Abstract
The Rubia genus includes major groups of medicinal plants such as Rubia cordifolia, Rubia tinctorum, and Rubia akane. They contain anthraquinones (AQs), particularly alizarin and purpurin, which have pharmacological effects that are anti-inflammatory, antioxidant, anticancer, hemostatic, antibacterial, and more. Alizarin and purpurin have been utilized as natural dyes for cotton, silk, and wool fabrics since the dawn of time. These substances have been used in the cosmetics and food industries to color products. The amount of AQs in different Rubia species is minimal. In order to produce these compounds, researchers have established cell and organ cultures. Investigations have been conducted into numerous chemical and physical parameters that affect the biomass and accumulation of secondary metabolites in a cell, callus, hairy root, and adventitious root suspension cultures. This article offers numerous techniques and approaches used to produce biomass and secondary metabolites from the Rubia species. Additionally, it has been emphasized that cells can be grown in bioreactor cultures to produce AQs.
Keywords:
anthraquinones; alizarin; bioreactors; cell cultures; dyes; purpurin; secondary metabolites 1. Introduction
There are more than 60 species in the genus Rubia (Family: Rubiaceae), which is found in Asia, Africa, America, and Europe. Among these, R. cordifolia is a significant medicinal plant whose roots have been utilized for centuries in Traditional Chinese Medicine (TCM), Traditional Indian Medicine (Ayurveda), and Oriental medicine. It is a blood purifier, immunostimulant, anti-inflammatory, and anti-platelet activating agent used in Ayurveda [1]. This plant has been used in Traditional Chinese medicine (TCM) and oriental medicine to treat illnesses such as heart problems, yellow fever, rheumatism, hematemesis, epistaxis, metrorrhagia, contusion, menoxenia, and arthralgia [2]. The climber and perennial herb Rubia cordifolia have long, grooved stems that turn woody at the base (Figure 1A), angular leaves clustered in four whorls (Figure 1B), and tiny flowers placed in terminal panicle cymes (Figure 1C,D). The roots are long, cylindrical, and red in hue (Figure 1E). Pigments are derived from roots. Due to the presence of secondary metabolites in them, several Rubia species are well known for their economic and commercial significance. Major secondary metabolites recovered from Rubia species include anthraquinones (AQs), naphthoquinones, terpenes, and iridoids; anthraquinones and their glycosides are prominent among them [1,2,3]. Since ancient times, AQs derived from various Rubia species have been utilized as natural colorants [4]. The term “Madder” refers to Rubia species that produce colorants from different plant parts, particularly roots. Five main species produce AQs and their derivatives: Rubia tinctorum L. (also known as Dyer’s madder or European madder), Rubia peregrina L. (also known as Wild madder), Rubia cordifolia L. (also known as Indian madder), Rubia sikkimensis Kurz (also known as Naga madder), and Rubia yunnanensis Diels (also known as Xiao hong) [4].

Figure 1.
Rubia cordifolia plant (A), leaves (B), flowering twigs (C,D), dried roots (E) (photos were taken by the authors).
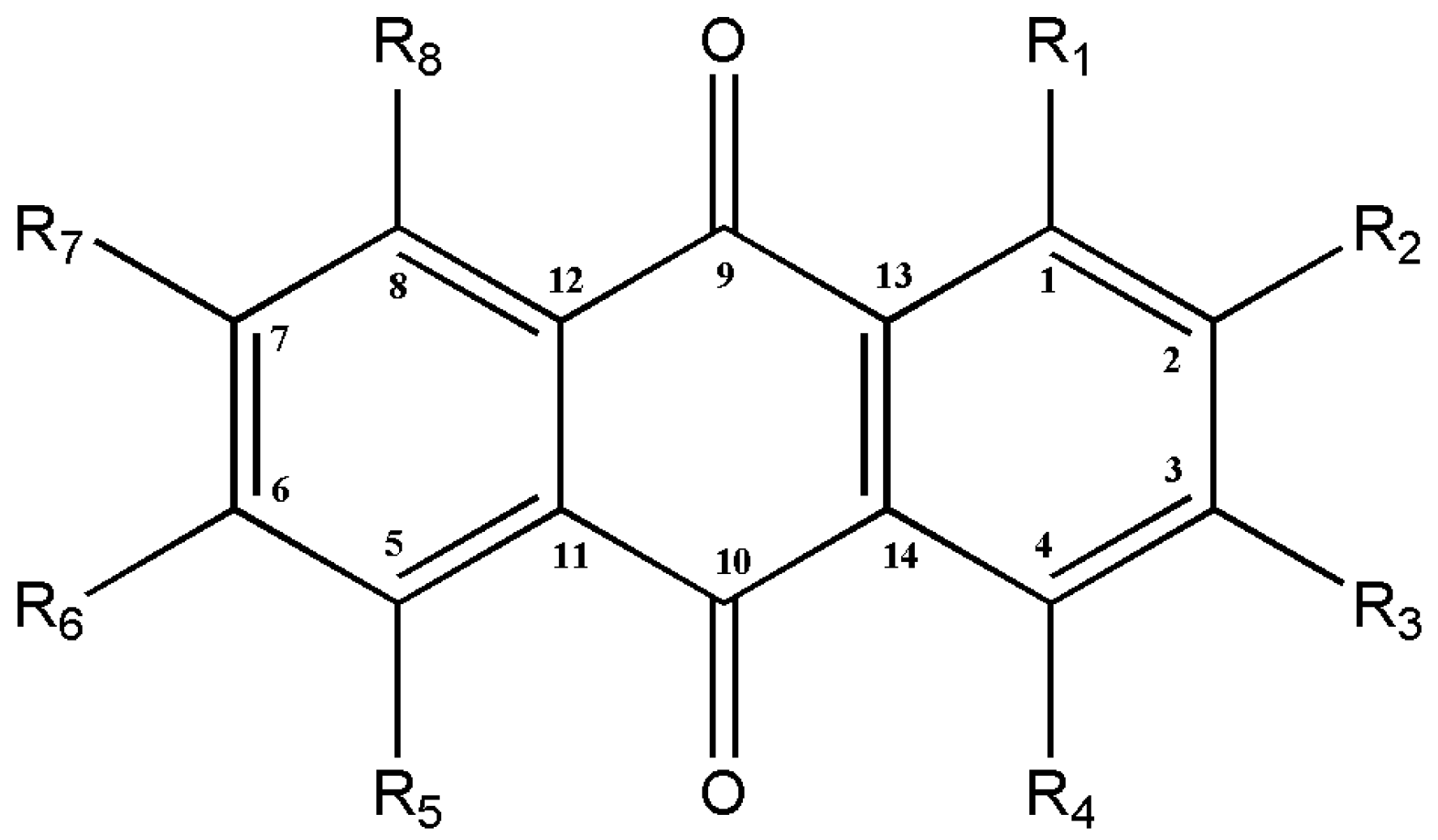
The quinone family contains several compounds known as AQs, which vary in the types and locations of their substituent groups. They are tricyclic aromatic chemical compounds with the formula C14H8O2, and the C-9 and C-10 positions on the central ring are where the ketone groups are located (Figure 2). In general, eight hydrogens can be swapped for each anthraquinone derivative. The term “hydroxyanthraquinoid derivatives” typically refers to 9,10-anthraquinone derivatives in which hydroxyl (-OH) groups have taken the place of numerous hydrogen atoms. The hydroxyanthraquinoid derivatives are pigmented and absorb visible light [5]. AQs are usually accumulated in the roots of Rubia species and can exist either in free form or as anthraquinone glycosides [4]. From the Rubia species, more than 35 anthraquinone compounds have been discovered; many of these compounds are thought to be artifacts created during extraction or drying [4,6]. Table 1 lists the principal anthraquinone compounds of R. tinctorum, R. peregrina, R. cordifolia, and R. akane; Figure 3 shows the structures of a few typical compounds. As natural dyes, these include alizarin (yellow to red), pseudopurpurin (orange), purpurin (dark red), lucidin-3-O-primeveroside (red), ruberythric acid (golden yellow), nordamnacanthal (orange), and munjistin (orange-red) [5]. Fabrics dyed with alizarin and purpurin include cotton, silk, wool, and jute. They are also employed as hair dyes and cosmetics. In Japan and Korea, these substances have been used to color products such as chewing gum, ice cream, and noodles [5].
Figure 2.
The general structure of anthraquinones.

Table 1.
Naturally occurring anthraquinonoid pigments in Rubia species.
