Dietary Patterns, Metabolomic Profile, and Nutritype Signatures Associated with Type 2 Diabetes in Women with Postgestational Diabetes Mellitus: MyNutritype Study Protocol
Abstract
:1. Introduction
2. Methods
2.1. Design
- (a)
- To determine dietary patterns associated with T2D and prediabetes;
- (b)
- To determine the metabolomic profile associated with T2D and prediabetes using one-dimensional proton nuclear magnetic resonance (1H NMR) spectroscopy;
- (c)
- To identify nutritype signatures (association between dietary patterns and metabolomic profile);
- (d)
- To determine the nutritype signatures associated with T2D in women post-GDM;
- (e)
- To compare other parameters in women post-GDM with normal glucose tolerance (NGT), pre-diabetes and T2D, which include:
- i.
- Anthropometric and clinical measurements;
- ii.
- Biochemical profile;
- iii.
- Energy and nutrient intake;
- iv.
- Lifestyle practices.
2.2. Study Setting
2.3. Study Population
2.4. Recruitment
2.5. Measures
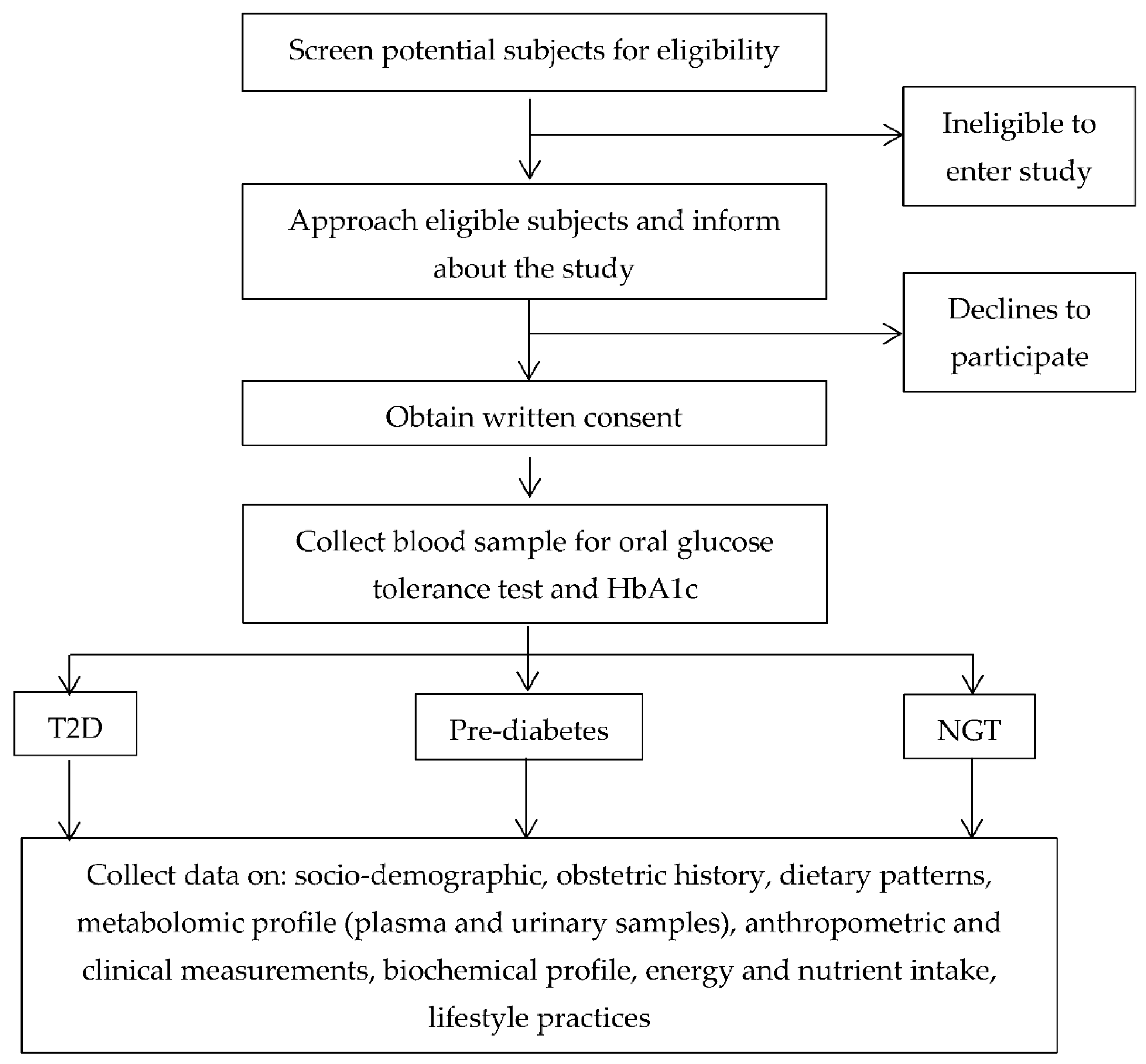
2.5.1. Diagnosis of Type 2 Diabetes and Prediabetes
2.5.2. Sociodemographic Characteristics and Obstetric History
2.5.3. Dietary Patterns
2.5.4. Energy and Nutrient Intake
2.5.5. Lifestyle Practices
2.5.6. Anthropometric and Clinical Measurements
2.5.7. Biochemical Profile
2.5.8. Metabolomic Profile
2.5.9. Nutritype Signatures
2.6. Governance and Ethics
2.7. Statistical Analysis
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar] [CrossRef]
- Institute for Public Health. National Health and Morbidity Survey (NHMS) 2019: NCDs-Non-Communicable Diseases: Risk Factors and Other Health Problems; Institute for Public Health, National Institutes of Health, Ministry of Health Malaysia: Shah Alam, Malaysia, 2019; Volume 1. [Google Scholar]
- Vounzoulaki, E.; Dipla, K.; Kintiraki, E.; Triantafyllou, A.; Grigoriadou, I.; Koletsos, N.; Zafeiridis, A.; Goulis, D.G.; Douma, S. Pregnancy and post-partum muscle and cerebral oxygenation during intermittent exercise in gestational diabetes: A pilot study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 232, 54–59. [Google Scholar] [CrossRef]
- Kwak, S.H.; Choi, S.H.; Jung, H.S.; Cho, Y.M.; Lim, S.; Cho, N.H.; Kim, S.Y.; Park, K.S.; Jang, H.C. Clinical and Genetic Risk Factors for Type 2 Diabetes at Early or Late Post Partum After Gestational Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2013, 98, E744–E752. [Google Scholar] [CrossRef]
