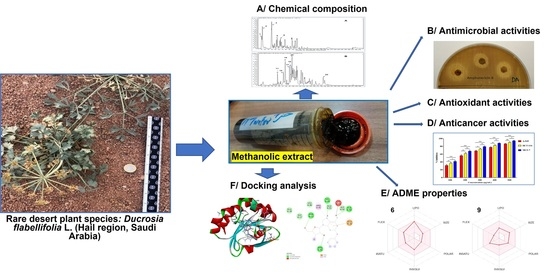
Chemical Composition of Ducrosia flabellifolia L. Methanolic Extract and Volatile Oil: ADME Properties, In Vitro and In Silico Screening of Antimicrobial, Antioxidant and Anticancer Activities
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of D. flabellifolia EO
2.2. Chemical Composition of D. flabellifolia Methanolic Extract
2.3. Antimicrobial Activities of D. flabellifolia Methanolic Extract
2.4. Antioxidant Activities of D. flabellifolia Methanolic Extract
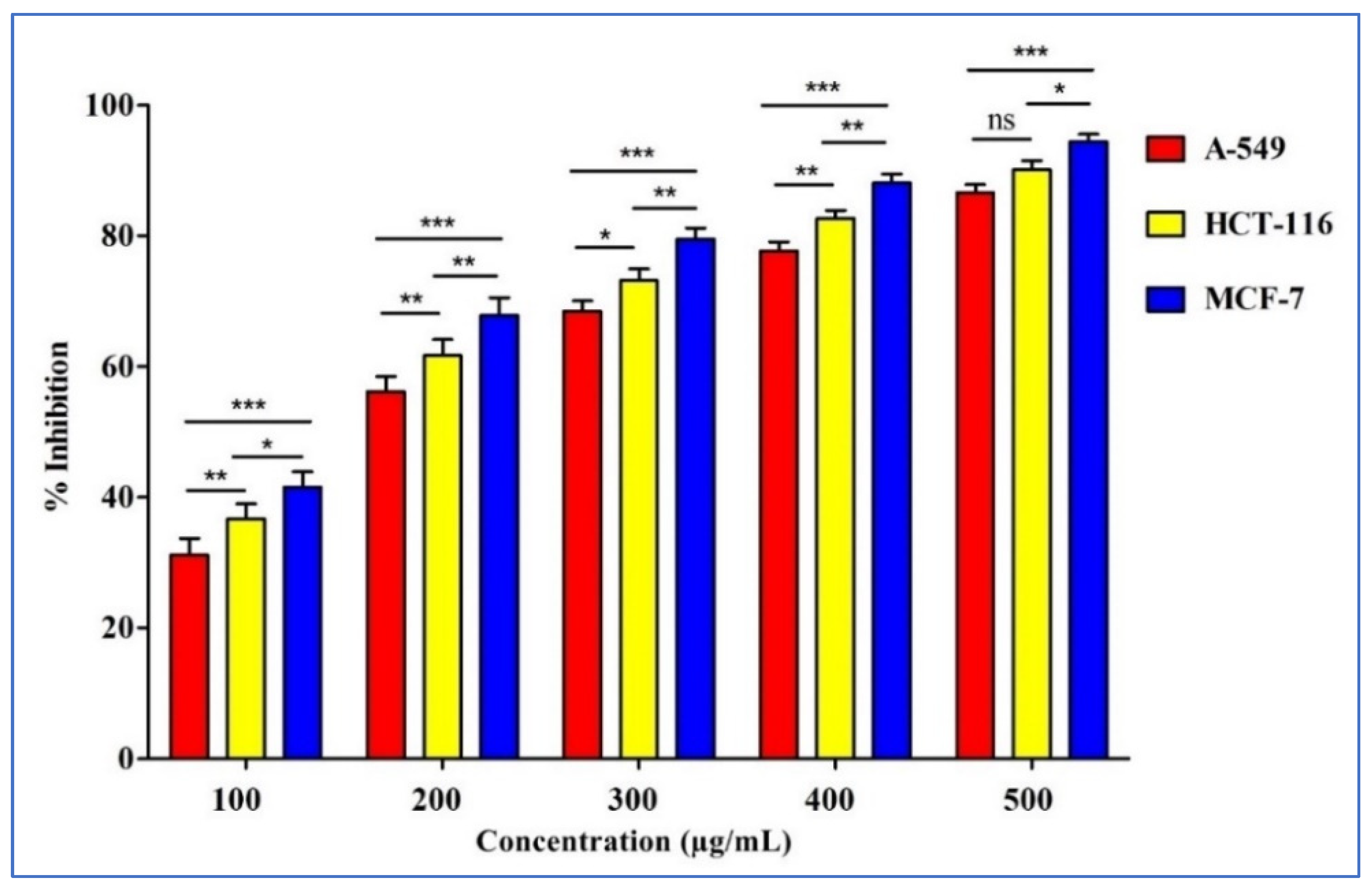
2.5. Anticancer Activities of D. flabellifolia Methanolic Extract
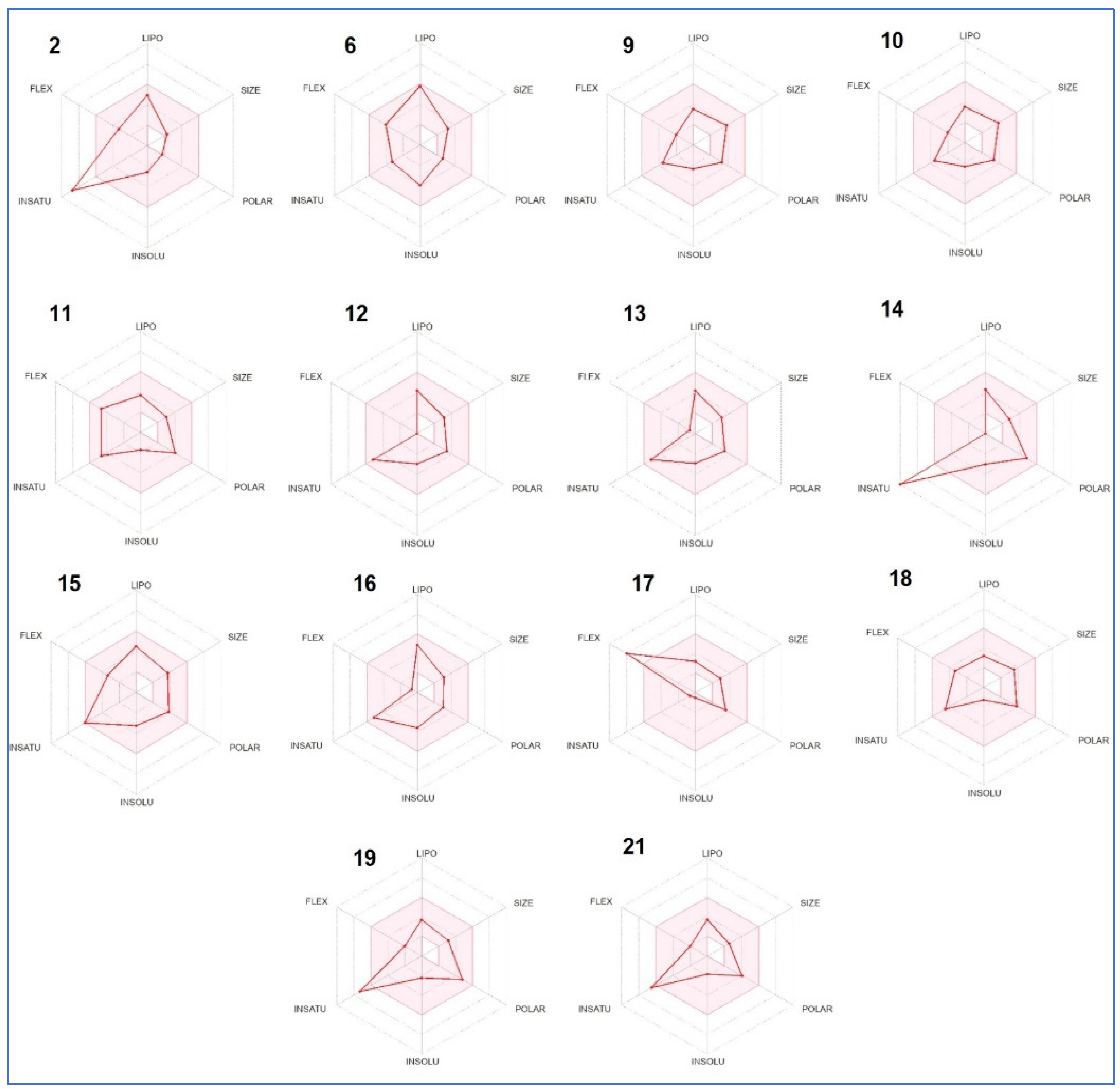
2.6. ADME Predictions
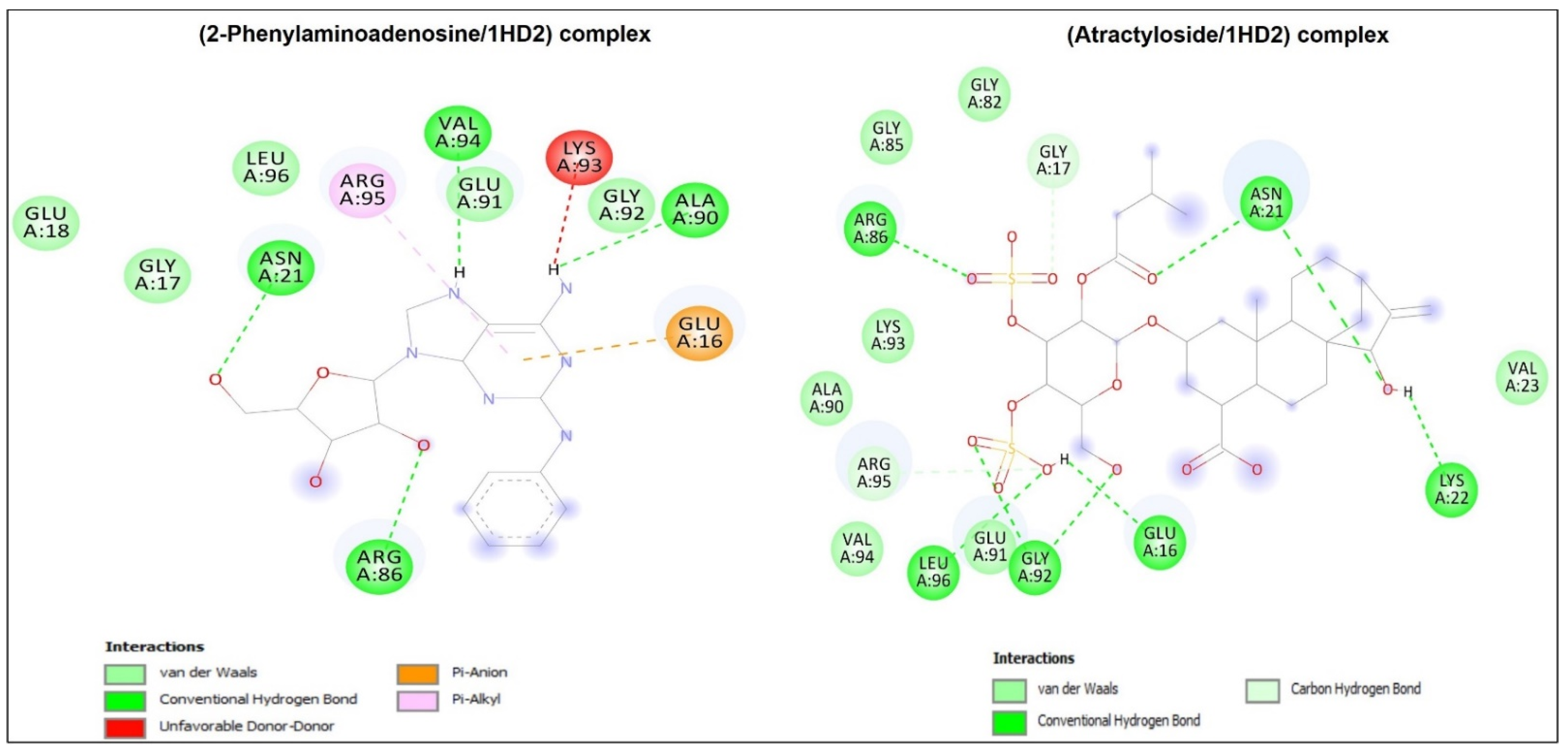
2.7. Molecular Docking Study
3. Discussion
4. Materials and Methods
4.1. Plant Material Sampling
4.2. Phytochemical Composition
4.2.1. Composition of the Essential Oil
4.2.2. Composition of the Methanolic Extract
4.3. Antimicrobial Activities of D. flabellifolia Methanolic Extract
4.4. Antioxidant Activities
4.5. Anticancer Activity
4.6. Computational Study
4.6.1. ADME Properties
4.6.2. Molecular Docking Study
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mottaghipisheh, J.; Boveiri Dehsheikh, A.; Mahmoodi Sourestani, M.; Kiss, T.; Hohmann, J.; Csupor, D. Ducrosia spp., Rare Plants with Promising Phytochemical and Pharmacological Characteristics: An Updated Review. Pharmaceuticals 2020, 13, 175. [Google Scholar] [CrossRef]
- Al-Shudiefat, M.; Al-Khalidi, K.; Abaza, I.; Afifi, F.U. Chemical composition analysis and antimicrobial screening of the essential oil of a rare plant from Jordan: Ducrosia flabellifolia. Anal. Lett. 2013, 47, 422–432. [Google Scholar] [CrossRef]
- Shahabipour, S.; Firuzi, O.; Asadollahi, M.; Faghihmirzaei, E.; Javidnia, K. Essential oil composition and cytotoxic activity of Ducrosia anethifolia and Ducrosia flabellifolia from Iran. J. Essent Oil Res. 2013, 25, 160–163. [Google Scholar] [CrossRef]
- Talib, W.H.; Issa, R.A.; Kherissat, F.; Mahasne, A.M. Jordanian Ducrosia flabellifolia inhibits proliferation of breast cancer cells by inducing apoptosis. Br. J. Med. Med. Res. 2013, 3, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Snoussi, M.; Ahmad, I.; Aljohani, A.M.A.; Patel, H.; Abdulhakeem, M.A.; Alhazmi, Y.S.; Tepe, B.; Adnan, M.; Siddiqui, A.J.; Sarikurkcu, C.; et al. Phytochemical Analysis, Antioxidant, and Antimicrobial Activities of Ducrosia flabellifolia: A Combined Experimental and Computational Approaches. Antioxidants 2022, 11, 2174. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Bergers, G.; Fendt, S.M. The metabolism of cancer cells during metastasis. Nat. Rev. Cancer 2021, 21, 162–180. [Google Scholar] [CrossRef]
- World Health Organization. Cancer. 2018. Available online: https://www.who.int/health-topics/cancer#tab=tab_1 (accessed on 27 September 2022).