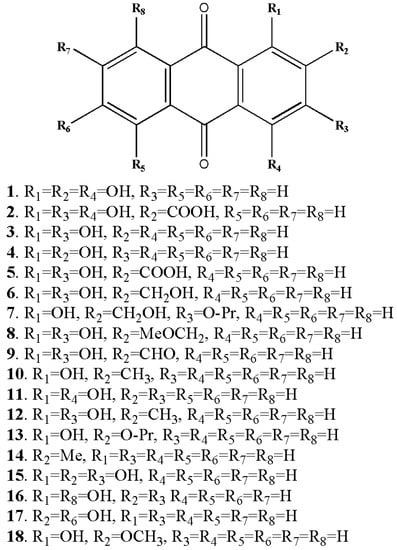
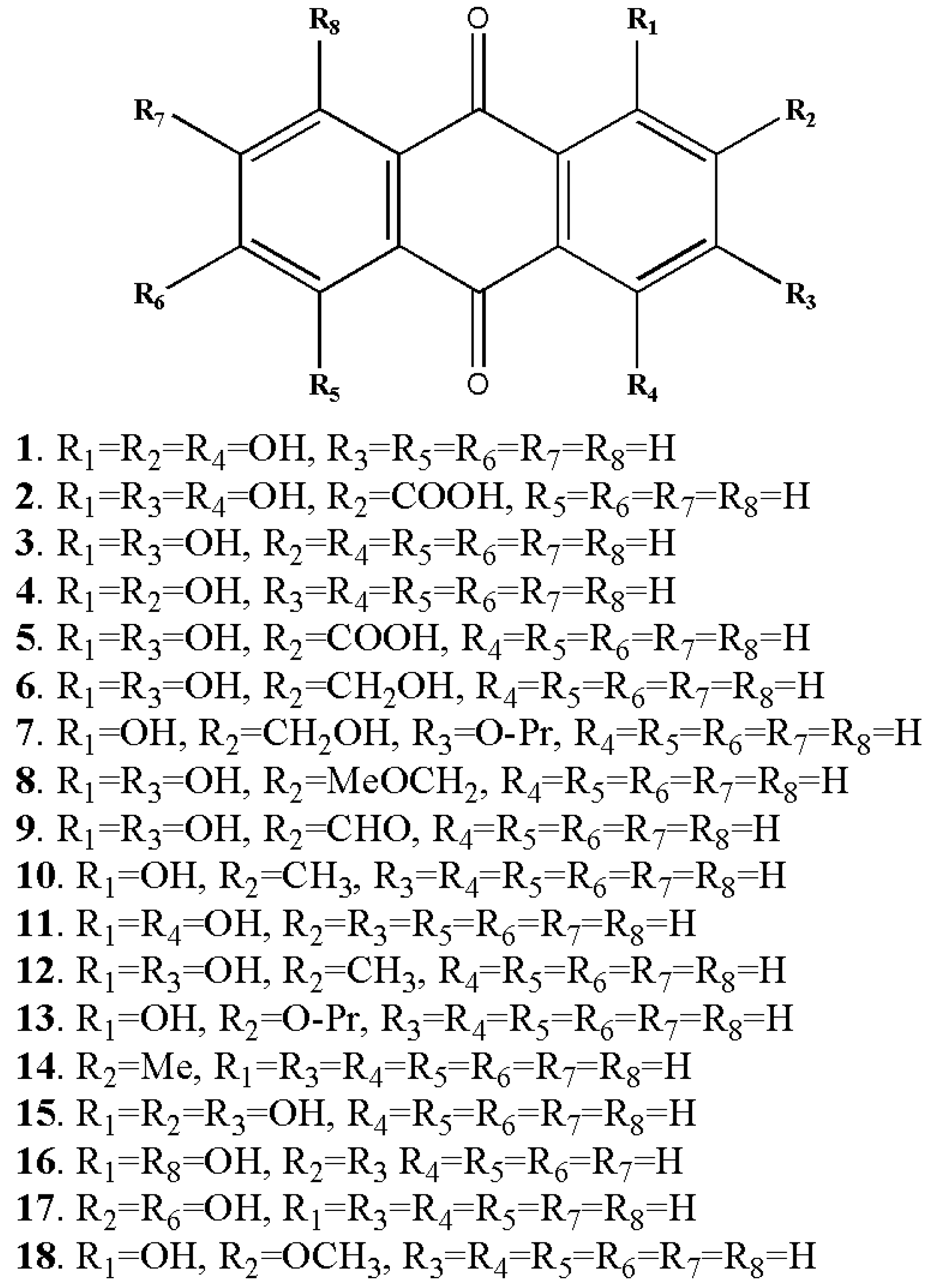
Figure 3.
Some of the anthraquinone derivatives isolated from Rubia cordifolia. Purpurin (1), pseudopurpurin (2), xanthopurpurin (3), alizarin (4), munjistin (5), lucidin (6), lucidin primeveroside (7), lucidin-Ω-ethyl-ether (8), nordamnacanthal (9), pachybasin (10), quinzarin (11), rubiadin (12), ruberythric acid (13), tectoquinone (14), anthragallol (15), danthron (16), anthrafalvin (17), alizarin-2-methyl-ether (18) (structures of metabolites were drawn by authors).
The biological effects of the anthraquinone derivatives, including their antibacterial, anticancer, anti-platelet aggregation, anti-inflammatory, antioxidant, and anti-hepatitis properties, have been documented [2]. In several in vitro and in vivo tests, the main chemicals alizarin and purpurin, which are isolated from Rubia species, have also shown diverse pharmacological activity, including antigenotoxic, anticancer, neuromodulatory, and antimicrobial effects [12,13]. Natural dyes such as alizarin and purpurin have been used to color silk, cotton, wool, nylon, and other textiles. Additionally, they were employed in the production of shampoos, lotions, and sprays, as well as natural hair colors [6].
Di- and tri-hydroxyanthraquinone-glycosides are present in around 2% of the dry weight of the roots of Rubia species, particularly R. tinctorum and R. cordifoila. The presence of anthraquinone molecules, however, may differ depending on the phenology, genotype, and age of plants [14,15]. Plant cell and tissue cultures are attractive alternatives for the production of valuable secondary metabolites, and they are independent of geographical, seasonal, and environmental variations. They offer a defined production system, which ensures a continuous supply of products, uniform quality, and yield. Moreover, cell and tissue cultures are free from contamination by other organisms, pesticides, and insecticides [16]. Many methods can be used to increase the production of secondary metabolites in tissue cultures, including strain enhancement, improvement of the culture conditions and environment, elicitation, feeding of nutrients and precursors, and other bioprocess technologies [16]. These days metabolic engineering techniques have been applied to promote the accumulation of desired compounds in tissue cultures [17]. Scale-up processes are also possible through the application of bioreactor technologies [18,19,20]. Given the foregoing, the Rubia species’ anthraquinone production has been adapted through cell and organ cultures. Many methods can be used to increase the production of secondary metabolites in vitro cell and organ cultures, including obtaining efficient cell lines for growth, screening of high-growth cell lines to produce metabolites, manipulation of nutrients to improve yield, optimization of culture environment, elicitation, and organ cultures for production of metabolites. In this review, the potential of employing plant cell and tissue culture methods to increase anthraquinone synthesis is investigated.