- Johnson, S.T.; Lynch, B.; Vallance, J.; Davenport, M.H.; Gardiner, P.A.; Butalia, S. Sedentary behavior, gestational diabetes mellitus, and type 2 diabetes risk: Where do we stand? Endocrine 2016, 52, 5–10. [Google Scholar] [CrossRef]
- D’Arcy, E.; Rayner, J.; Hodge, A.; Ross, L.; Schoenaker, D.A.M. The Role of Diet in the Prevention of Diabetes among Women with Prior Gestational Diabetes: A Systematic Review of Intervention and Observational Studies. J. Acad. Nutr. Diet. 2019, 120, 69–85.e7. [Google Scholar] [CrossRef]
- Ministry of Health Malaysia, Malaysian Endocrine & Metabolic Society, Perinatal Society of Malaysia, Family Medicine Specialists Association of Malaysia. Clinical Practice Guidelines Management of Type 2 Diabetes Mellitus, 6th ed.; Ministry of Health Malaysia: Putrajaya, Malaysia, 2020. [Google Scholar]
- Tobias, D.K.; Hu, F.B.; Chavarro, J.; Rosner, B.; Mozaffarian, D.; Zhang, C. Healthful Dietary Patterns and Type 2 Diabetes Mellitus Risk Among Women with a History of Gestational Diabetes Mellitus. Arch. Intern. Med. 2012, 172, 1566–1572. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Bulló, M.; Babio, N.; Martínez-González, M.; Ibarrola-Jurado, N.; Basora, J.; Estruch, R.; Covas, M.I.; Corella, D.; Arós, F.; et al. Reduction in the Incidence of Type 2 Diabetes with the Mediterranean Diet: Results of the PREDIMED-Reus Nutrition Intervention Randomized Trial. Diabetes Care 2010, 34, 14–19. [Google Scholar] [CrossRef]
- Norimah, A.K.; Safiah, M.; Jamal, K.; Haslinda, S.; Zuhaida, H.; Rohida, S.; Norazlin, S.; Poh, B.K.; Kandiah, M.; Zalilah, M.S.; et al. Food Consumption Patterns: Findings from the Malaysian Adult Nutrition Survey (MANS). Malays. J. Nutr. 2008, 14, 25–39. [Google Scholar]
- Mohamad Kasim, N.; Ahmad, M.H.; Azli Baharudin, S.; Naidu, B.M.; Chan, Y.Y.; Aris, T. Food Choices among Malaysian Adults: Findings from Malaysian Adults Nutrition Survey (MANS) 2003 and MANS 2014. Malays. J. Nutr. 2018, 24, 63–75. [Google Scholar]
- Zhang, A.; Sun, H.; Wang, X. Serum metabolomics as a novel diagnostic approach for disease: A systematic review. Anal. Bioanal. Chem. 2012, 404, 1239–1245. [Google Scholar] [CrossRef]
