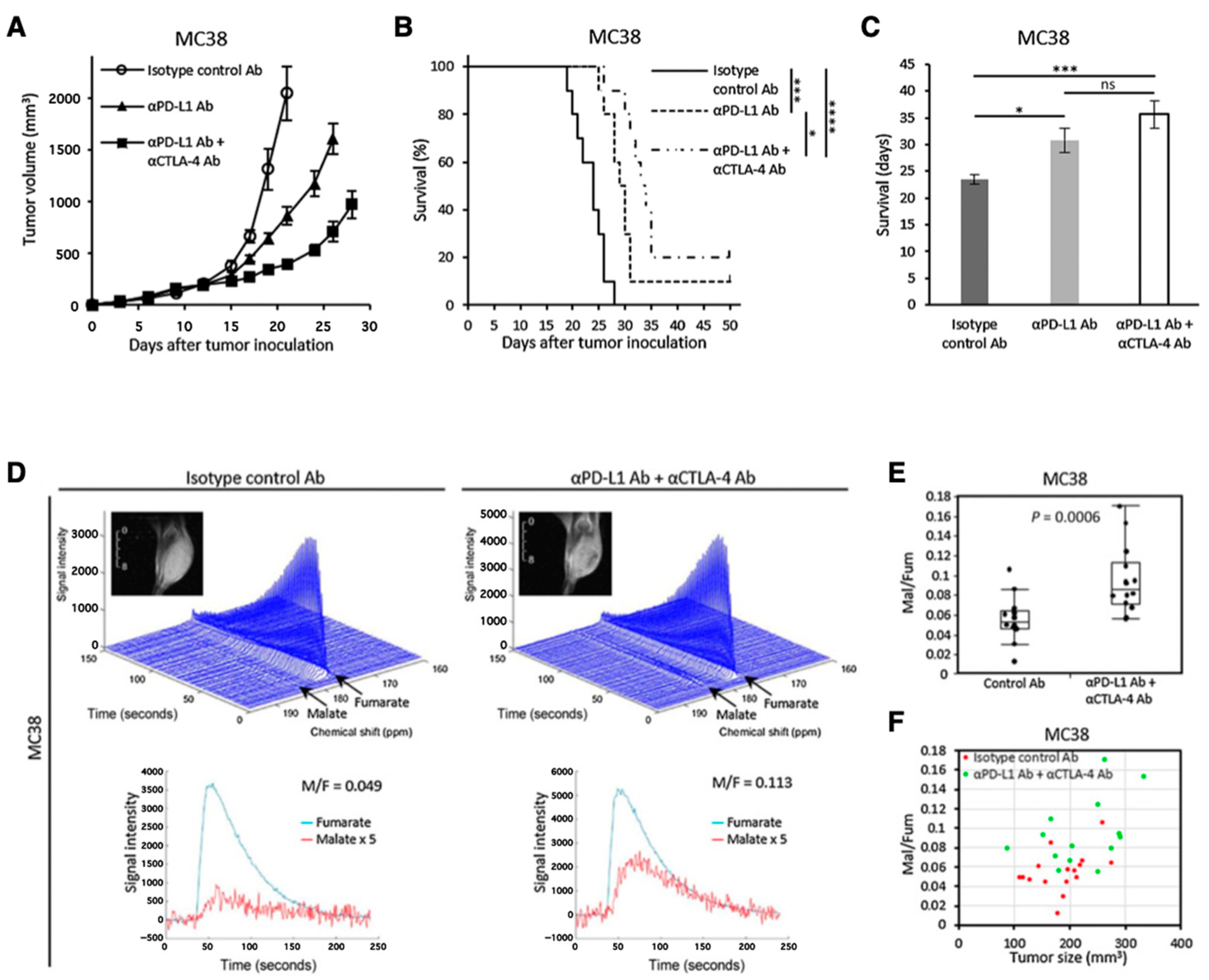
- Saida, Y.; Brender, J.R.; Yamamoto, K.; Mitchell, J.B.; Krishna, M.C.; Kishimoto, S. Multimodal Molecular Imaging Detects Early Responses to Immune Checkpoint Blockade. Cancer Res. 2021, 81, 3693–3705. [Google Scholar] [CrossRef]
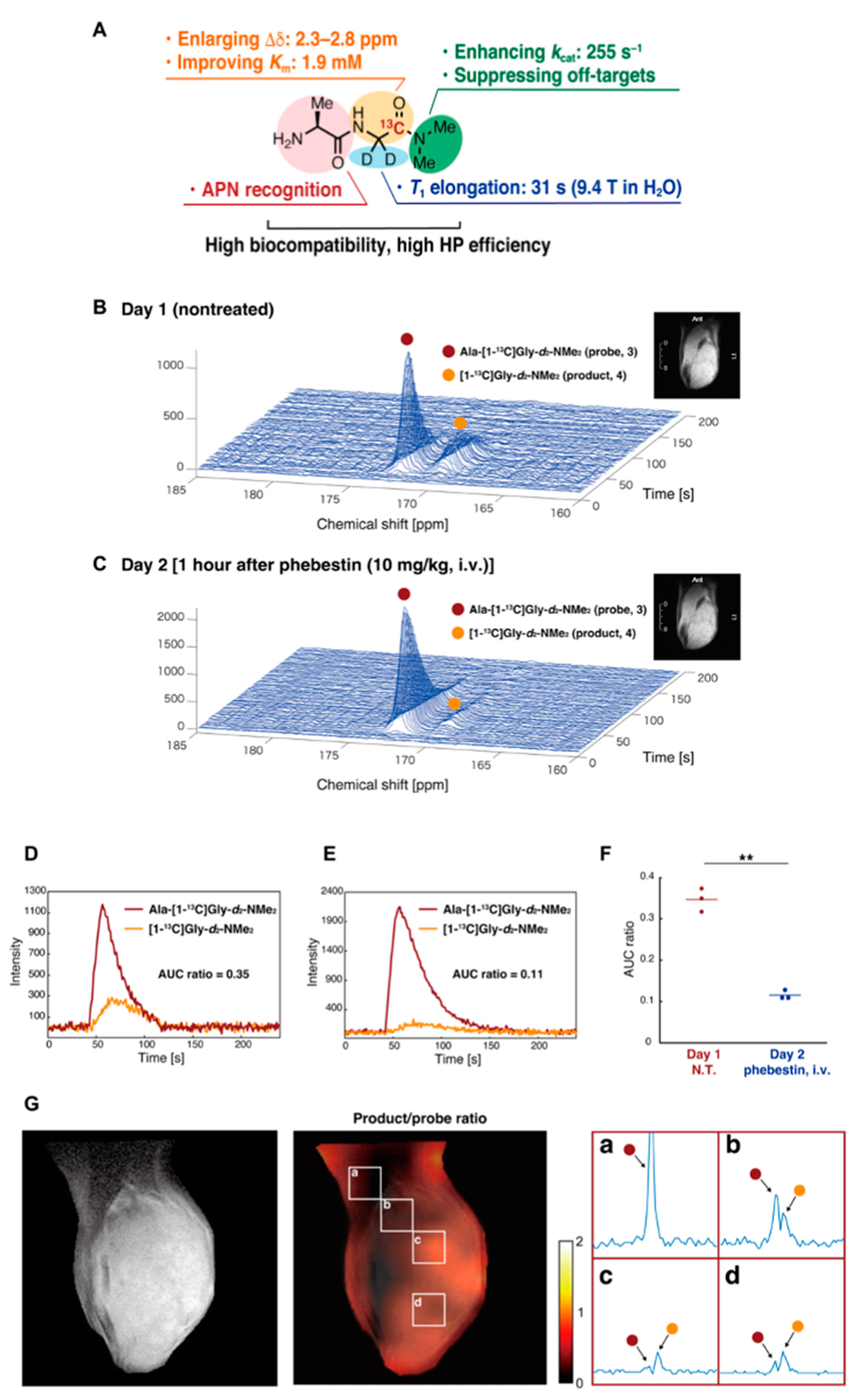
- Saito, Y.; Yatabe, H.; Tamura, I.; Kondo, Y.; Ishida, R.; Seki, T.; Hiraga, K.; Eguchi, A.; Takakusagi, Y.; Saito, K.; et al. Structure-guided design enables development of a hyperpolarized molecular probe for the detection of aminopeptidase N activity in vivo. Sci. Adv. 2022, 8, eabj2667. [Google Scholar] [CrossRef]
- Khan, N.; Hou, H.; Swartz, H.M.; Kuppusamy, P. Direct and repeated measurement of heart and brain oxygenation using in vivo EPR oximetry. Methods Enzymol. 2015, 564, 529–552. [Google Scholar] [CrossRef]
- Swartz, H.M.; Liu, K.J.; Goda, F.; Walczak, T. India ink: A potential clinically applicable EPR oximetry probe. Magn. Reson. Med. 1994, 31, 229–232. [Google Scholar] [CrossRef]
- Schaner, P.E.; Williams, B.B.; Chen, E.Y.; Pettus, J.R.; Schreiber, W.A.; Kmiec, M.M.; Jarvis, L.A.; Pastel, D.A.; Zuurbier, R.A.; DiFlorio-Alexander, R.M.; et al. First-in-human study in cancer patients establishing the feasibility of oxygen measurements in tumors using electron paramagnetic resonance with the OxyChip. Front. Oncol. 2021, 11, 743256. [Google Scholar] [CrossRef]
- Mignion, L.; Desmet, C.M.; Harkemanne, E.; Tromme, I.; Joudiou, N.; Wehbi, M.; Baurain, J.F.; Gallez, B. Noninvasive detection of the endogenous free radical melanin in human skin melanomas using electron paramagnetic resonance (EPR). Free Radic. Biol. Med. 2022, 190, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Pursley, R.; Enomoto, A.; Wu, H.; Brender, J.R.; Pohida, T.; Subramanian, S.; Krishna, M.C.; Devasahayam, N. Towards reduction of SAR in scaling up in vivo pulsed EPR imaging to larger objects. J. Magn. Reson. 