2. Biosynthesis of Anthraquinones
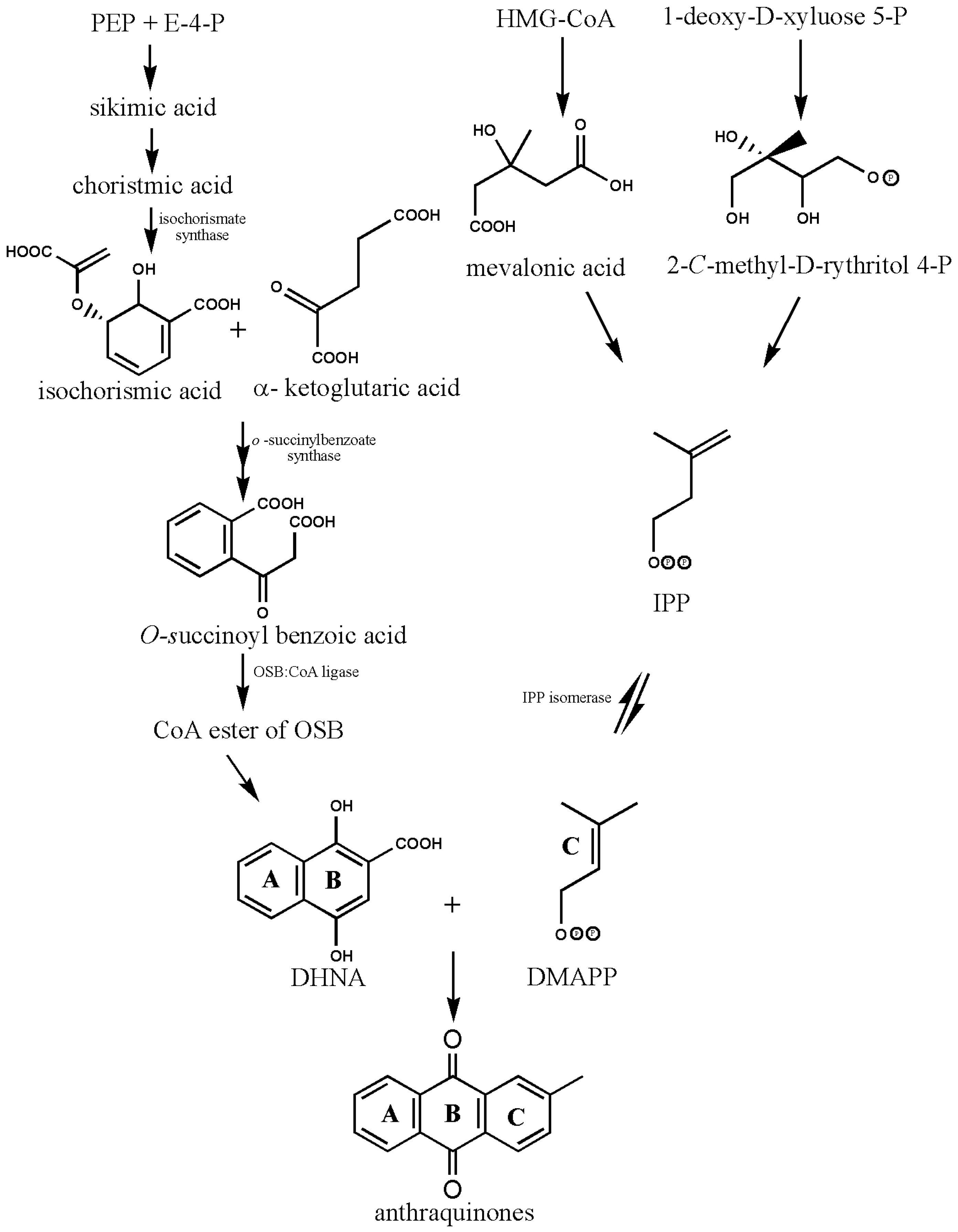
The structural basis of AQs is an anthracene ring with a basic core of a keto group at positions 9 and 10 and several functional groups at other positions, including -OH, -CH3, -OCH3, -CH2OH, -CHO, -COOH, and others (Figure 2). Plants synthesize AQs and their derivatives as secondary metabolites, and they can exist either as free or as glycosides [5]. When one or more sugar molecules, often glucose or rhamnose, are linked to an aglycone by an O-glycoside bond to a hydroxyl group, glycosides are formed. Some of these compounds are thought to be artifacts created during the extraction or drying of the dyestuff. The roots of Rubia tinctorum contain about 35 AQ derivatives [5]. In Rubia species, AQs are produced through the chorismite/o-succinylbenzoic acid or shikimate pathways (Figure 4). Ring C is created from isopentenyl diphosphate either by the mevalonic acid pathway or the 2-C-methyl-D-erythritol 4-phosphate pathway, whereas rings A and B are produced from chorismite and -ketoglutarate via o-succinylbenzoic acid [21,22,23]. The enzyme isochorismate synthase first transforms chorismate, a byproduct of the shikimate pathway, into isochorismate during the biosynthesis of A and B. o-succinylbenzoic acid (OSB) synthase, then catalyzes the conversion of isochorismate into OSB in the presence of ketoglutarate and thiamine diphosphate (TPP). Eventually, an OSB-CoA ester is created by activating OSB at the aliphatic carboxyl group. This reaction is catalyzed by OSB: CoA ligase. Ring closure of OSB: CoA produces 1,4-dihydroxy-2-naphtolic acid (DHNA), which gives rise to anthraquinones’ rings A and B. The eventual prenylation of DHNA results in naphthoquinone. The cyclization reaction between naphthoquinone and isoprene unit IPP or 3,3-dimethylallyl diphosphate (DMAPP), a byproduct of the 2-C-methyl-D-erythritol 4 phosphate (MEP) and mevalonic acid (MVA) pathways, results in the synthesis of ring C of anthraquinones [23].
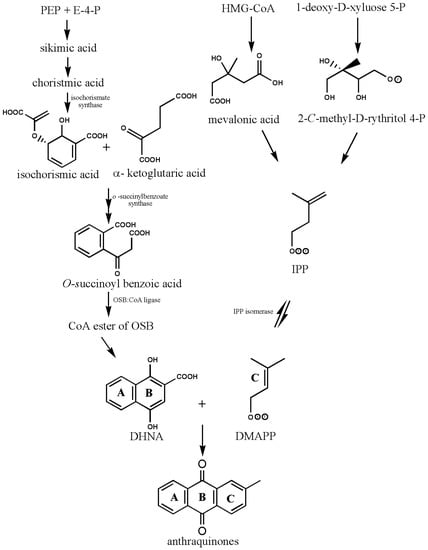
Figure 4.
Biosynthesis of anthraquinones. PEP—Phosphoenolpyruvate; E-4-P—Erythrose-4-phosphate; OSB—o-succinylbenzoic acid, DHNA—1,4-dihydroxy-2-naphthoic acid, HMG-CoA—3-hydroxy-3-methylglutaryl coenzyme A, IPP—isopentenyl diphosphate, DMAPP—3,3-dimethylallyl diphosphate (biosynthetic pathway and structures were drawn by authors).
3. Callus Cultures for the Production of Anthraquinones
The quantity of AQ accumulation in Rubia species is minimal (1.5–2%), and AQ concentrations also vary greatly with plant age, genotype, and phenology [15,21]. As a result, numerous researchers have established callus suspension culturing techniques in Rubia species, and the information is presented in Table 2.

Table 2.
Callus cultures for the production of anthraquinones (AQs) in Rubia species.
3.1. Optimization of Chemical and Physical Parameters
On Murashige and Skoog medium [42], Shin [24] established callus cultures of R. cordifolia and R. akane, and he examined the effects of 2,4-D or NAA (2 ppm), kinetin (5 ppm), temperature (20, 25, 30 °C), and α-ketoglutaric acid (10, 100 mg L−1) or shikimic acid (100 mg L−1) on the growth of callus and the accumulation of metabolites. According to Shin’s [24] findings, NAA promoted callus growth while incubating cultures at 25 °C and supplementing them with α-ketoglutaric acid encouraged the accumulation of alizarin and purpurin in callus cultures of both R. cordifolia and R. akane. In a different investigation, Mischenko et al. [25] established callus cultures of R. cordifolia on MS medium containing 0.5 mg L−1 BA and 2 mg L−1 NAA from the stem, stem apex, leaf, and leaf petiole explants, and selected clones. They were able to raise callus clones/lines from all the explants, but callus clones of stem and leaf petiole origin had the highest purpurin and munjistin contents. They chose clones with yellow and orange coloring and were successful in keeping them alive after extended cultivation.
3.2. Transformation of Root Loci (rolA, rolB, rolC) and Other Genes and Elicitation
Bulgakov et al. [26] transformed root loci genes (rolB and rolC genes) of Agrobacterium rhizogenes by cocultivation with R. cordifolia leaf and stem segments and raised transformed callus clones. They chose a few transgenic callus clones that outperformed non-transformed clones in terms of biomass accumulation as well as purpurin and munjistin levels. Additionally, they performed elicitation on a few different callus clones using salicylic acid (SA) or methyl jasmonate (MJ), as well as ethephon (0, 1, 10, and 100 μM), and the results revealed that SA and MJ stimulated purpurin and munjistin accumulation 1–1.5 times more than the untransformed clones. They also administered cantharidin, an inhibitor of protein phosphatase 2A, to control and transgenic callus clones, and the results showed that cantharidin supplementation stimulated the induction of AQ in R. cordifolia transgenic cells but failed to stimulate any response in the untransformed cultures. The combined action of MJ and cantharidin, which stimulates the accumulation of AQ in transgenic cells, was demonstrated in other studies. Through these tests, they were able to show that cantharidin and MJ work synergistically to change the regulatory balance of secondary metabolism in transformed callus clones of R. cordifolia. They also demonstrated that with such treatments, transformed clones had an AQ accumulation of 4% (dry weight) as opposed to non-transformed clones, whose AQ content was 1.5% dry weight. Further, they observed that ethephon (ethylene producer) did not affect AQ synthesis either in the transgenic or non-transformed strains. Moreover, MJ and SA similarly dynamically raised AQ content in both transgenic and non-transformed strains. They concluded that the pathways of ethylene, MJ, and SA are not engaged in the activator function of rol genes based on these experimental findings. Bulgakov et al. [27] employed the Ca2+ channel blockers verapamil and lanthanum (III) chloride (LaCl3) to confirm the involvement of the Ca2+-dependent NADPH oxidase signaling pathway in transformed cell lines. Verapamil and LaCl3 were used to treat both transformed (R. cordfiolia-Rc-rolC3 and Rc-rolB) and untransformed (Rc) clones/lines. They found that there were notable differences between the transformed cultures of rolC and rolB in terms of their sensitivity to Ca2+ channel blockers and calcium deficiency. Compared to the non-transformed culture, the rolC culture showed less resistance to the inhibitors, but the rolB culture was more resistant to the inhibitors. These findings led them to the conclusion that the Ca2+-dependent NADPH oxidase signaling pathway is not activated during the production of AQ in transgenic cultures. Okadaic acid and cantharidin, which are inhibitors of protein phosphatases 1 and 2A, were employed to treat both transformed and untransformed lines in a subsequent experiment to investigate the involvement of various phosphatases in the production of AQ [28]. Their findings demonstrated that cantharidin had no effect, while okadaic acid increased AQ accumulation in both transgenic and non-transformed cultures. According to these findings, AQ synthesis occurs in both normal and transgenic cultures of R. cordifolia using various phosphatases.