- Mattiuzzi, C.; Lippi, G. Current cancer epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217. [Google Scholar] [CrossRef]
- Othman, I.M.M.; Gad-Elkareem, M.A.M.; Anouar, E.H.; Snoussi, M.; Aouadi, K.; Kadri, A. Novel fused pyridine derivatives containing pyrimidine moiety as prospective tyrosyl-tRNA synthetase inhibitors: Design, synthesis, pharmacokinetics, and molecular docking studies. J. Mol. Struct. 2020, 1219, 128651. [Google Scholar] [CrossRef]
- Othman, I.M.M.; Gad-Elkareem, M.A.M.; Anouar, E.H.; Aouadi, K.; Kadri, A.; Snoussi, M. Design, synthesis ADMET and molecular docking of new imidazo[4,5-b]pyridine-5-thione derivatives as potential tyrosyl-tRNA synthetase inhibitors. Bioorg. Chem. 2020, 102, 104105. [Google Scholar] [CrossRef] [PubMed]
- Othman, I.M.M.; Gad-Elkareem, M.A.M.; Radwan, H.A.; Badraoui, R.; Aouadi, K.; Snoussi, M.; Kadri, A. Synthesis, Structure-Activity Relationship and in silico Studies of Novel Pyrazolothiazole and Thiazolopyridine Derivatives as Prospective Antimicrobial and Anticancer Agents. ChemistrySelect 2021, 6, 7860–7872. [Google Scholar] [CrossRef]
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.-M.; Dayem, A.A.; Cho, S.-G. Correlation between oxidative stress, nutrition, and cancer initiation. Int. J. Mol. Sci. 2017, 18, 1544. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Wu, Z.X.; Chen, Y.; Chen, Z.-S. Drug resistance: From bacteria to cancer. Mol. Biomed. 2021, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Van den Boogaard, W.M.C.; Komninos, D.S.J.; Vermeij, W.P. Chemotherapy side-effects: Not all DNA damage is equal. Cancers 2022, 14, 627. [Google Scholar] [CrossRef]
- Hajlaoui, H.; Arraouadi, S.; Mighri, H.; Ghannay, S.; Aouadi, K.; Adnan, M.; Kadri, A. HPLC-MS profiling, antioxidant, antimicrobial, antidiabetic, and cytotoxicity activities of Arthrocnemum indicum (Willd.) Moq. extracts. Plants 2022, 11, 232. [Google Scholar] [CrossRef]
- Alminderej, F.; Bakari, S.; Almundarij, T.I.; Snoussi, M.; Aouadi, K.; Kadri, A. Antimicrobial and wound healing potential of a new chemotype from Piper cubeba L. essential oil and in silico study on S. aureus tyrosyl-tRNA Synthetase Protein. Plants 2021, 10, 205. [Google Scholar] [CrossRef]
- Haddaji, F.; Papetti, A.; Noumi, E.; Colombo, R.; Deshpande, S.; Aouadi, K.; Adnan, M.; Kadri, A.; Selmi, B.; Snoussi, M. Bioactivities and in silico study of Pergularia tomentosa L. phytochemicals as potent antimicrobial agents targeting type IIA topoisomerase, TyrRS, and Sap1 virulence proteins. Environ. Sci. Poll. Res. 2021, 28, 25349–25367. [Google Scholar] [CrossRef]
- Eddouks, M.; Chattopadhyay, D.; De Feo, V.; Cho, W.C. Medicinal plants in the prevention and treatment of chronic diseases. Evid. Based Complement. Alternat. Med. 2012, 2012, 458274. [Google Scholar] [CrossRef]