2019, 299, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Elas, M.; Magwood, J.M.; Butler, B.; Li, C.; Wardak, R.; DeVries, R.; Barth, E.D.; Epel, B.; Rubinstein, S.; Pelizzari, C.A.; et al. EPR Oxygen Images Predict Tumor Control by a 50% Tumor Control Radiation Dose. Cancer Res. 2013, 73, 5328–5335. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, S.; Bernardo, M.; Saito, K.; Koyasu, S.; Mitchell, J.B.; Choyke, P.L.; Krishna, M.C. Evaluation of oxygen dependence on in vitro and in vivo cytotoxicity of photoimmunotherapy using IR-700–antibody conjugates. Free Radic. Biol. Med. 2015, 85, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, S.; Brender, J.R.; Chandramouli, G.V.; Saida, Y.; Yamamoto, K.; Mitchell, J.B.; Krishna, M.C. Hypoxia-activated prodrug evofosfamide treatment in pancreatic ductal adenocarcinoma xenografts alters the tumor redox status to potentiate radiotherapy. Antioxid. Redox Signal. 2021, 35, 904–915. [Google Scholar] [CrossRef] [PubMed]

| Modality | Application | Observation Object | Advantageous Feature | Reference | Possibility of Clinical Application |
|---|---|---|---|---|---|
| CW EPRI | Distribution mapping | Signal intensity | Direct and quantitative detection of free radical species | [41,61,62,63,64,96,97,98] | Applying EPR to whole or stem of human would be difficult because of lower intensity due to lower RF frequency worked on larger resonator [14]. However, a limited partial application would be available for human [130,131,132]. |
| Redox mapping | Signal decay rate | Reduction rate observed based on the direct and quantitative detection of free radical species | [41,97,98] | ||
| Oxygen mapping | EPR linewidth (Relaxation time) | Wide range of free radical species as O2-probe. | [88,89,94] | ||
| Signal intensity loss by RF power saturation (Relaxation behavior) | Simple acquisition process with only two images observed under different RF power | [67] | |||
| Separately mapping multiple free radical species | Difference of EPR resonant field | Wide spectral window has wide applicability. | [90,91] | ||
| Pulsed EPRI | Distribution mapping | Signal intensity | Rapid acquisition is available. | [74,77,78,79] | |