Using transformed (Rc-rolC3 and Rc-rolB) and non-transformed (Rc) clones/lines, Bulgakov et al. [29] investigated the function of the octadecanoid signaling pathway inhibitors diethyldithiocarbamate, propyl gallate, salicyl hydroxamic acid, and piroxicam. None of the investigated inhibitors of the octadecanoid pathway prevented AQ accumulation in both non-transformed and transformed cultures. Based on these findings, they concluded that the rolC and rolB gene-mediated increase in AQ synthesis does not include the octadecanoid pathway.
Shkryl et al. [32] transformed the R. cordifolia with rolA, rolB, and rolC genes using Agrobacterium tumefaciens strain GV3101 harboring constructs pPC002-A (rolA under the control of its native promoter), pPCV002-CaMVBT (rolB under the control of 35S CaMV promoter), pPCV002-CaMVC (rolC under the control of 35S CaMV promoter) and pPCV002-ABC (rolA, rolB, rolC under control of their native promoters) to assess the role of these genes (individually and synergistically) on AQ production in transformed calli. According to the findings of these investigations, the rolA, rolB, and rolC genes alone can increase the synthesis of AQS in transformed R. cordifolia calli. According to Shkryl et al. [32], the isochorismate synthase (ICS) gene, a crucial component of AQ biosynthesis, has higher transcription levels as a result of the stimulatory effect. In contrast to control and non-transformed calli, the transformed calli containing the rolB gene displayed vigorous AQ-stimulating activity, resulting in a 15-fold increase in AQ accumulation. Additionally, they demonstrated that a tyrosine phosphatase inhibitor reversed the rolB-induced rise in AQ production, demonstrating the role of tyrosine (de)phosphorylation in the stimulation of AQ. When compared to control calli, the rolA- and rolC-expressing cultures generated 2.8- and 4.3-fold more AQs, respectively. However, their results showed that the effect of rolA, rolB, and rolC on AQ biosynthesis was not synergistic because rolA and rolC attenuated the stimulatory effect of rolB on AQ synthesis.
To ascertain whether R. cordifolia cells transformed with the rolC gene are engaged in the formation of reactive oxygen species (ROS), which may result in enhanced synthesis of phytoalexins, Bulgakov et al. [31] experimented with R. cordifolia cells. They demonstrated lower steady-state levels of ROS in rolC-expressing R. cordifolia cells compared to control cells using single-cell tests based on confocal microscopy and fluorogenic dyes. While rolC-transformed cells were not significantly affected by the ROS inducer paraquat, normal cells saw a considerable increase in ROS. They also demonstrated that rolC-transformed cells had a two- to three-fold increase in tolerance to salt, heat, and cold treatments. These findings show that the rolC gene does not contribute to the synthesis of phytoalexins and instead functions as a ROS suppressor.
In multiple tests, Shkryl et al. [33] examined the expression of important antioxidant genes in the tissues of R. cordifolia leaves as well as in calli produced from leaves that were both non-transformed and pRiA4-transformed. They isolated partial cDNA (complementary DNA) sequences of ascorbate peroxidase, catalase, and Cu/Zn super oxidase dismutase genes (RcApx1, RcApx2, RcApx3, RcCAT1, RcCAT2, RcCSD1, RcCSD2, and RcCSD3) from plant tissues, as well as pRiA4-transformed and normal calli of R. cordifolia, and studied their expression by real-time PCR (polymerase chain reaction). Ascorbate peroxidase (RcApx1) and Cu/Zn superoxide dismutase (RcCSD1) were found to be the most prevalent transcripts in both plant tissues and untransformed calli, according to transcriptional profiling. In contrast, catalase genes were expressed in such samples. In pRiA4-transformed calli, they noticed the expression of numerous genes encoding ROS-detoxifying enzymes. These findings show that A. rhizogenes upregulates its antioxidant genes as a means of lowering ROS levels in the host cells.
In transgenic R. cordifolia cells, the effect of heterologous expression of the Arabidopsis calcium-dependent protein kinase (CDPK) gene, AtCPK1, on the production of AQ was examined by Shkryl et al. [34]. The agrobacterial transformation was employed to introduce a constitutively active, Ca2+-independent form and a non-active form (used as a negative control) into R. cordifolia cells. They discovered that overexpressing AtCPK1 in R. cordifolia cells resulted in a 10-fold increase in AQ content when compared to untransformed control cells but that AtCPK1 in the non-active form did not affect AQ production. Shkryl et al. [34] used real-time PCR analysis to demonstrate a correlation between the activation of the isochorismate synthase gene and the inhibition of anthraquinone production in transgenic calli. For up to several years, AtCPK1’s activator action remained constant when transgenic cells were grown for extended periods. These findings imply that secondary metabolism in plant cells can be engineered using the CDPK gene. The expression of the Arabidopsis CDPK gene, AtCPK1, in R. cordifolia cells led to a modest and permanent elevation of intracellular reactive oxygen species (ROS) levels, according to a study by Bulgakov et al. [35]. In another study, Bulgakov et al. [35] reported that the expression of the Arabidopsis CDPK gene, AtCPK1, in R. cordifolia cells caused moderate and stable elevation of intracellular reactive oxygen species (ROS) levels. In contrast, no such effect was produced by the mutant, inactive AtCPK1 gene. Veremeichik et al. [36] investigated the expression of the R. cordifolia RcPrx01-RcPrx07 peroxidase genes in aerial organs, cells transformed with the rolB and rolC genes, and cells transformed with the wild-type A. rhizogenes A4 strain. They demonstrated that all the examined peroxidase genes had significantly higher levels of expression in rolB-overexpressing cells than in other cells. These findings imply that the R. cordifolia native genes are overexpressed as a result of the agrobacterial rolB gene. Bulgakov et al. [37] showed that the rolB gene inhibits the production of reactive oxygen species (ROS) induced by paraquat, menadione, and light exposure in R. cordifolia cells that express this gene. These findings support the role of the rolB genes in the metabolism of ROS in transformed cells.
In order to measure the expression level and investigate their effects on AQ production, Shkryl et al. [39] generated native and constitutively active (Ca2+-independent) versions of AtCPK1 in Rubia cordifolia cells. In AtCPK1 lines, they measured the expression of genes encoding crucial AQ biosynthesis pathway enzymes such as isochorismate synthase (ICS), o-succinylbenzoate synthase (OSBS), o-succinyl benzoate ligase (OSBL), and isopentenyl diphosphate isomerase (IPPi). They reported enhanced expression of the ICS, OSBS, OSBL, and IPPI genes, as well as higher AQ synthesis in all AtCPK1-transgenic cell lines. These findings support the function of the R. cordifolia ICS, OSBS, OSBL, and IPPI genes in AQ biosynthesis. In a further investigation, Veremeichik [40] showed that the callus lines that were retained throughout long-term cultures expressed the rolA gene at a high level consistently.
3.3. Immobilization and Other Strategies
A method for immobilizing Rubia tinctorum-suspended cells was developed by Nartop et al. [38] using lignocellulosic material from jute, sisal, and loofa sponge. In comparison to control cell suspension, the immobilized cells produced 6.05 and 22.91 times more alizarin and purpurin when cultivated in a nutrient medium. In a different study, Mariadoss et al. [41] exposed the R. cordifolia callus to 2, 4, 6, 8, 10, 12, 14, and 16 Grays of gamma irradiation. The gamma-irradiated cells were then cultured in an MS medium that contained 1 mg L−1 of IAA, 1 mg L−1 of NAA, and 1 mg L−1 of BA, and growth and metabolite accumulation were evaluated. According to Mariadoss et al. [41], the callus cultures that underwent gamma irradiation at eight Grays accumulated a maximum alizarin level of 26.86 mg g−1 DW and a purpurin level of 44.85 mg g−1 DW during the fourth sub-cultures. These findings support the notion that mutant cells can accumulate more secondary metabolites. The presence of munjistin, purpurin, ruberythrinic acid, alizarine, xanthopurpurin, and munjistin methyl ester was reported by Mishchenko et al. [30] who carried out the qualitative and quantitative assessment of AQ in Rubia cordifolia cell cultures. In a model of edema, they also showed that the cell culture extract of R. cordifolia had anti-inflammatory properties.