- Mottaghipisheh, J.; Nové, M.; Spengler, G.; Kúsz, N.; Hohmann, J.; Csupora, D. Antiproliferative and cytotoxic activities of furocoumarins of Ducrosia anethifolia. Pharm. Biol. 2018, 56, 658–664. [Google Scholar] [CrossRef]
- Al-Ghamdi, F.A.; Abdelwahab, A.T. Volatile oil composition from stems, leaves and flowers of Ducrosia flabellifolia Boiss. From northern border of Saudi Arabia. Int. J. Appl. Biol. Pharm. Technol. 2014, 5, 296–300. [Google Scholar]
- Al-Whibi, M.; Moubayed, N.M.S.; Zahrani, H.; Mashhour, A. Antibacterial and cytotoxic activities of Ducrosia anethifolia: A potential biomedicine against selected human pathogens and cancer cell lines. Biomedica 2019, 35, 203–209. [Google Scholar]
- Gatsing, D.; Tchakoute, V.; Ngamga, D.; Kuiate, J.R.; Tamokou, J.D.D.; Nji-Nkah, B.F.; Tchouanguep, F.M.; Fodouop, S.P.C. In vitro antibacterial activity of Crinum purpurascens Herb. leaf extract against the Salmonella species causing typhoid fever and its toxicological evaluation. Iran. J. Med. Sci. 2009, 34, 126–136. [Google Scholar]
- Parveen, M.; Ghalib, R.M.; Khanam, Z.; Mehdi, S.H.; Ali, M. A Novel antimicrobial agent from the leaves of Peltophorum vogelianum (Benth.). Nat. Prod. Res. 2010, 24, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Moroh, J.L.; Bahi, C.; Dje, K.; Loukou, Y.G.; Guide Guina, F. Etude de l’activité antibactérienne de l’extrait acétatique de Morinda morindoides (Baker) Milne-Redheat (Rubiaceae) sur la croissance in vitro des souches d’Escherichia coli. Bull. Soc. R. Sci. Liege 2008, 77, 44–61. [Google Scholar]
- Elsharkawy, E.R.; Abdallah, E.M.; Shiboob, M.H.; Alghanem, S. Phytochemical, antioxidant and antibacterial potential of Ducrosia anethifolia in Northern Border region of Saudi Arabia. J. Pharm. Res. Int. 2019, 31, 1–8. [Google Scholar] [CrossRef]
- Alsaggaf, M.S. Application of wild Saudi plant extracts to control antibiotic resistant Staphylococcus aureus. Egypt. J. Exp. Biol. (Bot.) 2018, 14, 29–35. [Google Scholar] [CrossRef]
- Mothana, R.A.; Nasr, F.A.; Khaled, J.M.; Noman, O.M.; Abutaha, N.; Al-Rehaily, A.J.; Almarfadi, O.M.; Kurkcuoglu, M. Ducrosia ismaelis Asch. essential oil: Chemical composition profile and anticancer, antimicrobial and antioxidant potential assessment. Open Chem. 2020, 18, 175–184. [Google Scholar] [CrossRef]
- Mhadhbi, N.; Issaoui, N.; Hamadou, W.S.; Alam, J.M.; Elhadi, A.S.; Adnan, M.; Naїli, H.; Badraoui, R. Physico-Chemical Properties, Pharmacokinetics, Molecular Docking and In-Vitro Pharmacological Study of a Cobalt (II) Complex Based on 2-Aminopyridine. ChemistrySelect 2022, 7, e202103592. [Google Scholar] [CrossRef]
- Badraoui, R.; Adnan, M.; Bardakci, F.; Alreshidi, M.M. Chloroquine and hydroxychloroquine interact differently with ACE2 domains reported to bind with the coronavirus spike protein: Mediation by ACE2 polymorphism. Molecules 2021, 26, 673. [Google Scholar] [CrossRef]