| Oxygen mapping | T2* and/or T1 relaxation time | Quantitative and high resolution O2-mapping | [19,25,40,75,76,80,81,95,100,101,102,134,135,136] | ||
| OMRI/PEDRI | Oxygen mapping | Electron relaxation | Quantitative and high resolution O2-mapping | [26,103,104,105,106,107,108,112] | Application for human would be possible, when SAR of EPR excitation was reduced accordingly, and when the free radical compound used for hyperpolarization was approved [14]. |
| Redox mapping | Signal decay rate | High spatial and temporal resolution, and slice selection | [28,29,30,43,108] | ||
| Separately mapping multiple free radical species | Difference of EPR resonant field | High spatial and temporal resolution, and slice selection | [27,108] | ||
| Hyperpolarized 13C MRI | Mapping metabolic shift | Chemical shift of 13C-labeled compounds | Extracorporeally hyperpolarized 13C-labeled compound | [25,40,31,113,114,115,116,117,118,119,121,122,123,124,125,126,127,128] | Application for human patients has been reported [117,118,119]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takakusagi, Y.; Kobayashi, R.; Saito, K.; Kishimoto, S.; Krishna, M.C.; Murugesan, R.; Matsumoto, K.-i. EPR and Related Magnetic Resonance Imaging Techniques in Cancer Research. Metabolites 2023, 13, 69. https://doi.org/10.3390/metabo13010069
Takakusagi Y, Kobayashi R, Saito K, Kishimoto S, Krishna MC, Murugesan R, Matsumoto K-i. EPR and Related Magnetic Resonance Imaging Techniques in Cancer Research. Metabolites. 2023; 13(1):69. https://doi.org/10.3390/metabo13010069
Chicago/Turabian StyleTakakusagi, Yoichi, Ryoma Kobayashi, Keita Saito, Shun Kishimoto, Murali C. Krishna, Ramachandran Murugesan, and Ken-ichiro Matsumoto. 2023. "EPR and Related Magnetic Resonance Imaging Techniques in Cancer Research" Metabolites 13, no. 1: 69. https://doi.org/10.3390/metabo13010069
APA StyleTakakusagi, Y., Kobayashi, R., Saito, K., Kishimoto, S., Krishna, M. C., Murugesan, R., & Matsumoto, K.-i. (2023). EPR and Related Magnetic Resonance Imaging Techniques in Cancer Research. Metabolites, 13(1), 69. https://doi.org/10.3390/metabo13010069