4. Cell Suspension Cultures for the Production of Anthraquinones
The research conducted on cell suspension cultures in several Rubia species is shown in Table 3. Suzuki and colleagues [43] established cell suspension cultures in Rubia cordifolia and investigated the growth kinetics and effects of sucrose concentrations (2–7%), several sugars (sucrose, fructose, glucose, raffinose, lactose, and rhamnose at 5% level), myo-inositol concentrations (10–250 mg L−1), inorganic ions (KNO3, 0.1–1.0%), nitrogen source (NH4 or NO3 or combination of NH4:NO3, 1:3–3:1), auxins (NAA, IAA, 2,4-D; 0.2–1.0 mg L−1), and NAA (0.2–5.0 mg L−1) on cell growth and AQ content. According to their findings, there was a normal lag phase lasting 4 days, an exponential period lasting 20 days, and a stable phase with the accumulation of cell biomass and AQs after 20 days. The combination of 20 mg L−1 myoinositol, 5% sucrose, a 1:1 NH4:NO3 ratio, and 0.4 mg L−1 NAA was determined to be the most effective for biomass accumulation and AQ production among the numerous parameters examined. The effects of pH, white, blue, and red fluorescent light conditions on the development of cultivated cells and the synthesis of AQ were examined by Suzuki et al. [44]. They reported pH changes had not affected the growth and accumulation of AQs. Moreover, the incubation of cultures under dark conditions showed the highest accumulation of biomass and metabolites rather than monochromatic red, blue light, or fluorescent light conditions. On MS medium supplemented with 5 μM NAA+0.1 μM KN and MS +0.5 μM 2,4-D, respectively, Sato et al. [45] produced callus from leaf segments of R. tinctorum and R. akane. They used LS medium containing 0.5 μM NAA + 0.1 μM KN for 20 days in the dark to establish cell suspension cultures in 10 L fermenters. Multiple AQ were found in the cell suspension culture extracts, according to their findings. In elicitation experiments with cell cultures of R. akane, Jin et al. [46] investigated the effects of various polysaccharides, including chitosan, alginate, carrageenan, yeast extract, gum arabic, lichenan, xylan, and nigeran, at concentrations of 20–60 mg L–1. In the presence of 25 mg L–1 chitosan, the total production of AQ increased 1.3 times in an MS medium containing galactose. Pythium aphanidermatum (fungus) extract was employed by van Tegelen [47] to generate the R. tinctorum cell culture and as an elicitor, which increased AQ production by two-fold. They were able to isolate isochorismate synthase (EC 5.4.99.6) isoforms, the essential enzyme in the production of AQ, using these induced cells.

Table 3.
Cell suspension cultures for the production of AQs in Rubia species.
Eichinger et al. [48] established cell cultures of R. tinctorum using radiolabeled (1-13C)- or (U-13C6) glucose as a supplement. These investigations helped understand the AQ metabolic route because they used 13C labeling patterns to reconstitute the labeling patterns of acetyl CoA, pyruvate, phosphoenol pyruvate, erythrose 4-phosphate, and α-ketoglutarate through retrosynthesis.
The R. tinctorum cell culture system was employed by Vasconsuelo et al. [49,50,51,52] to examine signal transduction pathways. They established cell cultures of R. tinctorum by using B5 medium + 2% sucrose + 2 mg L−1 2,4-D + 0.5 mg L−1 NAA, 0.5 mg L−1 IAA, and 0.5 mg L−1 KN and elicit the cultures using 200 mg L−1 chitosan. They found that chitosan enhanced AQ production in R. tinctorum cell cultures via activating the Ca2+ messenger, phospholipase C, protein kinase C, phosphoinositide 3-kinase (PI3K), and mitogen-activated protein kinase (MAPK) pathways.
In cell suspensions of R. tintorium, Orban et al. [53] investigated the effects of various elicitors, including JA, SA, and polysaccharides of fungal origin, using MS media supplemented with 3% sucrose +1 mg L−1 IAA, 0.2 mg L−1 NAA, and 0.2 mg L−1 KN. They observed a three-fold increase in AQ accumulation, particularly in lucidin primeveroside and ruberythric acid, in the stimulated cells. In cell suspensions of R. tinctorum, Perassolo et al. [54] tested the impact of proline and aminoindan-2-phosphonic acid on AQ production and demonstrated that both substances enhanced AQ production. In a different investigation, Perassolo et al. [55] investigated the effects of glutamate and several proline analogs on the pentose phosphate pathway (PPP), the proline cycle, and AQ synthesis in R. tinctorum cell suspension cultures. According to these findings, PPP is not a limiting factor as a carbon donor to the shikimate pathway or for the synthesis of AQs because the treatments have not resulted in the induction of PPP.
5. Hairy and Adventitious Root Cultures for the Production of Anthraquinones
Alternatives to cell suspension cultures include cultures of hairy and adventitious roots because they are distinct organs and have a greater ability to participate in primary and secondary metabolism. In order to produce secondary metabolites, hairy and adventitious root cultures have been induced in numerous plants [59,60,61]. For the production of anthraquinones in Rubia species, hairy and adventitious root cultures were developed by several researchers. Such reports are compiled in Table 4. In Rubia cordifolia var. pratensis, Shin and Kim [62] produced hairy roots from stem segments by co-cultivating the plant with Agrobacterium rhizogenes A4 strain 15,834. In Nitsch and Nitsch’s medium enriched with 0.5 mg L−1 NAA, hairy root suspension cultures were developed, and such cultures were capable of accumulating AQs. To transform R. peregrina, Lodhi and Charlwood [63] co-cultivated callus cultures with Agrobacterium rhizogenes LBA 9402 and produced hairy roots. On Gamborg B5 medium with 30 g L−1 sucrose, they later cultivated hairy roots. In comparison to field-grown roots, they discovered a 2-fold increase in AQ accumulation. In a different experiment, Lodhi et al. [64] examined the expression of bacterial isochorismate synthase (EC 5.4.99.6) in transgenic hairy root cultures of R. peregrina. They found that after 10 days in culture, transgenic roots containing bacterial isochorismate synthase cDNA expressed twice as much isochorismate synthase activity (4.88 pkat/mg protein) as the control roots (2.45 pkat/mg protein). AQ levels accumulated to 20% after 30 days in culture.

Table 4.
Hairy/adventitious root cultures for the production of AQs in Rubia species.
By co-cultivating Agrobacterium rhizogens R1000, Park et al. [65] successfully induced hairy roots in R. akane and established hairy root cultures in MS liquid media for 25 days. They were able to produce 3.9 mg g−1 DW alizarin and 4.5 mg g−1 DW purpurin through such experiments. In a second experiment, Park and Lee [66] investigated the effects of various concentrations of IAA, IBA, or NAA (0, 0.1, 0.5, and 1 mg L−1) added to B5, MS, and SH media. Their findings demonstrated that hairy roots produced the maximum levels of alizarin (5.9 mg g−1 DW) and purpurin (7.2 mg g−1 DW) production when cultivated in full-strength SH medium with 0.5 mg L−1 NAA.
Sato et al. [67] generated hairy roots in R. tinctorum, which were then grown in an MS medium containing 3% sucrose. They investigated the effects of phytohormones 0.5 and 5.0 μM KN, IAA, NAA, or 2,4-D and found that 5 μM NAA was beneficial for AQ production. Additionally, they examined the effects of sucrose concentrations of 6, 8, 12, 15, and 18%, finding that 12% was the best concentration for AQ accumulation. Kino-oka et al. [68] tested the effect of fructose, galactose, lactose, maltose, and sucrose as well as nitrate (KNO3) or ammonium form of nitrogen (NH4NO3) added to MS medium on the synthesis of AQ. According to their findings, fructose was a superior carbon source, and nitrate from nitrogen was beneficial for AQ accumulation. With the hairy root cultures of R. tinctorum, Perassolo et al. [69] conducted elicitation tests. Hairy roots were cultivated in 1/2 strength B5 medium with 2% sucrose. They used 100 μM methyl jasmonate to stimulate the hairy root cultures. In comparison to control cells, they detected a 2.4-fold increase in intracellular and an 8.1-fold increase in extracellular AQ hyperaccumulation.
In Rubia tinctorum, Bicer et al. [70] developed adventitious roots from internode explants on an MS medium containing 15 μM IBA and 0.5 μM KN. The roots were grown in an MS liquid medium containing 3% sucrose, 1, 2, and 10 and 100 μM methyl jasmonate, as well as 1 and 2 mM caffeic acid. Among the treatments, 2 mM caffeic acid plus 100 mM methyl jasmonate helped AQs accumulate more effectively. Salicylic acid (20 and 40 μM) and L-phenylalanine (50 and 100 μM) were investigated by Demirci et al. [71] for their effects on the accumulation of AQs in adventitious root cultures of R. tinctorum. Their findings showed that salicylic acid considerably enhanced AQ accumulation while L-phenylalanine had no discernible effect on AQ accumulation. A 20 μM salicylic acid treatment led to the accumulation of 31.47 mg g−1 DW AQ, which was 1.5 times more than the control. The aforementioned findings demonstrate that numerous Rubia species can produce AQs using both hairy root and adventitious root cultures.