- Badraoui, R.; Saeed, M.; Bouali, N.; Hamadou, W.S.; Elkahoui, S.; Alam, M.J.; Siddiqui, A.J.; Adnan, M.; Saoudi, M.; Rebai, T. Expression Profiling of Selected Immune Genes and Trabecular Microarchitecture in Breast Cancer Skeletal Metastases Model: Effect of α–Tocopherol Acetate Supplementation. Calcif. Tissue Int. 2022, 110, 475–488. [Google Scholar] [CrossRef]
- Hamrita, B.; Noumi, B.; Papetti, A.; Badraoui, R.; Bouslama, L.; Ben Tekfa, M.I.; Hamdi, A.; Patel, M.; Elasbali, A.M.; Adnan, M.; et al. Phytochemical Analysis, Antioxidant, Antimicrobial, and Anti-Swarming Properties of Hibiscus sabdariffa L. Calyx Extracts: In Vitro and In Silico Modelling Approaches. Evid. Based Complement. Alternat. Med. 2022, 2022, 1252672. [Google Scholar] [CrossRef] [PubMed]
- Jedli, O.; Ben-Nasr, H.; Zammel, N.; Rebai, T.; Saoudi, M.; Elkahoui, S.; Jamal, A.; Siddiqui, A.J.; Sulieman, A.E.; Alreshidi, M.M.; et al. Attenuation of ovalbumin-induced inflammation and lung oxidative injury in asthmatic rats by Zingiber officinale extract: Combined in silico and in vivo study on antioxidant potential, STAT6 and TNF-pathways. 3 Biotech. 2022, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- Hchicha, K.; Korb, M.; Badraoui, R.; Naïli, H. A novel sulfate-bridged binuclear copper (II) complex: Structure, optical, ADMET and in vivo approach in a murine model of bone metastasis. New J. Chem. 2021, 45, 13775–13784. [Google Scholar] [CrossRef]
- Zammel, N.; Saeed, M.; Bouali, N.; Elkahoui, S.; Alam, J.M.; Rebai, T.; Kausar, M.A.; Adnan, M.; Siddiqui, A.J.; Badraoui, R. Antioxidant and Anti-Inflammatory Effects of Zingiber officinale roscoe and Allium subhirsutum: In Silico, Biochemical and Histological Study. Foods 2021, 10, 1383. [Google Scholar] [CrossRef]
- Akacha, A.; Badraoui, R.; Rebai, T.; Zourgui, L. Effect of Opuntia ficus indica extract on methotrexate-induced testicular injury: A biochemical, docking and histological study. J. Biomol. Struct. Dyn. 2022, 40, 4341–4351. [Google Scholar] [CrossRef] [PubMed]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy; Allured: Carol Stream, IL, USA, 2001. [Google Scholar]
- Noumi, E.; Snoussi, M.; Anouar, E.H.; Alreshidi, M.; Veettil, V.N.; Elkahoui, S.; Adnan, M.; Patel, M.; Kadri, A.; Aouadi, K.; et al. HR-LCMS-based metabolite profiling, antioxidant, and anticancer properties of Teucrium polium L. methanolic extract: Computational and in vitro study. Antioxidants 2020, 9, 1089. [Google Scholar] [CrossRef]
- Mseddi, K.; Alimi, F.; Noumi, E.; Veettil, V.N.; Deshpande, S.; Adnan, M.; Hamdi, A.; Elkahoui, S.; Alghamdi, A.; Kadri, A.; et al. Thymus musilii Velen. as a promising source of potent bioactive compounds with its pharmacological properties: In vitro and in silico analysis. Arab. J. Chem. 2020, 13, 6782–6801. [Google Scholar] [CrossRef]