- Allalou, A.; Nalla, A.; Prentice, K.J.; Liu, Y.; Zhang, M.; Dai, F.F.; Ning, X.; Osborne, L.R.; Cox, B.J.; Gunderson, E.P.; et al. A Predictive Metabolic Signature for the Transition from Gestational Diabetes Mellitus to Type 2 Diabetes. Diabetes 2016, 65, 2529–2539. [Google Scholar] [CrossRef]
- Andersson-Hall, U.; Carlsson, N.-G.; Sandberg, A.-S.; Holmäng, A. Circulating Linoleic Acid is Associated with Improved Glucose Tolerance in Women after Gestational Diabetes. Nutrients 2018, 10, 1629. [Google Scholar] [CrossRef] [Green Version]
- Fugmann, M.; Uhl, O.; Hellmuth, C.; Hetterich, H.; Kammer, N.N.; Ferrari, U.; Parhofer, K.G.; Koletzko, B.; Seissler, J.; Lechner, A. Differences in the Serum Nonesterified Fatty Acid Profile of Young Women Associated with a Recent History of Gestational Diabetes and Overweight/Obesity. PLoS ONE 2015, 10, e0128001. [Google Scholar] [CrossRef]
- Ott, R.; Pawlow, X.; Weiß, A.; Hofelich, A.; Herbst, M.; Hummel, N.; Prehn, C.; Adamski, J.; Römisch-Margl, W.; Kastenmül-ler, G.; et al. Intergenerational Metabolomic Analysis of Mothers with a History of Gestational Diabetes Mellitus and Their Offspring. Int. J. Mol. Sci. 2020, 21, 9647. [Google Scholar] [CrossRef]
- Tobias, D.K.; Clish, C.; Mora, S.; Li, J.; Liang, L.; Hu, F.B.; Manson, J.E.; Zhang, C. Dietary Intakes and Circulating Concentrations of Branched-Chain Amino Acids in Relation to Incident Type 2 Diabetes Risk Among High-Risk Women with a History of Gestational Diabetes Mellitus. Clin. Chem. 2018, 64, 1203–1210. [Google Scholar] [CrossRef]
- Khan, S.R.; Mohan, H.; Liu, Y.; Batchuluun, B.; Gohil, H.; Al Rijjal, D.; Manialawy, Y.; Cox, B.J.; Gunderson, E.P.; Wheeler, M.B. The discovery of novel predictive biomarkers and early-stage pathophysiology for the transition from gestational diabetes to type 2 diabetes. Diabetologia 2019, 62, 687–703. [Google Scholar] [CrossRef]
- O’Gorman, A.; Brennan, L. The role of metabolomics in determination of new dietary biomarkers. Proc. Nutr. Soc. 2017, 76, 295–302. [Google Scholar] [CrossRef]
- Aday, L.A.; Cornelius, L.J. Deciding How Many Will Be in the Sample. In Designing and Conducting Health Surveys: A Com-prehensive Guide; Jossey-Bass: San Francisco, CA, USA, 2006. [Google Scholar]
- Institute for Public Health. National Health and Morbidity Survey 2014: Malaysian Adult Nutrition Survey (MANS). Volume 1: Methodology and General Findings; Institute for Public Health, National Institutes of Health, Ministry of Health Malaysia: Kuala Lumpur, Malaysia, 2014. [Google Scholar]