6. Bioreactor Cultures for the Production of Anthraquinones
The bioreactor culture system offers more benefits than the conventional tissue culture system because the various factors such as aeration, gases such as oxygen, carbon dioxide, and ethylene levels, and hydrogen ion concentration could be regulated in the bioreactors. Continuous medium agitation can maximize nutrient concentration and also improve nutrient absorption. Enhancing cell proliferation and regeneration rates can also speed up production, decrease costs, improve product quality, eliminate pesticide contamination, and allow for year-round harvesting to satisfy the growing demand on a worldwide scale [17,73]. In plants, including Korean ginseng, Siberian ginseng, echinacea, and St. Jones wort, bioreactor culture systems have been effectively developed for the synthesis of important secondary metabolites [74,75,76,77]. Researchers conducted bioreactor experiments on the plant Rubia tinctorum, and data from those studies are shown in Table 5. R. tinctorum cells were cultivated in 12 and 24-L airlift fermenters using MS media by Laszlo et al. [78], who also produced AQs such as ruberythric acid, alizarin, and purpurin. Busto et al. [79] studied the hydrodynamic stress on biomass and AQ production while growing R. tinctorum cells in 1.5 L stirred tank bioreactors at 450 rpm and in Erlenmeyer flasks at 100 rpm. They discovered that although the biomass produced in the bioreactor was 29% less than that produced in the Erlenmeyer flasks, the production of hydrogen peroxide in the bioreactor was 15 times higher, which resulted in a 233% increase in the production of AQ. In a different investigation, Busto et al. [80] compared normal flasks and baffled shaking flasks and established the impact of light irradiation on the generation of biomass and AQ. The generation of AQ is significantly inhibited by light, and dark incubation has been determined to be ideal for R. tinctorum cell cultures. After gamma irradiation, Mariadoss et al. [41] chose superior R. tinctorum cell lines and cultured them in 8 L stirred bioreactors with different impellers, including helical ribbon impellers (length: 180 mm, width: 90 mm, shaft height: 360 mm), and Rushton turbine impellers (length: 200 mm, width: 50 mm), using MS medium supplemented with 1 mg L−1 BA + 1 mg L−1 NAA+ 1mg L−1 IAA. They discovered that, after 30 days, helical ribbon agitation at a speed of 60 rpm produced a uniform bulk flow of the suspension cultures in the bioreactor without causing shear damage to the cells and produced a maximum biomass of 32.53 g L−1 dry biomass. They also found that using Rushton turbine impeller cells produced 21.04 mg g−1 DW alizarin and 51.28 mg g−1 DW purpurin, but using helical ribbon impeller cultured cells produced 37.96 mg g−1 DW alizarin and 78.93 mg g−1 DW purpurin. These findings point to how crucial it is to choose the right cell lines and to continue to optimize the impellers and agitation speed of cell suspensions to produce bioactive compounds.

Table 5.
Production of AQs in Rubia species using bioreactor cultures.
7. Conclusions and Prospects
Anthraquinones (AQs), which are utilized as natural colors in the food, cosmetic, and textile industries, are produced by Rubia species. Additionally, some AQs have a variety of biological functions, which makes them useful to the pharmaceutical industry. For the production of AQs, scientists have developed cell and organ cultures, and other methods for the accumulation of these substances have also been devised. Few researchers have used hairy root and adventitious cultures; the majority of investigations have focused on callus and cell cultures. Research on R. cordifolia, R. tinctorum, and R. peregrina should focus on the induction of adventitious roots and hairy roots, the selection of superior clones, the optimization of the culture media, and physical parameters that affect the accumulation of biomass and AQs. It is important to standardize the cultivation of adventitious and hairy roots in bioreactors as well as the optimization of bioreactor bioprocess parameters. A detailed investigation of the biosynthesis pathway elucidation is urgently required to identify the critical enzymes and genes regulating their expression. As a result, a metabolic route for AQ chemicals produced specifically will be controlled and modified.
Author Contributions
H.N.M.: conceptualization; H.N.M., K.S.J. and K.Y.P.: investigation, data curation, and formalization; S.Y.P.: resources and validation; H.N.M., K.S.J., K.Y.P. and S.Y.P.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Hosakatte Niranjana Murthy is thankful to the National Foundation of Korea for the award Brain Pool Fellowship (2022H1D3A2A02056665).
Conflicts of Interest
The authors declare no competing interest.
References
- Singh, R.; Geetanjali; Chauhan, S.M.S. 9,10-anthraquinones and other biologically active compounds from the genus Rubia. Chem. Biodivers. 2004, 1, 1241–1264. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.X.; Wang, P.; Wang, L.; Liu, C.; Xu, S.; Cheng, Y.; Wang, Y.; Li, Q.; Lei, H. Quinone derivatives from the genus Rubia and their bioactivities. Chem. Biodivers. 2014, 11, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Chen, Q.; Chen, W.; Yang, J.; Zhou, X.; Zhang, C.; Wu, A.; Lai, J.; Chen, J.; Mei, Q.; et al. A comprehensive review of Rubia cordifolia L.: Traditional uses, phytochemistry, pharmacological activities, and clinical applications. Front. Pharmacol. 2022, 13, 965390. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, R.S. Natural dyes in madder (Rubia spp.) and their extraction and analysis in historical textiles. Color. Technol. 2017, 133, 449–462. [Google Scholar] [CrossRef]
- Caro, Y.; Anamale, L.; Fouillaud, M.; Laurent, P.; Petit, T.; Dufosse, L. Natural hydroxyanthraquinoid pigments as potent food grade colorants: An overview. Nat. Prod. Bioprospect. 2012, 2, 174–193. [Google Scholar] [CrossRef]
- Bechtold, T.; Mussak, R. Handbook of Natural Colorants; Wiley: Chichester, UK, 2009. [Google Scholar]
- Angelini, L.G.; Pistelli, L.; Belloni, P.; Bertoli, A.; Panconesi, S. Rubia tinctorum a source of natural dyes: Agronomic evaluation, quantitative analysis of alizarin and industrial assays. Ind. Crops Prod. 1997, 6, 303–311. [Google Scholar] [CrossRef]
- Banyai, P.; Kuzovkina, N.; Kursinszki, L.; Szoke, E. HPLC analysis of alizarin and purpurin produced by Rubia tinctorum L. hairy root cultures. Chromatographia 2006, 63, S111–S114. [Google Scholar] [CrossRef]
- Drivas, I.; Blackburn, R.S.; Rayner, C.M. Natural anthraquinoid colorants as platform chemicals in the synthesis of sustainable disperse for polyesters. Dyes Pigm. 2011, 88, 7–17. [Google Scholar] [CrossRef]
- Kaur, P.; Chandel, M.; Kumar, S.; Kumar, N.; Singh, B.; Kaur, S. Modulatory role of alizarin from Rubia cordifolia L. against genotoxicity of mutagens. Food Chem. Toxicol. 2010, 48, 320–325. [Google Scholar] [CrossRef]
- Rafaelly, L.; Heron, S.; Nowik, W.; Tchapla, A. Optimization of ESI-MS detection for HPLC of anthraquinone dyes. Dyes Pigm. 2008, 77, 19–203. [Google Scholar] [CrossRef]
- Singh, J.; Husain, Y.; Luqman, S.; Meena, A. Purpurin: A natural anthraquinone with multifaceted pharmacological activities. Phytother. Res. 2020, 35, 2418–2428. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, J.; Qi, W.; Su, R.; He, Z.; Peng, X. Alizarin and purpurin from Rubia tinctorum L. suppress insulin fibrillation and reduce the amyloid-induced cytotoxicity. ACS Chem. Neurosci. 2021, 12, 2182–2193. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.S.B.; Hulme, A.N.; McNab, H.; Quye, A. The natural constituents of historical textile dyes. Chem. Soc. Rev. 2004, 33, 329–336. [Google Scholar] [CrossRef]
- Baydar, H.; Karadogan, T. Agronomic potential and industrial value of madder (Rubia tinctorum L.) as a dye crop. Turk. J. Agric. For. 2006, 30, 287–293. [Google Scholar]