- Koleva, I.I.; van Beek, T.A.; Linssen, J.P.; de Groot, A.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef]
- Hamdi, A.; Viaene, J.; Mahjoub, M.A.; Majouli, K.; Gad, M.H.H.; Kharbach, M.; Demeyer, K.; Marzouk, Z.; Vander Heyden, Y. Polyphenolic contents, antioxidant activities and UPLC–ESI–MS analysis of Haplophyllum tuberculatum A. Juss leaves extracts. Int. J. Biol. Macromol. 2018, 106, 1071–1079. [Google Scholar] [CrossRef]
- Hamadou, W.S.; Bouali, N.; Badraoui, R.; Hadj Lajimi, R.; Hamdi, A.; Alreshidi, M.; Patel, M.; Adnan, M.; Siddiqui, A.J.; Noumi, E.; et al. Chemical composition and the anticancer, antimicrobial, and antioxidant properties of acacia honey from the Hail region: The in vitro and in silico investigation. Evid. Based Complement. Alternat. Med. 2022, 2022, 1518511. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]

| N° | Compound | RI * | D. flabellifolia EO | Molecular Weight | Chemical Formula |
|---|---|---|---|---|---|
| 1 | Nonane | 900 | 0.68 | 128.259 | C9H20 |
| 2 | α-pinene | 940 | 5.83 | 136.238 | C10H16 |
| 3 | Sabinene | 969 | 0.85 | 136.23 | C10H16 |
| 4 | o-cymene | 976 | 0.51 | 134.22 | C10H14 |
| 5 | β-pinene | 983 | 0.38 | 136.278 | C10H16 |
| 6 | β-myrcene | 992 | 2.87 | 136.238 | C10H16 |
| 7 | α-phellandrene | 1005 | 0.40 | 136.23 | C10H16 |
| 8 | β-phellandrene | 1029 | 5.76 | 136.23 | C10H16 |
| 9 | Fenchone | 1093 | 1.81 | 152.23 | C10H16O |
| 10 | 4-undecene | 1076 | 0.72 | 154.292 | C11H22 |
| 11 | Undecane | 1100 | 0.33 | 156.313 | C11H24 |
| 12 | Citronellal | 1152 | 1.81 | 154.25 | C10H18O |
| 13 | Decanal | 1202 | 28.31 | 153.26 | C10H20O |
| 14 | Citronellol | 1236 | 1.95 | 156.269 | C10H20O |
| 15 | Verbenyl acetate | 1269 | 2.84 | 194.270 | C12H18O2 |
| 16 | 1-decanol | 1274 | 4.34 | 158.28 | C10H21OH |
| 17 | Thymol | 1298 | 1.84 | 150.22 | C10H14O |
| 18 | Undecanal | 1307 | 0.26 | 170.296 | C11H22O |
| 19 | Geranyl acetate | 1382 | 0.64 | 196.29 | C10H20O2 |
| 20 | Dodecanal | 1412 | 16.93 | 184.32 | C12H24O |
| 21 | 2-dodecenal | 1476 | 0.80 | 182.3 | C12H22O |
| 22 | 1-hexadecene | 1593 | 1.59 | 224.42 | C16H32 |
| 23 | Tetradecanal | 1614 | 1.14 | 212.37 | C14H28O |
| 24 | β-eudesmol | 1654 | 6.87 | 222.37 | C15H26O |
| 25 | 1-heptadecne | 1697 | 8.30 | 238.5 | C17H34 |
| 26 | 2-Hydroxycyclopentadecanone | 1852 | 0.70 | 240.38 | C15H28O2 |
| N° | Compound Name | Chemical Class | RT (mn) | MW (g/mol) | Chemical Formula | [m/z]- | [m/z]+ |
|---|---|---|---|---|---|---|---|
| 1 | 10-Hydroxyloganin | Terpenoids | 0.963 | 406.1437 | C17 H26 O11 | 387.1277 | - |
| 2 | 2,4,6,8,10-dodecapentaenal | Fatty Acyls | 1.060 | 174.105 | C12 H14 O | 191.0638 | - |
| 3 | 2-Phenylaminoadenosine | Glycosides | 3.827 | 358.1398 | C16 H18 N6 O4 | 357.1324 | - |