- Suzana, S.; Nik Shanita, S.; Zahara, A.M. Atlas of Food Exchanges and Portion Sizes, 3rd ed.; MDC Publishers: Kuala Lumpur, Malaysia, 2015. [Google Scholar]
- Yong, H.Y.; Shariff, Z.M.; Yusof, B.N.M.; Rejali, Z.; Bindels, J.; Tee, Y.Y.S.; Van Der Beek, E.M. Associations between the dietary patterns of pregnant Malaysian women and ethnicity, education, and early pregnancy waist circumference: A prospective cohort study. Nutr. Res. Pract. 2019, 13, 230–239. [Google Scholar] [CrossRef]
- Yong, H.Y.; Shariff, Z.M.; Yusof, B.N.M.; Rejali, Z.; Appannah, G.; Bindels, J.; Tee, Y.Y.S.; Van Der Beek, E.M. The association between dietary patterns before and in early pregnancy and the risk of gestational diabetes mellitus (GDM): Data from the Malaysian SECOST cohort. PLoS ONE 2020, 15, e0227246. [Google Scholar] [CrossRef]
- Shyam, S.; Khor, G.-L.; Ambak, R.; Mahadir, B.; Hasnan, M.; Ambu, S.; Chu, W.-L.; Aris, T. Association between dietary patterns and overweight risk among Malaysian adults: Evidence from nationally representative surveys. Public Health Nutr. 2019, 23, 319–328. [Google Scholar] [CrossRef]
- Fakhruddin, N.N.I.N.M.; Malaysia, U.K.; Shahar, S.; Rajikan, R.; Omar, M.A.; Din, N.C.; Razali, R.; Harith, S.; Mohamed, H.J.J.; Hakim, B.N.A.; et al. Identification of dietary patterns associated with characteristics of successful aging. Malays. J. Nutr. 2019, 25, 47–57. [Google Scholar] [CrossRef]
- Pérez-Rodrigo, C.; Gil, A.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G.; Aranceta-Bartrina, J. Clustering of Dietary Patterns, Lifestyles, and Overweight among Spanish Children and Adolescents in the ANIBES Study. Nutrients 2016, 8, 11. [Google Scholar] [CrossRef]
- Shadman, Z.; Poorsoltan, N.; Akhoundan, M.; Larijani, B.; Soleymanzadeh, M.; Zhand, C.A.; Rohani, Z.A.S.; Nikoo, M.K. Ramadan Major Dietary Patterns. Iran. Red Crescent Med. J. 2014, 16, e16801. [Google Scholar] [CrossRef]
- Ministry of Health Singapore. Energy and Nutrient Composition of Food. Available online: https://focos.hpb.gov.sg/eservices/ENCF/ (accessed on 2 February 2022).
- U.S. Department of Agriculture, Agricultural Research Service. Food Data Central. Available online: https://fdc.nal.usda.gov (accessed on 2 February 2022).