- Murthy, H.N.; Lee, E.J.; Paek, K.Y. Production of secondary metabolites from cell and organ cultures: Strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tiss. Organ Cult. 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Verpoorte, R.; Memelink, J. Engineering secondary metabolite production in plants. Curr. Opin. Biotechnol. 2002, 13, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.N.; Kim, Y.S.; Jeong, C.S.; Kim, S.J.; Zhong, J.J.; Paek, K.Y. Production of Ginsenosides from Adventitious Root Cultures of Panax ginseng. In Production of Biomass and Bioactive Compounds Using Bioreactor Technology; Paek, K.Y., Murthy, H.N., Zhong, J.J., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 625–651. [Google Scholar]
- Murthy, H.N.; Dalawai, D. Biotechnological production of diterpenoid lactones from cell and organ cultures of Andrographis paniculata. Appl. Microbiol. Biotechnol. 2021, 105, 7683–7694. [Google Scholar] [CrossRef]
- Murthy, H.N. Biotechnological production of bacosides from cell and organ cultures of Bacopa monnieri. Appl. Microbiol. Biotechnol. 2022, 106, 1799–1811. [Google Scholar] [CrossRef]
- Han, Y.S.; Heijden, R.V.D.; Lefeber, A.W.M.; Erkelens, C.; Verpoorte, R. Biosynthesis of anthraquinones in cell cultures of Cinchona ‘Robusta’ proceeds via the methylerythritol-4-phosphate pathway. Phytochemistry 2002, 59, 45–55. [Google Scholar] [CrossRef]
- Han, Y.S.; Heijden, R.V.D.; Verpoorte, R. Biosynthesis of anthraquinones in cell cultures of the Rubiaceae. Plant Cell Tiss. Organ Cult. 2001, 67, 201–220. [Google Scholar] [CrossRef]
- Leistner, E. Biosynthesis of Chorismate-Derived Quinones in Plant Cell Cultures. In Primary and Secondary Metabolism of Plant Cell Cultures. Proceedings in Life Sciences; Neumann, K.H., Barz, W., Reinhard, E., Eds.; Springer: Berlin/Heidelberg, Germany, 1985; pp. 215–224. [Google Scholar]
- Shin, S.H. Studies on the production of anthraquinone derivatives by tissue culture of Rubia species. Arch. Pharm. Res. 1989, 12, 99–102. [Google Scholar] [CrossRef]
- Mischenko, N.P.; Fedoreyev, S.A.; Glazunov, V.P.; Chernoded, G.K.; Bulgakov, V.P.; Zhuravlev, Y.N. Anthraquinone production by callus cultures of Rubia cordifolia. Fitoterapia 1999, 70, 552–557. [Google Scholar] [CrossRef]
- Bulgakov, V.P.; Tchernoded, G.K.; Mischenko, N.P.; Khodakovskaya, M.V.; Glazunov, V.P.; Radchenko, S.V.; Zvereva, E.V.; Fedoreyev, S.A.; Zhuravlev, Y.N. Effect of salicylic acid, methyl jasmonate, ethephon, and cantharidin on anthraquinone production by Rubia cordifolia callus cultures transformed with the rolB and rolC genes. J. Biotechnol. 2002, 97, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Bulgakov, V.P.; Tehernoded, G.K.; Mischenko, N.P.; Shkryl, Y.N.; Glazunov, V.P.; Fedoreyev, S.A.; Zhuravlev, Y.N. Effects of Ca2+ channel blockers and protein kinase/phosphatase inhibitors on growth and anthraquinone production in Rubia cordifolia callus cultures transformed by rolB and rolC genes. Planta 2003, 217, 349–355. [Google Scholar] [CrossRef]
- Bulgakov, V.P.; Tchernoded, G.K.; Mischenko, N.P.; Shkryl, Y.N.; Glazunov, V.P.; Fedoreyev, S.A.; Zhuravlev, Y.N. Increase in anthraquinone content in Rubia cordifolia cells transformed by rol genes does not involve activation of the NADPH oxidase signaling pathway. Biochemistry 2003, 68, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Bulgakov, V.P.; Tchernoded, G.K.; Mischenko, N.P.; Shkryl, Y.N.; Fedoreyev, S.A.; Zhuravlev, Y.N. The rolB and rolC genes activate synthesis of anthraquinones in Rubia cordifolia cells by mechanism independent of octadecanoid signaling pathway. Plant Sci. 2004, 166, 1069–1075. [Google Scholar] [CrossRef]
- Mishchenko, N.P.; Fedoreev, S.A.; Bryukhanov, V.M.; Zverev, Y.F.; Lampatov, V.V.; Azarova, O.V.; Shkryl, Y.N.; Chernoded, G.K. Chemical composition and pharmacological activity of anthraquinones from Rubia cordifolia cell cultures. Pharm. Chem. J. 2007, 41, 605–609. [Google Scholar] [CrossRef]
- Bulgakov, V.P.; Aminin, D.I.; Shkryl, Y.N.; Gorpenchenko, T.Y.; Vermeichik, G.N.; Dmitrenok, P.S.; Zhuravlev, Y.N. Suppression of reactive oxygen species and enhanced stress tolerance in Rubia cordifolia cells expressing the rolC gene. Mol. Plant Microb. Interact. 2008, 21, 1561–1570. [Google Scholar] [CrossRef]
- Shkryl, Y.N.; Veremeichik, G.N.; Bulgakov, V.P.; Tchernoded, G.K.; Mischenko, N.P.; Fedoreyev, S.A.; Zhuravlev, Y.N. Individual and combined effects of the rolA, B, and C genes on anthraquinone production in Rubia cordifolia transformed calli. Biotechnol. Bioeng. 2008, 100, 118–125. [Google Scholar] [CrossRef]
- Shkryl, Y.N.; Veremeichik, G.N.; Bulgakov, V.P.; Gopenchenko, T.Y.; Aminin, D.L.; Zhuravlev, Y.N. Decreased ROS level and activation of antioxidant gene expression in Agrobacterium rhizhogenes pRiA4-transformed calli of Rubia cordifolia. Planta 2010, 232, 1023–1032. [Google Scholar] [CrossRef]
- Shkryl, Y.N.; Veremeichik, G.N.; Bulgakov, V.P.; Zhuravlev, Y.N. Induction of anthraquinone biosynthesis in Rubia cordifolia cells by heterologous expression of a calcium-dependent protein kinase gene. Biotechnol. Bioeng. 2011, 108, 1734–1738. [Google Scholar] [CrossRef]
- Bulgakov, V.P.; Gorpenchenko, T.Y.; Shkryl, Y.N.; Veremeichik, G.N.; Mischenko, N.P.; Aavarmenko, T.V.; Fedoreyev, S.A.; Zhuravlev, Y.N. CDPK-driven changes in the intracellular ROS level and plant secondary metabolism. Bioeng. Bugs. 2011, 2, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Veremeichik, G.N.; Shkryl, Y.N.; Bulgakov, V.P.; Avramenko, T.V.; Zhuravlev, Y.N. Molecular cloning and characterization of seven class III peroxidases induced by overexpression of agrobacterial rolB gene in Rubia cordifolia transgenic callus cultures. Plant Cell Rep. 2012, 31, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Bulgakov, V.P.; Gorpenchenko, T.Y.; Veremeichik, G.N.; Shkryl, Y.N.; Tcherenoded, G.K.; Bulgakov, D.; Aminin, D.L.; Zhuravlev, Y.N. The rolB gene suppresses reactive oxygen species in transformed plant cells through the sustained activation of antioxidant defense. Plant Physiol. 2012, 158, 1371–1381. [Google Scholar] [CrossRef]
- Nartop, P.; Akay, S.; Gurel, A. Immobilization of Rubia tinctorum L. suspension cultures and its effects on alizarin and purpurin accumulation and biomass production. Plant Cell Tiss. Organ Cult. 2013, 112, 123–128. [Google Scholar] [CrossRef]
- Shkryl, Y.N.; Veremeichik, G.N.; Makhazen, D.S.; Silantieva, S.A.; Mishchenko, N.P.; Vasileva, E.A.; Fedoreyev, S.A.; Bulgakov, V.P. Increase of anthraquinone content in Rubia cordifolia cells transformed by native and constitutively active forms of the AtCPK1 gene. Plant Cell Rep. 2016, 35, 1907–1916. [Google Scholar] [CrossRef]
- Veremeichik, G.N.; Bulgakov, V.P.; Shkryl, Y.N.; Silantieva, S.A.; Makhazen, D.S.; Tchnernoded, G.K.; Mischenko, N.P.; Fedoreyev, S.A.; Vasileva, E.A. Activation of anthraquinone biosynthesis in long-cultured callus culture of Rubia cordifolia transformed with the rolA plant oncogene. J. Biotechnol. 2019, 306, 38–46. [Google Scholar] [CrossRef]
- Mariadoss, A.; Satdive, R.; Fulzele, D.P.; Ramamoorthy, S.; Doss, G.P.C.; Zayed, H.; Younes, S.; Rajasekaran, C. Enhanced production of anthraquinones by gamma-irradiated cell cultures of Rubia cordifolia in a bioreactor. Ind. Crops Prod. 2020, 145, 111987. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Suzuki, H.; Matsumoto, T.; Mikami, Y. Effects of nutritional factors on the formation of anthraquinones by Rubia cordifolia plant cells in suspension culture. Agric. Biol. Chem. 1984, 48, 603–610. [Google Scholar] [CrossRef]
- Suzuki, H.; Matsumoto, T.; Mikami, Y. Effects of physical factors and surface-active agents on the formation of anthraquinones by Rubia cordifolia cells in suspension culture. Agric. Biol. Chem. 1985, 49, 519–520. [Google Scholar] [CrossRef]
- Sato, K.; Goda, Y.; Kawasaki, Y.; Okuyama, E.; Yoshihara, K.; Ozeki, Y.; Nakamura, M. Characteristic of anthraquinone production in plant roots and cell suspension cultures of Rubia tinctorum and R. akane. Plant Tiss. Cult. Lett. 1992, 9, 220–226. [Google Scholar] [CrossRef][Green Version]