| 4 | Atractyloside | Glycosides | 4.260 | 726.2192 | C30 H46 O16 S2 | 707.201 | - |
| 5 | Cortisol 21-sulfate | Sterols lipids | 5.549 | 442.1627 | C21 H30 O8 S | 459.1217 | - |
| 6 | 5,8,11-heptadecatriynoic acid | Fatty Acyls | 6.468 | 258.156 | C17 H22 O2 | 239.1382 | - |
| 7 | Galactan | Polysaccharides | 8.436 | 680.2045 | C24 H40 O22 | 679.199 | - |
| 8 | Harderoporphyrin | Pigment | 9.807 | 608.2509 | C35 H36 N4 O6 | 643.2205 | - |
| 9 | Ergoline-1,8-dimethanol, 10- methoxy-6-methyl-, (8b)- | Alkaloid | 26.965 | 316.1812 | C18 H24 N2 O3 | 297.1636 | - |
| 10 | Ecgonine-methyl ester | Alkaloid | 1.454 | 199.1195 | C10 H17 N O3 | - | 200.1268 |
| 11 | 2-Hydroxy-3-(4-methoxyethylphenoxy)- propanoic acid | Organic Acids | 5.585 | 240.101 | C12 H16 O5 | - | 263.0902 |
| 12 | Lomatin | Coumarins | 6.222 | 246.878 | C14 H14 O4 | - | 247.0951 |
| 13 | Marmesin | Coumarins | 7.088 | 246.0903 | C14 H14 O4 | - | 269.0794 |
| 14 | Purpurogallin | Natural Phenol | 7.210 | 220.0361 | C11 H8 O5 | - | 269.0794 |
| 15 | Atranorin | Polyphenol | 7.363 | 196.0387 | C9 H8 O5 | - | 203.0328 |
| 16 | Methyl 7- desoxypurpurogallin-7- carboxylate trimethyl ether | Natural Phenols | 7.777 | 304.0935 | C16 H16 O6 | - | 287.0903 |
| 17 | Dihydro-Obliquin | Coumarin | 8.789 | 246.0904 | C14 H14 O4 | - | 269.0796 |
| 18 | 13-amino-tridecanoic acid | Fatty Acid | 9.341 | 229.2031 | C13 H27 N O2 | - | 230.2104 |
| 19 | Gummiferol | Fatty Acyl | 9.417 | 286.0836 | C16 H14 O5 | - | 269.0803 |
| 20 | Farnesyl pyrophosphate | Isoprenoid | 9.786 | 382.128 | C15 H28 O7 P2 | - | 405.1171 |
| 21 | Syringic acid | Natural Phenols | 15.399 | 198.054 | C9 H10 O5 | - | 203.0326 |
| 22 | Khayanthone | Polyphenols | 18.482 | 570.2856 | C32 H42 O9 | - | 593.275 |
| Code | Bacterial Strain | D. flabellifolia Methanolic Extract | Ampicillin Mean ± SD (mm) | |||
| mGIZ ± SD (mm) | MIC a | MBC b | MBC/MIC Ratio | |||
| B1 | E. coli ATCC 35218 | 12.66 ± 1.15 bc | 12.50 | 200 | 16; bacteriostatic | 7.00 ± 0.00 d |
| B2 | P. aeruginosa ATCC 27853 | 11.33 ± 0.57 cde | 25 | 200 | 8; bacteriostatic | 7.33 ± 0.57 d |
| B3 | P. mirabilis ATCC 29245 | 12.67 ± 0.57 bc | 25 | 200 | 8; bacteriostatic | 6.33 ± 0.57 d |
| B4 | K. pneumoniae ATCC 27736 | 14.33 ± 0.57 a | 25 | 200 | 8; bacteriostatic | 6.66 ± 0.57 d |
| B5 | P. mirabilis (Environmental strain, 3) | 12.67 ± 0.57 bc | 25 | 200 | 8; bacteriostatic | 21.00 ± 1.00 a |
| B6 | S. sciuri (Environmental strain, 4) | 11.33 ± 1.52 cde | 25 | 200 | 8; bacteriostatic | 7.00 ± 0.00 d |
| B7 | S. pyogens (Clinical strain) | 11.33 ± 1.15 cde | 25 | 200 | 8; bacteriostatic | 16.00 ± 1.73 b |
| B8 | P. aeruginosa (Environmental strain, pf8) | 10.33 ± 0.57 e | 12.50 | 100 | 8; bacteriostatic | 6.66 ± 0.57 d |