- Boyko, E.J.; Seelig, A.D.; Jacobson, I.G.; Hooper, T.I.; Smith, B.; Smith, T.C.; Crum-Cianflone, N.F. Sleep Characteristics, Mental Health, and Diabetes Risk: A Prospective Study of U.S. Military Service Members in the Millennium Cohort Study. Diabetes Care 2013, 36, 3154–3161. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Gillani, S.W.; Syed, S.A.; Sari, Y.O.; Sarriff, A.; Amin, A.; Baig, M. Perceived Stress Scale Psychometric Validation for Malaysian Diabetic Patients. Br. J. Pharm. Res. 2011, 1, 156–163. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.L.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Am. Coll. Sport. Med. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Global Adult Tobacco Survey Collaborative Group. Tobacco Questions for Surveys: A Subset of Key Questions from the Global Adult Tobacco Survey (GATS), 2nd ed.; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2011. [Google Scholar]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- World Health Organisation. Waist Circumference and Waist–Hip Ratio. Report of a WHO Expert Consultation, Geneva, Switzerland, 8–11 December 2008; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Bohannon, R.W.; Peolsson, A.; Massy-Westropp, N.; Desrosiers, J.; Bear-Lehman, J. Reference values for adult grip strength measured with a Jamar dynamometer: A descriptive meta-analysis. Physiotherapy 2006, 92, 11–15. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults [M1]. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Pauzi, F.A.; Sahathevan, S.; Khor, B.-H.; Narayanan, S.S.; Zakaria, N.F.; Abas, F.; Karupaiah, T.; Daud, Z.A.M. Exploring Metabolic Signature of Protein Energy Wasting in Hemodialysis Patients. Metabolites 2020, 10, 291. [Google Scholar] [CrossRef]
- Nik Mohd Fakhruddin, N.N.I.; Shahar, S.; Ismail, I.S.; Ahmad Azam, A.; Rajab, N.F. Urine Untargeted Metabolomic Profiling Is Associated with the Dietary Pattern of Succesful Aging among Malaysian Elderly. Nutrients 2020, 12, 2900. [Google Scholar] [CrossRef]
- Peré-Trepat, E.; Ross, A.B.; Martin, F.-P.; Rezzi, S.; Kochhar, S.; Hasselbalch, A.L.; Kyvik, K.O.; Sørensen, T.I. Chemometric strategies to assess metabonomic imprinting of food habits in epidemiological studies. Chemom. Intell. Lab. Syst. 2010, 104, 95–100. [Google Scholar] [CrossRef]
- Playdon, M.C.; Moore, S.C.; Derkach, A.; Reedy, J.; Subar, A.F.; Sampson, J.N.; Albanes, D.; Gu, F.; Kontto, J.; Lassale, C.; et al. Identifying biomarkers of dietary patterns by using metabolomics. Am. J. Clin. Nutr. 2016, 105, 450–465. [Google Scholar] [CrossRef]
- Chew, W.F.; Rokiah, P.; Chan, S.P.; Chee, W.S.S.; Lee, L.F.; Chan, Y.M. Prevalence of Glucose Intolerance, and Associated Antenatal and Historical Risk Factors among Malaysian Women with a History of Gestational Diabetes Mellitus. Singapore Med. J. 2012, 53, 814–820. [Google Scholar]
- Logakodie, S.; Azahadi, O.; Fuziah, P.; Norizzati, B.I.B.; Tan, S.F.; Zienna, Z.Z.R.; Norliza, M.; Noraini, J.; Hazlin, M.; Noraliza, M.Z.; et al. Gestational Diabetes Mellitus: The Prevalence, Associated Factors and Foe-to-Maternal Outcome of Women Attending Antenatal Care. Malays. Fam. Physician 2017, 12, 9–17. [Google Scholar]