- Jin, J.H.; Shin, J.H.; Kim, J.H.; Chung, I.S.; Lee, H.J. Effect of chitosan elicitation and media components on the production of anthraquinone colorants in madder (Rubia akane Nakai) cell culture. Biotechnol. Bioprocess. Eng. 1999, 4, 300–304. [Google Scholar] [CrossRef]
- van Tegelen, L.J.P.; Bongaerts, R.J.M.; Croes, A.F.; Verpoorte, R.; Wullems, G.J. Isochorismate synthase isoforms from elicited cell cultures of Rubia tinctorum. Phytochemistry 1999, 51, 263–269. [Google Scholar] [CrossRef]
- Eichinger, D.; Bacher, A.; Zenk, M.H.; Eisenreich, W. Quantitative assessment of metabolic flux by 13C NMR analysis. Biosynthesis of anthraquinones in Rubia tinctorum. J. Am. Chem. Soc. 1999, 121, 7469–7475. [Google Scholar] [CrossRef]
- Vasconsuelo, A.; Giuletti, A.M.; Picotto, G.; Rodriguez-Talou, J.; Boland, R. Involvement of the PLC/PKC pathway in chitosan-induced anthraquinone production by Rubia tinctorum L. cell cultures. Plant Sci. 2003, 165, 429–436. [Google Scholar] [CrossRef]
- Vasconsuelo, A.; Giulietti, A.M.; Boland, R. Signal transduction events mediating chitosan stimulation of anthraquinone synthesis in Rubia tinctorum. Plant Sci. 2004, 166, 405–413. [Google Scholar] [CrossRef]
- Vasconsuelo, A.; Morelli, S.; Picotto, G.; Giulietti, A.M.; Boland, R. Intracellular calcium mobilization: A key step for chitosan-induced anthraquinone production in Rubia tinctorum. Plant Sci. 2005, 169, 712–720. [Google Scholar] [CrossRef]
- Vasconsuelo, A.; Picotto, G.; Giuletti, A.M.; Boland, R. Involvement of G-proteins in chitosan-induced anthraquinone synthesis in Rubia tinctorum. Physiol. Plant. 2006, 128, 29–37. [Google Scholar] [CrossRef]
- Orban, N.; Boldizsar, I.; Szucs, Z.; Danos, B. Influence of different elicitors on the synthesis of anthraquinone derivatives in Rubia tinctorum L. cell suspension cultures. Dyes Pigm. 2008, 77, 249–257. [Google Scholar] [CrossRef]
- Perassolo, M.; Quevedo, C.; Busto, V.; Ianone, F.; Giulietti, A.M.; Talou, J.R. Enhance of anthraquinone production by effect of proline and aminoindan-2-phosphonic acid in Rubia tinctorum suspension cultures. Enzyme Mirob. Technol. 2007, 41, 181–185. [Google Scholar] [CrossRef]
- Perassolo, M.; Quevedo, C.V.; Giulietti, A.M.; Talou, J.R. Stimulation of the proline cycle and anthraquinone accumulation in Rubia tinctorum cell suspension cultures in the presence of glutamate and two proline analogs. Plant Cell Tiss. Organ Cult. 2011, 106, 153–159. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Linsmaier, E.M.; Skoog, F. Organic growth factor requirements of tobacco tissue cultures. Physiol. Plant. 1965, 18, 100–127. [Google Scholar] [CrossRef]
- Schenk, R.U.; Hildebrandt, A.C. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous cell cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Srivastava, S.; Srivastava, A.K. Hairy root culture for mass-production of high-value secondary metabolites. Crit. Rev. Biotechnol. 2007, 27, 29–43. [Google Scholar] [CrossRef]
- Murthy, H.N.; Hahn, E.J.; Paek, K.Y. Adventitious roots and secondary metabolism. Chin. J. Biotechnol. 2008, 24, 711–716. [Google Scholar] [CrossRef]
- Murthy, H.N.; Dandin, V.S.; Paek, K.Y. Tools for biotechnological production of useful phytochemicals from adventitious root cultures. Phytochem. Rev. 2016, 15, 129–145. [Google Scholar] [CrossRef]
- Shin, S.W.; Kim, Y.S. Production of anthraquinone derivatives by hairy roots of Rubia cordifolia var. pratensis. Kor. J. Pharmacogn. 1996, 27, 301–308. [Google Scholar]
- Lodhi, A.H.; Charlwood, B.V. Agrobacterium rhizogenes-mediated transformation of Rubia peregrina L.: In vitro accumulation of anthraquinones. Plant Cell Tiss Organ Cult. 1996, 46, 103–108. [Google Scholar] [CrossRef]
- Lodhi, A.H.; Bongaerts, R.J.M.; Verpoorte, R.; Coomber, S.A.; Charlwood, B.V. Expression of bacterial isochorismate synthase (EC 5.4.99.6) in transgenic root cultures of Rubia peregrina. Plant Cell Rep. 1996, 16, 54–57. [Google Scholar] [CrossRef]
- Park, S.U.; Kim, Y.K.; Lee, S.Y. Establishment of hairy root culture of Rubia akane Nakai for alizarin and purpurin production. Sci. Res. Essays 2009, 4, 94–97. [Google Scholar] [CrossRef]
- Park, S.U.; Lee, S.Y. Anthraquinone production by hairy root culture of Rubia akane Nakai: Influence of media and auxin treatment. Sci. Res. Essays 2009, 4, 690–693. [Google Scholar] [CrossRef]
- Sato, K.; Yamazaki, T.; Okuyama, E.; Yoshihira, K.; Shimomura, K. Anthraquinone production by transformed root cultures of Rubia tinctorum: Influence of phytohormones and sucrose concentration. Phytochemistry 1991, 30, 1507–1509. [Google Scholar] [CrossRef]
- Kino-Oka, M.; Mine, K.; Taya, M.; Tone, S.; Ichi, T. Production and release of anthraquinone pigments by hairy roots of madder (Rubia tinctorum L.) under improved culture conditions. J. Ferment. Bioeng. 1994, 77, 103–106. [Google Scholar] [CrossRef]
- Perassolo, M.; Cardillo, A.B.; Mugas, M.L.; Montoya, S.C.N.; Giulietti, A.M.; Talou, J.R. Enhancement of anthraquinone production and release by combination of culture medium selection and methyl jasmonate elicitation in hairy root cultures of Rubia tinctorum. Ind. Crops Prod. 2017, 105, 124–132. [Google Scholar] [CrossRef]
- Bicer, P.O.; Demirci, T.; Asci, O.A.; Baydar, N.G. Effects of methyl jasmonate and caffeic acid applications on secondary metabolites production in madder (Rubia tinctorum) root cultures. Indian J. Pham. Educ. Res. 2017, 51, S508–S512. [Google Scholar] [CrossRef]
- Demicri, T.; Asci, O.A.; Baydar, N.G. Influence of salicylic acid and L-phenylalanine on the accumulation of anthraquinone and phenolic compounds in adventitious root cultures of madder (Rubia tinctorum L.). Plant Cell Tiss. Organ Cult. 2021, 144, 313–324. [Google Scholar] [CrossRef]
- Nitisch, J.P.; Nitsch, C. Haploid plants form pollen grains. Science 1969, 163, 65–87. [Google Scholar] [CrossRef]
- Murthy, H.N.; Dalawai, D.; Bhat, M.A.; Dandin, V.S.; Paek, K.Y.; Park, S.Y. Biotechnological Production of Useful Phytochemicals from Adventitious Root Cultures. In Plant Cell and Tissue Differentiation and Secondary Metabolites: Fundamentals and Applications; Ramawath, K.G., Ekiert, H.M., Goyal, S., Eds.; Springer Nature: Geneva, Switzerland, 2021; pp. 469–486. [Google Scholar] [CrossRef]
- Murthy, H.N.; Georgiev, M.I.; Kim, Y.S.; Jeong, C.S.; Kim, S.J.; Park, S.Y.; Paek, K.Y. Ginsenosides: Prospective for sustainable biotechnological production. Appl. Microbiol. Biotechnol. 2014, 98, 6243–6254. [Google Scholar] [CrossRef]
- Murthy, H.N.; Kim, Y.S.; Georgiev, M.I.; Paek, K.Y. Biotechnological production of eleutherosides: Current state and perspectives. Appl. Microbiol. Biotechnol. 2014, 98, 7319–7329. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.N.; Kim, Y.S.; Park, S.Y.; Paek, K.Y. Biotechnological production of caffeic acid derivatives from cell and organ cultures of Echinacea species. Appl. Microbiol. Biotechnol. 2014, 98, 7707–7717. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.N.; Kim, Y.S.; Park, S.Y.; Paek, K.Y. Hypericins: Biotechnological production from cell and organ cultures. Appl. Microbiol. Biotechnol. 2014, 98, 9187–9198. [Google Scholar] [CrossRef]
- Laszlo, M.; Kretovics, J.; Danos, B.; Szokan, G.; Liszt, K.; Hollosy, F.; Toth, Z.; Gyurjan, I. The production of secondary metabolites by plant cells of Rubia tinctorum cultivated in bioreactors. Plant Med. Suppl. 1992, 58, 613. [Google Scholar] [CrossRef]
- Busto, V.D.; Rodriguez-Talou, J.; Giulietti, A.M.; Merchuk, J.C. Effect of shear stress on anthraquinones production in Rubia tinctorum suspension cultures. Biotechnol. Prog. 2008, 24, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Busto, V.D.; Calabro-Lopez, A.; Rodriguez-Talou, J.; Giulietti, A.M.; Merchuk, J.C. Anthraquinones production in Rubia tinctorum cell suspension cultures: Down scale of shear effects. Biochem. Eng. J. 2013, 77, 119–128. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).