| B9 | S. aureus MDR (Clinical strain, 136) | 14.67 ± 0.57 a | 12.50 | 50 | 4; bactericidal | 7.33 ± 0.57 d |
| B10 | E. cloacae (Clinical strain, 115) | 14.33 ± 0.57 a | 12.50 | 100 | 8; bacteriostatic | 6.66 ± 0.57 d |
| B11 | S. paucimobilis (Clinical strain, 144) | 12.33 ± 0.57 cd | 25 | 100 | 4; bactericidal | 7.66 ± 0.57 d |
| B12 | A. baumannii (Clinical strain, 146) | 14.00 ± 0.00 ab | 12.50 | 50 | 4; bactericidal | 13.33 ± 0.57 c |
| Code | Yeasts and molds | mGIZ±SD (mm) | MIC a | MFC b | MFC/MIC Ratio | Amphotericin B Mean ± SD (mm) |
| Y1 | C. albicans ATCC 10231 | 16.33 ± 0.57 a | 25 | 50 | 2; fungicidal | 22.66 ± 1.15 a |
| Y2 | C. neoformans ATCC 14116 | 17.00 ± 1.73 a | 6.25 | 12.50 | 2; fungicidal | 15.33 ± 0.57 b |
| Y3 | C. vaginalis (Clinical strain) | 6.00 ± 0.00 d | 6.25 | 25 | 4; fungicidal | 6.66 ± 0.57 d |
| Y4 | Candida sp. (Clinical strain) | 6.67 ± 0.57 cd | 25 | 100 | 4; fungicidal | 12.33 ± 0.57 c |
| M1 | A. fumigatus ATCC 204305 | 8.33 ± 1.15 bc | - | - | - | 15.00 ± 1.00 b |
| M2 | A. niger | 8.67 ± 0.57 b | - | - | - | 6.00 ± 0.00 d |
| Tests | DPPH IC50 (mg/mL) | ABTS IC50 (mg/mL) | β-Carotene IC50 (mg/mL) |
|---|---|---|---|
| D. flabellifolia methanolic extract | 0.05 ± 0 a | 0.105 ± 0 a | 5.00 ± 0.78 a |
| BHT (Butylated hydroxytoluene) | 0.023 ± 0 b | 0.018 ± 0 b | 0.042 ± 0 b |
| Ascorbic Acid | 0.022 ± 0 b | 0.021 ± 0 b | 0.017 ± 0 b |
| Entry | Bioactive Compounds | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 6 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 21 | |
| Pharmacokinetics properties | ||||||||||||||
| GI absorption | High | High | High | High | High | High | High | High | High | High | High | High | High | High |
| BBB permeant | Yes | Yes | No | No | No | Yes | Yes | No | No | Yes | Yes | Yes | No | No |
| P-gp substrate | No | No | Yes | No | No | No | No | No | No | No | No | No | No | No |
| CYP1A2 inhibitor | No | Yes | No | No | No | Yes | Yes | No | No | Yes | Yes | No | No | No |
| CYP2C19 inhibitor | No | Yes | No | No | No | No | No | No | No | Yes | Yes | No | No | No |
| CYP2C9 inhibitor | No | Yes | No | No | No | No | No | No | No | Yes | No | No | No | No |
| CYP2D6 inhibitor | No | No | Yes | No | No | No | No | No | No | No | No | No | No | No |
| CYP3A4 inhibitor | No | No | No | No | No | No | No | Yes | No | No | No | No | No | No |
| Log Kp (cm/s) | −5.15 | −4.60 | −7.73 | −7.08 | −7.09 | −6.45 | −6.45 | −6.18 | −5.51 | −6.44 | −5.66 | −7.66 | −8.02 | −6.77 |
| Druglikeness properties | ||||||||||||||
| Lipinski | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Ghose | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Veber | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Egan | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Muegge | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Bioavailability Score | 0.55 | 0.85 | 0.55 | 0.55 | 0.56 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.56 |