- Fatin, A.A.B.; Alina, T.I. Proportion of Women with History of Gestational Diabetes Mellitus Who Performed an Oral Glucose Test at Six Weeks Postpartum in Johor Bahru with Abnormal Glucose Tolerance. Malays. Fam. Physician 2019, 14, 2–9. [Google Scholar]
- Guasch-Ferré, M.; Bhupathiraju, S.N.; Hu, F.B. Use of Metabolomics in Improving Assessment of Dietary Intake. Clin. Chem. 2018, 64, 82–98. [Google Scholar] [CrossRef]
- Shyam, S.; Arshad, F.; Ghani, R.A.; Wahab, A.N.; Safii, N.S.; Nisak, M.Y.B.; Chinna, K.; Kamaruddin, N.A. Low glycaemic index diets improve glucose tolerance and body weight in women with previous history of gestational diabetes: A six months randomized trial. Nutr. J. 2013, 12, 68. [Google Scholar] [CrossRef]
- Taylor, K.; Ferreira, D.L.S.; West, J.; Yang, T.; Caputo, M.; Lawlor, D.A. Differences in Pregnancy Metabolic Profiles and Their Determinants between White European and South Asian Women: Findings from the Born in Bradford Cohort. Metabolites 2019, 9, 190. [Google Scholar] [CrossRef]
- Brennan, L. Metabolomics in nutrition research: Current status and perspectives. Biochem. Soc. Trans. 2013, 41, 670–673. [Google Scholar] [CrossRef]
- LeVatte, M.; Keshteli, A.H.; Zarei, P.; Wishart, D.S. Applications of Metabolomics to Precision Nutrition. Lifestyle Genom. 2021, 15, 1–9. [Google Scholar] [CrossRef]
| Category | Oral Glucose Tolerance Test | HbA1c (%) | |
|---|---|---|---|
| Fasting Plasma Glucose (mmol/L) | 2-h Plasma Glucose (mmol/L) | ||
| Normal glucose tolerance (NGT) | <6.1 | <7.8 | <5.7 |
| Prediabetes, which includes: Impaired fasting glucose (IFG) Impaired glucose tolerance (IGT) | 6.1–6.9 | 7.8–11.0 | 5.7–<6.3 |
| Type 2 diabetes (T2D) | ≥7.0 | ≥11.1 | ≥6.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasbullah, F.Y.; Yusof, B.-N.M.; Ghani, R.A.; Daud, Z.’A.M.; Appannah, G.; Abas, F.; Shafie, N.H.; Khir, H.I.M.; Murphy, H.R. Dietary Patterns, Metabolomic Profile, and Nutritype Signatures Associated with Type 2 Diabetes in Women with Postgestational Diabetes Mellitus: MyNutritype Study Protocol. Metabolites 2022, 12, 843. https://doi.org/10.3390/metabo12090843
Hasbullah FY, Yusof B-NM, Ghani RA, Daud Z’AM, Appannah G, Abas F, Shafie NH, Khir HIM, Murphy HR. Dietary Patterns, Metabolomic Profile, and Nutritype Signatures Associated with Type 2 Diabetes in Women with Postgestational Diabetes Mellitus: MyNutritype Study Protocol. Metabolites. 2022; 12(9):843. https://doi.org/10.3390/metabo12090843
Chicago/Turabian StyleHasbullah, Farah Yasmin, Barakatun-Nisak Mohd Yusof, Rohana Abdul Ghani, Zulfitri ’Azuan Mat Daud, Geeta Appannah, Faridah Abas, Nurul Husna Shafie, Hannah Izzati Mohamed Khir, and Helen R. Murphy. 2022. "Dietary Patterns, Metabolomic Profile, and Nutritype Signatures Associated with Type 2 Diabetes in Women with Postgestational Diabetes Mellitus: MyNutritype Study Protocol" Metabolites 12, no. 9: 843. https://doi.org/10.3390/metabo12090843
APA StyleHasbullah, F. Y., Yusof, B.-N. M., Ghani, R. A., Daud, Z. ’A. M., Appannah, G., Abas, F., Shafie, N. H., Khir, H. I. M., & Murphy, H. R. (2022). Dietary Patterns, Metabolomic Profile, and Nutritype Signatures Associated with Type 2 Diabetes in Women with Postgestational Diabetes Mellitus: MyNutritype Study Protocol. Metabolites, 12(9), 843. https://doi.org/10.3390/metabo12090843