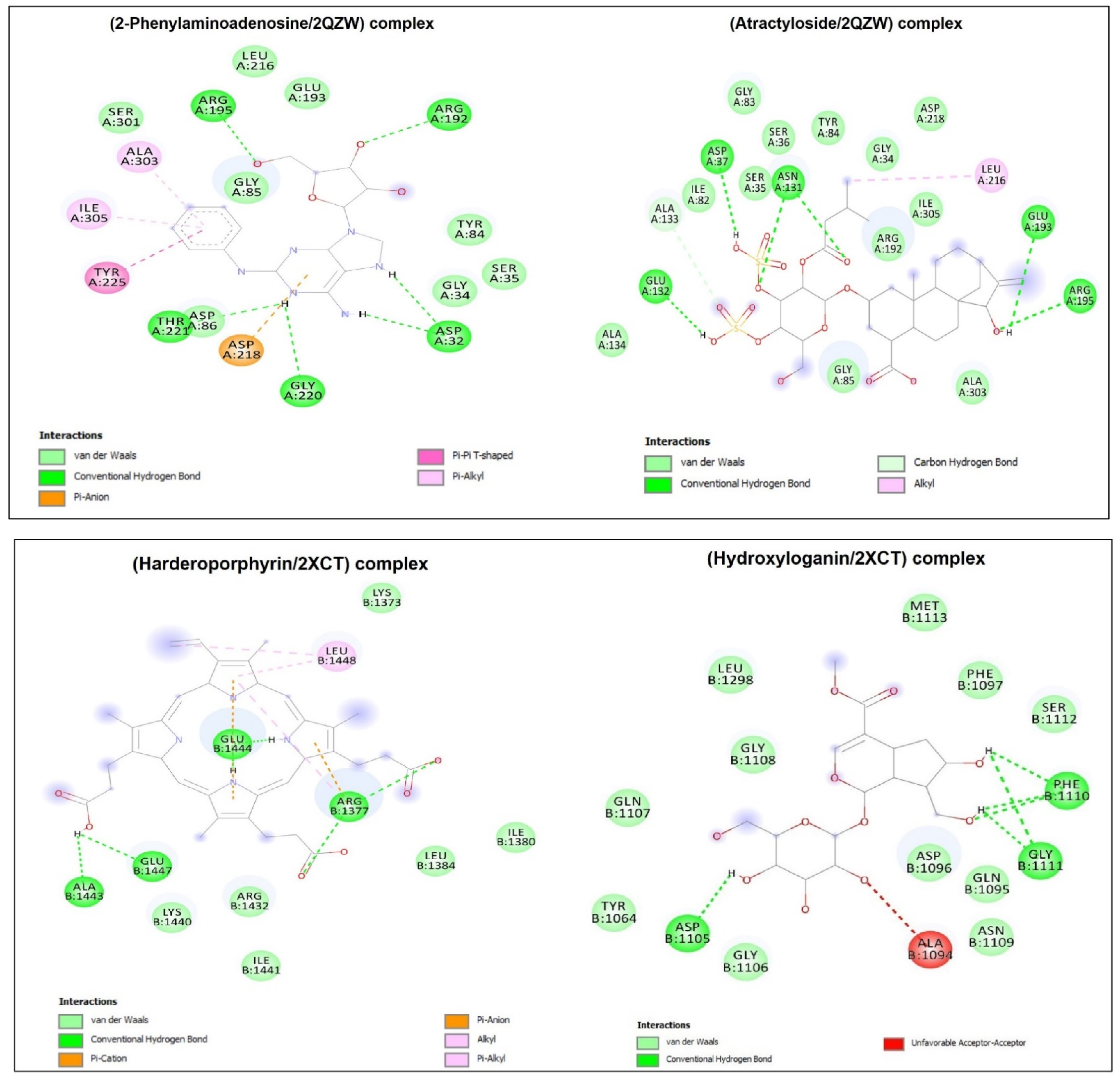
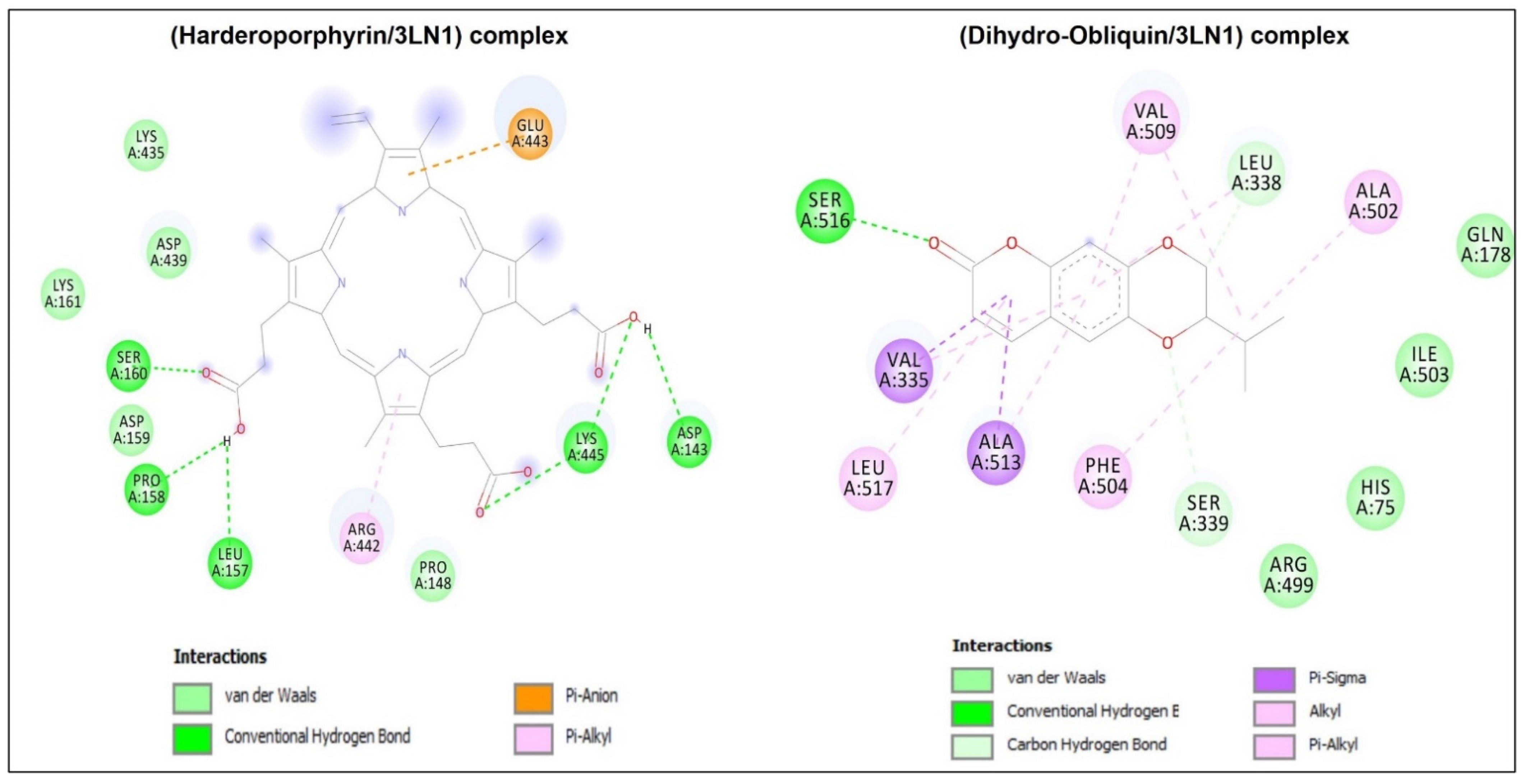
| Complexes | Binding Affinity (kcalxmol−1) | Conventional H-Bonds | No. Closest Interacting Residues | Closest Interacting Residue | |
|---|---|---|---|---|---|
| Residue | Distance (Å) | ||||
| 1HD2/2-Phenylaminoadenosine | −6.7 | 4 | 7 | Arg86 | 1.822 |
| 1HD2/Atractyloside | −6.8 | 8 | 8 | Gly92 | 2.153 |
| 2XCT/10-Hydroxyloganin | −7.6 | 6 | 4 | Asp1105 | 1.904 |
| 2XCT/Harderoporphyrin | −8.2 | 6 | 5 | Arg1377 | 2.186 |
| 2QZW/2-Phenylaminoadenosine | −8.3 | 6 | 9 | Arg195 | 2.223 |
| 2QZW/Atractyloside | −8.3 | 6 | 7 | Glu132 | 2.023 |
| 3LN1/Harderoporphyrin | −9.9 | 6 | 7 | Ser160 | 1.898 |
| 3LN1/Dihydro-Obliquin | −9.2 | 1 | 9 | Ser516 | 2.497 |
| D. flabellifolia/Tests | DPPH IC50 (mg/mL) | ABTS IC50 (mg/mL) | β-Carotene IC50 (mg/mL) |
|---|---|---|---|
| Methanolic extract | 0.05 ± 0 | 0.105 ± 0 | 5.00 ± 0.78 |
| Methanol/Water extract * | 0.014 ± 0.045 | 0.102 ± 0.024 | 7.80 ± 0.919 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snoussi, M.; Lajimi, R.H.; Badraoui, R.; Al-Reshidi, M.; Abdulhakeem, M.A.; Patel, M.; Siddiqui, A.J.; Adnan, M.; Hosni, K.; De Feo, V.; et al. Chemical Composition of Ducrosia flabellifolia L. Methanolic Extract and Volatile Oil: ADME Properties, In Vitro and In Silico Screening of Antimicrobial, Antioxidant and Anticancer Activities. Metabolites 2023, 13, 64. https://doi.org/10.3390/metabo13010064
Snoussi M, Lajimi RH, Badraoui R, Al-Reshidi M, Abdulhakeem MA, Patel M, Siddiqui AJ, Adnan M, Hosni K, De Feo V, et al. Chemical Composition of Ducrosia flabellifolia L. Methanolic Extract and Volatile Oil: ADME Properties, In Vitro and In Silico Screening of Antimicrobial, Antioxidant and Anticancer Activities. Metabolites. 2023; 13(1):64. https://doi.org/10.3390/metabo13010064
Chicago/Turabian StyleSnoussi, Mejdi, Ramzi Hadj Lajimi, Riadh Badraoui, Mousa Al-Reshidi, Mohammad A. Abdulhakeem, Mitesh Patel, Arif Jamal Siddiqui, Mohd Adnan, Karim Hosni, Vincenzo De Feo, and et al. 2023. "Chemical Composition of Ducrosia flabellifolia L. Methanolic Extract and Volatile Oil: ADME Properties, In Vitro and In Silico Screening of Antimicrobial, Antioxidant and Anticancer Activities" Metabolites 13, no. 1: 64. https://doi.org/10.3390/metabo13010064
APA StyleSnoussi, M., Lajimi, R. H., Badraoui, R., Al-Reshidi, M., Abdulhakeem, M. A., Patel, M., Siddiqui, A. J., Adnan, M., Hosni, K., De Feo, V., Polito, F., Kadri, A., & Noumi, E. (2023). Chemical Composition of Ducrosia flabellifolia L. Methanolic Extract and Volatile Oil: ADME Properties, In Vitro and In Silico Screening of Antimicrobial, Antioxidant and Anticancer Activities. Metabolites, 13(1), 64. https://doi.org/10.3390/metabo13010064